Abstract
Objectives
To assess the appropriateness of empirical antimicrobial therapy for sepsis and septic shock and determine factors associated with patient treatment outcomes at a Vietnamese national hospital.
Methods
A cross-sectional study was conducted on 134 patients diagnosed with sepsis and/or septic shock at Thong-Nhat Hospital, Ho Chi Minh City, Vietnam, from January 2018 to June 2018. Appropriateness of antimicrobial therapy was defined as physician adherence to antimicrobial guidelines using the Sanford Guide to Antimicrobial Therapy and the Vietnam national guidelines. Bayesian model averaging technique was used to identify the related factors associated with patient treatment outcomes.
Results
The median age of patients was 70 years. Organisms were identified in 54.5% of cases and predominated by Escherichia coli and staphylococci. Appropriate empirical antimicrobial agents were initiated in 56.6% (n = 73) of all cases. Of these patients, 31 cases (42.5%) and 61 cases (83.6%) received the antimicrobials in accordance with recommendations related to dosage and route of administration, respectively, bringing the overall rate of appropriate empirical antimicrobial therapy down to 23.3%. Patients who progressed to septic shock, received inappropriate antimicrobial therapy and required ICU admission were more likely to suffer treatment failure.
Conclusions
The study findings suggest that clinicians should appropriately adhere to antimicrobial guidelines, especially in patients with septic shock and those who require ICU care, to improve treatment outcomes.
Introduction
Sepsis is a clinical syndrome with physiological and biochemical abnormalities caused by a dysregulated inflammatory response to infection.1 Sepsis and the inflammatory response that ensues is a major healthcare problem that can lead to multiple organ dysfunction syndrome and death.1 The condition affects millions of people worldwide each year and kills as many as 25% of patients or more.2 A retrospective analysis of an international database reported a global incidence of 437 per 100 000 person-years for sepsis in the 10 years from 1995 to 2015.3 Patients older than 65 years of age account for the majority of all episodes of sepsis; with an increasingly ageing population, it is likely that the incidence of sepsis will continue to increase in the future.1
In 2016, Surviving Sepsis Campaign released the international guidelines for the management of sepsis and septic shock supported by the European Society of Intensive Care Medicine (ESICM) and Society of Critical Care Medicine (SCCM).2 Similar to polytrauma, acute myocardial infarction or stroke, early identification and appropriate management in the initial hours after sepsis develops improves outcomes.2,4 The cornerstone of initial resuscitation is the rapid restoration of perfusion and the early administration of antibiotics.2,5 Empirical antibiotic therapy is targeted at the suspected organism(s) and site of infection prior to culture results and preferably administered within the first hour.2,5 Surviving Sepsis Campaign 2016 stated that the effective use of antibiotics is central to the optimization of outcome in this life-threatening condition.2 Inappropriate antibiotics prescribed in patients with sepsis and/or septic shock, however, were surprisingly common, ranging from 20% to 50%.6–9 The association between initial empirical antimicrobial therapy and treatment outcomes has been reported.6–8,10 Kumar et al. (2009)6 reported that survival may decrease as much as 5-fold for septic shock treated with an empirical regimen that fails to cover the offending pathogen. In contrast, some studies noted that the association between the appropriateness of empirical antimicrobial therapy and patient outcome was not statistically significant.11–13
In Vietnam, such equivalent data on antimicrobial therapy in sepsis and septic shock have been limited, no matter how important it is in terms of developing strategies for sepsis management. We conducted this study to assess the appropriateness of empirical antimicrobial therapy for sepsis and septic shock and determine factors associated with patient’s treatment outcomes at Thong-Nhat hospital, a Vietnamese national hospital. We hypothesize that appropriate choice of antimicrobial therapy and adherence to empirical guidelines are related to better patient outcomes. Therefore, the hospital should establish strategies that ensure the appropriateness of antimicrobial therapy to improve treatment outcomes in patients with sepsis and other infectious diseases as well.
Methods
Study design and data collection
This was a cross-sectional study in in-patients with sepsis and/or septic shock at Thong-Nhat Hospital, a national hospital in South Vietnam, from January 2018 to June 2018. The study cohort was restricted to episodes of care that involved those aged 18 years and over. Cases were identified as admissions with a diagnosis of sepsis and/or septic shock based on the definitions of ESICM/SCCM 2016 (SEPSIS-3).14 The patients who were not treated with antibiotics for at least 3 days and/or whose medical records could not be accessed were excluded from the study.
We reviewed the medical records of included patients and collected data for analysis, including baseline socio-demographics, causative pathogens, antimicrobial therapy and treatment outcomes.
Definitions
In the present study, we use the 2016 consensus definitions for sepsis and septic shock.14 Sepsis was defined as life-threatening organ dysfunction—characterized by an acute increase of at least two SOFA scores, caused by a dysregulated host response to infection. Septic shock was a subset of sepsis in which patients met the sepsis definition and needed vasopressor therapy to maintain a mean arterial pressure (MAP) of 65 mmHg or greater and serum lactate greater than 2 mmol/L in the absence of hypovolemia.
We assessed the appropriateness of antibiotic therapy for choice of antibiotic drug (antibiotic indication), dosage and route of administration. Criteria for assessing the appropriateness of indication were physician adherence to guideline-recommended antimicrobials from either Vietnam Ministry of Health15 or the mobile app-based Sanford Guide (accessed in September 2018).16 The appropriateness of dosage and route of administration was assessed based on three databases: Lexicomp Lexi-Drugs Multinational,17 Micromedex Drug Reference,18 and the Sanford Guide to Antimicrobial Therapy.19 Overall appropriate antibiotic therapy was defined as patients receiving appropriate antibiotic indication, dosage and route of administration.
The primary outcome of interest in our study was treatment success, as manifested by a cure or a remission documented in the medical records at the time of discharge from the hospital. Otherwise, the case was identified as a treatment failure.
Statistical analysis
Sample characteristics were described using mean and SD for continuous variables with normal distribution, median and IQR for other continuous variables, and proportions for categorical variables.
We examined factors associated with the treatment outcomes, either treatment success or treatment failure. Explanatory variables used in the analysis comprised socio-demographic variables (age, gender and department of treatment), clinical variables [initial estimated glomerular filtration rate (eGFR), site of infection, presence of septic shock and blood culture], and antimicrobial therapy-related variable (the appropriateness of the empirical antimicrobial regimen) as suggested by the literature. A χ2 test and/or Fisher’s exact test were employed to compare treatment outcomes in subgroups of patients. To select the most optimal model predicting the treatment outcomes, we performed the Bayesian model averaging (BMA) technique with the following variables: age, gender, initial eGFR, department of treatment, site of infection, presence of septic shock, blood culture and overall appropriateness of empirical antimicrobial therapy. The BMA technique is an extension of the usual Bayesian inference methods. The technique not only models parameter uncertainty through the prior distribution but also models uncertainty obtaining posterior parameter and model posteriors using Bayes’ theorem, allowing for direct model selection, combined estimation and prediction.20 All analyses were performed using R software. The level of statistical significance was specified at P < 0.05.
Ethics
This study's protocol was approved by the Institutional Review Board of the Thong-Nhat Hospital (Project Number: 39 IRB/QD-BVTN). All personal information of patients was kept confidentially. Because we used retrospective data retrieved from patient medical records based on the IRB permission, the patients' informed consent was not obtained.
Results
Demographic and descriptive data
In total, over a 6 month period, 134 patients met the study criteria and were included in our research. The median age of the study population was 70 years, with 49.3% male patients. Most patients had an eGFR below 60 mL/min/1.73 m2 at the time of diagnosis. Of 134 patients, 13.4% (n = 18) patients were admitted to the ICU, and 14.9% (n = 20) progressed to septic shock. The most dominant primary site of sepsis and septic shock was urinary tract infection. Pathogens were identified in 54.5% of cases (n = 73) and were isolated from the blood in 39.6% of cases (n = 53) (Table 1).
Table 1.
Demographic characteristics of the study population
| Characteristics | Frequency | Percentage |
|---|---|---|
| Age, median (IQR) | 70 (58–82) | |
| ≥65 years old | 88 | 65.7 |
| <65 years old | 46 | 34.3 |
| Gender | ||
| male | 66 | 49.3 |
| female | 68 | 50.7 |
| Initial eGFRa | ||
| ≥60 mL/min/1.73 m2 | 41 | 31.3 |
| <60 mL/min/1.73 m2 | 90 | 68.7 |
| Department of treatment | ||
| ICU | 18 | 13.4 |
| infectious diseases department | 70 | 52.2 |
| others | 46 | 34.4 |
| Presence of septic shock | ||
| no | 114 | 85.1 |
| yes | 20 | 14.9 |
| Site of infection | ||
| unknown | 30 | 22.4 |
| urinary | 38 | 28.4 |
| intra-abdominal | 26 | 19.4 |
| respiratory | 22 | 16.4 |
| skin and soft tissue | 16 | 11.9 |
| others | 2 | 1.5 |
| Positive cultureb | ||
| yes | 73 | 57.0 |
| no | 55 | 43.0 |
| Blood culturec | ||
| positive | 53 | 44.5 |
| negative | 66 | 5.5 |
| Number of organisms isolated from bloodc | ||
| 0 | 66 | 55.5 |
| 1 | 49 | 41.2 |
| ≥2 | 4 | 3.7 |
Three patients did not have laboratory tests related to serum creatinine, thus we could calculate eGFR values of only the 131 remaining patients.
The cultures (blood and other cultures) were only collected from 128 patients.
The blood cultures were only collected from 119 patients.
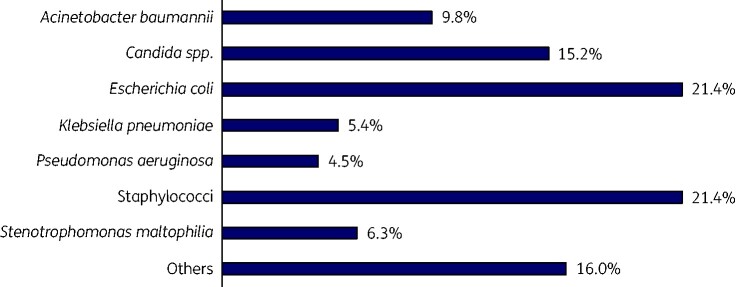
Gram-negative bacteria were most frequently identified in sepsis patients’ specimens (59.8%), while the incidence of fungal isolations was 15.2%. Escherichia coli and staphylococci predominated in cases of Gram-negative bacteraemia and Gram-positive bacteraemia, respectively (Figure 1).
Figure 1.
Suspected microbiological pathogens in 134 patients with sepsis and/or septic shock.
The median number of antibiotics prescribed in patients during the treatment course was 2 (IQR 2–3). The duration of antimicrobial therapy for sepsis was 11 days, with the longest of up to 33 days. β-Lactam and fluoroquinolone were the two most frequently prescribed antibiotic groups; particularly, 43.3% cases were treated with ceftriaxone and 38.1% with levofloxacin (Table 2).
Table 2.
Patterns of antimicrobial use in treatment of sepsis and/or septic shock
| Antibiotics | Frequency | Percentage cases prescribed |
|---|---|---|
| β-lactam | ||
| ceftriaxone | 58 | 43.3 |
| imipenem/cilastatin | 43 | 32.1 |
| meropenem | 25 | 18.7 |
| cefoperazone/sulbactam | 18 | 13.4 |
| piperacillin/tazobactam | 6 | 4.5 |
| othersa | 18 | 13.4 |
| Fluoroquinolone | ||
| levofloxacin | 51 | 38.1 |
| ciprofloxacin | 33 | 24.6 |
| othersb | 5 | 3.7 |
| Glycopeptide | ||
| teicoplanin | 21 | 15.7 |
| vancomycin | 12 | 9.0 |
| Aminoglycoside | ||
| netilmicin | 12 | 9.0 |
| amikacin | 5 | 3.7 |
| Polymyxin | ||
| colistin | 15 | 11.2 |
| Antifungal | ||
| fluconazole | 10 | 7.5 |
| 5-nitroimidazole | ||
| metronidazole | 9 | 6.7 |
| Othersc | 14 | 10.4 |
Other β-lactam: ampicillin/sulbactam, amoxicillin/clavulanic acid, cefaclor, cefuroxime, cefixime, ceftazidime, ertapenem and doripenem.
Other fluoroquinolone: moxifloxacin and norfloxacin.
Other classes: fosfomycin, linezolid, clindamycin, azithromycin and tigecycline.
Of the 134 included patients, 129 cases received antimicrobials as initial empirical therapy (5 remaining cases received targeted therapy based on the previous culture). The number of patients who received monotherapy, dual therapy and triple therapy was 33.3% (n = 43), 55.0% (n = 71) and 11.6% (n = 15), respectively. Regarding monotherapy, ceftriaxone was the most common antibiotic; regarding dual therapy, a β-lactam antibiotic was likely to be one of two agents, while the other antibiotic was generally a fluoroquinolone (Table S1, available as Supplementary data at JAC-AMR Online).
Appropriateness of empirical antimicrobial therapy and factors related to treatment outcome
Appropriate empirical antimicrobial agents were initiated in 56.6% (n = 73) of all sepsis cases and/or septic shock. Of these patients, 31 cases (42.5%) and 61 cases (83.6%) received the antimicrobials in accordance with recommendations related to dosage and route of administration, respectively, bringing the overall rate of appropriate empirical antimicrobial therapy down to 23.3% of all cases (Table 3). The most inappropriately prescribed antimicrobials included levofloxacin (15 cases), imipenem/cilastatin (9 cases), netilmicin (7 cases), metronidazole (5 cases) and teicoplanin (5 cases). These are agents that require adjustment for renal impairment or higher doses in sepsis treatment.
Table 3.
Appropriateness of empirical antimicrobial therapy
| Level of appropriateness | Frequency | Percentage |
|---|---|---|
| Antimicrobial agent | 73 | 56.6 |
| Dosage | 31 | 42.5 |
| Route of administration | 61 | 83.6 |
| Overall appropriateness | 30 | 23.3 |
Overall, the treatment success rate, which was defined as a cure or a remission documented in the medical records, was 80.6%. In patients with available laboratory results, we recorded that the patients whose WBC, C-reactive protein and procalcitonin values had returned to the normal ranges represented 65.2%, 16.7% and 60.3%, respectively.
Using the χ2 test and/or Fisher’s exact test, we discovered factors associated with treatment outcome in sepsis included age, department of treatment, presence of septic shock, site of infection and the overall appropriateness of initial empirical antimicrobial therapy (Table 4). Septic shock and ICU admission were significantly associated with a higher rate of treatment failure. Patients with sepsis that primarily developed from urinary tract infections had the highest chance of treatment success (92.1%), while the lowest rate was observed in those from respiratory infections (40.9%).
Table 4.
Factors related to treatment outcomesa
| Factors | Treatment success, n (%) | Treatment failure, n (%) | P value |
|---|---|---|---|
| Age | |||
| ≥65 years old | 66 (75.0) | 22 (25.0) | 0.042b |
| <65 years old | 42 (91.3) | 4 (8.7) | |
| Gender | |||
| male | 49 (74.2) | 17 (25.8) | 0.107 |
| female | 59 (86.8) | 9 (13.2) | |
| Initial eGFRc | |||
| ≥60 mL/min/1.73 m2 | 36 (87.8) | 5 (12.2) | 0.213 |
| <60 mL/min/1.73 m2 | 69 (76.7) | 21 (23.3) | |
| Department of treatment | |||
| ICU | 2 (11.1) | 16 (88.9) | <0.001b |
| infectious diseases department | 69 (98.6) | 1 (1.4) | |
| others | 37 (80.4) | 9 (19.6) | |
| Presence of septic shock | |||
| no | 103 (90.4) | 11 (9.6) | <0.001b |
| yes | 5 (25.0) | 15 (75.0) | |
| Site of infectiond | |||
| unknown | 27 (90.0) | 3 (10.0) | <0.001b |
| urinary | 35 (92.1) | 3 (7.9) | |
| intra-abdominal | 21 (80.8) | 5 (19.2) | |
| respiratory | 9 (40.9) | 13 (59.1) | |
| skin and soft tissue | 14 (87.5) | 2 (12.5) | |
| Blood culturee | |||
| positive | 42 (79.2) | 11 (20.8) | 0.739 |
| negative | 55 (83.3) | 11 (16.7) | |
| Appropriate empirical antimicrobial agentsf | |||
| yes | 58 (79.5) | 15 (20.5) | 0.874 |
| no | 46 (82.1) | 10 (17.9) | |
| Appropriate dosage (in 73 patients who received appropriate antibiotic agents) | |||
| yes | 30 (96.8) | 1 (3.2) | 0.004b |
| no | 28 (66.7) | 14 (33.3) | |
| Overallf | |||
| appropriate | 29 (96.7) | 1 (3.3) | 0.023b |
| inappropriate | 75 (75.8) | 24 (24.2) | |
Based on χ2 test and/or Fisher’s exact test.
Statistically significant at 95% CI.
Three patients did not have laboratory tests related to serum creatinine, thus we could calculate eGFR values of only the 131 remaining patients.
Two patients with sepsis that originated from other sites of infection were excluded from the analysis.
The blood cultures were only collected from 119 patients.
Empirical antimicrobial therapy were assessed in 129 patients whose microbiological tests were not available at the time of administering antibiotics; 5 other patients were prescribed targeted antimicrobial therapy.
The incidence of successful treatment was markedly increased in patients receiving appropriate initial empirical antibiotics compared with those who did not (96.7% versus 75.8%, P = 0.023). Of the 30 patients who received appropriate antimicrobial therapy, 29 had treatment success, and of the 99 patients who received inappropriate antimicrobial therapy, 75 had treatment success. In addition, the study showed that for patients initially treated with appropriate antibiotic agents, the rate of treatment success was also associated with the appropriateness of drug dosage.
Meanwhile, the incidence of treatment success did not significantly differ between genders, initial eGFR levels, and whether the blood culture was positive or not.
The BMA findings after adjusting for all variables showed that the consistent independently related factors for treatment outcome in sepsis patients were gender, department of treatment, presence of septic shock and overall appropriate of empirical antimicrobial therapy, with the posterior probability of 3.6%, 100.0%, 83.1% and 66.9%, respectively (Table 5).
Table 5.
The five best predictive models for sepsis/septic shock treatment outcome selected by the BMA technique
| Predictors | Probability (%) | Regression coefficient |
||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||
| Intercept | −18.6 | −1.3 | 19.2 | −18.6 | −2.5 | |
| Age | 0.0 | — | — | — | — | — |
| Gender | 3.6 | |||||
| male | — | — | — | −1.2 | — | |
| eGFR | 0.0 | |||||
| <60 mL/min/1.73 m2 | — | — | — | — | — | |
| Department of treatmenta | 100.0 | |||||
| infectious diseases department | 22.4 | 5.6 | 22.8 | 23.0 | 6.5 | |
| others | 20.3 | 3.3 | 20.4 | 20.4 | 3.8 | |
| Site of infectionb | 0.0 | |||||
| urinary tract | — | — | — | — | — | |
| intra-abdominal | — | — | — | — | — | |
| respiratory tract | — | — | — | — | — | |
| skin and soft tissue | — | — | — | — | — | |
| Presence of septic shock | 83.1 | |||||
| yes | −2.8 | −2.6 | — | — | — | |
| Blood culture | 0.0 | |||||
| positive | — | — | — | — | — | |
| Overall appropriate empirical antibiotic | 66.9 | |||||
| yes | 18.6 | — | 19.2 | 19.2 | — | |
| Number of variables | 3 | 2 | 2 | 3 | 1 | |
| BICc | −446.9 | −445.7 | −443.5 | −441.5 | −441.5 | |
| Posterior probability (%) | 53.6 | 29.6 | 9.8 | 3.6 | 3.5 | |
Reference group: ICU.
Reference group: unknown source.
Bayesian information criterion (BIC) or Schwarz information criterion is a criterion for model selection among a finite set of models; the model with the lowest BIC is preferred.
Discussion
This study found that β-lactam and fluoroquinolone were most frequently prescribed, with the predominance of ceftriaxone and levofloxacin (Table 2). Most sepsis patients were treated with an empirical regimen of one or two antimicrobial agents, whereas triple therapy is relatively low. Although broad-spectrum therapy was warranted to cover all possible causative pathogens,2,5 it does not mean that all sepsis patients need an empirical regimen with more than one antimicrobial agent, except for septic shock mentioned in important guidelines.2,5,15,16 Patients who received triple therapy presented risk factors for resistant organisms, anaerobic bacteria or fungi. For MRSA, teicoplanin was more likely to be prescribed than vancomycin in the present study. Although vancomycin is the drug of choice in treating MRSA infection, some areas favour using teicoplanin over vancomycin or if patients are not tolerant to vancomycin due to its availability, similar spectrum activity and efficacy and greater tolerance compared with vancomycin.21 For anaerobic and fungal infections, the antimicrobial agents were metronidazole and fluconazole, respectively. The median duration of antimicrobial therapy was 11 days, which was in line with recommendations from UpToDate and Surviving Sepsis Campaign 2016.2,5
Of the entire study population, 56.6% of patients received therapy in accordance with guidelines from either the Vietnam Ministry of Health or the Sanford Guide about the choice of agents. It is estimated that approximately 30%–50% of antibiotics used in hospitals are unnecessary or inappropriate.22 At the same time, in contrast to any other class of drugs, every antibiotic use has a potential public health consequence—inappropriate use may not harm only the individual patient, but contributes to societal harm by exerting an unnecessary selective pressure that may lead to antibiotic resistance among bacteria. Many previous studies also historically confirm a common situation of appropriate empirical antimicrobial therapy, such as Kumar et al. (2009),6 80.1%; Harbarth et al. (2003),8 77.0%; and Leibovici et al. (1997),9 68.5%. However, the rate of the appropriate use of antibiotics in our research is lower than that of these studies. This may be explained by the advances in the healthcare systems in Europe and North America where they were conducted. In addition, antibiotic resistance is globally increasing over time, making the choice of antibiotics more difficult. It may also be due to the different evaluation criteria between the studies. Accordingly, the empirical antimicrobial therapy was considered appropriate in their findings if microbiological culture results showed in vitro susceptibility to the prescribed empirical antibiotics. This approach ignores sepsis patients’ cases with unidentified pathogens accounting for a significant fraction of the sepsis.
Several pathways may explain our findings. The choice of empirical antimicrobial regimens in patients with sepsis and septic shock is complex, and several factors must be assessed at each medical centre and for each patient, while the emergency of initiating antimicrobial therapy made it more challenging to determine the appropriate regimen.2,5 Moreover, clinicians should consider the distribution of pathogens and their susceptibility patterns in the institution where the regimen is administered.2,5 Apart from choosing appropriate antibiotics, the selection of the optimal antibiotic regimens includes dosing, duration of therapy and route of administration. Our study revealed that the most common reasons for administering inappropriate dosage were related to adjustment for renal impairment and the necessity of higher doses of some agents in sepsis.
The incidence of treatment success was markedly increased in patients receiving appropriate initial empirical antibiotics compared with those who did not. In addition, the study showed that for patients initially treated with appropriate antibiotic choice, the rate of clinical success was associated with the appropriateness of antimicrobial dosage, which suggested that sepsis patients should be managed with optimal antimicrobial therapy of the right antibiotics and the right doses. After adjusting for all variables, the overall appropriateness of the initial antimicrobial therapy was consistently associated with the treatment outcome. These results are in line with previous studies that have linked the administration of appropriate empirical antimicrobial therapy to a higher rate of treatment success.6–8,10 In a 2010 systematic review and meta-analysis including 70 studies, inappropriate antimicrobial therapy was reported to be associated with a significant reduction in all-cause mortality.23 Surviving Sepsis Campaign 2016 also stated that selection of an optimal empirical antimicrobial regimen in sepsis and septic shock is one of the central determinants of outcome.2
On the other hand, some studies failed to demonstrate the beneficial effects of appropriate antimicrobial therapy in sepsis patients.11–13 Apart from the insufficient sample size, there are plausible scientific rationales to support that initial empirical antimicrobial therapy might not significantly affect survival in patients with sepsis and other severe infections. For one thing, sepsis may present a manifestation of an inflammatory and coagulation cascade triggered during the early stage of severe sepsis whose cause is likely multifactorial, not only the direct effects of invading microorganisms or their toxic products.24 Therefore, the syndrome may be expected to progress independently of the control of the underlying infection by initiating appropriate antimicrobial therapy. In addition, other interventions, including fluid resuscitation, source control, vasoactive medications, corticosteroids, blood products, immunoglobulins, blood purification, anticoagulants, mechanical ventilation and glucose control, are also important aspects of the treatment of infection.2,5 Antibiotic-resistant organisms, the severity of the underlying illness and comorbid condition of the patients also contribute to sepsis patients’ prognosis beyond the effect of antimicrobial therapy.
In the best model predicting treatment outcome selected by the BMA technique (Model 1), the fact that severe populations required ICU care and advanced towards septic shock may consistently link to poor outcomes. Patients who fit septic shock criteria tend to bear a greater than 40% hospital mortality rate, as mentioned in the third international consensus definitions for sepsis and septic shock (SEPSIS-3).14 Blood culture did not appear to be associated with treatment outcome, as previously reported, suggesting that prognosis is more closely related to the severity of sepsis than the severity of the underlying infection.25–27 Although the site of infection is an important predictor of prognosis1,28,29—informing the selection of antimicrobials capable of achieving therapeutic drug levels in infected tissue and fluid—our study did not confirm its significance in predicting outcome after adjusting other risk factors.
Limitations
The present study contains a few limitations. Firstly, we conducted an observational study that retrospectively collected data from patient medical records. Thus we did not state a causal effect of empirical antimicrobial therapy on outcome and did not consider all potential confounders that may affect the analysis. In addition, due to unavailable data, our study did not include the effect of time to antimicrobial administration, which is one of five crucial elements mentioned as a 1 h bundle in sepsis management according to the 2018 updated version of Surviving Sepsis Campaign.4 Secondly, we acknowledged that the present study included a modest sample size of sepsis patients and a small proportion of patients who required ICU care, which made our findings somehow not comprehensively reflect the effect of inappropriate antimicrobial therapy on the critically ill patients in the ICU setting. Further investigations with more data are warranted.
Conclusions
In summary, our data showed that inappropriate initial empirical antimicrobial therapy was of concern as it occurred in every four of five patients with sepsis and/or septic shock and was associated with adverse treatment outcomes. The empirical regimen with appropriate agents and dosage is one of the key elements of successful treatment. The study findings suggest that it is crucial for physicians to appropriately adhere to antimicrobial guidelines, particularly in patients who progressed to septic shock and were admitted to ICU, to improve treatment outcomes. Further studies investigating sepsis in specific settings need to be implemented to comprehensively assess antimicrobial therapy and its impacts on outcome.
Supplementary Material
Acknowledgements
We wish to thank clinical pharmacists in the Department of Pharmacy, Thong-Nhat Hospital and Ms Hao Quan Nhu Nguyen for their contribution to the collection of the data presented in this paper.
Funding
This study was conducted as part of our routine work.
Transparency declarations
None to declare.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. UpToDate. Sepsis Syndromes in Adults: Epidemiology, Definitions, Clinical Presentation, Diagnosis, and Prognosis. https://www.uptodate.com/contents/sepsis-syndromes-in-adults-epidemiology-definitions-clinical-presentation-diagnosis-and-prognosis.
- 2. Rhodes A, Evans LE, Alhazzani W. et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43: 304–77. [DOI] [PubMed] [Google Scholar]
- 3. Fleischman C, Scherag A, Adhikari NK. et al. Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med 2016; 193: 259–72. [DOI] [PubMed] [Google Scholar]
- 4. Levy MM, Evans LE, Rhodes A.. The Surviving Sepsis Campaign bundle: 2018 update. Crit Care Med 2018; 46: 997–1000. [DOI] [PubMed] [Google Scholar]
- 5. UpToDate. Evaluation and Management of Suspected Sepsis and Septic Shock in Adults. https://www.uptodate.com/contents/evaluation-and-management-of-suspected-sepsis-and-septic-shock-in-adults.
- 6. Kumar A, Ellis P, Arabi Y. et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009; 136: 1237–48. [DOI] [PubMed] [Google Scholar]
- 7. Kang CI, Kim SH, Park WB. et al. Bloodstream infections caused by antibiotic-resistant Gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 2005; 49: 760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harbarth S, Garbino J, Pugin J. et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med 2003; 115: 529–35. [DOI] [PubMed] [Google Scholar]
- 9. Leibovici L, Paul M, Poznanski O. et al. Monotherapy versus β-lactam–aminoglycoside combination treatment for Gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother 1997; 41: 1127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gradel KO, Jensen US, Schønheyder HC. et al. Impact of appropriate empirical antibiotic treatment on recurrence and mortality in patients with bacteraemia: a population-based cohort study. BMC Infect Dis 2017; 17: 122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zaragoza R, Artero A, Camarena JJ. et al. The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin Microbiol Infect 2003; 9: 412–8. [DOI] [PubMed] [Google Scholar]
- 12. Kim SH, Park WB, Lee CS. et al. Outcome of inappropriate empirical antibiotic therapy in patients with Staphylococcus aureus bacteraemia: analytical strategy using propensity scores. Clin Microbiol Infect 2006; 12: 13–21. [DOI] [PubMed] [Google Scholar]
- 13. Osih RB, McGregor JC, Rich SE. et al. Impact of empiric antibiotic therapy on outcomes in patients with Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 2007; 51: 839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singer M, Deutschman CS, Seymour C. et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vietnam Ministry of Health. Sepsis. In: Guidelines for Antibiotic Use [in Vietnamese]. Hanoi Medical Publishing House, 2015; 117–23.
- 16. Sanford Guide. Sepsis, adult. In: The Sanford Guide to Antimicrobial Therapy [mobile app]. Antimicrobial Therapy Inc., 2018.
- 17. Lexi-Drugs Multinational. Drugs. In: Lexicomp [mobile app]. Wolters Kluwer, 2019.
- 18. Drug name. In: IBM Micromedex Drug Ref [mobile app]. IBM Corporation, 2019.
- 19. Anti-infectives. In: The Sanford Guide to Antimicrobial Therapy [mobile app]. Antimicrobial Therapy Inc., 2019.
- 20. Fragoso TM, Bertoli W, Louzada F.. Bayesian model averaging: a systematic review and conceptual classification. Int Stat Rev 2017; 86: 1–28. [Google Scholar]
- 21. UpToDate. Methicillin-Resistant Staphylococcus aureus (MRSA) in Adults: treatment of Bacteremia. https://www.uptodate.com/contents/methicillin-resistant-staphylococcus-aureus-mrsa-in-adults-treatment-of-bacteremia.
- 22. CDC. Antibiotic Prescribing and Use in Hospitals and Long-Term Care. https://www.cdc.gov/antibiotic-use/healthcare/index.html.
- 23. Paul M, Shani V, Muchtar E. et al. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54: 4851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. UpToDate. Pathophysiology of Sepsis. https://www.uptodate.com/contents/pathophysiology-of-sepsis.
- 25. Zahar JR, Timsit JF, Garrouste-Orgeas M. et al. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med 2011; 39: 1886–95. [DOI] [PubMed] [Google Scholar]
- 26. Sigakis MJG, Jewell E, Maile MD. et al. Culture-negative and culture-positive sepsis: a comparison of characteristics and outcomes. Anesth Analg 2019; 129: 1300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brun-Buisson C, Doyon F, Carlet J.. Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am J Respir Crit Care Med 1996; 154: 617–24. [DOI] [PubMed] [Google Scholar]
- 28. Krieger JN, Kaiser DL, Wenzel RP.. Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis 1983; 148: 57–62. [DOI] [PubMed] [Google Scholar]
- 29. Leligdowicz A, Dodek PM, Norena M. et al. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med 2014; 189: 1204–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.