Two recent clinical trials with sodium glucose co-transporter-2 (SGLT2) inhibitors, dapagliflozin and empagliflozin, have now demonstrated a reduction in heart failure (HF) hospitalization or cardiovascular mortality risk in patients with HF and reduced ejection fraction (HFrEF), independent of the presence of comorbid diabetes.1 While the pre-specified subgroup analyses examining the efficacy of SGLT2 inhibitors in patients with HFrEF show a consistent benefit across subgroups irrespective of race-ethnicity, there appears to be a signal of a potentially greater effect in Black and Asian patients randomized to treatment compared to other groups (Figure 1A). The ability to show this degree of benefit in Black patients, who only made up 4–7% of the patients enrolled, is compelling and merits further investigation given the overwhelming disparities in risk, prevalence, and clinical outcomes for Black patients with HFrEF. The ~50% relative risk reduction in the primary endpoint demonstrates an effect size in Black patients that is striking, unlikely to be due to chance, and should not be ignored.
Figure 1.
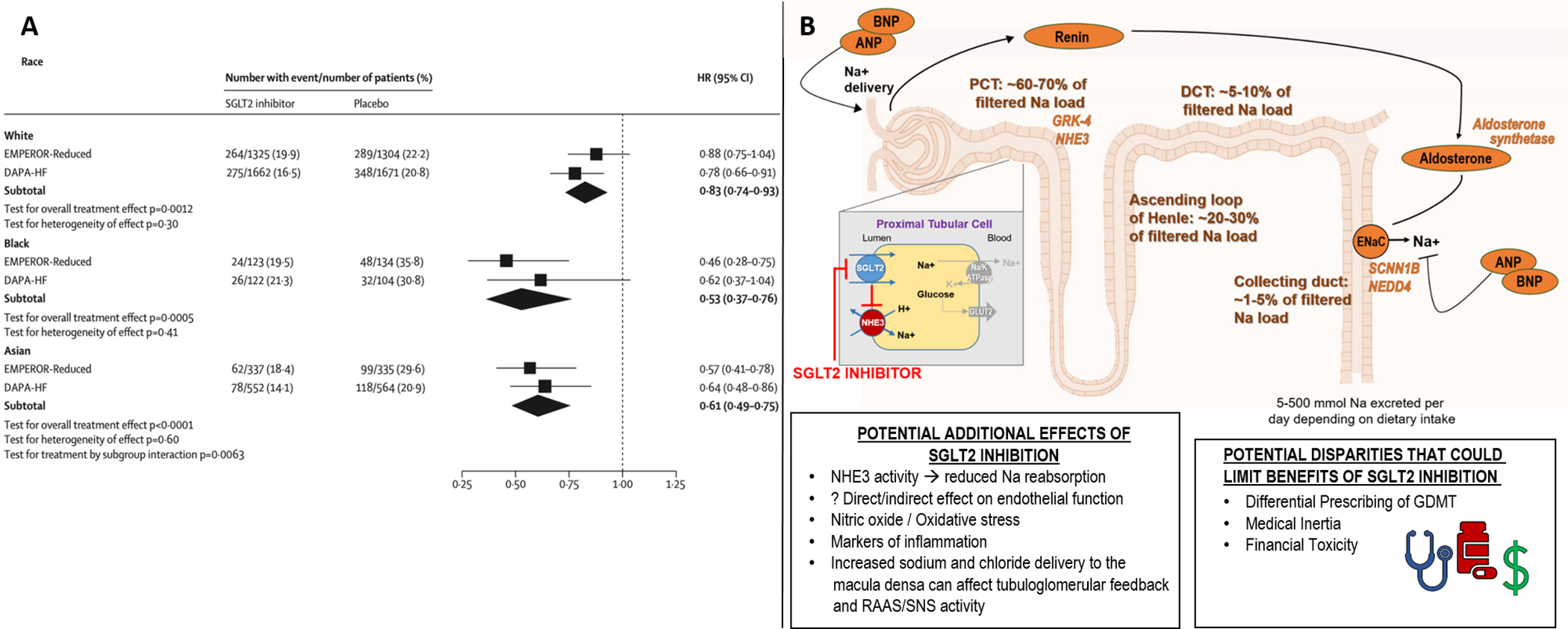
Effect of SGLT2 inhibitors on primary composite outcome according to pre-specified subgroup analyses by race-ethnicity (A); and potential mechanisms for racial differences that could explain overwhelming benefit of SGLT2-inhibitors in Black patients with HFrEF, as well as racial disparities that could impact real-world access to SGLT2-inhibitors for Black patients (B).
A meta-analysis of the pooled treatment effects of empagliflozin and dapagliflozin on the composite endpoint of cardiovascular death or first hospitalization for HF in subgroups by race-ethnicity demonstrates a greater effect in Black patients (Panel A, modified with permission from Zannad et al. Lancet 2020; 396: 819–29). Numerous mechanisms have been suggested that may contribute to higher renal reabsorption of sodium in subjects of African ancestry, with biomarkers or receptors with known racial differences depicted in orange (Panel B). The putative decongestive properties induced through use of SGLT2 inhibitors are outlined, and may help overcome this pathophysiology, translating to improved clinical outcomes for Black patients enrolled in these clinical trials. However, various mechanisms could reduce access to SGLT2 inhibitors for Black patients, serving to actually worsen pre-existing healthcare disparities. ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; ENaC, epithelial sodium channel; GDMT, guideline directed medical therapy; GLUT2, glucose transporter 2; GRK-4, G protein--coupled receptor kinase 4; NEDD4, neural precursor cell expressed, developmentally downregulated 4; NHE3, sodium-hydrogen exchanger 3; RAAS, renin angiotensin aldosterone system; SNS, sympathetic nervous system; SCNN1B, sodium channel epithelial 1 beta subunit.
To understand the potential benefits of SGLT2 inhibition, it is worth noting the distinction between racial differences and disparities, both of which contribute to the unique epidemiology of HF in Black patients. According to the Institute of Medicine, health disparities are differences that result from the operation of healthcare systems, legal and regulatory climates, as well as discriminatory biases, stereotyping and uncertainty.2 Conversely, racial differences are defined as clinical, biological, genetic, or epigenetic factors associated with disease risk, outcome, or treatments not caused by social factors.2 Our central hypothesis is that the pleiotropic effects on decongestion, blood pressure, and other factors induced by treatment with SGLT2 inhibitors are of particular benefit for HF patients with impaired natriuresis, particularly Black patients whose renal sodium handling may be uniquely influenced by ancestry and predispose to volume retention. Without proper attention, however, health disparities including differential prescribing patterns by clinicians, financial toxicity of novel therapeutic agents, and other factors may attenuate the clinical efficacy of SGLT2 inhibitors in a real-world setting.
Low urinary sodium concentration predicts adverse outcomes in HF. SGLT2 inhibitors induce natriuresis by blocking sodium reabsorption in the proximal convoluted tubule (Figure 1B), resulting in decreased blood volume due to both osmotic and natriuretic diuretic effects, particularly when combined with loop diuretics.3 In subjects with hypertension and/or HF, hyperactivity of the sympathetic nervous and the renin-angiotensin-aldosterone systems leads to subtle renal microvascular and tubulointerstitial injury, and an imbalance in the expression of vascular tone in favor of vasoconstriction, impairing urinary sodium excretion. Although this pathophysiology occurs in many patients, it may be particularly relevant for Black individuals who have the highest prevalence of salt sensitivity (up to 75% of Black individuals with hypertension) and the highest prevalence of HF. Prior studies have documented a higher renal reabsorption of sodium in persons of African ancestry (Figure 1B), suggestive of higher renal sodium avidity.4 After similar states of sodium and volume loading, Black subjects excrete less urinary sodium than White subjects, with greater elevations in blood pressure and more fluid retention. Although no single genotype has been determined to be causative, this SS phenotype is characterized by lower levels of plasma renin and aldosterone in normotensives, hypertensives, as well as patients with HF. The additional natriuresis, reductions in plasma volume and blood pressure, and improvements in inflammation, oxidative stress, and overall metabolism induced through the use of SGLT2 inhibitors may help to overcome this pathophysiology, translating to markedly improved clinical outcomes for Black patients.
With the explosion of new therapeutic agents showing morbidity and mortality benefit in HFrEF, clinicians will have an unprecedented number of drugs to choose from to treat patients with HF. Despite robust trial data showing drug efficacy, medical inertia and other barriers often lead to slow uptake of novel therapies among providers. Despite higher rates of death and hospitalization from HF, Black patients are less likely to receive novel pharmacologic and device-based therapies, and are unlikely to receive established guideline-directed medical therapy with proven benefit, namely hydralazine-isosorbide dinitrate which remains grossly underutilized in this population. A recent retrospective analysis of Medicare claims data on >1 million US adults with diabetes demonstrated that Black patients were less likely than White patients to be prescribed SGLT2 inhibitors.5 Although the relative cost and potential for financial toxicity with these agents must be taken into account, failure to prescribe novel therapeutic compounds with proven efficacy in high-risk populations will only serve to exacerbate pre-existing healthcare disparities.
As Black patients have a high prevalence of HF, diabetes, and chronic kidney disease, as well as a high risk of adverse outcomes, the identification of a pharmaceutical class with such great potential for therapeutic benefit should be of great interest for patients, clinicians, and scientists alike. In this respect, further mechanistic research is warranted. Future studies might investigate racial differences in renal sodium avidity. Although the exact mechanisms underpinning SS are not entirely clear and there have been mixed results using other diuretics in HF, this physiology is a well-accepted etiology for racial differences in hypertension, but has been largely unexplored as a mechanistic contributor to volume overload in patients with HF, and may be associated with the disparate rates of HF hospitalization between Black individuals and other race-ethnic groups. An ongoing clinical trial will examine the financial burden associated with novel therapeutics for patients with HF (NCT04793880). Much work remains to achieve health equity in HF, however the results from these recent SGLT2 inhibitor trials offer a glimmer of hope.
DISCLOSURES
AAM has received grants from NHLBI (R03 HL146874), the Woodruff Foundation, and the Association of Black Cardiologists. JMT has received grants and personal fees on unrelated projects from Sequana Medical, BMS, 3ive labs, Boehringer Ingelheim, Sanofi, FIRE1, and personal fees from Astra Zeneca, Novartis, Cardionomic, Bayer, MagentaMed, Renalguard, W.L. Gore, and grants from Otsuka and Abbott. JB is a consultant for Abbott, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, Eli Lilly, G3 Pharmaceutical, Impulse Dynamics, Innolife, Janssen, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana, StealthPeptide, Vifor.
REFERENCES
- 1.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W and Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. The Lancet. 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 2.Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care 2003. [Google Scholar]
- 3.Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE and Testani JM. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation. 2020;142:1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spence JD and Rayner BL. Hypertension in Blacks. Hypertension. 2018;72:263–269. [DOI] [PubMed] [Google Scholar]
- 5.McCoy RG, Dykhoff HJ, Sangaralingham L, Ross JS, Karaca-Mandic P, Montori VM and Shah ND. Adoption of New Glucose-Lowering Medications in the U.S.—The Case of SGLT2 Inhibitors: Nationwide Cohort Study. Diabetes Technology & Therapeutics. 2019;21:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]


