Abstract
Objective:
Local activation of B cells and antibody production is important for protective and pathogenic immune responses. There is also evidence that local activation of B cells and antibody production is important in the pathogenesis of chronic rhinosinusitis with nasal polyps (CRSwNP), and a severe subset of this disease, aspirin-exacerbated respiratory disease (AERD). This review aims to summarize these findings and the potential role of B cells and antibodies in disease pathogenesis.
Data Sources:
Published literature from PubMed searches
Study Selections:
Studies relevant to B cell development and to the role of B cells and antibodies in the pathogenesis of CRSwNP and AERD.
Results:
Formation of tertiary lymphoid structures plays a key role in the local activation of B cells and antibody production. This process is important for fighting infections but also contributes to autoimmune disease. There is also evidence to support a role for local B cell activation and antibody production in a variety of allergic diseases. Nasal polyp tissues from patients with CRSwNP and AERD have elevated levels of activated B cell subsets and locally produced antibodies. These locally produced antibodies may contribute to disease pathogenesis in a variety of ways, including activation of innate effector cells, while locally activated B cells may contribute to pathogenesis through the activation of T cells.
Conclusions:
More studies are needed to determine the role of B cells and antibodies in driving disease in these patients. However, targeting the processes that drive local B cell activation and antibody production may provide new therapeutic approaches and could help to reduce chronic inflammation.
I. Introduction
Aspirin-exacerbated respiratory disease (AERD) is an adult onset respiratory disorder characterized by the triad of severe chronic rhinosinusitis with nasal polyps (CRSwNP), eosinophilic asthma, and pathognomonic upper and lower respiratory reactions to aspirin and other cyclooxygenase (COX)-1 inhibitors1. Chronic rhinosinusitis with nasal polyps is a sub classification of chronic rhinosinusitis (CRS) where patients have inflammatory outgrowths of sinus mucosa leading to nasal obstruction and anosmia2. Nasal polyps are markedly severe in AERD, often do not respond to standard therapies, and are associated with substantial medical resource utilization3–5. Following endoscopic sinus surgery patients with AERD often have rapid recurrence of nasal polyposis, and 85% of patients with AERD have regrowth of nasal polyps within two years of surgery6.
Nasal polyps in AERD and CRSwNP are characterized by dysregulated epithelium, activated B cells and plasma cells, T helper type 2 (Th2) inflammation, and mast cell and eosinophil infiltration7–13. Understanding the mechanisms underlying AERD and CRSwNP are necessary to develop improved diagnostic and therapeutic tools. Recent evidence supports a role for activated B cells and local antibody production in the pathogenesis of AERD and CRSwNP 12–14. Here we will review the immunobiology of B cell activation and antibody production in secondary and tertiary lymphoid organs, local antibody production in AERD and CRSwNP, and possible mechanisms by which locally produced antibodies may contribute to disease pathogenesis.
II. B cell activation and antibody production in secondary lymphoid organs
Peripheral B cell subsets
B cells develop in the bone marrow through a highly regulated and coordinated process. The details of their development is beyond the scope of this review, but an excellent review of this process has previously been published15. While the majority of B cells are B2 B cells, or follicular B cells, two other populations of peripheral B cell subsets also exist and play key roles in early immune responses, the marginal zone B cell and the B1 B cell16, but these will not be discussed further in this review. Once mature naïve B cells leave the bone marrow, they rapidly undergo two distinct transitional stages in the spleen before they become mature naïve B cells that circulate throughout the body in search of their cognate antigen. Once they encounter their cognate antigen, B cells then undergo the process of maturation and differentiation.
B cell activation and antibody production
B cell activation can occur via either a T cell-dependent or independent mechanism, depending on the nature of the antigen17, 18. There are two types of T cell-independent antigens. The first type, called a TI-1 antigen, can activate B cells independently of their B cell receptor specificity, and are commonly composed of TLR agonists such as LPS19. At high concentrations, these TI-1 antigens can provide a strong enough signal to induce B cell activation and antibody production, and this mechanism may be relevant in the context of local inflammation, such as in nasal polyps. The second type, called a TI-2 antigen, is a highly repetitive antigen that can crosslink multiple B cell receptors on the same cell, such as bacterial polysaccharides, which in the presence of pro-inflammatory cytokines, provides sufficient signal to induce activation19. In the case of TI-2 antigens, while T cells are not explicitly required, they may provide key activation signals through production of pro-inflammatory cytokines, like IL-220. These additional signals may also be provided through innate immune cells, such as NK cells or dendritic cells20, and this could also be an important mechanism for B cell activation within the inflammatory environment of nasal polyps.
While TI B cell activation can play an important part in the generation of antibody responses, the majority of antibodies are produced via T cell-dependent activation mechanisms. Classically, this B cell activation occurs in secondary lymphoid organs (SLOs). After their maturation in the bone marrow, mature naïve B cells generally circulate through SLOs due to their high expression of CXCR5, whose ligand CXCL13 is highly expressed by follicular stromal and dendritic cells in SLOs21, 22. SLOs, including the lymph nodes, Peyer’s patches, and spleen, develop during embryogenesis, are critical for immune homeostasis, and play an important role in the immune response to invading pathogens and inflammation23. Importantly, similar mechanisms may contribute to the local activation of B cells and antibody production at sites of inflammation. Once a naïve B cell encounters its cognate antigen, it upregulates expression of CCR7 allowing it to traffic to the T cell zone, where it will receive additional signals from cognate helper T cells that are required for its differentiation and production of antibodies22. Cognate antigen encounter also induces expression of co-stimulatory molecules by the B cells, including CD40 and MHCII, which enhance the interactions between cognate B and T cell pairs in the T cell zone24.
After this initial interaction with Th cells in the T cell zone, B cells can further differentiate into one of 3 subsets: extrafollicular plasmablasts, early memory cells, or germinal center (GC) B cells16. The first two of these are formed independently of the germinal center, and therefore produce lower affinity antibodies that are important for controlling early phases of infections, and they are similar to B cells activated by T-independent mechanisms16. B cells with BCRs that strongly recognize antigen are more likely to differentiate down the extrafollicular pathway, and these cells are also characterized by expression of Epstein-Barr Virus-Induced protein 2 (EBI2), which prevents them from trafficking back into the follicle, and they generally have a short life span of about 3–5 days25, 26. This extrafollicular mechanism may also promote the activation of autoreactive B cells and production of autoantibodies at local sites of inflammation27.
GC B cells, on the other hand, maintain expression of CXCR5 and traffic back into the follicle to participate in the GC reaction28. During this phase, B cells with the highest affinity BCRs will be selected for survival, undergo somatic hypermutation, and class switch recombination to generate high affinity IgG, IgA and IgE antibodies. These positively selected B cells will eventually exit the GC reaction as either long-lived memory B cells or plasma cells. Memory B cells will continue to circulate throughout SLOs and can be rapidly re-activated upon secondary encounter with their cognate antigen to become antibody-secreting plasma cells or new memory cells. Plasma cells can traffic to sites of inflammation to produce large quantities of high-affinity antibodies needed to control infections, or they can traffic to the bone marrow where they can survive for years in specialized niches and continually produce high affinity antibody that can be found in the circulation. While these mechanisms of B cell activation and antibody production have been well-established and are critical for the generation of protective immune responses to a variety of infections, there are other mechanisms that can contribute to B cell activation and antibody production at local sites of inflammation. These local activation events may play a critical role in the control of infections, such as influenza, but they may also contribute to the pathogenesis of a variety of inflammatory diseases and will be discussed in detail below.
III. Tertiary lymphoid structures have a role in local immunoglobulin production during acute inflammation
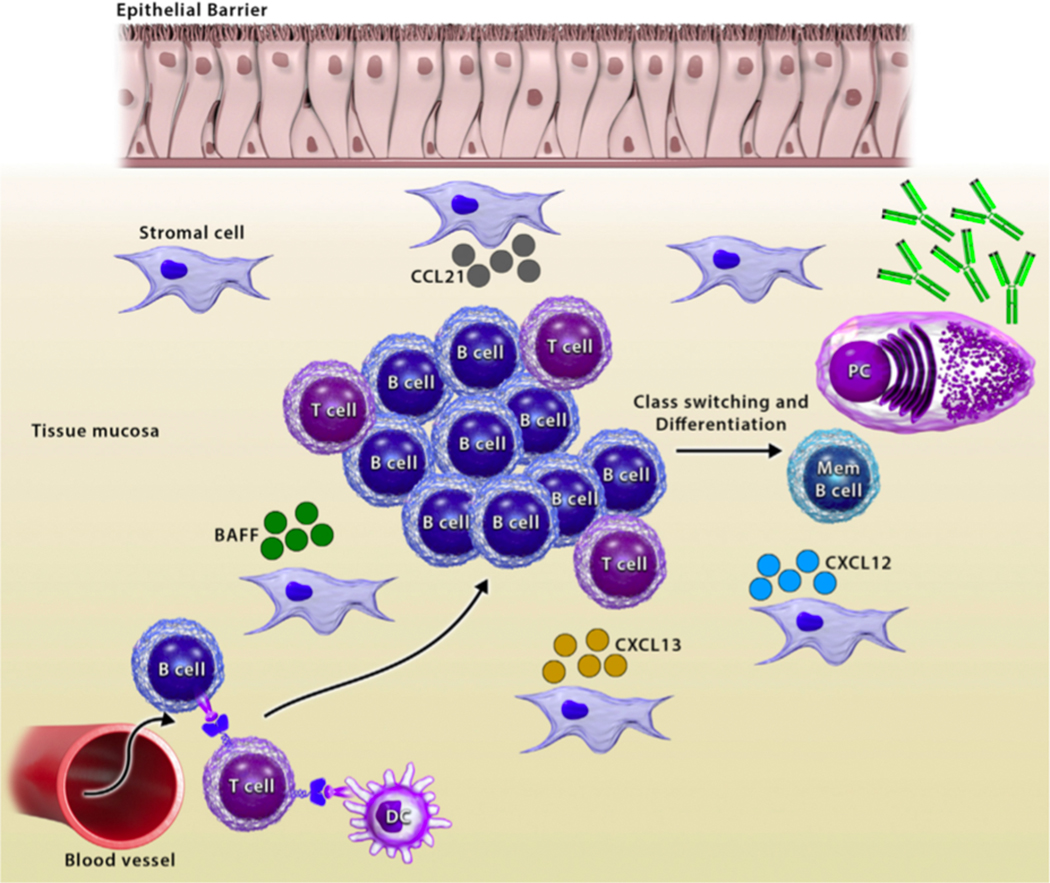
As discussed above, B cells generally become activated in SLOs through the GC reaction and subsequently traffic to sites of infection or inflammation to participate in the immune response, which results in formation of memory B cells and plasma cells that produce high affinity antibodies29. In contrast, tertiary lymphoid organs (TLOs), which are also referred to as ectopic lymphoid tissues, are not generated during embryonic development, but are induced by antigenic stimuli including infections, environmental insults, and self-antigens (Figure 1). Both the gut and the airways are common sites for the development of TLOs, referred to as gut- and bronchus-associated lymphoid tissues, respectively (GALT and BALT), in response to inflammation30, 31. Homeostatic chemokines and cytokines that are important for the formation of SLOs have also been shown to be necessary for formation of TLOs, including IL-7, CCL19, CCL20, CCL21, and CXCL1332-35. These homeostatic chemokines and cytokines can be induced in nonlymphoid tissues during periods of inflammation or infection, and they may help to recruit lymphocytes to the inflamed tissue34. B cells accumulate within TLOs in GC-like structures and can express high levels of activation induced cytidine deaminase, one of the key enzymes required for antibody class switching33, 36. Further, TLOs can form memory B cells that can be rapidly re-activated following reinfection37. The structure of TLOs is less organized than SLOs16, possibly resulting in reduced clonal selection and subsequent activation of autoreactive B- and T-cell clones, which may contribute to the development of inflammation or autoimmunity, similar to extrafollicular B cell responses31, 38.
Figure 1.
Formation of tertiary lymphoid structures. Lymphocytes are recruited into the tissue via chemokines. T cells are activated by dendritic cells (DCs), and they activate B cells, along with cytokines, like BAFF. Activated B and T cells form lymphoid aggregates where B cells proliferate, undergo class switching, and differentiate.
IV. Local antibody production CRSwNP, and AERD
Local antibody production in type 2 disease
It is well established that formation of TLOs can facilitate the local activation of B cells and antibody production that can contribute to control of infection as well as autoimmunity31, 39. This mechanism has also been implicated in the pathogenesis of type 2 inflammatory diseases, such as allergic rhinitis, asthma and CRSwNP. In allergic rhinitis, local B cell activation results in the activation of class switching to not only IgE, but also multiple IgG isotypes40–42. Interestingly, it has also been shown that this local class switching is elevated during the pollen season in pollen-allergic patients but is generally absent outside of the pollen season in those same patients43. This suggests that the local production of IgE plays a key role in driving allergic symptoms in these patients. Similarly in asthma, it has been demonstrated that local class switching to IgE occurs in the lungs, and that this IgE is functional and can induce the degranulation of mast cells in vitro42, 44, 45. Further, there is evidence for local class switching to IgA in asthmatic lungs, which may also play a role in the activation of eosinophils in disease46. Not surprisingly then, there is also evidence for local class switching to IgE in the gut of patients with peanut allergies47. A recent study utilized high throughput sequencing of antibodies obtained from gut biopsies and found evidence for local class switching to IgE in the gut of food allergic patients48. Interestingly, while many groups have shown that IgG+ B cells can be induced to undergo an additional round of class switching, called sequential switching, to IgE after local activation, this study found evidence that IgA+ B cells can undergo similar sequential switching events to become IgE-expressing cells48.
Local antibody production in CRSwNP and AERD
Both CRSwNP and AERD are characterized by local type 2 inflammatory environments. Over the last several years there has been accumulating evidence to support a key role for the local activation of B cells and antibody production in the pathogenesis of CRSwNP and AERD. Early studies found evidence for increased expression of B cell and plasma cell markers in nasal polyp tissues based on gene expression and IHC analyses8, 49. Later studies using flow cytometry analysis also found evidence for elevated levels of B cells, plasma cells, and plasmablasts in nasal polyps from CRSwNP patients12, 14. This work also found that plasmablasts in CRSwNP nasal polyps were more likely to be extrafollicular, based on their expression of EBI2, and that B cells from nasal polyps were potent antibody-secreting cells12. More recently, it has been shown that nasal polyps from AERD patients have even higher frequencies of plasma cells, in addition to elevated levels of antibodies, compared to polyps from CRSwNP patients13. The plasma cells in polyps from AERD patients were also more likely to express the IL-5Ra, suggesting that IL-5 may play an important role in their activation and/or antibody production13. In addition to elevated levels of antibody-secreting cells, elevated expression of germline transcripts, which are markers of local class switching events, have been reported in nasal polyps from CRSwNP patients12, 50, 51. Other studies have provided evidence that some of the local production of antibodies may be driven by super antigens derived from Staphylococcus aureus (SA)8, 51–53. These studies have demonstrated a link between levels of SA enterotoxins and IgE levels in nasal polyps, and they have identified enterotoxin-specific antibodies, which may play a role in promoting inflammation in some patients54. Altogether, these studies suggest that local switching not only to IgE, but also to IgG and IgA, may play important roles in the pathogenesis of nasal polyposis.
V. Local nasal polyp immunoglobulins may contribute disease pathogenesis
Immunoglobulin mediated activation of Fc receptor-expressing innate immune effector cells
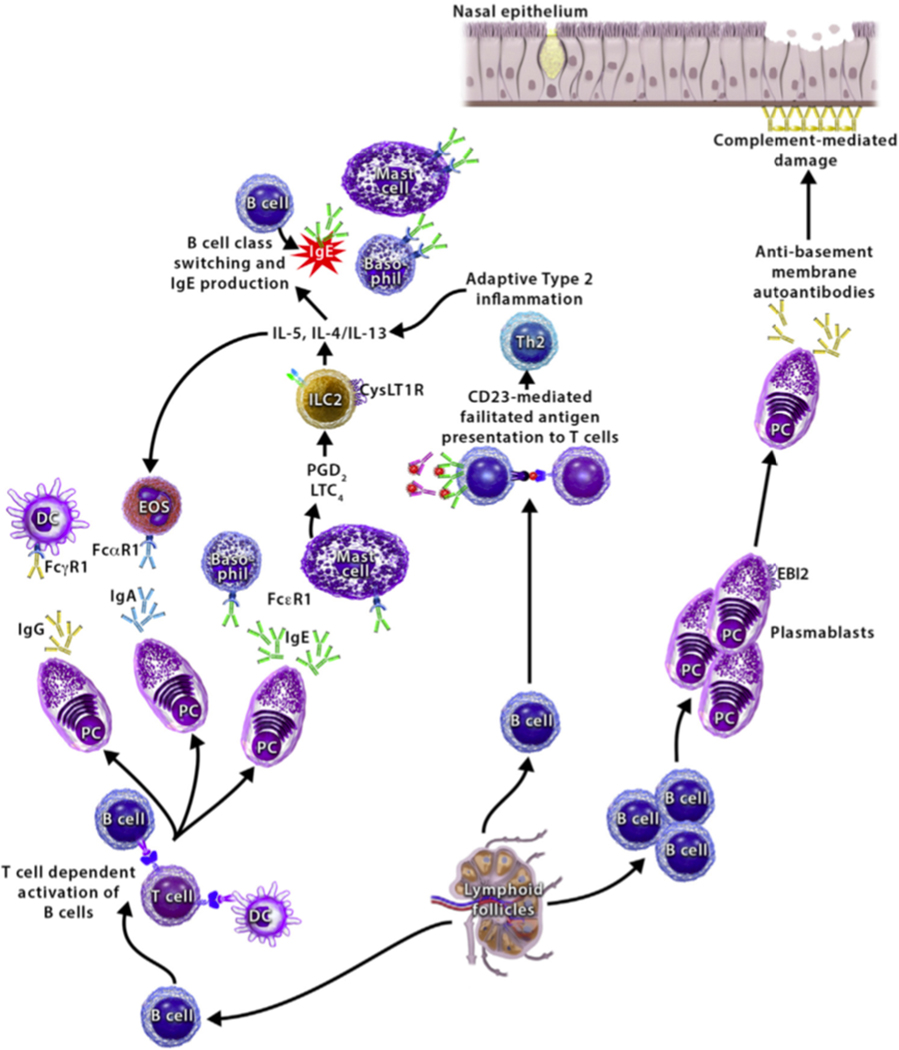
Given the increased local production of nasal polyp immunoglobulins in AERD, specifically IgE and IgG4, understanding the role these immunoglobulins have in disease pathogenesis may provide greater understanding of underlying immune dysregulation in AERD (Figure 2). One possible role of locally produced immunoglobulins in CRSwNP and AERD pathobiology is activation of Fc receptor-expressing innate immune effector cells. Locally generated IgE may lead to activation of mast cells, basophils, and other FcεRI-bearing effector cells in the nasal polyp tissue. Analysis of microparticles has shown increased activation of mast cells and basophils in subjects with AERD compared to aspirin-tolerant subjects with CRSwNPs55. It is known that mast cells infiltrate the nasal polyp tissue and bronchial mucosa in patients with AERD56–58. Mast cells release inflammatory mediators such as tryptase, histamine, leukotrienes, and prostaglandin(PG) D2 at high levels at baseline in AERD, and they are further activated during aspirin-induced reactions7, 59–61. Subjects with AERD who have the highest levels of urinary PGD-M, a metabolite of PGD2, have the most severe aspirin-induced reactions7, indicating that mast cell-derived PGD2, and other products of mast cell activation, likely contribute to the upper and lower airway tissue inflammation and bronchoconstriction seen in AERD. Further, previous studies have shown that use of drugs such as cromolyn and nedocromil, can block chronic inflammation and aspirin-induced reactions in AERD62, 63.
Figure 2.
Mechanisms of local antibody production in nasal polyps. (Left) locally produced antibodies activate innate effector cells, furthering type 2 inflammation. (Center) CD23-mediated facilitated antigen presentation by local B cells activates Th2 cells. (Right) autoreactive B cells and plasma cells produce autoantibodies leading to complement-mediated epithelial cell damage.
The cause of mast cell activation in AERD is unknown. As discussed above, patients with AERD have elevated serum64 and nasal13 IgE levels but lack classic atopy64. Recent studies have reported that treatment with omalizumab, a monoclonal antibody that binds IgE, decreases products of mast cell activation including PGD-M and leukotriene E4 in subjects with AERD65 and blunts aspirin-induced reactions66. These findings support a role for local IgE in driving the persistent mast cell activation in AERD. A recent study found that patients with AERD who have the highest nasal polyp IgE levels have the most rapid nasal polyp recurrence13. Polyclonal nasal polyp IgE has been shown to induce histamine release in nasal polyp tissue fragments, suggesting that the polyclonal IgE is functional53. Taken together, these findings suggest that tissue IgE could be instrumental to the mast cell and basophil activation in the nasal polyp tissue of subjects with AERD.
Subjects with recurrent nasal polyposis have elevated total nasal polyp IgA and IgG14, 52, which may also mediate nasal polyp inflammation through Fc receptor signaling. Significant tissue eosinophilia is characteristic of nasal polyposis in AERD67–69. Drugs targeting IL-5, a key survival factor for eosinophils, have shown promise in the treatment of nasal polyposis and AERD70–72. While many factors contribute to the dense tissue eosinophilia in the nasal polyps of patients with AERD, IgA may play a role in sustaining eosinophil survival73 and may lead to eosinophil degranulation74 further exacerbating nasal polyp inflammation in AERD. Similarly, IgG isotypes may contribute to nasal polyp pathogenesis through multiple mechanisms. IgG isotypes may direct local complement activation in nasal polyp tissue75, activate innate effector cells including macrophages and dendritic cells through Fc receptor signaling76, and lead to tissue destruction through auto-antibodies77.
IgG4 is elevated in the serum78 and polyp tissue13, 79 of subjects with AERD and is associated with an inferior post-operative course after endoscopic sinus surgery and longer duration of nasal polyposis13, 79. Yet, the role that IgG4 plays in nasal polyp pathogenesis, if any, is not well understood. IgG4 is thought to have an immunoregulatory role in patients with allergic sensitization80 and may reflect chronic antigen exposure81, as occurs in the upper airway where there is constant contact with microbial organisms and airborne allergens. IgG4 is also seen in pathologic conditions such as IgG4-related disease81 and eosinophilic esophagitis82, although the pathologic role of IgG4 in these disease is not well defined. IgG4-related disease can manifest as chronic rhinosinusitis leading to fibrotic disease in the sinuses83, 84, and B cells play a prominent role in IgG4-related disease as evidenced by IgG4+ plasma cell infiltration in affected tissues and clinical response to B-cell depleting therapies85. Another explanation of the relationship between local IgG4 production and worse disease outcomes in AERD could be that IgG4+ cells represent an intermediate step in class switching to IgE86 and do not have a direct pathologic effect themselves. Future study of the role of IgG4 in AERD may help to further our understanding of its role underlying disease pathogenesis.
Immunoglobulin mediated complement activation leading to destruction of epithelial barrier
TLOs are associated with a variety of autoimmune and inflammatory diseases31, possibly due to reduced organization of TLOs compared to SLOs and impaired clonal selection. In the sinonasal mucosal tissue of patients with CRS, a number of autoreactive IgG and IgA antibodies have been identified, including antibodies to double-stranded DNA and anti-basement membrane autoantibodies77, 87, 88. These autoreactive antibodies are associated with more severe phenotypes of nasal polyposis such as AERD. Antibody-mediated complement activation, as measured by levels of C5b – 9, C4d, and activated C1, is increased in the nasal polyp tissue89. Activated compliment product deposition occurs linearly on the epithelial basement membrane, suggesting the possibility that complement-mediated epithelial destruction is caused by anti-basement membrane antibodies89. Given the association of nasal polyp autoreactive antibodies and elevated tissue eosinophilia, IgE, and more severe nasal polyposis, further investigation of autoreactive antibodies in AERD is an important area for future study.
Immunoglobulin facilitated antigen presentation to T cells
In allergic disease, facilitated antigen presentation is the process by which an antigen-IgE complex is taken up by B cells through the low-affinity IgE receptor (CD23) and is presented to naïve T cells. Following antigen presentation, the naïve T cells undergo differentiation into allergen-specific Th2 cells capable of producing type 2 cytokines such as IL-4 and IL-13. This can further promote the allergic response by stimulating IgE class switching of allergen-specific B cells90, 91. Additional type 2 cytokines produced by the Th2 cells, such as IL-5 and IL-9, lead to further migration and activation of effector cells such as eosinophils, basophils and mast cells to the site of inflammation92, 93. Shami et al demonstrated that polyclonal IgE idiotypes in nasal polyp tissue complex with antigen to bind to CD23 on the surface of B cells, and they can elicit facilitated antigen presentation and subsequent proallergic T-cell response94. By depleting IgG from nasal homogenates, they also showed an increase in IgE-allergen binding to B cells through CD23, resulting in greater FcεRI-mediated basophil activation and subsequent histamine release94. As discussed above, inhibiting signaling of the type 2 cytokines IL-4 and IL-13, has shown efficacy in the treatment of nasal polyposis and AERD95, 96. Facilitated antigen presentation by IgE in the nasal polyp tissue of patients with AERD may have a role in nasal polyp pathogenesis and merits further investigation.
VI. Implications for management of CRSwNP and AERD
Many patients with CRSwNP and AERD are unable to achieve sufficient disease control with standard medical and surgical therapies, requiring revision surgery and high-dose corticosteroids to control inflammation. Given that locally produced nasal polyp immunoglobulins may facilitate disease pathogenesis in CRSwNP and AERD, modification with therapeutics targeting local nasal tissue immunoglobulins and/or B cells and plasma cells is of interest.
One area that may be amenable to therapeutic modification is targeting local IgE driven by S. aureus super antigens by treating the underlying S. aureus infection 8, 53. Van Zele et al. conducted a 12 week, randomized, double-blind, placebo-controlled study of oral methylprednisolone compared to oral doxycycline and found that both regimens led to some reduction in nasal polyp size compared to placebo. They did not assess S. aureus colonization or IgE to staphylococcal enterotoxins but did find that total nasal IgE levels were lower in the subjects treated with methylprednisolone compared to doxycycline97. Studies of anti-staphylococcal topical therapies have been conducted in subjects with CRS but have not been conducted specifically in subjects with nasal polyposis98, 99. Further study of antimicrobial therapies to eradicate S. aureus are required to assess how this impacts staphylococcal enterotoxin-mediated inflammation.
Several monoclonal antibodies approved for severe asthma and/or CRSwNP are known to affect serum and tissue IgE levels. Dupilumab, a fully human monoclonal antibody targeting IL-4Rα, a shared receptor subunit between IL-4 and IL-13, reduces nasal polyp size, improves sinonasal symptoms and sense of smell, and reduces the need for oral corticosteroids and/or surgery in patients with CRSwNP95. In a post hoc analysis of a phase II study of dupilumab vs placebo in subjects with CRSwNP, the subgroup of patients with aspirin sensitivity on dupilumab had significant improvement in nasal polyp score and sense of smell compared to placebo96. While the inhibition of IL-4 and IL-13 can modify type 2 inflammation in multiple ways, it is known to reduce serum IgE levels in patients with CRSwNP and asthma, and nasal secretion and nasal polyp tissue IgE levels in patients with CRSwNP 95, 100, 101. Omalizumab, a monoclonal antibody targeting IgE, has also been shown to reduce nasal polyp size and nasal congestion and lead to improvement of sinonasal symptom scores and sense of smell in subjects with CRSwNP102, 103. In a study of 21 adults with AERD, 12 months of treatment with omalizumab reduced levels of urinary LTE4 and PGD-M. In addition, subjects with AERD had reduction in sinonsal symptom scores65. The use of monoclonal antibodies to target IgE and other local tissue antibodies requires further study with biomarker-based endotyping and responder analyses to allow for optimization of biologic selection in patients with CRSwNP.
Autoantibodies have been identified in nasal polyp tissue and are associated with more severe disease 77, 87, 88. Therapies specifically targeting nasal polyp B cells and plasma cells have not been studied for treatment of CRSwNP. Rituximab, a monoclonal antibody targeting CD20 104, 105, is known to reduce autoantibodies in multiple autoimmune diseases. While rituximab has not been studied for treatment of CRSwNP, it has been used to treat sinus manifestations of IgG4-related disease 84. Further understanding of autoantibodies in the nasal polyp tissue of patients AERD and CRSwNP may lead to the identification of new therapeutic targets in patients with refractory disease.
VI. Conclusions
Activation of B cells and production of antibodies at local sites of inflammation plays a critical role in both protective and pathogenic B cell responses. There is accumulating evidence that highly activated B cell subsets are elevated in the nasal polyp tissues of CRSwNP and AERD patients. Moreover, the local production of antibodies likely plays a key role in the pathogenesis of these diseases, via multiple mechanisms. More studies are needed to determine the precise role of B cells and antibodies in driving disease in these patients. However, targeting the processes that drive this local B cell activation and antibody production may provide new therapeutic approaches for these patients and could help to reduce chronic inflammation.
Key Messages.
Local activation of B cells plays a key role in both protective and pathogenic immune responses
Local production of IgE has been shown to play an important role in the pathogenesis of allergic disease
Aspirin-exacerbated respiratory disease (AERD) is a severe subset of chronic rhinosinusitis with nasal polyps (CRSwNP) and both diseases are characterized by elevations in activated B cell subsets and antibodies in nasal polyp tissues
Local production of antibodies in AERD and CRSwNP may contribute to disease pathogenesis through multiple mechanisms
Acknowledgments
Funding sources: NIH grant K23AI139352
Abbreviations
- AERD
Aspirin-exacerbated respiratory disease
- BALT
Bronchus-associated lymphoid tissue
- CRS
Chronic rhinosinusitis
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- EBI2
Epstein-Barr virus-induced protein 2
- GALT
Gut-associated lymphoid tissue
- GC
Germinal center
- iBALT
Induced bronchus-associated lymphoid tissue
- ILF
Isolated lymphoid follicle
- SA
Staphylococcus aureus
- SLO
Secondary lymphoid organ
- TLO
Tertiary lymphoid organ
Footnotes
Disclosures: KB has served on scientific advisory boards for Regeneron, Genentech, AstraZeneca, and GlaxoSmithKline. KH has no disclosures.
References
- 1.White AA, Stevenson DD. Aspirin-Exacerbated Respiratory Disease. N Engl J Med. 2018;379:1060–1070. [DOI] [PubMed] [Google Scholar]
- 2.Laidlaw TM, Buchheit KM. Biologics in chronic rhinosinusitis with nasal polyposis. Ann Allergy Asthma Immunol. 2020;124:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: A systematic review. The Laryngoscope. 2015;125:1547–1556. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya N Assessing the additional disease burden of polyps in chronic rhinosinusitis. The Annals of otology, rhinology, and laryngology. 2009;118:185–189. [DOI] [PubMed] [Google Scholar]
- 5.Campbell AP, Phillips KM, Hoehle LP, et al. Depression symptoms and lost productivity in chronic rhinosinusitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017;118:286–289. [DOI] [PubMed] [Google Scholar]
- 6.McMains KC, Kountakis SE. Medical and surgical considerations in patients with Samter’s triad. American journal of rhinology. 2006;20:573–576. [DOI] [PubMed] [Google Scholar]
- 7.Buchheit KM, Cahill KN, Katz HR, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:1566–1576 e1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–79. [DOI] [PubMed] [Google Scholar]
- 9.Bachert C, Zhang N, Holtappels G, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. The Journal of allergy and clinical immunology. 2010;126:962–968, 968 e961–966. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Peters A, Suh L, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2008;121:1385–1392, 1392 e1381–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ordovas-Montanes J, Dwyer DF, Nyquist SK, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman S, Kasjanski R, Poposki J, et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin Exp Allergy. 2017;47:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchheit KM, Dwyer DF, Ordovas-Montanes J, et al. IL-5Ralpha marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2020;145:1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–1083, 1083 e1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busslinger M Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. [DOI] [PubMed] [Google Scholar]
- 16.Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol. 2013;131:933–957; quiz 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLennan IC, Gulbranson-Judge A, Toellner KM, et al. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. [DOI] [PubMed] [Google Scholar]
- 18.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. [DOI] [PubMed] [Google Scholar]
- 19.Mosier DE, Subbarao B. Thymus-independent antigens: complexity of B-lymphocyte activation revealed. Immunol Today. 1982;3:217–222. [DOI] [PubMed] [Google Scholar]
- 20.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. [DOI] [PubMed] [Google Scholar]
- 21.Reif K, Ekland EH, Ohl L, et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. [DOI] [PubMed] [Google Scholar]
- 22.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. [DOI] [PubMed] [Google Scholar]
- 23.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. [DOI] [PubMed] [Google Scholar]
- 24.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. [DOI] [PubMed] [Google Scholar]
- 25.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gatto D, Wood K, Brink R. EBI2 operates independently of but in cooperation with CXCR5 and CCR7 to direct B cell migration and organization in follicles and the germinal center. J Immunol. 2011;187:4621–4628. [DOI] [PubMed] [Google Scholar]
- 27.Jenks SA, Cashman KS, Woodruff MC, Lee FE, Sanz I. Extrafollicular responses in humans and SLE. Immunol Rev. 2019;288:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan TD, Gardam S, Gatto D, Turner VM, Silke J, Brink R. In vivo control of B-cell survival and antigen-specific B-cell responses. Immunol Rev. 2010;237:90–103. [DOI] [PubMed] [Google Scholar]
- 29.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koboziev I, Karlsson F, Grisham MB. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann N Y Acad Sci. 2010;1207 Suppl 1:E86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol. 2008;20:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Timmer TC, Baltus B, Vondenhoff M, et al. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 2007;56:2492–2502. [DOI] [PubMed] [Google Scholar]
- 33.Perros F, Dorfmuller P, Montani D, et al. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. [DOI] [PubMed] [Google Scholar]
- 34.Lo JC, Chin RK, Lee Y, et al. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest. 2003;112:1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. [DOI] [PubMed] [Google Scholar]
- 36.GeurtsvanKessel CH, Willart MA, Bergen IM, et al. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. [DOI] [PubMed] [Google Scholar]
- 38.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. [DOI] [PubMed] [Google Scholar]
- 39.Sorini C, Falcone M. Shaping the (auto)immune response in the gut: the role of intestinal immune regulation in the prevention of type 1 diabetes. Am J Clin Exp Immunol. 2013;2:156–171. [PMC free article] [PubMed] [Google Scholar]
- 40.Payne SC, Chen PG, Borish L. Local class switching in nonallergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2011;19:193–198. [DOI] [PubMed] [Google Scholar]
- 41.Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J Immunol. 2003;171:5602–5610. [DOI] [PubMed] [Google Scholar]
- 42.Smurthwaite L, Durham SR. Local IgE synthesis in allergic rhinitis and asthma. Curr Allergy Asthma Rep. 2002;2:231–238. [DOI] [PubMed] [Google Scholar]
- 43.Takhar P, Smurthwaite L, Coker HA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005;174:5024–5032. [DOI] [PubMed] [Google Scholar]
- 44.Ying S, Humbert M, Meng Q, et al. Local expression of epsilon germline gene transcripts and RNA for the epsilon heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol. 2001;107:686–692. [DOI] [PubMed] [Google Scholar]
- 45.Mouthuy J, Detry B, Sohy C, Pirson F, Pilette C. Presence in sputum of functional dust mite-specific IgE antibodies in intrinsic asthma. Am J Respir Crit Care Med. 2011;184:206–214. [DOI] [PubMed] [Google Scholar]
- 46.Nahm DH, Park HS. Correlation between IgA antibody and eosinophil cationic protein levels in induced sputum from asthmatic patients. Clin Exp Allergy. 1997;27:676–681. [PubMed] [Google Scholar]
- 47.Hoh RA, Boyd SD. Gut Mucosal Antibody Responses and Implications for Food Allergy. Front Immunol. 2018;9:2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoh RA, Joshi SA, Lee JY, et al. Origins and clonal convergence of gastrointestinal IgE(+) B cells in human peanut allergy. Sci Immunol. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. [DOI] [PubMed] [Google Scholar]
- 50.De Schryver E, Devuyst L, Derycke L, et al. Local immunoglobulin e in the nasal mucosa: clinical implications. Allergy Asthma Immunol Res. 2015;7:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gevaert P, Nouri-Aria KT, Wu H, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68:55–63. [DOI] [PubMed] [Google Scholar]
- 52.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37:1840–1847. [DOI] [PubMed] [Google Scholar]
- 53.Zhang N, Holtappels G, Gevaert P, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66:141–148. [DOI] [PubMed] [Google Scholar]
- 54.Chen JB, James LK, Davies AM, et al. Antibodies and superantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;139:1195–1204 e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi T, Kato A, Berdnikovs S, et al. Microparticles in nasal lavage fluids in chronic rhinosinusitis: Potential biomarkers for diagnosis of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2017;140:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balzar S, Fajt ML, Comhair SA, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oskeritzian CA, Zhao W, Min HK, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nasser SM, Pfister R, Christie PE, et al. Inflammatory cell populations in bronchial biopsies from aspirin-sensitive asthmatic subjects. Am J Respir Crit Care Med. 1996;153:90–96. [DOI] [PubMed] [Google Scholar]
- 59.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2015;135:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kowalski ML, Sliwinska-Kowalska M, Igarashi Y, et al. Nasal secretions in response to acetylsalicylic acid. J Allergy Clin Immunol. 1993;91:580–598. [DOI] [PubMed] [Google Scholar]
- 61.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol. 2003;111:743–749. [DOI] [PubMed] [Google Scholar]
- 62.Imokawa S, Sato A, Taniguchi M, et al. [Sodium cromoglycate nebulized solution has an acute bronchodilative effect in patients with aspirin-intolerant asthma (AIA)]. Arerugi. 1992;41:1515–1520. [PubMed] [Google Scholar]
- 63.Robuschi M, Gambaro G, Sestini P, et al. Attenuation of aspirin-induced bronchoconstriction by sodium cromoglycate and nedocromil sodium. Am J Respir Crit Care Med. 1997;155:1461–1464. [DOI] [PubMed] [Google Scholar]
- 64.Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2014;28:287–289. [DOI] [PubMed] [Google Scholar]
- 65.Hayashi H, Mitsui C, Nakatani E, et al. Omalizumab reduces cysteinyl leukotriene and 9alpha,11beta-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137:1585–1587 e1584. [DOI] [PubMed] [Google Scholar]
- 66.Lang DM, Aronica MA, Maierson ES, Wang XF, Vasas DC, Hazen SL. Omalizumab can inhibit respiratory reaction during aspirin desensitization. Ann Allergy Asthma Immunol. 2018;121:98–104. [DOI] [PubMed] [Google Scholar]
- 67.Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med. 2015;192:682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Payne SC, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope. 2011;121:2262–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–842. [DOI] [PubMed] [Google Scholar]
- 70.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. The Journal of allergy and clinical immunology. 2011;128:989–995 e981–988. [DOI] [PubMed] [Google Scholar]
- 71.Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: Randomized trial. J Allergy Clin Immunol. 2017;140:1024–1031 e1014. [DOI] [PubMed] [Google Scholar]
- 72.Tuttle KL, Buchheit KM, Laidlaw TM, Cahill KN. A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. 2018;6:1045–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartemes KR, Cooper KM, Drain KL, Kita H. Secretory IgA induces antigen-independent eosinophil survival and cytokine production without inducing effector functions. J Allergy Clin Immunol. 2005;116:827–835. [DOI] [PubMed] [Google Scholar]
- 74.Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyle RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–2400. [PubMed] [Google Scholar]
- 75.Van Zele T, Coppieters F, Gevaert P, Holtappels G, Van Cauwenberge P, Bachert C. Local complement activation in nasal polyposis. Laryngoscope. 2009;119:1753–1758. [DOI] [PubMed] [Google Scholar]
- 76.Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. [DOI] [PubMed] [Google Scholar]
- 77.Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–1206 e1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szczeklik A, Schmitz-Schumann M, Nizankowska E, Milewski M, Roehlig F, Virchow C. Altered distribution of IgG subclasses in aspirin-induced asthma: high IgG4, low IgG1. Clin Exp Allergy. 1992;22:283–287. [DOI] [PubMed] [Google Scholar]
- 79.Koyama T, Kariya S, Sato Y, et al. Significance of IgG4-positive cells in severe eosinophilic chronic rhinosinusitis. Allergol Int. 2019;68:216–224. [DOI] [PubMed] [Google Scholar]
- 80.Santos AF, James LK, Bahnson HT, et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. 2015;135:1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–551. [DOI] [PubMed] [Google Scholar]
- 82.Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–609. [DOI] [PubMed] [Google Scholar]
- 83.Moteki H, Yasuo M, Hamano H, Uehara T, Usami S. IgG4-related chronic rhinosinusitis: a new clinical entity of nasal disease. Acta Otolaryngol. 2011;131:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vandjelovic ND, Humphreys IM. Immunoglobulin G4-related sclerosing disease of the paranasal sinuses: A case report and literature review. Allergy Rhinol (Providence). 2016;7:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carruthers MN, Topazian MD, Khosroshahi A, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171–1177. [DOI] [PubMed] [Google Scholar]
- 86.Cameron L, Gounni AS, Frenkiel S, Lavigne F, Vercelli D, Hamid Q. S epsilon S mu and S epsilon S gamma switch circles in human nasal mucosa following ex vivo allergen challenge: evidence for direct as well as sequential class switch recombination. J Immunol. 2003;171:3816–3822. [DOI] [PubMed] [Google Scholar]
- 87.Jeffe JS, Seshadri S, Hamill KJ, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123:2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Schryver E, Calus L, Bonte H, et al. The quest for autoreactive antibodies in nasal polyps. J Allergy Clin Immunol. 2016;138:893–895 e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Roey GA, Vanison CC, Wu J, et al. Classical complement pathway activation in the nasal tissue of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2017;140:89–100 e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Punnonen J, de Vries JE. IL-13 induces proliferation, Ig isotype switching, and Ig synthesis by immature human fetal B cells. J Immunol. 1994;152:1094–1102. [PubMed] [Google Scholar]
- 91.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986;136:949–954. [PubMed] [Google Scholar]
- 92.Soussi-Gounni A, Kontolemos M, Hamid Q. Role of IL-9 in the pathophysiology of allergic diseases. J Allergy Clin Immunol. 2001;107:575–582. [DOI] [PubMed] [Google Scholar]
- 93.Ohnishi T, Sur S, Collins DS, Fish JE, Gleich GJ, Peters SP. Eosinophil survival activity identified as interleukin-5 is associated with eosinophil recruitment and degranulation and lung injury twenty-four hours after segmental antigen lung challenge. J Allergy Clin Immunol. 1993;92:607–615. [DOI] [PubMed] [Google Scholar]
- 94.Shamji MH, Thomsen I, Layhadi JA, et al. Broad IgG repertoire in patients with chronic rhinosinusitis with nasal polyps regulates proinflammatory IgE responses. J Allergy Clin Immunol. 2019;143:2086–2094 e2082. [DOI] [PubMed] [Google Scholar]
- 95.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–1650. [DOI] [PubMed] [Google Scholar]
- 96.Laidlaw TM, Mullol J, Fan C, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019;7:2462–2465 e2461. [DOI] [PubMed] [Google Scholar]
- 97.Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010;125:1069–1076 e1064. [DOI] [PubMed] [Google Scholar]
- 98.Rudmik L, Soler ZM. Medical Therapies for Adult Chronic Sinusitis: A Systematic Review. JAMA. 2015;314:926–939. [DOI] [PubMed] [Google Scholar]
- 99.Jervis-Bardy J, Boase S, Psaltis A, Foreman A, Wormald PJ. A randomized trial of mupirocin sinonasal rinses versus saline in surgically recalcitrant staphylococcal chronic rhinosinusitis. Laryngoscope. 2012;122:2148–2153. [DOI] [PubMed] [Google Scholar]
- 100.Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castro M, Corren J, Pavord ID, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018;378:2486–2496. [DOI] [PubMed] [Google Scholar]
- 102.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- 103.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–116 e111. [DOI] [PubMed] [Google Scholar]
- 104.Lee S, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol. 2010;125:814–820. [DOI] [PubMed] [Google Scholar]
- 105.Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5:433–441. [DOI] [PubMed] [Google Scholar]