Abstract
Antibiotics are the primary drugs for combating Neisseria gonorrhoeae infections, but with evolving antibiotic resistance of this bacterium, new druggable molecules are needed to stem the tide of this impending public health crisis. Propolis has long been recognized for its antimicrobial properties, being composed of secondary metabolites with antibacterial potential. We herein describe the evaluation of a Jamaican multifloral propolis for antibacterial activity against N. gonorrhoeae. The bioassay-guided evaluation of the ethyl acetate extract yielded (+)-medicarpin (1), whose final structure was elucidated based on spectral analysis and comparison with the known metabolites. Compound (1) selectively inhibited N. gonorrhoeae with a minimum inhibitory concentration value of 0.25 mg/mL, showing an additive effect against N. gonorrhoeae when combined with vancomycin.
Introduction
Neisseria gonorrhoeae (GC) is a Gram-negative bacterium that causes the third most reported sexually transmitted infection, with 800 000 cases reported in the United States and over 87 million cases estimated worldwide.1 Due to its frequency of infection, ability to evade the host immune system, and increasing resistance to antibiotics, N. gonorrhoeae continues to be a public health problem. The increased number of GC infections is due in part to its increased antibiotic resistance. There is a growing concern regarding its antibiotic resistance, especially in underdeveloped countries that require expensive drugs, such as ceftriaxone and azithromycin, for effective treatment.2 If left untreated, gonorrhea can cause pelvic inflammatory disease and sequelae such as chronic pelvic pain, infertility, and ectopic pregnancy. To combat this impending public health crisis, more effective antimicrobials are needed to effectively inhibit the proliferation of N. gonorrhoeae. Natural products continue to be the single most encouraging model for the discovery and development of new therapeutics.3−5 Over the past 39 years, natural products or natural product-derived entities have accounted for 48% of the new antibacterial drugs approved for use in the Unites States.3
Propolis is well recognized for its natural antimicrobial properties6−8 and, more recently, propolis has been used to treat infections and potentiate wound healing.9 Propolis is a resin composed of beeswax, saliva, and plant exudates collected by bees (Apis mellifera) that serve as a waterproofing layer to protect the hive from microbial invasion and oxidative damage.10 The phytochemical content of propolis reflects the metabolite profile of the plants from which the bees foraged.11 Studies have shown significant chemogeographical variations in the composition and antibacterial properties of propolis from tropical regions. Central and South American collections harbor prenylated benzophenones, which are absent in North American, European, and Asian propolis.12−14 There are over 300 known compounds from various collections of propolis.15 Terpenes, polyphenolic compounds, flavonoids, and their derivatives isolated from various propolis collections have displayed a vast array of biological activities ranging from antibacterial to antitumor, antiviral, and immunomodulatory activity.16−18 There have been various studies addressing the effectiveness of propolis against commonly encountered bacterial strains such as Escherichia coli, Salmonellaenterica, Staphylococcus aureus, Aspergillus niger, and Candida albicans,9.19 However, there are currently no data on the effectiveness of Caribbean propolis against N. gonorrhoeae. In our continuing efforts to expand the range of metabolites active against N. gonorrhoeae, we have investigated a Jamaican collection of multiflora propolis against E. coli, S. aureus, and N. gonorrhoeae. The initial bioautography screens of crude extracts followed by a bioassay-guided separation of the ethyl acetate extract resulted in the isolation of (+)-medicarpin (1). We herein describe the isolation and biological evaluation of (+)-medicarpin (1) against N. gonorrhoeae, E. coli, and S. aureus.
Results and Discussion
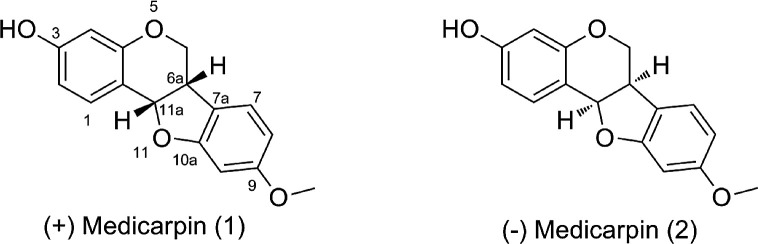
The ethyl acetate extract displayed antibacterial activity in preliminary bioautography screening. The bioassay-guided fractionation of the ethyl acetate extract resulted in the isolation of the known pterocarpan (+)-medicarpin (1). Compound 1 displayed a [M + H]+ peak of 271.09753 in the high-resolution ESI TOF consistent with molecular formula C16H14O4. The petrocarpan core was established based on the characteristic four contiguous protons: 3.56 ppm (m, H-6β), 4.22 ppm (dd, 10.8, 4.8, H-6α), 3.51 ppm (m, H-6a), and 5.50 ppm (d, 6.7, H-11a) and 13C NMR signals at 66.5 for C6, 40.0 for C6a, and 78.9 for C11a. Additionally, analysis of the 2D spectra (heteronuclear single-quantum coherence, heteronuclear multiple bond correlation, correlation spectroscopy, and total correlation spectroscopy) enabled the complete assignment of 1 (Table 1). Finally, the comparison of the experimental and literature values for (+)-medicarpin (1) is almost identical with the only noticeable variations occurring at positions 6a and 11a of (−)-medicarpin (2) (Figure 1).20
Table 1. Comparison of the 13C NMR Data of Medicarpin (1) with Synthetic (+)-Medicarpin (1) and (−)-Medicarpin (2).
| (+)-medicarpin (1) | synthetic (+)-medicarpin (1) | (−)-medicarpin (2) | |
|---|---|---|---|
| 1a | 112.2 | 112.8 | 112.8 |
| 1 | 132.2 | 132.4 | 133.1 |
| 2 | 106.4 | 106.7 | 107.3 |
| 3 | 157.5 | 157.3 | 160.0 |
| 4 | 103.7 | 103.9 | 104.4 |
| 4a | 156.6 | 156.8 | 158.8 |
| 6 | 66.5 | 66.7 | 67.5 |
| 6a | 39.5 | 39.7 | 40.8 |
| 7a | 119.2 | 119.3 | 120.8 |
| 7 | 124.8 | 125.0 | 125.9 |
| 8 | 110.0 | 110.0 | 110.7 |
| 9 | 160.6 | 160.8 | 162.0 |
| 10 | 96.9 | 97.1 | 97.5 |
| 10a | 161.1 | 161.3 | 162.5 |
| 11a | 78.7 | 78.8 | 80.0 |
| 12 | 55.9 | 55.7 | 55.9 |
Figure 1.
Structures of (+)-medicarpin (1) and (−)-medicarpin (2).
(+)-Medicarpin (1) has been isolated from several sources including Machaerium aristulatum,21Sophora japonica,22 and Brazilian red propolis.23 It inhibits the proliferation of osteoclasts, indicating some potential as a treatment for estrogen-sensitive osteoporosis.24 It also displays a wide range of antimicrobial activity and is responsible for much of the antifungal activity observed in red Brazilian propolis.25
Antibacterial Activity of Jamaican Propolis Extracts
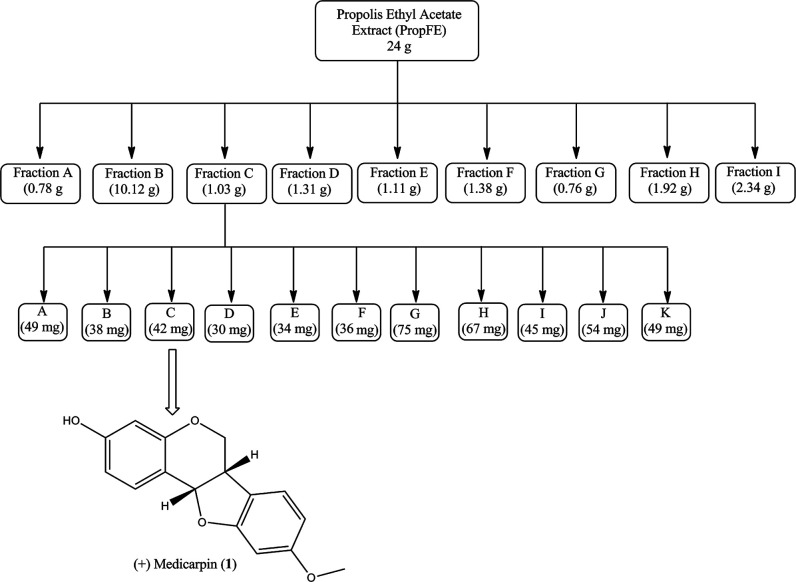
In this study, the antibacterial activity of ethyl acetate extracts from the propolis was determined by a twofold minimum inhibitory concentration (MIC) spot dilution assay. The extract was separated into several fractions (A–I; Figure 2 and Table 2) that were assayed for antibacterial activity against S. aureus, E. coli, and N. gonorrhoeae. Fractions A and B did not demonstrate antibacterial activity against any of the tested strains. However, S. aureus was inhibited by fractions F and G at 12.5 and 25 mg/mL, respectively (Figure 2 and Table 2). Consequently, other studies have shown that S. aureus was more susceptible (0.032–1.2 mg/mL) to propolis extracts from a variety of geographical locations.12,26 It has been suggested that geographical locations may have an enormous impact on the composition of propolis, which in turn may affect its antibacterial activity.12 Unlike S. aureus, the propolis extracts (25 mg/mL) did not inhibit the growth of E. coli. However, N. gonorrhoeae was the only strain tested that was susceptible to fractions C–I over a broad concentration range (0.5–1.79 mg/mL) (Table 2). The lowest level of susceptibility determined was to fraction F for N. gonorrhoeae and S. aureus at 0.3 and 12.5 mg/mL, respectively. This indicates that N. gonorrhoeae is significantly more susceptible to the propolis extract than S. aureus and E. coli (∼42-fold and 83-fold, respectively). We did not test E. coli above 25 mg/mL; therefore, it is possible that E. coli may be susceptible to the extract at a higher concentration. Therefore, N. gonorrhoeae is significantly more susceptible to this multifloral Jamaican propolis sample than S. aureus and E. coli.
Figure 2.
Schematic representation of the normal phase chromatographic isolation of (+)-medicarpin (1).
Table 2. Antibacterial Activity of Ethyl Acetate Extracted Fractionsa.
| fractions | MIC (mg/mL)b |
||
|---|---|---|---|
| N. gonorrhoeae | E. coli | S. aureus | |
| A | – | – | – |
| B | – | – | – |
| C | 0.50 | – | – |
| D | 0.78 | – | – |
| E | 0.92 | – | – |
| F | 0.30 | – | 12.5 |
| G | 0.56 | – | 25 |
| H | 0.85 | – | – |
| I | 1.79 | – | – |
Notes: −, not inhibited at the highest tested concentration (25 mg/mL).
Values were the averages of three or more readings.
Based on the initial selectivity, sample size, and initial metabolite profile, we prioritized fraction C for further purification to identify the compound responsible for the observed antibacterial activity. As a result, (+)-medicarpin (1) was isolated and the MIC was determined for S. aureus, E. coli, and N. gonorrhoeae in a separate assay (Table 3). Compound 1 does not display antibacterial activity against S. aureus and E. coli at the highest concentration tested (5 mg/mL) but does selectively inhibit N. gonorrhoeae at 0.25 mg/mL (Table 3).
Table 3. Antibacterial Activity of the Purified Metabolite, (+)-Medicarpin (1), against Gram-Positive and Gram-Negative Bacteriaa.
| purified compound | MIC (mg/mL)b |
||
|---|---|---|---|
| N. gonorrhoeae | E. coli | S. aureus | |
| (+)-medicarpin (1) | 0.25 | – | – |
Notes: −, not inhibited at the highest tested concentration (5 mg/mL).
Values were the averages of three or more readings and a standard error of the mean of 0.02.
Evaluating the Antibacterial Activity of (+)-Medicarpin (1) Combined with Vancomycin
The increasing resistance to single-dose and multidrug therapeutics suggests that bacteria are becoming a major public health issue. Gram-negative bacteria present possibly an even larger public health problem because many antibiotics are unable to penetrate their outer membrane. The use of natural products combined with conventional antibiotics may help increase the number of treatment options and improve efficacy. To this end, a checkerboard assay was performed to evaluate whether (+)-medicarpin (1) would reduce the concentration of vancomycin, glycopeptide antibiotic not normally used to treat Gram-negative bacteria, required to inhibit the growth of N. gonorrhoeae. A fractional inhibitory concentration index (FICI) was determined for (+)-medicarpin (1) that ranged from 0.7 to 1 mg/mL for 4 and 24 h incubation periods, respectively. As a result, (+)-medicarpin (1) used in combination with vancomycin was additive in inhibiting N. gonorrhoeae at low concentrations. Although the use of 1 and vancomycin were only additive (FICI of 0.5–4) against N. gonorrhoeae, the FICI value of 0.7 was close to the FICI synergistic value of <0.5, which suggests a modest impact on the efficacy of vancomycin. Wink and co-workers demonstrated that propolis collected from different European countries acted in synergy with vancomycin against several Gram-positive bacteria.12 In the previous study, Helicobacter pylori were the only Gram-negative bacteria tested using propolis from European countries in combination with levofloxacin that showed susceptibility. This is not surprising given that several studies have indicated that propolis is more effective against Gram-positive than Gram-negative bacteria.27 However, we demonstrate that N. gonorrhoeae is significantly more susceptible than S. aureus and E. coli, and when vancomycin is used with propolis, the MIC of vancomycin required to inhibit N. gonorrhoeae was reduced. Although the FICI values of combining both compounds were not synergistic, there appears to be an impact on the efficacy of vancomycin against N. gonorrhoeae. The additive activity observed with vancomycin is promising and could potentially lead to improved drug combinations to combat the resistance being observed in the treatment of gonorrhea worldwide.
Materials and Methods
General Experimental Procedure
All one-dimensional and two-dimensional NMR spectra were recorded in CDCl3 on a Bruker AVANCE III NMR spectrometer at 400 MHz for 1H and 100 MHz for 13C. LCMS was performed on a reversed-phase analytical column (4.6 × 250 mm2, 5 μm) using a photodiode array detector and with an electrospray single quadrupole mass spectrometer. High-resolution mass measurements were obtained on an Agilent 6230 ESI-TOF mass spectrometer. The samples were run in positive-mode ionization with a capillary voltage of 4000 V. The drying gas (nitrogen) temperature was 325 °C delivered at 10 L/min, and the fragmentation voltage was set to 150 eV. Optical rotation was determined on a Jasco DIP 370 polarimeter. MPLC separation was performed on a Reveleris system equipped with UV and ELSD detectors. All solvents were of HPLC grade with 0.1% TFA or ACS grade.
Collection
Propolis was collected from a commercial bee farm in July 2017 from Buff Bay, Portland Jamaica (GPS coordinates 18° 13′48″ N; 76° 39′53″ W). The samples were stored at −18 °C in an airtight container until analysis. A voucher specimen (JP17A) is preserved in the Department of Chemistry and Biochemistry at North Carolina Central University, Durham.
Organic Extraction
The propolis sample (750 g) was sequentially extracted with hexane (3 × 2 L), EtOAc (3 × 2 L), and MeOH (3 × 2 L). Evaporation of the solvents yielded three crude extracts hexane (100 g), EtOAc (55 g), and MeOH (60 g). A portion of the EtOAc extract (24 g) was adsorbed onto the silica gel and subjected to flash chromatography using a EtOAc–hexane gradient (0%–100%), and nine (9) major fractions (A–I) were collected. Further purification of fraction C via normal phase chromatography eluting with mixtures of EtOAc–hexane yielded compound 1.
Bioautography Assay
Bioautography assay was adapted from Galindo-Cuspinera and Rankin.28 Briefly, thin-layer chromatograms (TLCs) were developed at a solvent ratio of 80/20 or 50/50 (Hex/EtOAc). The dried TLC plates were placed facing down on the agar plates inoculated with 3 × 108 cfu/mL of gonococci for 30 min before being incubated overnight at 5% CO2, 37 °C. After 24 h, the TLC plates were removed and the agar plates were visualized under a dissecting scope for clear zones, which indicated bacterial inhibition.
Antibacterial Susceptibility Assay
The MIC for N. gonorrhoeae was determined using a broth dilution method described by the National Committee for Clinical Laboratory Standards (NCCLS). Briefly, the NCCLS method was modified for growth of N. gonorrhoeae as follows: the cells were grown overnight on gonococcal agar plates containing Kellogg’s supplements29 at 37 °C, 5% CO2 for 18 h. After overnight growth, the cells were inoculated in gonococcal broth (GCB) containing Kellogg’s supplements and then diluted to a 0.5 McFarland turbidity standard of 1.5 × 108 cfu/mL. The cell suspension (90 μL) was seeded into a 96-well plate containing either the purified secondary metabolite medicarpin (1) or enriched fractions isolated from the propolis sample, which was serially diluted 2-fold (0.002–25 μg/mL). After 18 h of incubation, 2 μL from each well was spotted on GC agar plates containing Kellogg’s supplements and then incubated overnight at 37 °C, 5% CO2 for 18 h. The MIC was recorded as the lowest concentration of the propolis extract that inhibited bacterial growth. Statistical significance was calculated based on replicates from three or more independent biological assays.
Checkerboard Dilution Assay
The synergistic effect between the purified propolis compound (+)-medicarpin (1) and vancomycin (Van), a glycopeptide antibiotic not usually effective against Gram-negative bacteria, was determined using a checkerboard dilution assay (Table 4). Cell cultures were prepared as described above for the antibacterial susceptibility assay. A 96-well plate containing gonococcal cells at a 0.5 McFarland turbidity standard of 1.5 × 108 cfu/mL supplemented with 2-fold serially diluted (+)-medicarpin (1) and vancomycin. All assays were incubated at 37 °C, 5% CO2 for 18 h and spotted on GC agar plates. The FICI was determined using the following equation: FICI = (MIC of compound 1 and Van in combination/MIC of compound 1 alone) + (MIC of Van and compound 1 in combination/Van alone). Synergistic effects were grouped as follows: antagonistic (>4), indifferent (≥1–4.0), additive (≥0.5–1), and synergistic (≤0.5) based on the FICI.12
Table 4. Antibacterial Activity of (+)-Medicarpin (1) and Vancomycin against N. gonorrhoeae.
| strain | incubation time (h) | agent | MIC (mg/mL)a |
||||
|---|---|---|---|---|---|---|---|
| alone | combined | FIC | FICI | interpretation | |||
| N. gonorrhoeae | 4 | (+)Med | 0.750 | 0.313 | 0.4 | 0.7 | additive |
| Van | 0.060 | 0.021 | 0.3 | ||||
| 24 | (+)Med | 0.305 | 0.156 | 0.5 | 1 | additive | |
| Van | 0.033 | 0.018 | 0.5 | ||||
Values were the averages of three or more readings.
Acknowledgments
This project was supported by funds from the North Carolina Central University’s Office of the Academic Affairs, Innovation Initiative Award. The multifloral propolis sample was provided by Earl G. Christian, Buff Bay Portland, Jamaica. The art work was rendered by Daniel Christian. The authors acknowledge the contributions of Juliet Udozimma and Julianna Acosta to this project. They are grateful to Dr. Lee-Hsuing, UNC Chapel Hill, for providing a reference sample of synthetic (+)-medicarpin (1).
Author Contributions
The manuscript was written through contributions of all authors. All authors have revised and approved the final version of the manuscript.
The authors declare no competing financial interest.
References
- Turner J. M.; Connolly K. L.; Aberman K. E.; Fonseca II J. C.; Singh A.; Jerse A. E.; Nicholas R. A.; Davies C. Molecular Features of Cephalosporins Important for Activity against Antimicrobial-Resistant Neisseria gonorrhoeae. ACS Infect. Dis. 2021, 7, 293–308. 10.1021/acsinfecdis.0c00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr S. S.; Barbee L.; Workowski K. A.; Bachmann L. H.; Pham C.; Schlanger K.; Torrone E.; Weinstock H.; Kersh E. N.; Thorpe P. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1911–1916. 10.15585/mmwr.mm6950a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Cragg G. M.; Newman D. J. Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G. M.; Newman D. J.; Snader K. M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- Castaldo S.; Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. 10.1016/S0367-326X(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Pajor M.; Worobo R. W.; Milewski S.; Szweda P. The Antimicrobial Potential of Bacteria Isolated from Honey Samples Produced in the Apiaries Located in Pomeranian Voivodeship in Northern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2002 10.3390/ijerph15092002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecka K.; Kus P. M.; Worobo R. W.; Szweda P. Study of the Anti-Staphylococcal Potential of Honeys Produced in Northern Poland. Molecules 2018, 23, 260 10.3390/molecules23020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Waili N. Mixing two different propolis samples potentiates their antimicrobial activity and wound healing property: A novel approach in wound healing and infection. Vet. World 2018, 11, 1188–1195. 10.14202/vetworld.2018.1188-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocot J.; Kielczykowska M.; Luchowska-Kocot D.; Kurzepa J.; Musik I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell. Longevity 2018, 2018, 1–29. 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharsany K.; Viljoen A.; Leonard C.; Van Vuuren S. The new buzz: Investigating the antimicrobial interactions between bioactive compounds found in South African propolis. J. Ethnopharmacol. 2019, 238, 111867 10.1016/j.jep.2019.111867. [DOI] [PubMed] [Google Scholar]
- Al-Ani I.; Zimmermann S.; Reichling J.; Wink M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2 10.3390/medicines5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viuda-Martos M.; Ruiz-Navajas Y.; Fernandez-Lopez J.; Perez-Alvarez J. A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Omar R. M.; Igoli J.; Gray A. I.; Ebiloma G. U.; Clements C.; Fearnley J.; Ebel R. A.; Zhang T.; De Koning H. P.; Watson D. G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma Brucei. Phytochem. Anal. 2016, 27, 107–115. 10.1002/pca.2605. [DOI] [PubMed] [Google Scholar]
- Bankova V.; Popova M.; Trusheva B. The phytochemistry of the honeybee. Phytochemistry 2018, 155, 1–11. 10.1016/j.phytochem.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Governa P.; Cusi M. G.; Borgonetti V.; Sforcin J. M.; Terrosi C.; Baini G.; Miraldi E.; Biagi M. Beyond the Biological Effect of a Chemically Characterized Poplar Propolis: Antibacterial and Antiviral Activity and Comparison with Flurbiprofen in Cytokines Release by LPS-Stimulated Human Mononuclear Cells. Biomedicines 2019, 7, 73 10.3390/biomedicines7040073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho F. M. A.; Schneider J. K.; De Jesus C. V. F.; De Andrade L. N.; Amaral R. G.; David J. M.; Krause L. C.; Severino P.; Soares C. M. F.; Bastos E. C.; Padilha F. F.; Gomes S. V. F.; Capasso R.; Santini A.; Souto E. B.; De Albuquerque-junior R. L. C. Brazilian Red Propolis: Extracts Production, Physicochemical Characterization, and Cytotoxicity Profile for Antitumor Activity. Biomolecules 2020, 10, 726 10.3390/biom10050726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Beltran N. P.; Balderrama-Carmona A. P.; Umsza-Guez M. A.; Souza Machado B. A. Antiviral effects of Brazilian green and red propolis extracts on Enterovirus surrogates. Environ. Sci. Pollut. Res. Int. 2020, 27, 28510–28517. 10.1007/s11356-019-07458-z. [DOI] [PubMed] [Google Scholar]
- Okinczyc P.; Paluch E.; Franiczek R.; Widelski J.; Wojtanowski K. K.; Mroczek T.; Krzyzanowska B.; Skalicka-Wozniak K.; Sroka Z. Antimicrobial activity of Apis mellifera L. and Trigona sp. propolis from Nepal and its phytochemical analysis. Biomed. Pharmacother. 2020, 129, 110435 10.1016/j.biopha.2020.110435. [DOI] [PubMed] [Google Scholar]
- Yang X.; Zhao Y.; Hsieh M. T.; Xin G.; Wu R. T.; Hsu P. L.; Horng L. Y.; Sung H. C.; Cheng C. H.; Lee K. H. Total Synthesis of (+)-Medicarpin. J. Nat. Prod. 2017, 80, 3284–3288. 10.1021/acs.jnatprod.7b00741. [DOI] [PubMed] [Google Scholar]
- Seo E. K.; Kim N. C.; Mi Q.; Chai H.; Wall M. E.; Wani M. C.; Navarro H. A.; Burgess J. P.; Graham J. G.; Cabieses F.; Tan G. T.; Farnsworth N. R.; Pezzuto J. M.; Kinghorn A. D. Macharistol, a new cytotoxic cinnamylphenol from the stems of Machaerium aristulatum. J. Nat. Prod. 2001, 64, 1483–1485. 10.1021/np0103158. [DOI] [PubMed] [Google Scholar]
- VanEtten H. D.; Matthews P. S.; Mercer E. H. (+)-Maackiain and (+)-Medicarpin as phytoalexins in Sophora japonica and identification of the (−) isomers by biotransformation. Phytochemistry 1983, 22, 2291–2295. 10.1016/S0031-9422(00)80164-8. [DOI] [Google Scholar]
- Rufatto L. C.; Luchtenberg P.; Garcia C.; Thomassigny C.; Bouttier S.; Henriques J. A. P.; Roesch-Ely M.; Dumas F.; Moura S. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 2018, 214, 74–82. 10.1016/j.micres.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Tyagi A. M.; Gautam A. K.; Kumar A.; Srivastava K.; Bhargavan B.; Trivedi R.; Saravanan S.; Yadav D. K.; Singh N.; Pollet C.; Brazier M.; Mentaverri R.; Maurya R.; Chattopadhyay N.; Goel A.; Singh D. Medicarpin inhibits osteoclastogenesis and has nonestrogenic bone conserving effect in ovariectomized mice. Mol. Cell Endocrinol. 2010, 325, 101–109. 10.1016/j.mce.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Dudoit A.; Mertz C.; Chillet M.; Cardinault N.; Brat P. Antifungal activity of Brazilian red propolis extract and isolation of bioactive fractions by thin-layer chromatography-bioautography. Food Chem. 2020, 327, 127060 10.1016/j.foodchem.2020.127060. [DOI] [PubMed] [Google Scholar]
- Grecka K.; Kus P. M.; Okinczyc P.; Worobo R. W.; Walkusz J.; Szweda P. The Anti-Staphylococcal Potential of Ethanolic Polish Propolis Extracts. Molecules 2019, 24, 1732 10.3390/molecules24091732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylek I.; Karpinski T. M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047 10.3390/molecules24112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Cuspinera V.; Rankin S. A. Bioautography and chemical characterization of antimicrobial compound(s) in commercial water-soluble annatto extracts. J. Agric. Food Chem. 2005, 53, 2524–2529. 10.1021/jf048056q. [DOI] [PubMed] [Google Scholar]
- Chandler R. W.; Rendtorff R. C.; Curran J. W.; Kellogg D. S. Jr. Evaluation of media used for cultures of Neisseria gonorrhoeae and comparison of commercial and laboratory prepared supplements. J. Am. Vener. Dis. Assoc. 1974, 1, 14–19. [PubMed] [Google Scholar]