Abstract
Background:
Trimethylamine N-oxide (TMAO), a gut-derived metabolite, is elevated in heart failure (HF) and linked to poor prognosis. We investigated variations in TMAO in HF, left ventricular assist device (LVAD) and heart transplant (HT), and assessed its relation with inflammation, endotoxemia, oxidative stress and gut dysbiosis.
Methods:
We enrolled 341 patients. TMAO, C-reactive protein, interleukin-6, tumor necrosis factor-α, endothelin-1, adiponectin, lipopolysaccharide, soluble-CD14 and isoprostane were measured in 611 blood samples in HF (New York Heart Association (NYHA) Class I- IV) and at multiple time points post-LVAD and post-HT. Gut microbiota were assessed via 16S rRNA sequencing among 327 stool samples. Multivariable regression models were used to assess the relationship between TMAO and: i) NYHA Class, ii) pre- vs post-LVAD or post-HT; iii) biomarkers of inflammation, endotoxemia, oxidative stress, and microbial diversity.
Results:
ln-TMAO was lower among HF NYHA Class I (1.23 (95%CI:0.52–1.94)μM) vs either Class II, III or IV (1.99 (95%CI:1.68–2.30)μM, 1.97 (95%CI:1.71–2.24)μM, 2.09 (95%CI:1.83–2.34)μM, respectively, all p<0.05). In comparison to Class II-IV, ln-TMAO was lower 1-month post-LVAD (1.58 (95%CI:1.32–1.83)μM), and 1-week and 1-month post-HT (0.97 (95%CI:0.60–1.35)μM & 1.36 (95%CI:1.01–1.70)μM). ln-TMAO levels in long-term LVAD (>6month: 1.99 (95%CI:1.76–2.22)μM and HT (>6month: 1.86 (95%CI:1.66–2.05)μM) were not different from symptomatic HF. After multivariable adjustments, TMAO was not associated with biomarkers of inflammation, endotoxemia, oxidative stress or microbial diversity.
Conclusion:
TMAO levels are increased in symptomatic HF patients and remain elevated long-term after LVAD and HT. TMAO levels were independent from measures of inflammation, endotoxemia, oxidative stress and gut dysbiosis.
Journal Subject Terms: Heart Failure, Inflammation, Biomarkers, Translational Studies
Heart Failure (HF) is a prevalent disease that is associated with high mortality and morbidity1. Over the last decades, multiple therapeutic modalities have been developed to prevent adverse ventricular remodeling, reduce rehospitalizations and improve survival in HF2–4. However, current interventions rarely reverse the underlying disease process, and progression from chronic stable HF to advanced disease still occurs. Left ventricular assist devices (LVADs) and heart transplantation (HT) are the only treatment modalities that can improve survival in advanced HF.
Systemic inflammation and oxidative stress are commonly observed in HF and may directly relate to its pathogenesis5. Recently, perturbations in the gut microbiota known as “gut dysbiosis” and impairment of gut mucosal barriers, facilitating entry of endotoxins and gut metabolites into the circulation, have been observed in HF patients6, 7. Elevated levels of circulating endotoxins and bacterial byproducts enhance systemic inflammation and oxidative stress, thereby contributing to progression of HF to more advanced state8. Moreover, gut bacteria are the main producers of uremic toxins, most notably trimethylamine N-oxide (TMAO).
Trimethylamine N-oxide is a gut-derived metabolite that has been linked to cardiovascular disease (CVD) outcomes9 and to HF pathogenesis and prognosis in both humans and animal models10, 11. Dietary sources and microbial composition of the gut flora regulate TMAO production, while the kidneys12–15 mediate TMAO clearance. Accordingly, renal function is a determinant of circulating TMAO levels16. Changes in renal function are commonly observed among patients with advanced HF. However, in these patients, interpretation of serum creatinine (sCr) can be confounded by changes in creatinine production that are independent of renal function (e.g. changes in muscle mass related to cardiac cachexia). Cystatin C (CysC) is an endogenous biomarker of renal function produced by all nucleated cells at a near constant rate that is independent of muscle mass. Cystatin C-estimated glomerular filtration rate (eGFRCys) outperforms sCr-based estimates in chronic diseases including HF and in critically ill patients17–22.
To date, no study has measured TMAO levels across a broad spectrum of systolic HF progression and after treatment with LVAD or HT. In addition, eGFRCys has never been used to adjust TMAO levels for renal function in this patient population. Furthermore, the relation of TMAO with established biomarkers of inflammation remains to be established. Lastly, little is known about the association between TMAO levels and variation in gut microbial communities among HF, LVAD and HT patients.
Thus, we aimed to: i) compare TMAO levels among distinct clinical subgroups of HF patients before and after LVAD or HT; ii) evaluate the relationship between TMAO and two indices of renal function (sCr vs CysC) as well as biomarkers of inflammation, endotoxemia and oxidative stress; ii) investigate the relationship between TMAO and gut dysbiosis; and, lastly, iv) explore the predictive value of TMAO on clinical outcomes in a subset of HF and LVAD patients.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files.
Study population
Patients were enrolled between June 2016 and February 2019 at Columbia University Irving Medical Center (CUIMC) during routine clinical visits or index hospitalization for LVAD or HT surgery. Exclusion criteria were: i) HF with preserved left ventricular ejection function (LVEF) >40%; ii) infiltrative and hypertrophic cardiomyopathy; iii) advanced renal disease requiring dialysis; iv) liver cirrhosis or active hepatitis; and v) active malignancy.
Patients were classified into the following categories: HF: NYHA Class I, II, III, IV; post-LVAD: 1month, 3–6 month, >6month; and post-HT: 1week, 1month, 3 month, and >6month. All HF patients were treated according to current guidelines, and HT patients received standard immunosuppression per institutional protocol. Clinical information was extracted from electronic medical records (EMRs). Antibiotics use 1 month before stool and/or blood sample collection were recorded, including data for treatment of confirmed/suspected infection or chronic prophylaxis among HT patients. The study was approved by CUIMC Institutional Review Board (AAAP8204) and participants gave written informed consent.
A convenience sample of controls free of CVD enrolled in the Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS)23,24 at CUIMC was used as a comparison group to confirm that TMAO levels in our cohort were elevated relative to CVD-free individuals.
TMAO measurement and calculation of renal function
Plasma TMAO was measured using ultra performance Liquid Chromatography-tandem Mass Spectrometry (Supplemental); eGFRCys and eGFRCr were calculated as previously described25, 26,27 (Supplemental).
Measurements of biomarkers of inflammation, endotoxemia and oxidative stress
Biomarkers of inflammation (C-reactive protein[CRP], interleukin-6[IL-6], tumor necrosis factor-α[TNF-α], endothelin-1[ET-1], adiponectin), endotoxemia (lipopolysaccharide[LPS] and soluble CD14[sCD14])), and oxidative stress [isoprostane] were measured in plasma or serum (Supplemental).
Stool analysis
Patients provided non-fasting stool samples in sterile stool hats28. Details on stool collection and DNA extraction and sequencing are provided in Supplemental. All collected stool samples had a corresponding blood sample (median difference between blood and stool collection dates was 2 (Interquartile interval(IQI) =19.5) days.
Statistical analysis
All statistical analyses were conducted using R version 3.6.2. Difference in means or proportions of potential confounders according to categories were assessed using one-way ANOVA for continuous variables and chi-square for categorical variables. Multivariable mixed-effects linear models regressed natural log transformed TMAO across categories. Patients were modeled as random effects to account for within patient correlation. Ln-transformed TMAO values were utilized in regression models to enhance normality. Multivariable adjusted mean values of ln-TMAO are presented across categories. Paired t-tests were used to compare mean eGFR values calculated using CycC vs. sCr-based equations; for these paired comparisons, we adjusted for multiple comparisons using the false discovery rate. Pearson correlations and mixed-effect models were used to inform the degree of linear relationship between TMAO levels and different assessments of renal function, biomarkers of inflammation, endotoxemia, and oxidative stress, and metrics of gut microbial diversity. Time-varying Cox proportional hazards models were used to assess the relationship between variations in ln-transformed TMAO levels and clinical outcomes.
For gut microbiota analyses, demultiplexed sequence files were processed in R version 3.6.2, using DADA2 pipeline to identify exact sequence variants (ESVs)29, 30.Reads were truncated at forward and reverse lengths of 260 and 220. After processing, a total of 5,697,709 sequence reads were included of median library size=13,625 and 30,959 ESVs were identified. Phyloseq package was used for 16S analyses. Microbial alpha diversity (i.e., number and distribution of bacterial taxa within samples) was defined using Shannon Index and number of observed ESVs. DESeq package28 was used to evaluate whether specific taxa differed by categories after multivariable adjustment and adjustment for multiple comparisons using the false discovery rate.
RESULTS
Baseline characteristics
Among 341 enrolled patients, 611 blood samples (HF: 9 NYHA Class I, 46 Class II, 61 Class III, 68 Class IV; post-LVAD: 64 (1month), 52 (3–6month), 85 (>6month); post-HT: 29 (1week), 34 (1month), 37 (3 month), 126 (>6month) were collected. In a subset of 225 patients, 327 stool samples for microbiome assessments were also collected (HF: 7 NYHA Class I, 29 Class II, 31 Class III, 30 Class IV; post-LVAD: 37 (1month), 31 (3–6month), 52 (>6month); post-HT: 13 (1week), 12 (1month), 13 (3month), 72 (>6month)). Characteristics of patients providing blood samples are reported in Table 1–3 and characteristics of patients providing stool samples are shown in Table I–III. All study patients were predominantly white males, with no difference in body mass index, HF etiology, history of diabetes across categories (Table 1–3).
Table 1:
Baseline Characteristics of HF Patients Providing Blood Samples
| Study cohorts | NYHA Class I | NYHA Class II | NYHA Class III | NYHA Class IV | P-value |
|---|---|---|---|---|---|
| Number of samples (N=184) | 9 | 46 | 61 | 68 | |
| Demographic and clinical characteristics | |||||
| Age (years) | 54.8 ± 19.4 | 59.3 ± 13.5 | 60.6 ± 12.7 | 58.8 ± 14.3 | 0.68 |
| Male | 7 (77.8%) | 30 (65.2%) | 50 (82.0%) | 60 (88.2%) | 0.03 |
| Race | 0.02 | ||||
| White | 1 (11.1%) | 20 (43.5%) | 35 (57.4%) | 36 (52.9%) | |
| Black | 2 (22.2%) | 11 (23.9%) | 18 (29.5%) | 14 (20.6%) | |
| Hispanic | 3 (33.3%) | 5 (10.9%) | 6 (9.8%) | 5 (7.4%) | |
| Other | 3 (33.3%) | 10 (21.7%) | 2 (3.3%) | 13 (19.1%) | |
| BMI (kg/m2) Median [IQI] | 31.1 [28.5,36.7] | 30.2 [25.4, 34.6] | 30.1 [26.1,35.0] | 29.0 [25.0,31.5] | 0.44 |
| Smoking | 3 (33.3%) | 20 (43.5%) | 35 (57.4%) | 35 (51.5%) | 0.37 |
| Etiology, Ischemic | 2 (22.2%) | 19 (41.3%) | 29 (47.5%) | 31 (45.6%) | 0.52 |
| Hypertension | 4 (44.4%) | 33 (71.7%) | 36 (59.0%) | 43 (63.2%) | 0.35 |
| Diabetes | 1 (11.1%) | 14 (30.4%) | 21 (34.4%) | 21 (30.9%) | 0.57 |
| Atrial fib/flutter | 1 (11.1%) | 15 (32.6%) | 32 (52.5%) | 30 (44.1%) | 0.05 |
| Stroke | 0 (0.0%) | 1 (2.2%) | 5 (8.2%) | 3 (4.4%) | 0.45 |
| Laboratory parameters | |||||
| BUN (mg/dl) | 17.1 ± 3.8 | 25.5 ± 13.2 | 23.9 ± 9.2 | 32.0 ± 19.1 | 0.008 |
| Serum Cr (mg/dl) | 1.1 ± 0.2 | 1.6 ± 1.7 | 1.3 ± 0.3 | 1.4 ± 0.5 | 0.39 |
| Serum CysC (mg/liter) | 1.0 ± 0.1 | 1.5 ± 1.3 | 1.4 ± 0.5 | 1.7 ± 0.6 | <0.001 |
| eGFRCr (ml/min/1.73m2) | 76.3 ± 19.4 | 63.2 ± 26.5 | 63.7 ± 18.6 | 59.7 ± 23.1 | 0.31 |
| eGFRCys (ml/min/1.73m2) | 77.3 ± 16.0 | 62.0 ± 23.9 | 56.3 ± 23.3 | 48.6 ± 24.8 | 0.001 |
| NT-ProBNP (ng/l) Median [IQI] | 515 [271, 694] | 967[219, 2048] | 1541 [557,4017] | 2863 [1817,4606] | <0.001 |
| Na (mmol/l) | 141.6 ± 3.2 | 140.3 ± 3.4 | 140.3 ± 3.2 | 136.6 ± 4.8 | <0.001 |
| AST (U/L) | 25.0 ± 15.9 | 28.0 ± 21.7 | 25.9 ± 13.6 | 41.0 ± 66.8 | 0.32 |
| ALT (U/L) | 23.3 ± 15.4 | 27.5 ± 21.3 | 29.3 ± 25.3 | 66.9 ± 147.7 | 0.12 |
| Total Bilirubin (mg/dl) | 0.5 ± 0.1 | 0.6 ± 0.5 | 0.7 ± 0.4 | 0.9 ± 0.4 | <0.001 |
| LDH (U/L) | 215.0 ± 96.1 | 241.6 ± 74.3 | 272.7 ± 99.8 | 343.9 ± 185.8 | 0.03 |
| Biomarkers | |||||
| ln-CRP (mg/L) | 0.5 ± 1.7 | 0.8 ± 1.2 | 1.2 ± 1.3 | 2.7 ± 1.4 | <0.001 |
| ln-IL-6 (pg/mL) | 0.8 ± 0.6 | 1.2 ± 0.7 | 1.7 ± 0.8 | 2.5 ± 1.0 | <0.001 |
| ln-TNF-α (pg/mL) | 0.2 ± 0.4 | 0.4 ± 0.5 | 0.5 ± 0.4 | 0.8 ± 0.4 | <0.001 |
| ln-ET-1 (pg/mL) | 0.6 ± 0.5 | 0.8 ± 0.5 | 0.9 ± 0.5 | 1.1 ± 0.5 | 0.001 |
| ln-Adiponectin (ng/mL) | 8.6 ± 0.8 | 9.1 ± 0.8 | 9.2 ± 0.6 | 9.7 ± 0.7 | <0.001 |
| ln-Isoprostane (pg/mL) | 4.4 ± 0.4 | 4.5 ± 0.5 | 4.6 ± 0.4 | 4.8 ± 0.5 | 0.008 |
| ln-sCD14 (ng/mL) | 7.1 ± 0.3 | 7.3 ± 0.3 | 7.3 ± 0.3 | 7.5 ± 0.4 | 0.002 |
| LPS (EU/mL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.4 ± 0.3 | 0.04 |
| Medications | |||||
| ASA | 5 (55.6%) | 31 (67.4%) | 31 (50.8%) | 40 (58.8%) | 0.39 |
| Coumadin | 0 (0.0%) | 3 (6.5%) | 17 (27.9%) | 6 (8.8%) | 0.002 |
| ACE inhibitors | 3 (33.3%) | 18 (39.1%) | 15 (24.6%) | 3 (4.4%) | <0.001 |
| ARB | 5 (55.6%) | 16 (34.8%) | 31 (50.8%) | 6 (8.8%) | <0.001 |
| Aldosterone antagonists | 6 (66.7%) | 27 (58.7%) | 40 (65.6%) | 36 (52.9%) | 0.51 |
| β-blockers | 9 (100.0%) | 45 (97.8%) | 55 (90.2%) | 36 (52.9%) | <0.001 |
| Statins | 5 (55.6%) | 24 (52.2%) | 32 (52.5%) | 39 (57.4%) | 0.93 |
| Loop diuretics | 6 (66.7%) | 33 (71.7%) | 52 (85.2%) | 49 (72.1%) | 0.23 |
| Digoxin | 2 (22.0%) | 5 (10.9%) | 19 (31.1%) | 14 (20.6%) | 0.1 |
| Antibiotics* | 0 (0.0%) | 4 (8.7%) | 8 (13.1%) | 17 (25.0%) | 0.04 |
NYHA, New York Heart Association; BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase, LDH, lactate dehydrogenase; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; Cr, Creatinine; CysC, Cystatin C; eGFR, estimated glomerular filtration rate; TNF-α, tumor necrosis factor alpha; sCD 14, soluble CD14; IL-6, interleukin-6; CRP, C-reactive protein; ET-1, endothelin-1; LPS, lipopolysaccharide; ASA, acetylsalicylic acid; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Data presented n (%) or mean ± SD as appropriate, unless otherwise noted.
Antibiotic use is defined as any antibiotic use that was indicated for infection that was estimated to have occurred 1 month before stool collection.
Table 3:
Baseline Characteristics of HT Patients Providing Blood Samples
| Study cohorts | HT (1wk) | HT (1mo) | HT (3mo) | HT (>6mo) | P-value |
|---|---|---|---|---|---|
| Number of samples (N=226) | 29 | 34 | 37 | 126 | |
| Demographic and clinical characteristics | |||||
| Age, (years) | 51.9 ± 12.4 | 51.4 ± 11.3 | 51.3 ± 11.7 | 56.5 ± 12.3 | <0.001 |
| Male | 27 (93.1%) | 31 (91.2%) | 33 (89.2%) | 104 (82.5%) | 0.31 |
| Race | 0.99 | ||||
| White | 14 (48.3%) | 16 (47.1%) | 18 (48.6%) | 65 (51.6%) | |
| Black | 10 (34.5%) | 12 (35.3%) | 13 (35.1%) | 39 (31.0%) | |
| Hispanic | 3 (10.3%) | 3 (8.8%) | 3 (8.1%) | 16 (12.7%) | |
| Other | 2 (6.9%) | 3 (8.8%) | 3 (8.1%) | 6 (4.8%) | |
| BMI, (kg/m2) Median [IQI] | NA [NA, NA] | 26.3 [22.5,28.7] | 27.1 [24.4,29.5] | 28.1 [25.5,32.4] | 0.01 |
| Smoking | 13 (44.8%) | 14 (41.2%) | 18 (48.6%) | 58 (46.0%) | 0.94 |
| Etiology, Ischemic | 11 (37.9%) | 9 (26.5%) | 13 (35.1%) | 55 (43.7%) | 0.30 |
| Hypertension | 17 (58.6%) | 21 (61.8%) | 25 (67.6%) | 108 (85.7%) | 0.001 |
| Diabetes | 13 (44.8%) | 13 (38.2%) | 13 (35.1%) | 54 (42.9%) | 0.81 |
| Atrial fib/flutter | 14 (48.3%) | 14 (41.2%) | 15 (40.5%) | 41 (32.5%) | 0.38 |
| Stroke | 5 (17.2%) | 7 (20.6%) | 5 (13.5%) | 15 (11.9%) | 0.59 |
| Time after HT, months Median [IQI] |
0.3 [0.2,0.3] |
0.7 [0.6,0.9] |
2.7 [1.9,3.6] |
24.2 [7.2,83.0] |
<0.001 |
| Laboratory parameters | |||||
| BUN (mg/dl) | 30.4 ± 15.4 | 32.6 ± 13.1 | 29.6 ± 12.4 | 27.7 ± 11.9 | 0.27 |
| Serum Cr (mg/dl) | 1.3 ± 0.6 | 1.4 ± 1.1 | 1.5 ± 1.0 | 1.6 ± 1.2 | 0.03 |
| Serum CysC (mg/l) | 1.7 ± 0.7 | 1.6 ± 1.0 | 1.4 ± 0.6 | 1.6 ± 0.9 | 0.11 |
| eGFRCr (ml/min/1.73m2) | 79.3 ± 39.3 | 69.1 ± 29.9 | 64.1 ± 22.2 | 59.1 ± 34.4 | <0.001 |
| eGFRcys (ml/min/1.73m2) | 50.3 ± 24.3 | 54.5 ± 23.7 | 59.6 ± 22.1 | 54.8 ± 24.2 | 0.01 |
| Na (mmol/l) | 138.8 ± 3.1 | 139.1 ± 2.9 | 139.8 ± 3.9 | 141.5 ± 2.8 | <0.001 |
| AST (U/L) | 24.2 ± 10.3 | 22.0 ± 11.5 | 23.0 ± 8.5 | 24.8 ± 15.3 | 0.58 |
| ALT (U/L) | 31.9 ± 28.9 | 33.4 ± 22.7 | 32.6 ± 22.5 | 22.8 ± 13.5 | 0.01 |
| Total Bilirubin (mg/dl) | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.34 |
| LDH (U/L) | 603.7 ± 236.4 | 391.0 ± 137.8 | 351.4 ± 125.3 | 251.1 ± 86.3 | <.0001 |
| Biomarkers | |||||
| ln-CRP (mg/L) | 2.5 ± 1.4 | 1.3 ± 1.7 | 0.4 ± 1.5 | 1.1 ± 1.3 | <0.001 |
| ln-IL-6 (pg/mL) | 2.3 ± 1.1 | 1.7 ± 1.1 | 1.2 ± 1.0 | 1.4 ± 1.0 | <0.001 |
| ln-TNF-α (pg/mL) | 0.1 ± 0.5 | 0.1 ± 0.4 | 0.3 ± 0.5 | 0.5 ± 0.4 | <0.001 |
| ln-ET-1 (pg/mL) | 1.0 ± 0.5 | 0.8 ± 0.6 | 0.6 ± 0.6 | 0.7 ± 0.5 | 0.003 |
| ln-Adiponectin (ng/mL) | 9.7 ± 0.7 | 9.6 ± 0.8 | 9.4 ± 0.6 | 9.3 ± 0.7 | <0.001 |
| ln-Isoprostane (pg/mL) | 4.2 ± 0.5 | 4.3 ± 0.4 | 4.5 ± 0.5 | 4.5 ± 0.5 | 0.01 |
| ln-sCD14 (ng/mL) | 7.2 ± 0.5 | 7.2 ± 0.3 | 7.2 ± 0.3 | 7.3 ± 0.4 | 0.03 |
| LPS (EU/mL) | 0.5 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.4 ± 0.3 | 0.55 |
| Medications | |||||
| ASA | 8 (27.6%) | 28 (82.4%) | 35 (94.6%) | 117 (92.9%) | <0.001 |
| Coumadin | 0 (0.0%) | 1 (2.9%) | 0 (0.0%) | 4 (3.2%) | 0.55 |
| ACE inhibitors | 1 (3.4%) | 1 (2.9%) | 0 (0.0%) | 8 (6.3%) | 0.38 |
| ARB | 0 (0.0%) | 0 (0.0%) | 1 (2.7%) | 29 (23.0%) | <0.001 |
| Aldosterone Antagonists | 1 (3.4%) | 2 (5.9%) | 0 (0.0%) | 3 (2.4%) | 0.48 |
| β-blockers | 1 (3.4%) | 4 (11.8%) | 1 (2.7%) | 24 (19.0%) | 0.02 |
| Statins | 12 (41.4%) | 30 (88.2%) | 34 (91.9%) | 102 (81.0%) | <0.001 |
| Loop diuretics | 11 (37.9%) | 13 (38.2%) | 18 (48.6%) | 29 (23.0%) | 0.02 |
| Antibiotics* | 26 (89.7%) | 34 (100.0%) | 35 (94.6%) | 40 (31.7%) | <0.001 |
| Immunosuppression | |||||
| Tacrolimus | 25 (86.2%) | 31 (91.2%) | 35 (94.6%) | 100 (79.4%) | 0.08 |
| Cyclosporine | 0 (0.0%) | 1 (2.9%) | 1 (2.7%) | 21 (16.7%) | 0.004 |
| Prednisone | 25 (86.2%) | 31 (91.2%) | 36 (97.3%) | 93 (73.8%) | 0.003 |
| Sirolimus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.6%) | 0.66 |
| Everolimus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (4.0%) | 0.26 |
| Mycophenolate Mofetil | 24 (82.8%) | 30 (88.2%) | 34 (91.9%) | 92 (73.0%) | 0.04 |
HT, heart transplant; BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase, LDH, lactate dehydrogenase; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; Cr, Creatinine; CysC, Cystatin C; eGFR, estimated glomerular filtration rate; TNF-α, tumor necrosis factor alpha; sCD14, soluble CD14; IL-6, interleukin-6; CRP, C-reactive protein; ET-1, endothelin-1; LPS, lipopolysaccharide; ASA, acetylsalicylic acid; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; NA, not available.
Data presented n (%) or mean ± SD as appropriate, unless otherwise noted.
Antibiotic use is defined as any antibiotic use that was indicated for infection or prophylaxis as per HT protocol that was estimated to have occurred 1 month before stool collection.
Among HF patients, NYHA Class IV (vs Class I-III) patients had lower sodium, higher total bilirubin, N-terminal prohormone B-type natriuretic peptide (NT-proBNP), blood urea nitrogen and CysC (lower eGFRCys) levels. HF NYHA Class IV patients were less likely to be treated according to HF guidelines and more likely to receive antibiotics (Table 1).
Among LVAD patients, majority were INTERMACS profile 2 and 3. LVAD (1month) patients vs LVAD (3–6 and >6month)) had lower sodium, higher liver enzymes and NT-proBNP levels as well as lower sCr (higher eGFRCr). LVAD (1month) patients were less likely to be treated with HF medications and more likely to receive antibiotics (Table 2). Renal function by eGFRCys remained largely unchanged over time after LVAD (Figure 1).
Table 2:
Baseline Characteristics of LVAD Patients Providing Blood Samples
| Study cohorts | LVAD (1mo) | LVAD (3–6mo) | LVAD (>6mo) | P-value |
|---|---|---|---|---|
| Number of samples (N=201) | 64 | 52 | 85 | |
| Demographic and clinical characteristics | ||||
| Age (years) | 58.7 ± 13.0 | 57.7 ± 14.6 | 57.8 ± 14.3 | <0.001 |
| Male | 57 (89.1%) | 45 (86.5%) | 74 (87.1%) | 0.90 |
| Race | 0.05 | |||
| White | 43 (67.2%) | 31 (59.6%) | 55 (64.7%) | |
| Black | 8 (12.5%) | 10 (19.2%) | 22 (25.9%) | |
| Hispanic | 2 (3.1%) | 5 (9.6%) | 5 (5.9%) | |
| Other | 11 (17.2%) | 6 (11.5%) | 3 (3.5%) | |
| BMI (kg/m2) Median [IQI] | 28.5 [22.9, 29.4] | 27.6 [25.0, 30.6] | 28.0 [24.8, 32.5] | 0.35 |
| Smoking | 41 (64.1%) | 31 (59.6%) | 43 (50.6%) | 0.24 |
| Etiology, Ischemic | 38 (59.4%) | 26 (50.0%) | 37 (43.5%) | 0.16 |
| INTERMACS Profile | 0.58 | |||
| 1 | 7 (10.9%) | 4 (7.7%) | 6 (7.1%) | |
| 2 | 35 (54.7%) | 32 (61.5%) | 42 (49.4%) | |
| 3 | 12 (18.8%) | 12 (23.1%) | 21 (24.7%) | |
| 4 | 4 (6.2%) | 3 (5.8%) | 5 (5.9%) | |
| 5–7 or not provided | 6 (9.4%) | 1 (1.9%) | 11 (12.9%) | |
| Hypertension | 39 (60.9%) | 31 (59.6%) | 49 (57.6%) | 0.92 |
| Diabetes | 22 (34.4%) | 19 (36.5%) | 36 (42.4%) | 0.58 |
| Atrial fib/flutter | 28 (43.8%) | 20 (38.5%) | 39 (45.9%) | 0.69 |
| Stroke | 2 (3.1%) | 2 (3.8%) | 9 (10.6%) | 0.13 |
| Time after LVAD, months Median [IQI] |
0.6 [0.5, 0.8] | 4.5 [3.6, 6.0] | 12.1 [9.2, 17.6] | <0.001 |
| Laboratory parameters | ||||
| BUN (mg/dl) | 19.0 ± 12.4 | 22.6 ± 10.4 | 24.6 ± 13.3 | 0.01 |
| Serum Cr (mg/dl) | 1.2 ± 0.6 | 1.3 ± 0.5 | 1.4 ± 0.8 | 0.01 |
| Serum CysC (mg/l) | 1.6 ± 0.6 | 1.6 ± 0.6 | 1.5 ± 0.7 | 0.09 |
| eGFRCr (ml/min/1.73m2) | 77.9 ± 39.0 | 67.5 ± 25.5 | 62.0 ± 24.8 | <0.001 |
| eGFRcys (ml/min/1.73m2) | 51.3 ± 22.4 | 48.6 ± 21.0 | 58.1 ± 29.1 | 0.03 |
| NT-ProBNP (ng/l) Median [IQI] | 2654 [1999, 4205] | 1336 [573, 2177] | 976 [528, 1916] | <0.001 |
| Na (mmol/l) | 136.1 ± 4.2 | 140.6 ± 3.0 | 140.2 ± 3.3 | <0.001 |
| AST (U/L) | 28.1 ± 14.0 | 27.0 ± 17.5 | 26.9 ± 17.2 | 0.97 |
| ALT (U/L) | 29.1 ± 19.2 | 19.1 ± 9.8 | 21.8 ± 12.8 | 0.002 |
| Total Bilirubin (mg/dl) | 0.8 ± 0.8 | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.01 |
| LDH (U/L) | 349.1 ± 114.2 | 301.3 ± 92.7 | 329.3 ± 174.1 | 0.14 |
| Biomarkers | ||||
| ln-CRP (mg/L) | 4.0 ± 0.9 | 1.7 ± 1.0 | 1.8 ± 1.2 | <0.001 |
| ln-IL-6 (pg/mL) | 3.2 ± 0.8 | 1.7 ± 0.8 | 1.7 ± 0.9 | <0.001 |
| ln-TNF-α (pg/mL) | 0.9 ± 0.4 | 0.7 ± 0.4 | 0.6 ± 0.6 | 0.003 |
| ln-ET-1 (pg/mL) | 0.9 ± 0.5 | 0.7 ± 0.5 | 0.9 ± 0.5 | 0.23 |
| ln-Adiponectin (ng/mL) | 9.6 ± 0.5 | 9.3 ± 0.6 | 9.2 ± 0.7 | <0.001 |
| ln-Isoprostane (pg/mL) | 4.3 ± 0.4 | 4.5 ± 0.4 | 4.5 ± 0.5 | 0.04 |
| ln-sCD14 (ng/mL) | 7.5 ± 0.5 | 7.5 ± 0.3 | 7.4 ± 0.3 | 0.10 |
| LPS (EU/mL) | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.4 ±0.2 | 0.02 |
| Medications | ||||
| ASA | 56 (87.5%) | 39 (75.0%) | 59 (69.4%) | 0.03 |
| Coumadin | 38 (59.4%) | 47 (90.4%) | 75 (88.2%) | <0.001 |
| ACE inhibitors | 6 (9.4%) | 16 (30.8%) | 27 (31.8%) | 0.003 |
| ARB, | 1 (1.6%) | 5 (9.6%) | 15 (17.6%) | 0.01 |
| Aldosterone Antagonists | 23 (35.9%) | 26 (50.0%) | 25 (29.4%) | 0.05 |
| β-blockers | 34 (53.1%) | 44 (84.6%) | 80 (94.1%) | <0.001 |
| Statins | 20 (31.2%) | 18 (34.6%) | 25 (29.4%) | 0.82 |
| Loop diuretics | 41 (64.1%) | 40 (76.9%) | 51 (60.0%) | 0.12 |
| Digoxin | 16 (25.0%) | 18 (34.6%) | 34 (40.0%) | 0.16 |
| *Antibiotics | 35 (54.7%) | 11 (21.2%) | 29 (34.1%) | 0.001 |
LVAD, left ventricular assist device; BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase, LDH, lactate dehydrogenase; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; Cr, Creatinine; CysC, Cystatin C; eGFR, estimated glomerular filtration rate; TNF-α, tumor necrosis factor alpha; sCD14, soluble CD14; IL-6, interleukin-6; CRP, C-reactive protein; ET-1, endothelin-1; LPS, lipopolysaccharide; ASA, acetylsalicylic acid; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
Data presented n (%) or mean ± SD as appropriate, unless otherwise noted.
Antibiotic use is defined as any antibiotic use that was indicated for infection that was estimated to have occurred 1 month before stool collection.
Figure 1:
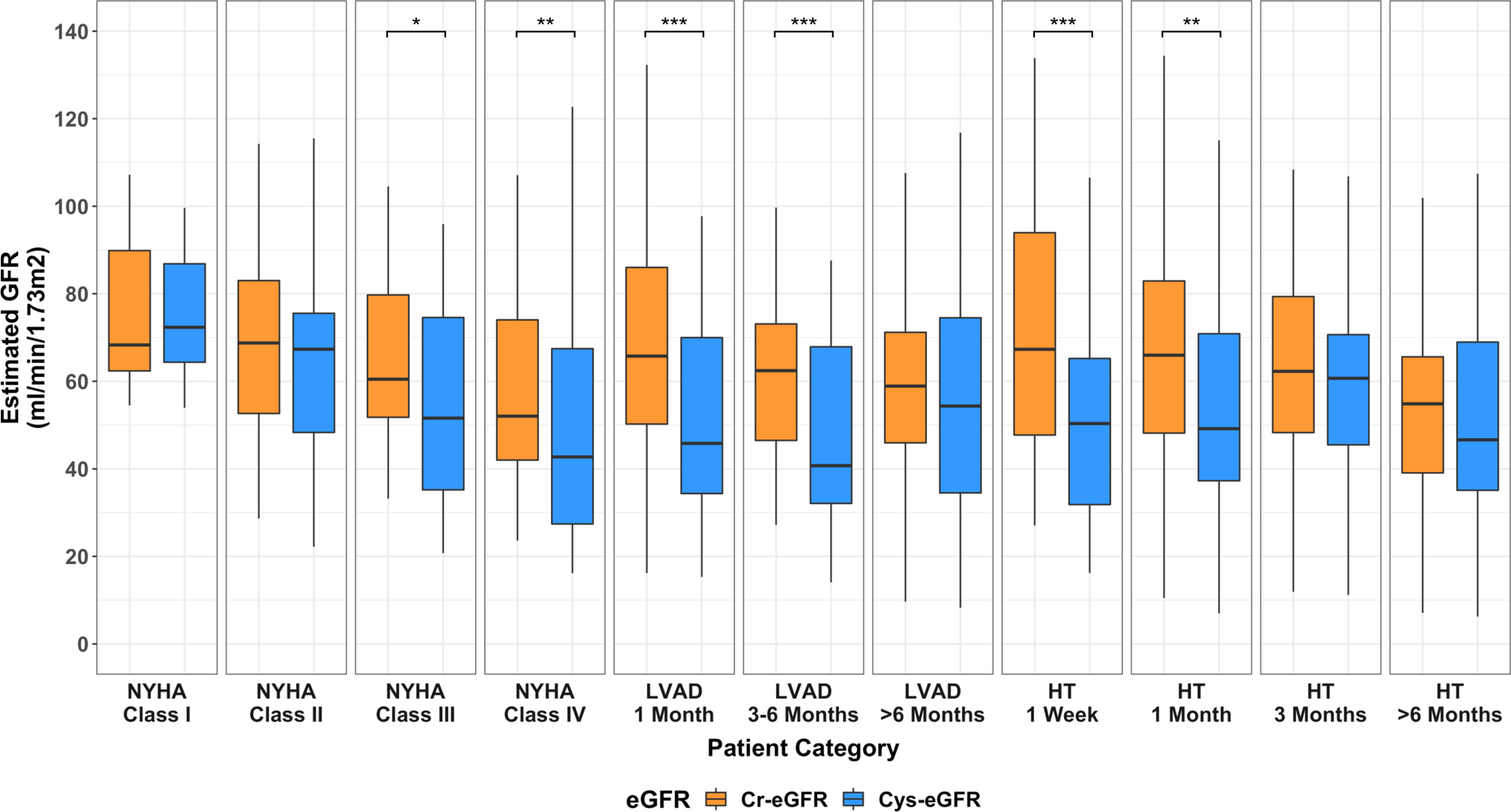
Comparison of renal function assessment using serum Cystatin C- and serum Creatinine-eGFR among Heart Failure, LVAD and Heart Transplant patients.
p-value: * 0.05–0.001; ** 0.001–0.0001; *** <0.0001; all comparisons denoted with any * had a false discovery rate <0.05. HF, heart failure; HT, heart transplant, LVAD, left ventricular assist device.
Among HT patients, early after HT (1week, 1month) patients had higher eGFRCr while eGFRCys remained largely unchanged over time post-HT. HT(>6month) were less likely to be on antibiotics as compared to the earlier time points (Table 3). Of note, the proportion of HT vs LVAD or HF patients treated with statins was significantly higher (>80% after 1 month of HT). Tacrolimus was the predominant calcineurin inhibitor (CNI) used.
When comparing eGFRCr vs eGFRCys, significant differences in renal function assessment were present in patients with advanced HF (NYHA Class IV), early after LVAD (1month; 3–6month) and HT (1week; 1month) (Figure 1).
Circulating TMAO levels across HF, LVAD and HT patients
Median plasma TMAO levels (IQI) of the entire cohort were 5.86 (3.49–10.50) μM. For individual subgroups, levels were as follows: HF: 6.96 (3.96–12.73)μM, LVAD: 5.81 (3.50–9.87)μM and HT: 5.35 (2.85–9.76)μM. These levels were significantly higher than in healthy controls at our and other institutions24, 31, and were comparable to levels previously reported in patients with CVD13, chronic kidney disease (CKD)16 and HF11 (Figure 2).
Figure 2:
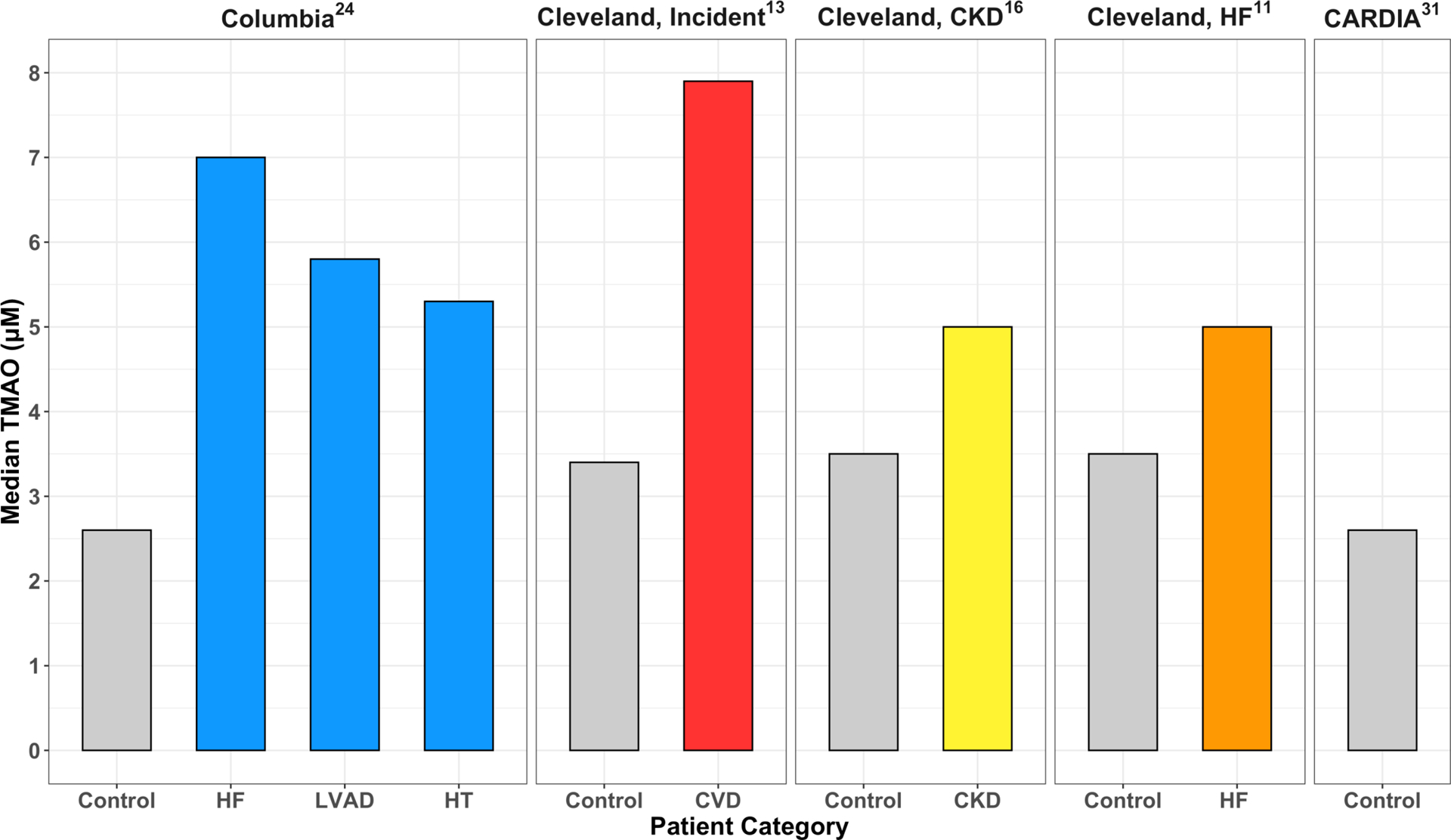
Comparison of TMAO levels between Healthy Controls and Heart Failure, LVAD, Heart Transplant Patients from Columbia University Irving Medical Center and from published data from other institutions. CVD, cardiovascular disease; CKD, chronic kidney disease; HF, heart failure; HT, heart transplant; LVAD, left ventricular assist device.
When analyzing the entire cohort of HF, LVAD and HT patients, ln-TMAO demonstrated a moderate inverse correlation with eGFR, irrespective of the biomarker studied: eGFRCr (Rho=−0.41; p<0.001) and eGFRCys (Rho=−0.37; p<0.0001) (Figure I).
In unadjusted model (M0), mean follow up ln-TMAO was lowest among asymptomatic HF NYHA Class I patients 1.23(0.52–1.94)μM and significantly increased among Class II, III and IV patients (1.99(1.68–2.30)μM, 1.97(1.71–2.24)μM, 2.09(1.83–2.34)μM, respectively, p<0.05 for comparison between Class I vs Class II-IV). In comparison to Class II-IV patients, levels were lower early after LVAD (1month: 1.58(1.32–1.83)μM and after HT (1week: 0.97(0.60–1.35)μM; 1month: 1.36(1.01–1.70)μM). Notably, long-term LVAD (>6month: 1.99(1.76–2.22)μM and HT (>6month: 1.86(1.76–2.22)μM) ln-TMAO levels were similar to those of symptomatic Class II-IV HF patients (Figure 3A).
Figure 3.
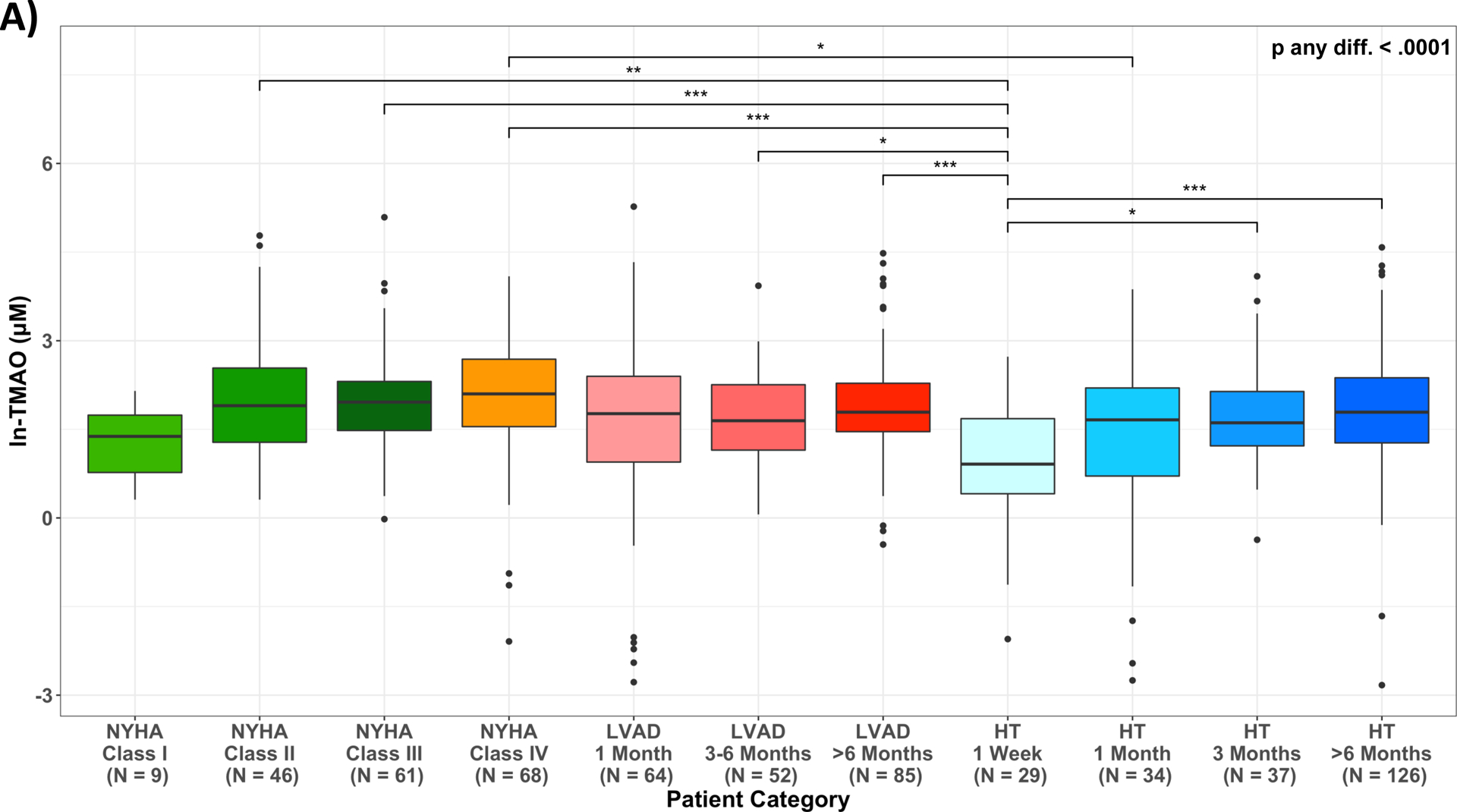
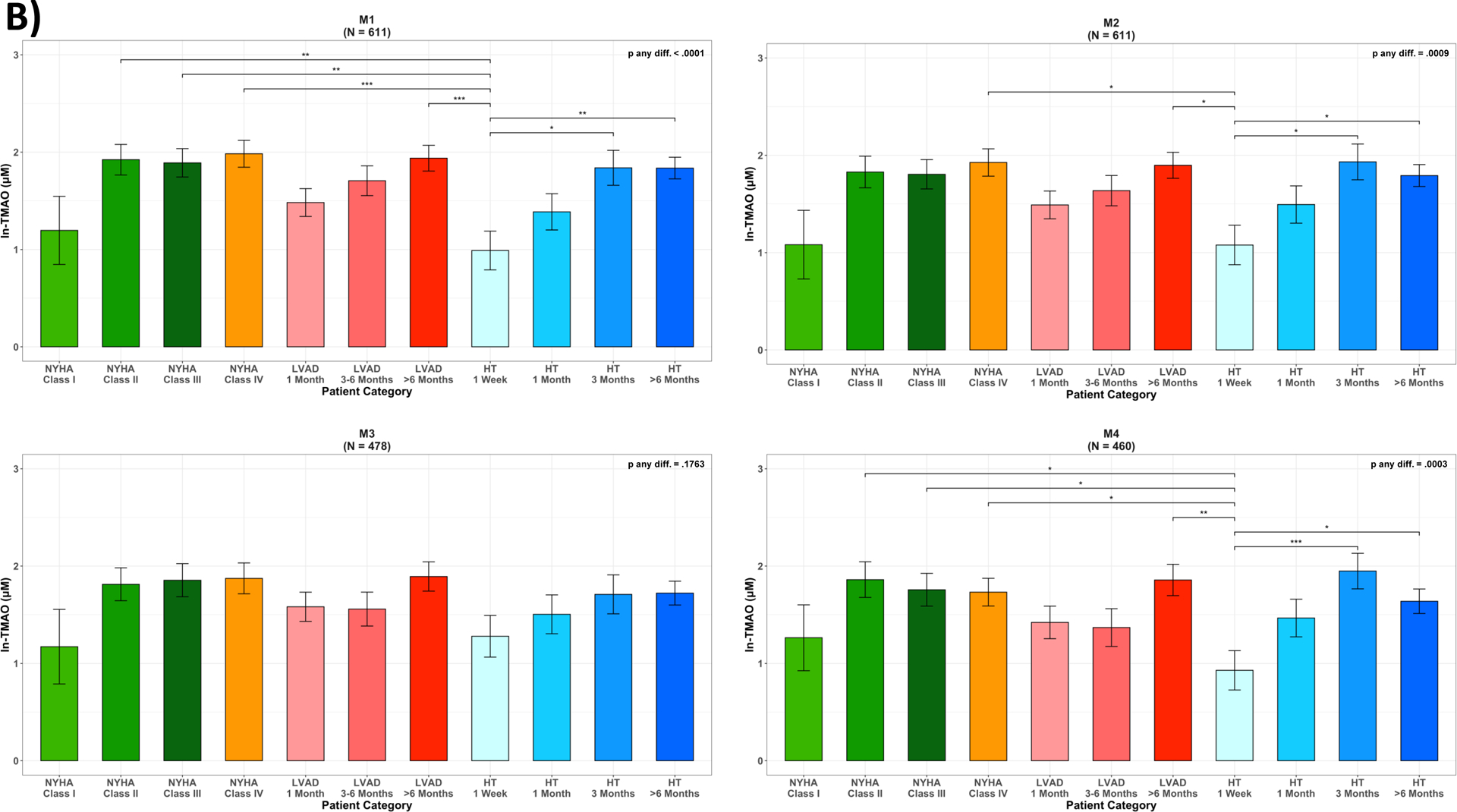
Variation in circulating TMAO across disease categories of Heart Failure, LVAD and Heart Transplant patients. (A) unadjusted ln-TMAO levels (M0); (B) adjusted least squared means: M1: adjusted for age, sex, race/ethnicity; M2: adjusted for Model 1 plus antibiotics use one month prior to stool collection; M3: adjusted for Model 2 plus serum creatinine estimated glomerular filtration rate (eGFRCr); M4: adjusted for Model 2 plus Cystatin C estimated glomerular filtration rate (eGFRCys); p-value: * 0.05–0.01; ** 0.01–0.001; *** <0.001; HF, heart failure; HT, heart transplant; LVAD, left ventricular assist device.
After multivariable adjustment for age, sex, race/ethnicity (M1) and antibiotics use (M2), variations in mean values of ln-TMAO across categories did not critically change. These patterns were attenuated and lost statistical significance after adjustment for renal function using eGFRCr (M3). However, when using eGFRCys (M4), the reductions observed early after HT remained statistically significant (Figure 3B). Results were similar in the sensitivity analysis that utilized data from those patients who had eGFRCr and eGFRCys (N=389) measured concurrently (Figure IIA&IIB).
A subgroup of 57 LVAD and 26 HT patients had TMAO levels measured longitudinally both pre-LVAD and at least once post-LVAD for a total of 162 samples collected, and pre-HT and at least once post-HT for a total of 102 samples. The patterns observed confirmed our cross-sectional findings, showing a trend in which TMAO levels decrease early following LVAD or HT and slowly rebound to pre-intervention levels (Figure 3A&3B; Table IV&V). Additionally, in a small subgroup of 13 LVAD and 10 HT patients with TMAO measured longitudinally at all time points pre- and post-LVAD/HT, the results were confirmatory of the above findings (Figure IIIA&IIIB).
Relation between circulating TMAO levels and biomarkers of inflammation, endotoxemia and oxidative stress
All biomarkers of inflammation (CRP, IL-6, TNF-α, ET-1, adiponectin), endotoxemia (LPS, sCD14) and oxidative stress (isoprostane) increased as HF progressed from Class I to IV. Among LVAD patients, biomarkers of inflammation (CRP, IL-6, TNF-α, adiponectin) were progressively lower with time post-LVAD, whereas endotoxemia (LPS) and oxidative stress (isoprostane) increased. Among HT patients, similarly to LVAD patients, biomarkers of inflammation (CRP, IL-6, TNF-α, adiponectin, ET-1) were progressively lower with time post-HT, whereas endotoxemia (LPS, sCD14) and oxidative stress (isoprostane) remained elevated (Table1, Figure IV&V).
For the entire cohort, Table VI summarized the relationship between ln-TMAO and the above-mentioned biomarkers. In unadjusted model (M0), ln-TMAO levels were significantly associated with levels on inflammation (TNF-α, ET-1), endotoxemia (sCD14) and oxidative stress (isoprostane). These associations were lost after multiple adjustments for baseline characteristics and renal indices (M3&M4).
Relation between circulating TMAO levels and gut microbial diversity metrics
Mean values of Shannon Index and number of observed ESVs for the entire cohort were 5.24±0.64 and 343.15±192.08, respectively. For individual subgroups, they were as follows: HF: 5.37±0.64, 391±223; LVAD: 5.23±0.64, 332±176, HT: 5.13±0.63, 313±172, respectively.
After multivariable adjustment for age, sex, race/ethnicity and antibiotics use (M2), mean levels of the Shannon Index decreased across worsening NYHA Class among HF patients; the number of observed ESVs followed the same pattern. For LVAD and HT, adjusted mean levels of the Shannon Index increased with time post LVAD and HT (Figure 4, Table VII).
Figure 4:
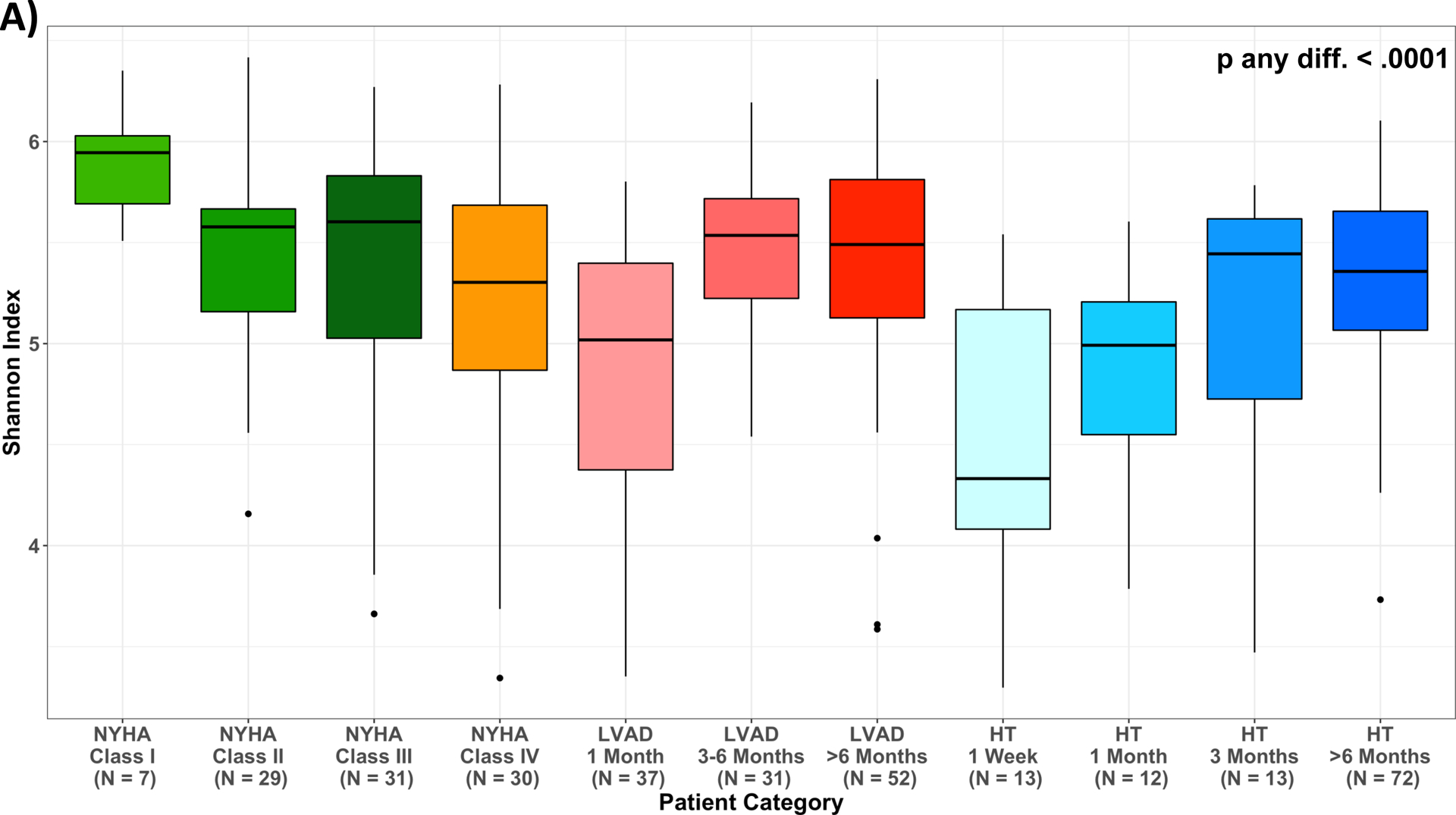
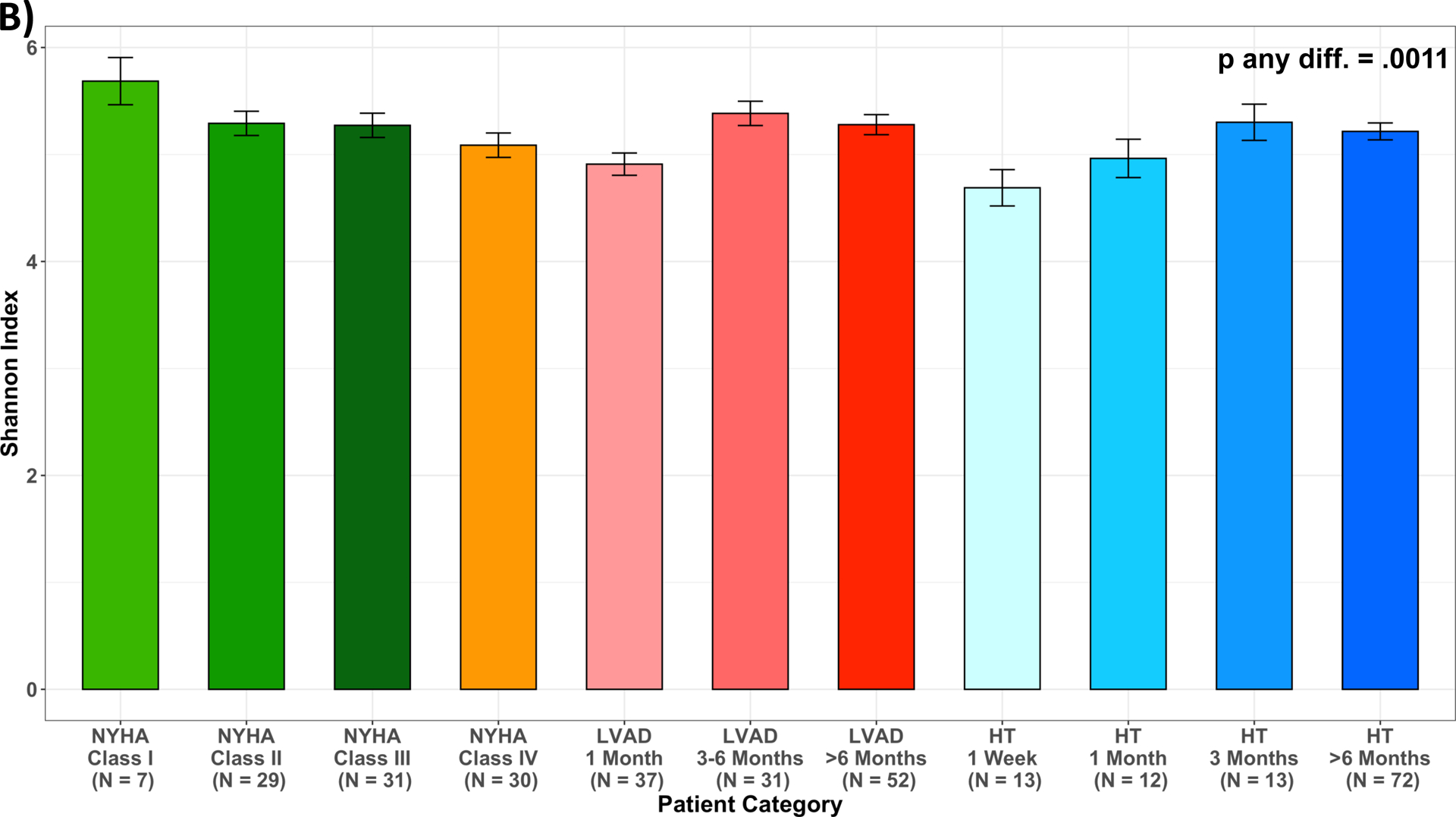
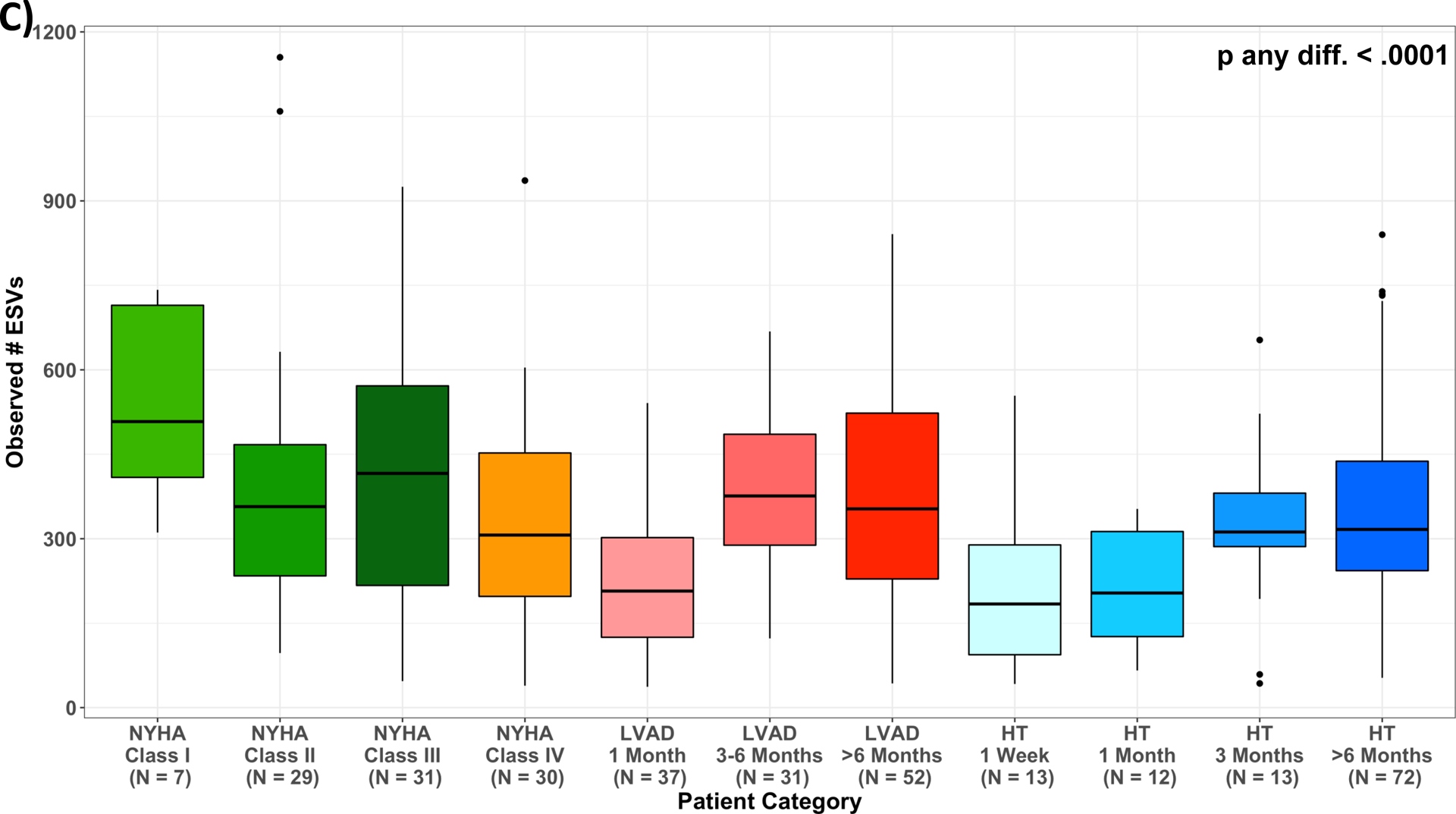
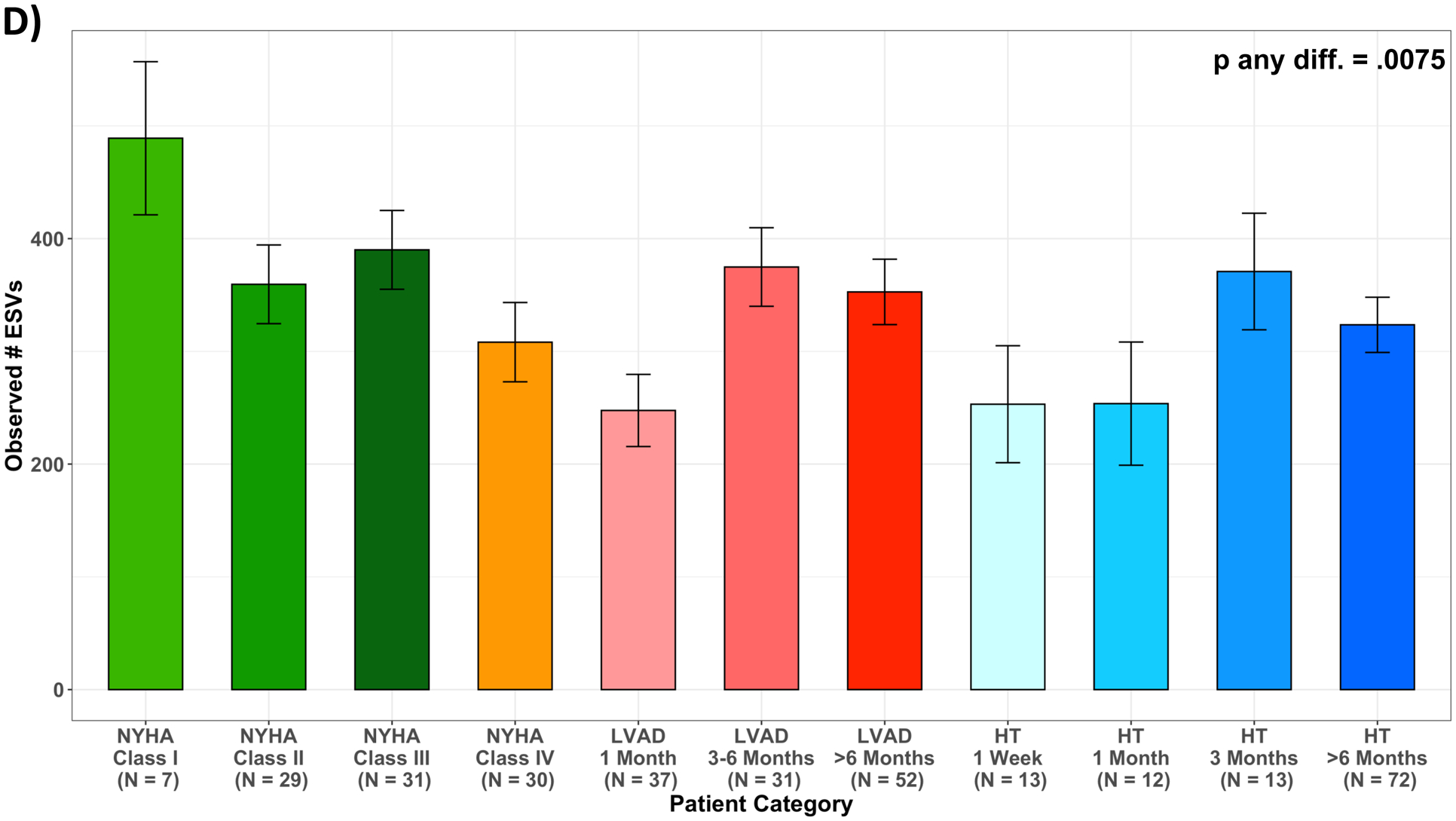
Measures of alpha diversity across patient categories. (A) unadjusted Shannon Index; (B) least squared means of Shannon Index, adjusted for age, sex, race/ethnicity, and antibiotics use one month prior to stool collection (M2); (C) unadjusted number of Observed ESVs; (D) least squared means of number of Observed ESVs, adjusted for age, sex, race/ethnicity, and antibiotics use one month prior to stool collection (M2); Multiple pairwise comparisons had p-value < 0.05, summarized in Supplemental Table 3; HF, heart failure; HT, heart transplant; LVAD, left ventricular assist device.
No significant correlation was found between Shannon Index or number of observed ESVs with ln-TMAO levels for the entire cohort (r=0.07, p=0.21; r=0.02, p=0.71, respectively) (Figure VIA&VIB). Similarly, no significant correlation was found after adjustment for age, sex, race/ethnicity, antibiotics use and renal indices (M3 (eGFRCr) (p=0.11, p=0.46, respectively) and M4 (eGFRCys) (p=0.10, p=0.37, respectively - data not shown). In analyses at the individual taxa level, we found no ESVs to be associated with TMAO levels after multivariable adjustment and multiple comparisons correction (data not shown).
Circulating TMAO as predictor of clinical outcomes in HF and LVAD patients
Among 112 NYHA Class I-III patients, 16 underwent LVAD implant, 3 were transplanted and 11 died over a median follow up of 1006 [IQR: 609–1278] days. The hazard ratio summarizing the rate of adverse events related to a one unit increase in ln-TMAO levels follows and was not statistically significant: HR 1.24 (CI 0.84–1.83), p=0.28. This result remained non-significant after adjusting for age and sex: HR 1.28 (0.85–1.92), p=0.22.
Among 75 NYHA Class IV patients: 49 underwent LVAD surgery, 10 were transplanted and 10 died during follow up. Of the 49 LVAD patients, 8 died after implant at a median follow up of 470 [IQR: 335–777] days. The hazard ratio summarizing the rate of death related to a one unit increase in ln-TMAO levels follows and was not statistically significant: HR 1.26 (0.52–3.09), p=0.60. This result remined non-significant after adjusting for age and sex: HR 1.68 (0.67–4.25), p =0.27.
DISCUSSION
The present study compared TMAO levels across a large cohort of patients with systolic HF at various stages of disease progression (NYHA Class I–IV) and after treatment with LVAD or HT. The main findings are:
TMAO levels progressively increased with HF severity and were similarly elevated, long-term after LVAD and HT. Notably, these levels were comparable to those previously reported in CVD and CKD.
TMAO levels inversely related to renal function using both sCr- or CysC-based equations although significant discordance in eGFR, between these two methods were observed. Specifically, eGFRCys was significantly lower than eGFRCr in advanced HF (class IV) as well as early after LVAD (1 month, 3–6 month) and HT (1week, 1 month).
Following LVAD and HT, TMAO levels transiently declined early postoperatively then progressively increased. These differences in TMAO early after HT were statistically significant after multivariable adjustments that included renal function based on CysC but were meaningfully attenuated when sCr was used.
TMAO levels positively related to biomarkers of inflammation (TNF-α, ET-1), endotoxemia (sCD14) and oxidative stress (isoprostane), although these associations lost significance after adjustment for baseline characteristics and renal function.
There was no association between TMAO and gut alpha diversity metrics for the entire cohort studied, and this remained unchanged after the above-mentioned adjustments.
Lastly, among a small subset of NYHA Class I-III and of Class IV HF (pre-LVAD), patients with higher TMAO levels had an empirically higher risk for adverse events, although findings were not statistically significant in these exploratory analyses and require confirmation in future studies.
Elevated TMAO levels have been consistently linked to increased risk of cardiovascular events such as death and myocardial infarction9, 32, 33. In HF, circulating TMAO has been shown to be independent predictor of survival and rehospitalization11, 34, 35. The mechanisms implicating TMAO in the pathophysiology of cardiac dysfunction are not fully understood, although in a recent study using a transaortic constriction model of HF, mice fed on a treated high-choline diet had adverse ventricular remodeling and marked increase in cardiac fibrosis10. Other factors, such as direct suppressive effects of TMAO on myocardial function through inhibition of actomyosin activity have also been implicated36. However, the observation that TMAO levels remain elevated long-term after cardiac replacement therapy, albeit preliminary, are associated with premature mortality after LVAD, has not been previously reported. A single study examined the relationship between TMAO and post-HT related outcomes such as cardiac allograft vasculopathy (CAV)37. In that report, half of the patients (n=30) were maintained on a CNI regimen with cyclosporine and half (n=32) were on everolimus based immunosuppression. Consistent with our findings, TMAO levels were elevated 3 years after HT in patients with CNIs when compared to healthy controls. However, in contrast to our results, a progressive decline in TMAO was observed among HT patients treated with everolimus. This inconsistency might be attributed to inherent differences in patients’ baseline characteristics and immunosuppressive regimen (e.g. the majority of our HT patients were maintained on tacrolimus - an alternative CNI). Finally, while the prior report did not find an association between TMAO and CAV, our study did not examine this relationship due to small sample size and limited longitudinal data. Larger studies are warranted to further test the potential pathogenic and prognostic role of TMAO in this unique population and investigate the differential effects of immunosuppressive regimens on TMAO levels.
It is well established that circulating TMAO levels are inversely related to renal function, and our findings are in agreement with prior reports16, 38. TMAO clearance is largely dependent on urinary excretion15. However, in animal models, elevated TMAO levels and its primary source, dietary choline, led directly to progressive renal tubule-interstitial fibrosis16; while, select targeting of TMAO metabolism may prevent or retard CKD development39. Advanced HF is commonly associated with significant CKD, the etiology of which is thought to be multifactorial. While hemodynamically driven reduction in renal perfusion is a well-known contributor to CKD, renal dysfunction often persists or continues to progress even after LVAD and HT despite improvement or normalization of hemodynamics40, 41. Although it is true that years of HF may lead to irreversible kidney damage, it is conceivable that after LVAD and HT, an ongoing renal insult is occurring not only from nephrotoxic medications such as CNIs, but also from the detrimental effects of circulating gut metabolites like TMAO. Notably, after multiple adjustments (including eGFRcys), TMAO levels were at least 2-fold higher in our cohort of long-term LVAD and HT patients compared with those previously reported in healthy controls24, potentially translating in sustained and progressive nephrotoxicity.
The concentration of sCr is influenced by changes in muscle metabolism and protein intake, which are prevalent in advanced HF and early after LVAD or HT. Conversely., Cystatin C is not affected by muscle mass or diet42, and is less impacted by age, sex and race than sCr, and, therefore, is the renal biomarker recommended to be used in advanced disease states43, 44. Our study was the first to analyze the association of TMAO with both eGFRCr and eGFRCys in a large cohort of HF, LVAD and HT patients. Not surprisingly, the eGFR was consistently higher across all study groups when calculated using sCr rather than CysC. We also observed a transient decline in TMAO levels early after LVAD and HT that remained significant only after adjustment for eGFRCys rather than eGFRCr in HT patients. Dietary changes and antibiotic use for prophylaxis or treatment of infections during the early postoperative period may account, at least partially, for these findings. Wang et al pointed to a major contributing role for the intestinal microbiota in the production of TMAO through its suppression by means of oral antibiotics and then reacquisition of TMAO production from dietary phosphatidylcholine after withdrawal of antibiotics and subsequent intestinal recolonization45. More generally, antibiotics have been shown to alter the gut microbiota and reduce community diversity in the days and weeks following treatment. However, these alterations are ephemeral and while recovery may be incomplete, diversity shifts back to partially resemble pre-treatment microbial community structure within one to six months46, 47. Our results are in agreement with these findings, as they indicate that the observed declines in TMAO levels are accompanied by transient declines in standard metrics of gut microbial diversity, such as Shannon Index and number of observed ESVs, early after LVAD and HT surgery. Interestingly, no significant correlation was observed between microbial diversity metrics and TMAO levels. This is not surprising as gut microbial diversity metrics are broad and reflect community characteristics, but do not precisely capture nuanced variation in the taxa that are responsible for TMAO production (only a subset of taxa express TMA lyases48). Our lack of findings even at the individual taxa level could be due to low power in our current study or lack of metagenomic and/or strain level information necessary to more directly interrogate the functional capacity of the microbiota to produce TMAO. Future mechanistic studies are warranted to test these hypotheses more rigorously.
The relationship between TMAO and markers of inflammation and endotoxemia, which are commonly associated with severity and progression of HF, has been elusive. Troseid et al reported on the prognostic relevance of TMAO among chronic HF patients35, and concordant with our results, found a graded increase in unadjusted TMAO as the disease severity rises. Although the levels of endotoxemia (LPS) and inflammation (CRP) were elevated among these patients, no association with TMAO was found. Similarly, in chronic HF patients, Tang et al did not find any correlations between TMAO and other substrates of TMAO formation (choline, betaine) with hsCRP11. Our findings are in agreement with these reports, as we also show no significant correlation between TMAO and hsCRP or IL-6. In addition, while TMAO levels were associated with other biomarkers of systemic inflammation, endotoxemia and oxidative stress (TNF-α, ET1, sCD14, isoprostane), these associations lost significance when baseline characteristics and renal function were accounted for. Although these results suggest that TMAO might be a surrogate marker of renal dysfunction and/or other co-morbidities, we cannot exclude that this metabolite has direct pro-inflammatory and even cardio- and nephrotoxic effects based on mechanisms shown in animal models (see above).
Some limitations should be acknowledged. No dietary intake (including red meat and fish that may influence TMAO levels) or other behavioral variables were collected. The use of food frequency questionnaire should be included in future studies aiming to provide knowledge on habitual dietary intake over a longer period. We did not directly measure GFR using renal clearance of iothalamate or iohexol, thereby limiting our ability to definitively conclude which filtration marker provides the most accurate eGR. However, several reports indicate that CysC outperforms sCr, when compared with the above gold standards, in other patient populations that are relevant to the present study such as HF, CKD and cardiovascular surgery. Although several inflammatory biomarkers associated with HF severity were studied, interleukin-1 was not part of our panel. There is a clear difference in medication use among groups. As expected, HT patients adhere to an immunosuppression regimen, and we observed an uneven distribution of HF medications utilization among NYHA Class IV and LVAD (1 month) patients, likely due to reduced tolerability to these drugs. Notably, concurrent medications, including statins and immunosuppressive agents such as mycophenolic acid, corticosteroids and everolimus, may alter the gut microbiota and affect TMAO production49, 50. Finally, the observational nature of our study precludes any inferences regarding causality. Prospective studies, controlled for the differential effects of drugs, are warranted to address all these key issues.
In conclusion, we demonstrated that long-term LVAD or HT therapy does not normalize TMAO levels in HF patients. There were clear discrepancies in estimation of GFR using sCr vs. CysC, particularly in early postoperatively (LVAD and HT), that meaningfully influenced interpretation of TMAO results. TMAO levels do not correlate with biomarkers of inflammation, endotoxemia and oxidative stress after adjustment for renal function. No relationship was found between TMAO and established metrics of microbial gut diversity. Whether pharmaceutical and/or nutritional interventions that modify TMAO levels may improve outcomes in HF, LVAD and HT patients remains to be investigated.
Supplementary Material
What is new?
This study investigated, for the first time, circulating levels of trimethylamine N-Oxide (TMAO) across a broad spectrum of heart failure (HF) disease progression and following treatment with left ventricular assist devices (LVADs) and heart transplantation (HT).
We found that TMAO levels progressively increase with HF severity and remain elevated long-term after LVAD and HT; inversely relate to renal function using both serum creatinine- or Cystatin C-based estimations; and do not correlate with established measures of inflammation, endotoxemia, oxidative stress and gut dysbiosis.
WHAT ARE THE CLINICAL IMPLICATIONS?
Trimethylamine N-Oxide is a gut-derived metabolite that has been linked to HF pathogenesis and prognosis. In the present study, we demonstrate that hemodynamic improvements after LVAD or HT do not translate into reductions in TMAO levels.
Future studies are warranted to: 1) investigate clinical implications of elevated TMAO levels following LVAD and HT; and 2) identify novel therapeutics and/or potential nutritional interventions that may modify TMAO levels with the goal to improve outcomes among HF, LVAD and HT patients
Sources of Funding
This research has been supported by funds from the Susan and Lowell McAdam Heart Failure Research Initiative and the Lisa and Mark Schwartz Program to Reverse Heart Failure at New York-Presbyterian Hospital/Columbia University.
Non-standard Abbreviations and Acronyms
- CysC
Cystatin C
- eGFRCys
Cystatin C-estimated glomerular filtration rate
- eGFRCr
Creatinine-estimated glomerular filtration rate
- IL-6
Interleukin-6
- ET-1
Endothelin-1
- LPS
lipopolysaccharide
- sCD14
soluble CD14
Footnotes
Disclosures
Dr Colombo is recipient of a research grant from Abbott; he also serves as a consultant (with no honoraria) for the same company. Dr Naka serves as a consultant for Abbott. Dr Uriel serves on advisory boards for Leviticus and Livemetric/Cormetric. The other authors report no conflicts. Dr. Mohan is supported by the NIH (R01 MD014161, R01 DK114893 and U01 DK116066), NSF (2032726) and is a member of the scientific advisory board for Angion Biomedica
Supplemental Materials:
Supplemental Methods
Supplemental Figures I-VI
Supplemental Tables I-VII
REFERENCES
- 1.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM et al. American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention and Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K et al. Investigators P-H and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J et al. Committees D-HT and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 4.van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, Zimetbaum PJ, Gerritse B, Kristiansen N and Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470–7. [DOI] [PubMed] [Google Scholar]
- 5.Levine B, Kalman J, Mayer L, Fillit HM and Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. [DOI] [PubMed] [Google Scholar]
- 6.Kummen M, Mayerhofer CCK, Vestad B, Broch K, Awoyemi A, Storm-Larsen C, Ueland T, Yndestad A, Hov JR and Troseid M. Gut Microbiota Signature in Heart Failure Defined From Profiling of 2 Independent Cohorts. J Am Coll Cardiol. 2018;71:1184–1186. [DOI] [PubMed] [Google Scholar]
- 7.Luedde M, Winkler T, Heinsen FA, Ruhlemann MC, Spehlmann ME, Bajrovic A, Lieb W, Franke A, Ott SJ and Frey N. Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail. 2017;4:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, Schroedl W, Karhausen T, Doehner W, Rauchhaus M et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–9. [DOI] [PubMed] [Google Scholar]
- 9.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y and Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL et al. Choline Diet and Its Gut Microbe-Derived Metabolite, Trimethylamine N-Oxide, Exacerbate Pressure Overload-Induced Heart Failure. Circ Heart Fail. 2016;9:e002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y and Hazen SL. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64:1908–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang WH, Wang Z, Shrestha K, Borowski AG, Wu Y, Troughton RW, Klein AL and Hazen SL. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2015;21:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL and Tang WH. Plasma Trimethylamine N-Oxide, a Gut Microbe-Generated Phosphatidylcholine Metabolite, Is Associated With Atherosclerotic Burden. J Am Coll Cardiol. 2016;67:2620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bain MA, Faull R, Milne RW and Evans AM. Oral L-carnitine: metabolite formation and hemodialysis. Curr Drug Metab. 2006;7:811–6. [DOI] [PubMed] [Google Scholar]
- 15.Bell JD, Lee JA, Lee HA, Sadler PJ, Wilkie DR and Woodham RH. Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: identification of trimethylamine-N-oxide. Biochim Biophys Acta. 1991;1096:101–7. [DOI] [PubMed] [Google Scholar]
- 16.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS and Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassus J, Harjola VP, Sund R, Siirila-Waris K, Melin J, Peuhkurinen K, Pulkki K, Nieminen MS and group F-AS. Prognostic value of cystatin C in acute heart failure in relation to other markers of renal function and NT-proBNP. Eur Heart J. 2007;28:1841–7. [DOI] [PubMed] [Google Scholar]
- 18.Shlipak MG, Katz R, Fried LF, Jenny NS, Stehman-Breen C, Newman AB, Siscovick D, Psaty BM and Sarnak MJ. Cystatin-C and mortality in elderly persons with heart failure. J Am Coll Cardiol. 2005;45:268–71. [DOI] [PubMed] [Google Scholar]
- 19.Ravn B, Prowle JR, Martensson J, Martling CR and Bell M. Superiority of Serum Cystatin C Over Creatinine in Prediction of Long-Term Prognosis at Discharge From ICU. Crit Care Med. 2017;45:e932–e940. [DOI] [PubMed] [Google Scholar]
- 20.Tang WHW, Dupont M, Hernandez AF, Voors AA, Hsu AP, Felker GM, Butler J, Metra M, Anker SD, Troughton RW et al. Comparative assessment of short-term adverse events in acute heart failure with cystatin C and other estimates of renal function: results from the ASCEND-HF trial. JACC Heart Fail. 2015;3:40–49. [DOI] [PubMed] [Google Scholar]
- 21.Dupont M, Wu Y, Hazen SL and Tang WH. Cystatin C identifies patients with stable chronic heart failure at increased risk for adverse cardiovascular events. Circ Heart Fail. 2012;5:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, Krankel N, Widera C, Sonnenschein K, Haghikia A et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Predicts Risk of Cardiovascular Events in Patients With Stroke and Is Related to Proinflammatory Monocytes. Arterioscler Thromb Vasc Biol. 2018;38:2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goh CE, Trinh P, Colombo PC, Genkinger JM, Mathema B, Uhlemann AC, LeDuc C, Leibel R, Rosenbaum M, Paster BJ et al. Association Between Nitrate-Reducing Oral Bacteria and Cardiometabolic Outcomes: Results From ORIGINS. J Am Heart Assoc. 2019;8:e013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy S, Yuzefpolskaya M, Nandakumar R, Colombo PC and Demmer RT. Plasma Trimethylamine-N-oxide and impaired glucose regulation: Results from The Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS). PLoS One. 2020;15:e0227482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL et al. Investigators C-E. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F and Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- 27.Pinsino A, Mondellini GM, Royzman EA, Hoffman KL, D’Angelo D, Mabasa M, Gaudig A, Zuver AM, Masoumi A, Garan AR et al. Cystatin C- Versus Creatinine-Based Assessment of Renal Function and Prediction of Early Outcomes Among Patients With a Left Ventricular Assist Device. Circ Heart Fail. 2020;13:e006326. [DOI] [PubMed] [Google Scholar]
- 28.Yuzefpolskaya M, Bohn B, Nasiri M, Zuver AM, Onat DD, Royzman EA, Nwokocha J, Mabasa M, Pinsino A, Brunjes D et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J Heart Lung Transplant. 2020;39:P880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callahan T, Barnard J, Helmkamp L, Maertens J and Kahn M. Reporting Data Quality Assessment Results: Identifying Individual and Organizational Barriers and Solutions. EGEMS (Wash DC). 2017;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callahan TJ, Bauck AE, Bertoch D, Brown J, Khare R, Ryan PB, Staab J, Zozus MN and Kahn MG. A Comparison of Data Quality Assessment Checks in Six Data Sharing Networks. EGEMS (Wash DC). 2017;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer KA, Benton TZ, Bennett BJ, Jacobs DR Jr., Lloyd-Jones DM, Gross MD, Carr JJ, Gordon-Larsen P and Zeisel SH. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan Y, Sheng Z, Zhou P, Liu C, Zhao H, Song L, Li J, Zhou J, Chen Y, Wang L et al. Plasma Trimethylamine N-Oxide as a Novel Biomarker for Plaque Rupture in Patients With ST-Segment-Elevation Myocardial Infarction. Circ Cardiovasc Interv. 2019;12:e007281. [DOI] [PubMed] [Google Scholar]
- 33.Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki T, Yazaki Y, Voors AA, Jones DJL, Chan DCS, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL et al. Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure: results from BIOSTAT-CHF. Eur J Heart Fail. 2019;21:877–886. [DOI] [PubMed] [Google Scholar]
- 35.Troseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjorndal B, Halvorsen B et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med. 2015;277:717–26. [DOI] [PubMed] [Google Scholar]
- 36.Kumemoto R, Yusa K, Shibayama T and Hatori K. Trimethylamine N-oxide suppresses the activity of the actomyosin motor. Biochim Biophys Acta. 2012;1820:1597–604. [DOI] [PubMed] [Google Scholar]
- 37.Troseid M, Mayerhofer CCK, Broch K, Arora S, Svardal A, Hov JR, Andreassen AK, Gude E, Karason K, Dellgren G et al. The carnitine-butyrobetaine-TMAO pathway after cardiac transplant: Impact on cardiac allograft vasculopathy and acute rejection. J Heart Lung Transplant. 2019;38:1097–1103. [DOI] [PubMed] [Google Scholar]
- 38.Missailidis C, Hallqvist J, Qureshi AR, Barany P, Heimburger O, Lindholm B, Stenvinkel P and Bergman P. Serum Trimethylamine-N-Oxide Is Strongly Related to Renal Function and Predicts Outcome in Chronic Kidney Disease. PLoS One. 2016;11:e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta N, Buffa JA, Roberts AB, Sangwan N, Skye SM, Li L, Ho KJ, Varga J, DiDonato JA, Tang WHW et al. Targeted Inhibition of Gut Microbial Trimethylamine N-Oxide Production Reduces Renal Tubulointerstitial Fibrosis and Functional Impairment in a Murine Model of Chronic Kidney Disease. Arterioscler Thromb Vasc Biol. 2020;40:1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshioka D, Takayama H, Colombo PC, Yuzefpolskaya M, Garan AR, Topkara VK, Han J, Kurlansky P, Naka Y and Takeda K. Changes in End-Organ Function in Patients With Prolonged Continuous-Flow Left Ventricular Assist Device Support. Ann Thorac Surg. 2017;103:717–724. [DOI] [PubMed] [Google Scholar]
- 41.Lindelow B, Bergh CH, Herlitz H and Waagstein F. Predictors and evolution of renal function during 9 years following heart transplantation. J Am Soc Nephrol. 2000;11:951–7. [DOI] [PubMed] [Google Scholar]
- 42.Tangri N, Stevens LA, Schmid CH, Zhang YL, Beck GJ, Greene T, Coresh J and Levey AS. Changes in dietary protein intake has no effect on serum cystatin C levels independent of the glomerular filtration rate. Kidney Int. 2011;79:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T and Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006;21:660–4. [DOI] [PubMed] [Google Scholar]
- 44.White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N and Knoll GA. Estimating glomerular filtration rate in kidney transplantation: a comparison between serum creatinine and cystatin C-based methods. J Am Soc Nephrol. 2005;16:3763–70. [DOI] [PubMed] [Google Scholar]
- 45.Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y and Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3:1255–1265. [DOI] [PubMed] [Google Scholar]
- 47.Dethlefsen L and Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rath S, Heidrich B, Pieper DH and Vital M. Uncovering the trimethylamine-producing bacteria of the human gut microbiota. Microbiome. 2017;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flannigan KL, Taylor MR, Pereira SK, Rodriguez-Arguello J, Moffat AW, Alston L, Wang X, Poon KK, Beck PL, Rioux KP et al. An intact microbiota is required for the gastrointestinal toxicity of the immunosuppressant mycophenolate mofetil. J Heart Lung Transplant. 2018;37:1047–1059. [DOI] [PubMed] [Google Scholar]
- 50.Tuteja S and Ferguson JF. Gut Microbiome and Response to Cardiovascular Drugs. Circ Genom Precis Med. 2019;12:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, Parikh CR and Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crespo-Leiro MG, Delgado JF, Paniagua MJ, Vazquez de Prada JA, Fernandez-Yanez J, Almenar L, Diaz-Molina B, Roig E, Arizon JM, Alonso-Pulpon L et al. Prevalence and severity of renal dysfunction among 1062 heart transplant patients according to criteria based on serum creatinine and estimated glomerular filtration rate: results from the CAPRI study. Clin Transplant. 2010;24:E88–93. [DOI] [PubMed] [Google Scholar]
- 53.Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


