Abstract
This article addresses the Human T-lymphotropic virus (HTLV). This subject comprises the Clinical Protocol and Therapeutic Guidelines for Comprehensive Care for People with Sexually Transmitted Infections, published by the Brazilian Ministry of Health. HTLV-1/2 infection is a public health problem globally, and Brazil has the largest number of individuals living with the virus. HTLV-1 causes several clinical manifestations of neoplasm (adult T-cell leukemia/lymphoma) and inflammatory nature, such as HTLV-1-associated myelopathy and other manifestations such as uveitis, arthritis, and infective dermatitis. These pathologies have high morbidity and mortality and negatively impact the quality of life of infected individuals. This review includes relevant information for health authorities professionals regarding viral transmission, diagnosis, treatment, and monitoring of individuals living with HTLV-1 and 2 in Brazil.
Keywords: Human T-Cell lymphotropic virus 1, Sexually transmitted diseases, Diagnosis, Signs and symptoms, Disease prevention
Highlighted excerpt:
HTLV-1/2 transmission can occur through blood transfusion and derivatives, injectable drug use, organ transplantation, unprotected sexual intercourse, and vertical transmission.
FOREWORD
This article addresses Human T-lymphotropic virus (HTLV) infection. This subject comprises the Clinical Protocol and Therapeutic Guidelines (PCDT) for Comprehensive Care for People with Sexually Transmitted Infections (STI), published by the Health Surveillance Department of the Brazilian Ministry of Health. To elaborate the PCDT, selection and analysis of the evidence available in the literature were performed, and a panel of specialists discussed it. The document was approved by the National Committee for the Incorporation of Technologies in the Brazilian National Health System (Conitec) 1 and updated by the team of specialists in STI in 2020 2 .
EPIDEMIOLOGICAL ASPECTS
HTLV-1 was described in patients with adult T-cell leukemia/lymphoma and, like HTLV-2 3 - 6 , classified in the Retroviridae family, genus Deltaretrovirus 7 . There are six molecular subtypes (a, b, c, d, e, f) of HTLV-1 8 - 10 and four (a, b, c, d) of HTLV-2 11 - 14 ; and two other types, HTLV-3 and HTLV-4, which have been described in isolated areas of forests in Cameroon, a country in the western region of Central Africa, and not yet associated with clinical manifestations 15 - 17 .
HTLV-1/2 infection results from the transmission of infected lymphocytes, present in body fluids (blood, semen, vaginal secretion, and mother's milk), by transfusion of blood and derivatives, intravenous drug use, organ transplantation, unprotected sexual intercourse, and vertical transmission. Vertical transmission can occur by the placental route, during birth, and mainly by breastfeeding 18 - 25 . HTLV-1 proviral load and exposure time are related to the increased risk of transmission, especially during sexual intercourse or breastfeeding 26 . The risk associated with the transfusion of blood and its derivatives was significantly reduced, with the introduction of systematic screening of blood and organs and blood components' leukoreduction 27 , 28 .
Sexual contact is an important route of HTLV-1 and HTLV-2 dissemination in urban, rural, and indigenous areas 12 , 29 , 30 . In urban areas, infection is most common among women 31 - 33 . However, among indigenous communities, the transmission effectiveness shows no difference between the sexes 12 , 29 , 34 . Sexual transmission is associated with unprotected sex practices, sexual partnership with intravenous drug users, and the presence of other STI 35 - 37 .
HTLV-1 and HTLV-2 are distributed worldwide 18 . Brazil has variable frequencies, ranging from 0.01 to 1.35% in the general population 28 , 38 , 39 , according to the geographical area and behavioral risk factors 12 , 18 , 40 , 41 . Groups with higher vulnerability to infection by both viruses include (i) intravenous drug users, (ii) sex workers, (iii) men who have sex with men, (iv) individuals submitted to blood transfusion before 1993, and (v) sexual partners of individuals with known HTLV infection. The decrease in HTLV-1 prevalence among blood donors throughout the years 28 , 38 is a privileged situation in Brazil, promoted since 1993 42 with the mandatory screening regulation of blood and its products.
The seroepidemiological studies for HTLV-1/2 are based on the detection of specific antibodies. It is important to emphasize that few population studies were conducted adequately. Therefore, a significant part of the epidemiological information about HTLV-1/2 derived from old studies, which often do not sufficiently define incidence and prevalence rates, shows conflicting results and does not allow the definition of precise prevention and control measures 18 , 39 .
HTLV-2, considered an ancestral infection, is apparently well adapted to humans, with rare clinical manifestations 5 , 43 - 48 . HTLV-2 is usually used as a marker of human migrations after the departure from the African continent 49 , 50 .
CLINICAL ASPECTS
Retroviruses integrate with the nucleic acid in the infected cell and establish a viral persistence, leading to the virus maintenance and the different outcomes of the infection. HTLV-1 is associated with an aggressive malignant disease, adult T-cell leukemia/lymphoma (ATL) 51 , 52 , and the neurodegenerative disease HTLV-1 associated myelopathy (HAM) 53 - 57 .
HTLV-1 infection shows a great variety of interactions with the human host and important clinical manifestations have been recognized in the eye 58 - 61 , skin 61 , 62 , lung 61 , 63 - 65 , joints 66 - 68 , thyroid 69 , 70 , heart 61 , 71 , 72 , intestines 61 , 73 and bladder 61 , 74 , 75 , among others. The broad spectrum of diseases reveals the infection's clinical complexity, which requires multidisciplinary attention for the infected patients' care. Although the clinical outcome of the HTLV-1 infections is considered low (5%), the number of clinical cases associated with HTLV-1 infection can reach a higher level and still needs to be better defined 55 . Intermediate clinical manifestations can be frequent before HAM occurs 76 , 77 . The proviral load in HTLV-1 infection is important in disease progression 78 , 79 , and is usually lower in asymptomatic individuals compared with those who present HTLV-1 associated diseases.
HTLV-1 ASSOCIATED MYELOPATHY
HAM occurs in about 4% of HTLV carriers, although clinical manifestations may affect more than 10% of them 77 . HAM manifests predominantly in the fourth and fifth decades of life, being uncommon before 20 or after 70 years of age. Generally, it starts insidiously and progresses slowly, especially among women: HAM cases in women are two to three times higher than that observed among men. Gait disturbances are a consequence to the gradual decrease in muscle strength and spasticity of the lower limbs 80 , leading to the need, over time, for walking aids (with the support of canes and walkers) and may evolve into the use of a wheelchair. The time of evolution varies, from months to decades. The symptoms of vesicointestinal and sexual dysfunction can be the initial complaints of the affected individual. Generally, HAM is characterized by urinary urge incontinence, intestinal constipation, and erectile dysfunction in the male population. The neurological clinical picture may be associated with multisystemic processes such as dermatitis, uveitis, pneumonia, besides cognitive alterations 81 , 82 . The diagnosis of HAM is rather critical since its early treatment may lead to a more effective therapeutic response 83 and better prognosis when instituted up to five years after the first symptoms.
Proviral load levels correlate with the progression of the disease, especially with muscle weakness. Although the magnitude of the proviral load in peripheral blood is associated with HAM, it is not the sole diagnostic or prognostic factor of the pathology 84 . Proviral load in cerebrospinal fluid is important to define the progression of HAM since HTLV-1 infected cells in the central nervous system accelerate the local inflammatory process 26 , 85 - 87 . However, other prognostic value markers should be evaluated to identify people at higher risk of illness 88 - 90 .
ADULT T-CELL LEUKEMIA/LYMPHOMA
The neoplasm of peripheral T-cells caused by HTLV-1 presents itself with leukocytosis, characterized by the presence of abnormal lymphocytes (flower cells) and, clinically, by lymphadenopathies, skin lesions, dysfunction of multiple organs resulting from the invasion of the neoplastic cells, in addition to the presence of opportunistic infections. Elevated levels of the enzyme lactate dehydrogenase and hypercalcemia are characteristic. In Japan, there are over one million carriers and the incidence of ATL varies from 0.6 to 0.7 per 1000 persons/year 91 . The risk of illness is higher in men, and symptoms begin 20 to 30 years after infection 92 . Rarely, ATL occurs before 30 years of age; however, its frequency tends to increase to reach those with 70 years of age. In Japan, where the probability of developing ATL is 5%, risk factors are: (i) maternal transmission, (ii) older age, (iii) increased proviral load in peripheral blood, (iv) family history of ATL, and (v) prior positive testing for anti-HTLV-1 93 , 94 . ATL is rare in other countries, not reaching 2% of cases 95 , despite evidence of lack of diagnosis 96 , 97 .
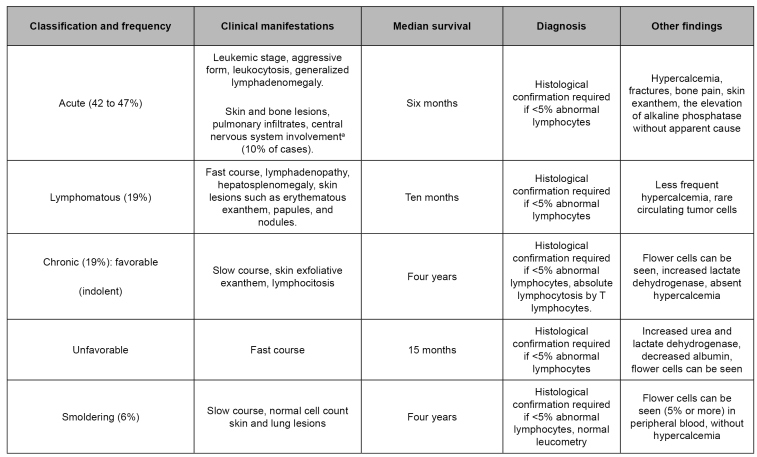
Four clinical forms of ATL are recognized 98 , which take into account the presence and severity of the leukemic manifestations, in addition to altered laboratory tests, such as increased lactate dehydrogenase and hypercalcemia. This classification is described in Figure 1, and the factors that predict worse prognosis, including those mentioned above, are found in Figure 2 51 , 98 - 101 .
FIGURE 1: Classification and characteristics of adult T-cell leukemia/lymphoma.
Source: adapted from Nosaka et al. 2017 93 ; Iwanaga et al. 2010 94 ; Phillips et al. 2010 95 ; Rosadas et al. 2020 96 .
Note: a) Need for intrathecal chemotherapy.
FIGURE 2: Adult T-cell leukemia/lymphoma worst prognosis predictors.
Source: adapted from Iwanaga et al. 2010 94 .
DERMATOLOGICAL ALTERATIONS IN INDIVIDUALS WITH HTLV
In addition to the clinical manifestations classically associated with HTLV-1 in the skin, such as infective dermatitis and the cutaneous manifestations of ATL, other dermatological affections attributed to the infection have been described as serious forms of scabies (especially in HIV-1 coinfected individuals) 102 , ichthyoses, seborrheic dermatitis, and dermatophytoses 103 .
At first, infective dermatitis was described in Jamaican children infected by HTLV-1 104 , mainly when vertical transmission occurs, although the disease can also affect adolescents and adults 105 . Infective dermatitis is characterized by erythematous-desquamative lesions, which generally involve the scalp, retro auricular regions, neck, face, armpits, and inguinal region. Typically, it is associated with infection by Gram-positive bacteria such as Streptococcus beta-hemoliticus and Staphylococcus aureus. According to a case series study, almost half of the individuals who had long-term follow-up were also diagnosed with HAM 106 . The differential diagnosis includes other causes of chronic eczemas, such as atopic dermatitis and seborrheic dermatitis 106 . Presence of the characteristic lesions, chronic rhinorrhea, recurrent chronic dermatitis, and positive serology for HTLV are the main criteria for diagnosing infective dermatitis, whose treatment consists of administering antibiotics with topical use of corticosteroids, combined or not with antifungals.
Dermatological alterations in ATL vary in presentation (erythroderma, papules, nodules, infiltrating lesions, or erythematous plaques) and depend on the disease stage; nodulations are more frequent in severe forms, especially in the acute, lymphomatous, or cutaneous primary tumoral form 107 . The lesions may evolve indolently and modify with the use of corticosteroids. Histopathological evaluation is essential for specific diagnosis.
UVEITIS IN INDIVIDUALS WITH HTLV-1
In Japan, uveitis was first reported in 1989 108 . Most common in people in age up to 50 years and a little more frequent in women, its exact incidence among HTLV-1 carriers remains uncertain. The disease is manifested by visual disorders, including 'floaters' and blurred or hazy vision, and it is bilateral in almost half of the affected people 109 . Eye signs include iritis, vitreous opacities, retinal vasculitis, and retinal hemorrhages and exudates. There is a good patient response to topical or systemic corticosteroids, although recurrence is common with therapy discontinuation.
COINFECTIONS IN INDIVIDUALS WITH HTLV
HTLV-infected individuals may present some coinfections, more frequently than the general population, either by sharing infection routes or as a consequence of the immunological alterations induced by the infection itself. Moreover, HTLV can alter the natural course of some coinfections.
In HIV coinfection, for example, the evidence suggests a neutral or even protective role for those coinfected by HTLV-2 110 . However, if the coinfection is HIV-1/HTLV-1, the existing data show a higher risk of death, both in adults and in children 111 . The reasons for these findings are not very clear. A hypothesis for the lack of clinical benefit is the delay in introducing the antiretroviral therapy due to the increase in the T-CD4+ cells count caused by HTLV-1. Coinfected individuals treated with antiretroviral therapy and with HIV-1 viral suppression present similar survival time to those monoinfected under the same conditions; however, in those with a detectable viral load, the survival of coinfected individuals is significantly lower 112 .
Regarding coinfection with hepatitis C virus (HCV), existing data are conflicting: while some studies show an increase in HCV viremia and a lower probability of spontaneous clearance of the infection 113 , others suggest a higher chance of elimination of this virus in HIV-1 and HTLV-coinfected individuals, probably due to the immunomodulation caused by HTLV in this group of individuals, resulting from the high production of proinflammatory cytokines 114 . Moreover, studies are suggesting less hepatic damage in triple infected individuals - with HIV, HTLV, and HCV- and a greater chance of spontaneous clearance of HCV 115 , 116 .
Individuals with HTLV-1 and Strongyloides stercoralis coinfection suffer a negative impact in the course of both infections, becoming more susceptible to more severe forms of strongyloidiasis, therapeutic resistance, in addition to presenting a higher HTLV-1 proviral load and a higher risk of HTLV-1 vertical transmission 117 - 126 .
Individuals with HTLV-1 present a higher risk of infection by Mycobacterium tuberculosis 127 - 132 , but the clinical impact is not clear.
DIAGNOSIS
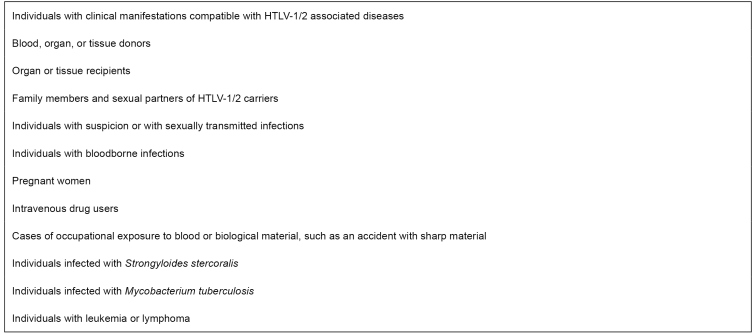
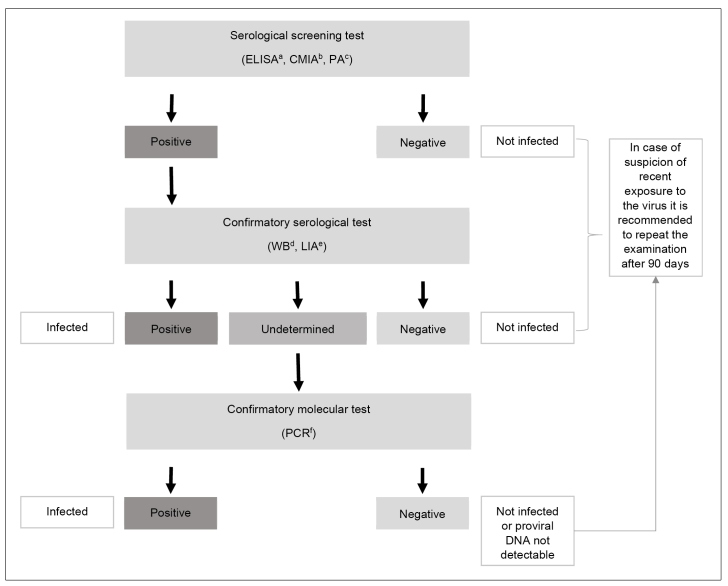
In Brazil, routine testing for HTLV-1/2 in blood and organ donors has been performed since 1993 and 2009, respectively 42 , 133 . In both cases, the infection is a criterion for donor exclusion. Although there is no national policy for HTLV-1/2 antenatal screening in Brazil, the test is done as a routine in some states. The MS/SCTIE Portaria no. 23, of May 31, 2016, included the Western blot (Wb) test and the polymerase chain reaction (PCR) to confirm HTLV-1 infection among patients suspected of ATL assisted by the Brazilian National Health System (SUS) 134 . Figure 3 shows the indications for HTLV-1/2 testing. Laboratory diagnosis must be performed using screening tests, followed by confirmatory tests in a different blood sample when screening test results are positive 135 - 137 (Figure 4).
FIGURE 3: Indications for laboratory testing for the human T-cell lymphotropic virus (HTLV-1/2).
FIGURE 4: Recommendations for human T-cell lymphotropic virus (HTLV-1/2) infection laboratory diagnosis.
Notes: a) ELISA: immunoenzymatic assay; b) CMIA: chemiluminescence; c) PA: particle agglutination; d) WB: Western blot; e) LIA: line immunoassay; f) PCR: polymerase chain reaction.
The screening tests are used for detecting antibodies against HTLV-1/2 in plasma or serum. The laboratory techniques for performing these tests include (i) immunoenzymatic reaction, (ii) chemiluminescence, and (iii) particle agglutination 136 . The screening tests present high sensitivity. The negative result excludes infection - unless there is evidence of recent exposure to the virus when it is recommended to repeat the test after 90 days 24 , 25 . The specificity of screening tests in Brazil varies from 92 to 99.5%. It is highly recommended to perform confirmatory tests to exclude false-positive results in the screening tests 136 - 138 .
The confirmatory tests identify antibodies against different HTLV-1 and HTLV-2 antigens or amplify and identify proviral genetic material, usually in peripheral blood lymphocytes. Confirmatory and viral typing tests are (i) Wb, (ii) line immunoassay (LIA), and (iii) PCR 136 .
Usually, Wb and LIA are sufficient for diagnosis; however, in some cases, undetermined or undefined results may occur regarding the type of HTLV 139 - 149 , more frequently in individuals infected by HTLV-2 or HIV-1 or both 141 , 150 . LIA presents greater accuracy in confirming HTLV-1 and HTLV-2 infection when compared to Wb 151 , 152 . Indeterminate or untyped results by Wb or LIA must be submitted to qualitative or quantitative PCR: nested PCR (nPCR) and real-time PCR (RT-PCR) are used. RT-PCR enables not only the quantification of the HTLV-1/2 proviral load but also the stratification of the risk of developing HTLV-1 associated diseases 26 , 93 , 94 , 142 , 153 - 155 . The detection of viral RNA is not used in the clinical routine, since viremia is low or absent, even in individuals with HAM 156 , 157 .
At the time of this publication, a molecular test for HTLV-1/2 is not commercially available. The tests used are in-house, requiring prior validation 155 , 158 - 161 . The absence of commercial tests and standardization of national protocols makes the implementation of molecular testing in the routine and the comparison of results obtained in different laboratories difficult 162 , 163 . Some individuals infected by HTLV-1/2 may present undetectable proviral load 164 - 166 . In these cases, it is possible to perform nPCR of higher sensitivity than RT-PCR. Another alternative is to perform a confirmatory serological test (if not yet performed) or to request consecutive samples for follow-up 148 .
There is evidence that the duration of the immunological window period in HTLV-1/2 infection for antibody detection varies from 16 to 39 days after organ transplantation, and for the proviral genetic material, from 16 to 23 days after infection 167 . A study conducted with individuals infected by blood transfusion showed a median seroconversion of 51 days (36 to 72 days) 25 . It is important to emphasize that the methodologies available when this study was developed did not have the same sensitivity as the current diagnostic methods 168 .
TREATMENT
The therapy for HTLV-1 infection consists of interventions directed to the complications resulting from the disease 169 , 170 . In 2016, Conitec 170 , and in 2019-2020, the International Retrovirology Association published recommendations for ATL and HAM treatment 171 , 172 . The use of zidovudine associated with interferon-alpha was authorized for the treatment of ATL by the publication of MS/SVS Portaria no. 54 on Jul 18, 2016 2 , 170 . The therapeutic regimens vary according to clinical presentation, progression of symptoms, and local availability of medications.
Infected people must be accompanied in the specialized service to receive psychological support, with particular attention to the early diagnosis of clinical manifestations associated with the infection.
SURVEILLANCE, PREVENTION, AND CONTROL
Despite being described some decades ago, HTLV infection remains relatively unknown to the general population and health professionals. In the services that assist the infected individuals, the approach should focus not only on the aspects of the risk of becoming sick 173 but also on preventing the transmission of infection.
After a positive diagnosis for HTLV-1/2 infection, the sexual partners should be invited to undergo serological screening, and those with positive tests must be referred for counseling and appropriate follow-up. Such counseling should include information about the chronicity of the infection and the relevance of long-term clinical follow-up 169 , 174 . It is important to clarify the initial clinical manifestations and their progression, the transmission mechanisms, and their prevention. The donation of blood, semen, solid organs or tissues and breastfeeding are strongly discouraged.
In HIV and other STI specialized clinical centres, it is important to include HTLV screening in the routine of care. HTLV-infected individual must be oriented about the risk of sexual transmission, serodiscordant sexual partners, and condom use - which may be interrupted during the fertile period when there is a firm decision to become pregnant and following medical counselling and recommendation 174 .
In Brazil, given the scarcity of material available for health professionals and the general population, several initiatives have been developed by academic groups and non-governmental organizations to disseminate information about HTLV-1/2. Among the organizations and initiatives with this purpose, the following should be highlighted: the Research Support Center on Retroviruses (NAP-Retroviruses) of the University of São Paulo; the Hemominas Foundation Journals on HTLV infection; the HTLVida Association; and the Vitamóre Group - Association of HTLV Carriers.
The lack of a national register system impairs the identification of the actual scenario of the infection in the country and, therefore, the implementation of specific public health policies. It is essential to highlight that case notification is one of the pillars of confrontation and research about HTLV-1 in countries like Japan, England, Spain, and Martinique island 175 - 178 .
SPECIAL POPULATIONS
Pregnant women
In Brazil, HTLV-1/2 prevalence in pregnant women can reach 1% in certain regions of the country (Table 1) 159 , 179 - 196 . Despite reports about the development of HTLV-associated diseases in pregnancy (HAM, ATL), there is no consistent evidence about the impact on the pregnancy-puerperium cycle 23 . However, childhood infection is associated with an increased risk of developing diseases associated with HTLV-1, especially ATL that has a high lethality 23 , 197 , 198 . Therefore, prevention of mother to child transmission is essential to reduce the incidence of diseases associated with the virus 23 , 96 , 137 .
TABLE 1: Prevalence of HTLV-1/2 infection in pregnant women in different Brazilian states.
| Region/State | Prevalence (%) | n | Referencesa |
|---|---|---|---|
| North | |||
| Pará | 0.6 | 324 | Guerra et al. 2018 188 b |
| 0.3 | 13,382 | Sequeira et al. 2012 192 | |
| Amazonas | 0 | 674 | Machado Filho et al. 2010 194 |
| Northeast | |||
| Alagoas | 0.2 | 54,813 | Moura et al. 2015 179 |
| Bahia | 0.14 | 692 | Boa-Sorte et al. 2014 190 c |
| 1.05 | 2,766 | Mello et al. 2014 191 | |
| 0.98 | 408 | Magalhães et al. 2008 195 | |
| 0.84 | 6,754 | Bittencourt et al. 2001 183 | |
| 0.88 | 1,024 | Santos et al. 1995 185 | |
| Maranhão | 0.7 | 713 | Mendes et al. 2020 186 |
| 0.3 | 2,044 | Guimarães de Souza et al. 2012 193 | |
| Ceará | 0.12 | 814 | Broutet et al. 1996 184 |
| Midwest | |||
| Mato Grosso do Sul | 0.13 | 116,689 | Dal Fabbro et al. 2008 196 |
| 0.1 | 32,512 | Figueiró Filho et al. 2007 180 | |
| Goiás | 0.1 | 15,485 | Oliveira et al. 2006 181 |
| Southeast | |||
| Rio de Janeiro | 0.74 | 1,628 | Barmpas et al. 2019 187 |
| 0.66 | 1,204 | Monteiro et al. 2014 189 | |
| São Paulo | 0.1 | 913 | Olbrich Neto et al, 2004 182 |
| South | |||
| Paraná | 0.31 | 643 | Medeiros et al. 2018 159 d |
a) Only studies with confirmatory tests for HTLV-1/2 infection were included; b) Adolescent pregnant women; c) Study with blood samples on filter paper; d) High-risk pregnant women.
Since breastfeeding is the main mother to child transmission route of HTLV-1/2 135 , 199 - 204 and there is no vaccine against the infection or even any curative treatment, breastfeeding is contraindicated in mothers infected by the virus. For these women, the use of lactation inhibitors is recommended and the provision of infants with milk formula substitutes 2 . Universal antenatal HTLV-1/2 infection screening is not provided by the SUS, but it is recommended to test all pregnant women, followed by counselling for those infected and their relatives, allowing the effective implementation of prevention strategies.
Indigenous peoples
The vertical and sexual transmission routes are essential for HTLV maintenance in epidemiologically closed or semi-closed communities, as it occurs with HTLV-2c, which is prevalent among indigenous people residing in the Brazilian Amazon and urban areas 12 , 13 , 205 - 209 . It is worth remembering that intrafamiliar infection in the Kayapó communities is important and it is observed the transmission of the virus between two or three generations and in more than 20% of infected children under nine years old 12 . Vertical transmission maintains the virus in high endemicity since the usual nonbreastfeeding procedures by infected mothers are not followed regularly 205 . The increasing number of reports associating diseases with HTLV-2 5 , 43 - 48 infections requires special attention to the indigenous communities located in areas of high virus endemicity in the Brazilian Amazon 39 .
CONCLUSIONS
Although HTLV infection is neglected, Brazil has produced several initiatives directed towards the prevention of HTLV-1 infection and disease. The complications with relevant clinical consequences, such as HTLV-1 associated myelopathy and T-cell leukemia/lymphoma, can be minimized with access to services offered by the SUS. The low complexity cases can be assisted at the health centers and, when necessary, forwarded to the specialized centers for treatment, rehabilitation, and social support. Despite the severe consequences that the infection can have on people's lives, its control still represents a public health challenge. National epidemiological studies, development and validation of diagnostic tests, and elaboration of clinical protocols with new therapeutic options can define public policies and specific actions towards the approach, prevention, control, and adequate treatment of HTLV-1/2 infection in Brazil.
ACKNOWLEDGMENTS
The authors are grateful to the technical panel of specialists responsible for elaborating the 2020 PCDT for Comprehensive Care for People with Sexually Transmitted Infections.
Referências
- 1.Ministério da Saúde (BR) Diário Oficial da União. Brasília (DF): Oct, 2018. [2020 out 15]. Portaria MS/SCTIE no 42, de 5 de outubro de 2018. Torna pública a decisão de aprovar o Protocolo Clínico e Diretrizes Terapêuticas para Atenção Integral às Pessoas com Infecções Sexualmente Transmissíveis (IST), no âmbito do Sistema Único de Saúde - SUS. [Internet] Seção I:88. Available from: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/44303574/do1-2018-10-08-portaria-n-42-de-5-de-outubro-de-2018-44303438. [Google Scholar]
- 2.Ministério da Saúde (BR) Protocolo clínico e diretrizes terapêuticas para atenção integral às pessoas com infeções sexualmente transmissíveis (IST) Brasília: Ministério da Saúde; 2020. [2020 jun 14]. [Internet] Available from: http://www.aids.gov.br/pt-br/pub/2015/protocolo-clinico-e-diretrizes-terapeuticas-para-atencao-integral-pessoas-com-infeccoes. [Google Scholar]
- 3.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. [2020 Oct 15];Proc Natl Acad Sci U S A. 1980 77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poiesz BJ, Ruscetti FW, Reitz MS, Kalyanaraman VS, Gallo RC. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sézary T-cell leukaemia. [2020 Oct 15];Nature. 1981 Nov;294:268–271. doi: 10.1038/294268a0. [Internet] [DOI] [PubMed] [Google Scholar]
- 5.Kalyanaraman VS, Sarngadharan MG, Robert-Guroff M, Miyoshi I, Golde D, Gallo RC. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. [2020 Oct 15];Science. 1982 Nov;218(4572):571–573. doi: 10.1126/science.6981847. [Internet] [DOI] [PubMed] [Google Scholar]
- 6.Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. [2020 Oct 15];Oncogene. 2005 Sep;24(39):5926–5930. doi: 10.1038/sj.onc.1208980. [Internet] [DOI] [PubMed] [Google Scholar]
- 7.International Commitee on Taxonomy of Viruses - ICTV . Taxonomy history: primate T-lymphotropic virus 1. [S.l.]: ICTV; 2017. [2020 Oct 15]. [Internet] Available from: https://talk.ictvonline.org/taxonomy/p/taxonomy-history?taxnode_id=19911434&src=NCBI&ictv_id=19911434 . [Google Scholar]
- 8.Miura T, Fukunaga T, Igarashi T, Yamashita M, Ido E, Funahashi S, et al. Phylogenetic subtypes of human T-lymphotropic virus type I and their relations to the anthropological background. [2020 Oct 15];Proc Natl Acad Sci U S A. 1994 Feb;91(3):1124–1127. doi: 10.1073/pnas.91.3.1124. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal AU, Gessain A, Yoshida M, Mahieux R, Nishioka K, Tekaia F, et al. Molecular epidemiology of HTLV type I in Japan: evidence for two distinct ancestral lineages with a particular geographical distribution. [2020 Oct 15];AIDS Res Hum Retroviruses. 1994 Nov;10(11):1557–1566. doi: 10.1089/aid.1994.10.1557. [Internet] [DOI] [PubMed] [Google Scholar]
- 10.Van Dooren S, Salemi M, Vandamme AM. Dating the origin of the African human T-cell lymphotropic virus type-i (HTLV-I) subtypes. [2020 Oct 15];Mol Biol Evol. 2001 Apr;18(4):661–671. doi: 10.1093/oxfordjournals.molbev.a003846. [Internet] [DOI] [PubMed] [Google Scholar]
- 11.Hall WW, Takahashi H, Liu C, Kaplan MH, Ijichi S, Nagashima K, et al. Multiple isolates and characteristics of human T-cell leukemia virus type II. [2020 Oct 15];J Virol. 1992 Apr;66(4):2456–2463. doi: 10.1128/jvi.66.4.2456-2463.1992. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishak R, Harrington WJ, Azevedo VN, Eiraku N, Ishak MO, Guerreiro JF, et al. Identification of human T cell lymphotropic virus type IIa infection in the Kayapo, an indigenous population of Brazil. [2020 Oct 15];AIDS Res Hum Retroviruses. 1995 Jul;11(7):813–821. doi: 10.1089/aid.1995.11.813. [Internet] [DOI] [PubMed] [Google Scholar]
- 13.Eiraku N, Novoa P, Costa Ferreira M, Monken C, Ishak R, Costa Ferreira O, et al. Identification and characterization of a new and distinct molecular subtype of human T-cell lymphotropic virus type 2. [2020 Oct 15];J Virol. 1996 Mar;70(3):1481–1492. doi: 10.1128/jvi.70.3.1481-1492.1996. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandamme AM, Salemi M, Van Brussel M, Liu HF, van Laethem K, van Ranst M, et al. African origin of human T-lymphotropic virus type 2 (HTLV-2) supported by a potential new HTLV-2d subtype in Congolese Bambuti Efe Pygmies. [2020 Oct 15];J Virol. 1998 May;72(5):4327–4340. doi: 10.1128/jvi.72.5.4327-4340.1998. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe ND, Heneine W, Carr JK, Garcia AD, Shanmugam V, Tamoufe U, et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. [2020 Oct 15];Proc Natl Acad Sci U S A. 2005 May;102(22):7994–7999. doi: 10.1073/pnas.0501734102. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perzova R, Benz P, Abbott L, Welch C, Thomas A, Ghoul RW, et al. Short communication: no evidence of HTLV-3 and HTLV-4 infection in New York State subjects at risk for retroviral infection. [2020 Oct 15];AIDS Res Hum Retroviruses. 2010 Nov;26(11):1229–1231. doi: 10.1089/aid.2010.0079. [Internet] [DOI] [PubMed] [Google Scholar]
- 17.Duong YT, Jia H, Lust JA, Garcia AD, Tiffany AJ,, Heneine W, et al. Short communication: Absence of evidence of HTLV-3 and HTLV-4 in patients with large granular lymphocyte (LGL) leukemia. [2020 Oct 15];AIDS Res Hum Retroviruses. 2008 Dec;24(12):1503–1505. doi: 10.1089/aid.2008.0128. [Internet] [DOI] [PubMed] [Google Scholar]
- 18.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. [2020 Oct 15];Front Microbiol. 2012 Nov;3:388–388. doi: 10.3389/fmicb.2012.00388. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishak R, Vallinoto ACR, Azevedo VN, Lewis M, Hall WW, Ishak MO. Molecular evidence of mother-to-child transmission of HTLV-IIc in the Kararao Village ( Kayapo ) in the Amazon Region of Brazil. [2020 Oct 15];Rev Soc Bras Med Trop. 2001 34(6):519–525. doi: 10.1590/S0037-86822001000600004. [Internet] [DOI] [PubMed] [Google Scholar]
- 20.Moriuchi M, Moriuchi H. Seminal fluid enhances replication of human T-cell leukemia virus type 1: implications for sexual transmission. [2020 Oct 15];J Virol. 2004 Nov;78(22):12709–12711. doi: 10.1128/JVI.78.22.12709-12711.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lairmore MD, Anupam R, Bowden N, Haines R, Haynes RAH, Ratner L, et al. Molecular determinants of human T-lymphotropic virus type 1 transmission and spread. [2020 Oct 15];Viruses. 2011 Jul;3(7):1131–1165. doi: 10.3390/v3071131. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendoza C, Roc L, Benito R, Reina G, Ramos JM, Gómez C, et al. HTLV-1 infection in solid organ transplant donors and recipients in Spain. [2020 Oct 15];BMC Infect Dis. 2019 Aug;19:706–706. doi: 10.1186/s12879-019-4346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosadas C, Taylor GP. Mother-to-child HTLV-1 transmission: unmet research needs. [2020 Oct 15];Front Microbiol. 2019 May;10:999–999. doi: 10.3389/fmicb.2019.00999. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook LBM, Melamed A, Demontis MA, Laydon DJ, Fox JM, Tosswill JHC, et al. Rapid dissemination of human T-lymphotropic virus type 1 during primary infection in transplant recipients. [2020 Oct 15];Retrovirology. 2016 Jan;13:3–3. doi: 10.1186/s12977-015-0236-7. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manns A, Wilks RJ, Murphy EL, Haynes G, Barnett M, Hanchard B, et al. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. [2020 Oct 15];Int J Cancer. 1992 Jul;51(6):886–891. doi: 10.1002/ijc.2910510609. [Internet] [DOI] [PubMed] [Google Scholar]
- 26.Nagai M, Usuku K, Matsumoto W, Kodama D, Takenouchi N, Moritoyo T, Hashiguchi S, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. [2020 Oct 15];J Neurovirol. 1998 Dec;4(6):586–593. doi: 10.3109/13550289809114225. [Internet] [DOI] [PubMed] [Google Scholar]
- 27.Dias-Bastos MR, Oliveira CDL, Carneiro-Proietti ABF. Decline in prevalence and asymmetric distribution of human T cell lymphotropic virus 1 and 2 in blood donors, State of Minas Gerais, Brazil, 1993 to 2007. [2020 Oct 15];Rev Soc Bras Med Trop. 2010 Nov-Dec;43(6):615–619. doi: 10.1590/S0037-86822010000600002. [Internet]. [DOI] [PubMed] [Google Scholar]
- 28.Carneiro-Proietti ABF, Sabino EC, Leão S, Salles NA, Loureiro P, Sarr M, et al. Human T-lymphotropic virus type 1 and type 2 seroprevalence, incidence, and residual transfusion risk among blood donors in Brazil during 2007-2009. [2020 Oct 15];AIDS Res Hum Retroviruses. 2012 Oct;28(10):1265–1272. doi: 10.1089/aid.2011.0143. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lairmore MD, Jacobson S, Gracia F, De BK, Castillo L, Larreategui M, et al. Isolation of human T-cell lymphotropic virus type 2 from Guaymi Indians in Panama. [2020 Oct 15];Proc Natl Acad Sci U S A. 1990 Nov;87(22):8840–8844. doi: 10.1073/pnas.87.22.8840. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunes D, Boa-Sorte N, Grassi MFR, Taylor GP, Teixeira MG, Barreto ML, et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. [2020 Oct 15];PLoS One. 2017 Feb;12:e0171303. doi: 10.1371/journal.pone.0171303. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa CA, Furtado KCYO, Ferreira LSC, Almeida DS, Linhares AC, Ishak R, et al. Familial Transmission of Human T-cell Lymphotrophic Virus: Silent Dissemination of an Emerging but Neglected Infection. [2020 Oct 15];PLoS Negl Trop Dis. 2013 Jun;7:e2272. doi: 10.1371/journal.pntd.0002272. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV-1 in Japan as determined by screening of blood donors. [2020 Oct 15];J Med Virol. 2012 Feb;84(2):327–335. doi: 10.1002/jmv.23181. [Internet] [DOI] [PubMed] [Google Scholar]
- 33.Hananiya HS, Ella EE, Aminu M, Anyanwu NCJ. Prevalence of human T-cell lymphotropic virus and the socio-demographic and risk factors associated with the infection among post-natal clinics women in Zaria, Nigeria. [2020 Oct 15];J Immunoassay Immunochem. 2019 40(5):485–494. doi: 10.1080/15321819.2019.1636817. [Internet] [DOI] [PubMed] [Google Scholar]
- 34.Braço ILJ, Sá KSG, Waqasi M, Queiroz MAF, Silva ANR, Cayres-Vallinoto IMV, et al. High prevalence of human T-lymphotropic virus 2 (HTLV-2) infection in villages of the Xikrin tribe (Kayapo), Brazilian Amazon region. [2020 Oct 15];BMC Infect Dis. 2019 May;19(1):459–459. doi: 10.1186/s12879-019-4041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy EL, Figueroa JP, Gibbs WN, Brathwaite A, Holding-Cobham M, Waters D, et al. Sexual transmission of human T-lymphotropic virus type I (HTLV-I) [2020 Oct 15];Ann Intern Med. 1989 Oct;111(7):555–560. doi: 10.7326/0003-4819-111-7-555. [Internet] [DOI] [PubMed] [Google Scholar]
- 36.La Rosa AM, Zunt JR, Peinado J, Lama JR, Ton TGN, Suarez L, et al. Retroviral infection in Peruvian men who have sex with men. [2020 Oct 15];Clin Infect Dis. 2009 Jul;49(1):112–117. doi: 10.1086/599609. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zunt JR, La Rosa AM, Peinado J, Lama JR, Suarez L, Pun M, et al. Risk factors for HTLV-II infection in Peruvian men who have sex with men. [2020 Oct 15];Am J Trop Med Hyg. 2006 May;74(5):922–925. [Internet] Available from: https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&retmode=ref&cmd=prlinks&id=16687704 . [PMC free article] [PubMed] [Google Scholar]
- 38.Galvão-Castro B, Loures L, Rodriques LG, Sereno A, Ferreira OC, Júnior , Franco LG, et al. Distribution of human T-lymphotropic virus type I among blood donors: a nationwide Brazilian study. [2020 Oct 15];Transfusion. 1997 Feb;37(2):242–243. doi: 10.1046/j.1537-2995.1997.37297203532.x. [Internet] [DOI] [PubMed] [Google Scholar]
- 39.Ishak R, Ishak MO, Vallinoto ACR. The challenge of describing the epidemiology of HTLV in the Amazon region of Brazil. [2020 Oct 15];Retrovirology. 2020 Feb;17:4–4. doi: 10.1186/s12977-020-0512-z. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Einsiedel L, Woodman RJ, Flynn M, Wilson K, Cassar O, Gessain A. Human T-lymphotropic virus type 1 infection in an indigenous Australian population: epidemiological insights from a hospital-based cohort study. [2020 Oct 15];BMC Public Health. 2016 Aug;16:787–787. doi: 10.1186/s12889-016-3366-5. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paiva AM, Assone T, Haziot MEJ, Smid J, Fonseca LAM, Luiz OC, et al. Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. [2020 Oct 15];Sci Rep. 2018 8:7742–7742. doi: 10.1038/s41598-018-25939-y. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brasil. Ministério da Saúde . Diário Oficial da União. Brasília (DF): Dec, 1993. Portaria GM/MS n. 1376, de 19 de novembro de 1993. Aprova alterações na Portaria no 721/GM, de 09.08.89, que aprova Normas Técnicas para coleta, processamento e transfusão de sangue, componentes e derivados, e dá outras providências.http://redsang.ial.sp.gov.br/site/docs_leis/ps/ps29.pdf [Internet] Seção I:18405. [Google Scholar]
- 43.Hjelle B, Appenzeller O, Mills R, Appenzeller O, Jahnke R, Alexander S, et al. Chronic neurodegenerative disease associated with HTLV-II infection. [2020 Oct 15];Lancet. 1992 Mar;339(8794):645–646. doi: 10.1016/0140-6736(92)90797-7. [Internet] [DOI] [PubMed] [Google Scholar]
- 44.Zucker-Franklin D, Hooper WC, Evatt BL. Human lymphotropic retroviruses associated with mycosis fungoides: evidence that human T-cell lymphotropic virus type II (HTLV-II) as well as HTLV-I may play a role in the disease. [2020 Oct 15];Blood. 1992 Sep;80(6):1537–1545. [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/1520878. [PubMed] [Google Scholar]
- 45.Maytal J, Horowitz S, Lipper S, Poiesz B, Wang CY, Siegal FP. Progressive nemaline rod myopathy in a woman coinfected with HIV-1 and HTLV-2. Mt Sinai J Med. 1993 May;60(3):242–246. [PubMed] [Google Scholar]
- 46.Peters AA, Oger JJ, Coulthart MB, Waters DJ, Cummings HJ, Dekaban GA. An apparent case of human T-cell lymphotropic virus type II (HTLV-II)-associated neurological disease: a clinical, molecular, and phylogenetic characterisation. [2020 Oct 15];J Clin Virol. 1999 Sep;14(1):37–50. doi: 10.1016/S1386-6532(99)00041-4. [Internet] [DOI] [PubMed] [Google Scholar]
- 47.Araujo A, Hall WW. Human T-Lymphotropic virus type ii and neurological disease. [2020 Oct 15];Ann Neurol. 2004 Jul;56(1):10–19. doi: 10.1002/ana.20126. [Internet] [DOI] [PubMed] [Google Scholar]
- 48.Rosadas C, Vicente ACP, Zanella L, Cabral-Castro MJ, Peralta JM, Puccioni-Sohler M. Human T-lymphotropic virus type 2 subtype b in a patient with chronic neurological disorder. [2020 Oct 15];J Neurovirol. 2014 Dec;20(6):636–639. doi: 10.1007/s13365-014-0280-4. [Internet] [DOI] [PubMed] [Google Scholar]
- 49.Black FL. Tracing prehistoric migrations by the viruses they carry: human T-cell lymphotropic viruses as markers of ethnic relationships. Hum Biol. 1997 Aug;69(4):467–482. [PubMed] [Google Scholar]
- 50.Ishak R, Machado LFA, Cayres-Vallinoto I, Ishak MO, Vallinoto ACR. Infectious agents as markers of human migration toward the Amazon Region of Brazil. [2020 Oct 15];Front Microbiol. 2017 Aug;8:1663–1663. doi: 10.3389/fmicb.2017.01663. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, et al. Treatment and survival among 1594 patients with ATL. [2020 Oct 15];Blood. 2015 Dec;126(24):2570–2577. doi: 10.1182/blood-2015-03-632489. [Internet] [DOI] [PubMed] [Google Scholar]
- 52.Taniguchi H, Imaizumi Y, Takasaki Y, Nakashima J, Kato T, Itonaga H, et al. Clinical features at transformation in adult T-cell leukemia-lymphoma with smoldering and chronic types. [2020 Oct 15];Int J Hematol. 2019 Apr;109(4):402–408. doi: 10.1007/s12185-019-02602-4. [Internet] [DOI] [PubMed] [Google Scholar]
- 53.Rodgers-Johnson P, Gajdusek DC, Morgan OS, Zaninovic V, Sarin PS, Graham DS. HTLV-I and HTLV-III antibodies and tropical spastic paraparesis. [2020 Oct 15];Lancet (London, England) 1985 Oct;2(8466):1247–1248. doi: 10.1016/s0140-6736(85)90778-0. [Internet] [DOI] [PubMed] [Google Scholar]
- 54.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, et al. HTLV-I associated myelopathy, a new clinical entity. [2020 Oct 15];Lancet. 1986 May;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [Internet] [DOI] [PubMed] [Google Scholar]
- 55.Araujo A. Update on neurological manifestations of HTLV-1 infection. [2020 Oct 15];Curr Infect Dis Rep. 2015 Feb;17(2):459–459. doi: 10.1007/s11908-014-0459-0. [Internet] [DOI] [PubMed] [Google Scholar]
- 56.Bangham CRM, Araujo A, Yamano Y, Taylor GP. HTLV-1-associated myelopathy/tropical spastic paraparesis. [2020 Oct 15];Nat Rev Dis Primers. 2015 Jun;1:15012. doi: 10.1038/nrdp.2015.12. [Internet] [DOI] [PubMed] [Google Scholar]
- 57.Nozuma S, Jacobson S. Neuroimmunology of human T-Lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis. [2020 Oct 15];Front Microbiol. 2019 Apr;10:885–885. doi: 10.3389/fmicb.2019.00885. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chew R, Henderson T, Aujla J, Whist E, Einsiedel L. Turning a blind eye: HTLV-1-associated uveitis in Indigenous adults from Central Australia. [2020 Oct 15];Int Ophthalmol. 2018 Oct;38(5):2159–2162. doi: 10.1007/s10792-017-0659-3. [Internet] [DOI] [PubMed] [Google Scholar]
- 59.Nakao K, Abematsu N, Sakamoto T. Systemic diseases in patients with HTLV-1-associated uveitis. [2020 Oct 15];Br J Ophthalmol. 2018 Mar;102(3):373–376. doi: 10.1136/bjophthalmol-2017-310658. [Internet] [DOI] [PubMed] [Google Scholar]
- 60.Kamoi K, Okayama A, Izumo S, Hamaguchi I, Uchimaru K, Tojo A, et al. Tackling HTLV-1 infection in ophthalmology: a nationwide survey of ophthalmic care in an endemic country, Japan. [2020 Oct 15];Br J Ophthalmol. 2020 Mar; doi: 10.1136/bjophthalmol-2019-315675. [Internet] [DOI] [PubMed] [Google Scholar]
- 61.Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. [2020 Oct 15];Lancet Infect Dis. 2019 Jan;20(1):133–143. doi: 10.1016/s1473-3099(19)30402-5. [Internet] [DOI] [PubMed] [Google Scholar]
- 62.Bimbi C, Brzezinski P, Sokolowska-Wojdylo M. Crusted (Norwegian) scabies as a strong marker of adult T-cell leukemia/lymphoma in HTLV-1 infection. [2020 Oct 15];Clin Case Reports. 2019 Mar;7(3):474–476. doi: 10.1002/ccr3.1983. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magno Falcão LF, Falcão ASC, Medeiros Sousa RC, Vieira WB, Oliveira RTM, Normando VMF, et al. CT Chest and pulmonary functional changes in patients with HTLV-associated myelopathy in the Eastern Brazilian Amazon. [2020 Oct 15];PLoS One. 2017 Nov;12(11):e0186055. doi: 10.1371/journal.pone.0186055. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dias ARN, Falcão LFM, Falcão ASC, Normando VMF, Quaresma JAS. Human T lymphotropic virus and pulmonary diseases. [2020 Oct 15];Front Microbiol. 2018 Aug;9:1879–1879. doi: 10.3389/fmicb.2018.01879. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kako S, Joshita S, Matsuo A, Kawaguchi K, Umemura T, Tanaka E. A case of adult T-Cell leukemia/lymphoma complicated with bilateral chylothorax. [2020 Oct 15];Case Rep Oncol Med. 2019 Feb;2019:8357893. doi: 10.1155/2019/8357893. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. [2020 Oct 15];Lancet. 1989 Feb;1(8635):441–441. doi: 10.1016/s0140-6736(89)90038-x. [Internet] [DOI] [PubMed] [Google Scholar]
- 67.Sato K, Maruyama I, Maruyama Y, Kitajima I, Nakajima Y, Higaki M, et al. Arthritis in patients infected with human T lymphotropic virus type I. Clinical and immunopathologic features. [2020 Oct 15];Arthritis Rheum. 1991 Jun;34(6):714–721. doi: 10.1002/art.1780340612. [Internet] [DOI] [PubMed] [Google Scholar]
- 68.Dennis G, Chitkara P. A case of human T lymphotropic virus type I-associated synovial swelling. [2020 Oct 15];Nat Clin Pract Rheumatol. 2007 Nov;3:675–680. doi: 10.1038/ncprheum0648. [Internet] [DOI] [PubMed] [Google Scholar]
- 69.Kawai H, Inui T, Kashiwagi S, Tsuchihashi T, Masuda K, Kondo A, et al. HTLV-I infection in patients with autoimmune thyroiditis (Hashimoto’s thyroiditis) [2020 Oct 15];J Med Virol. 1992 Oct;38(2):138–141. doi: 10.1002/jmv.1890380212. [Internet] [DOI] [PubMed] [Google Scholar]
- 70.Matsuda T, Tomita M, Uchihara J-N, Okudaira T, Ohshiro K, Tomoyose T, et al. Human T cell leukemia virus type I-infected patients with Hashimoto’s thyroiditis and Graves’ disease. [2020 Oct 15];J Clin Endocrinol Metab. 2005 Oct;90(10):5704–5710. doi: 10.1210/jc.2005-0679. [Internet] [DOI] [PubMed] [Google Scholar]
- 71.Abolbashari S, Darroudi S, Tayefi M, Khashyarmaneh Z, Zamani P, Haghighi HM, et al. Association between serum zinc and copper levels and antioxidant defense in subjects infected with human T-lymphotropic virus type 1. [2020 Oct 15];J Blood Med. 2018 Dec;10:29–35. doi: 10.2147/jbm.s184913. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohammadi FS, Mosavat A, Shabestari M, Shabestari M, Ghezeldasht SA, Shabestari M, et al. HTLV-1-host interactions facilitate the manifestations of cardiovascular disease. [2020 Oct 15];Microb Pathog. 2019 Sep;134:103578. doi: 10.1016/j.micpath.2019.103578. [Internet] [DOI] [PubMed] [Google Scholar]
- 73.Oliveira TSS, Andrade RCP, Santos DN, Orrico KF, Abraão J, Neto , Oliveira CJV, et al. Prevalence of Bowel Symptoms in Patients Infected with Human T-Lymphotropic type 1 Virus. [2020 Oct 15];Rev Soc Bras Med Trop. 2019 Nov;52:e20180486. doi: 10.1590/0037-8682-0486-2018. [Internet] [DOI] [PubMed] [Google Scholar]
- 74.Silva MT, Coutinho F, Leite AC, Harab RC, Araújo A, Andrada-Serpa MJ. Isolated bladder dysfunction in human T lymphotropic virus type 1 infection. [2020 Oct 15];Clin Infect Dis. 2009 Feb;48(3):e34–e36. doi: 10.1086/595855. [Internet] [DOI] [PubMed] [Google Scholar]
- 75.Nayar S, Pawar B, Einsiedel L, Fernandes D, George P, Thomas S, et al. Isolated neurogenic bladder associated with human T-Lymphotropic virus type 1 infection in a renal transplant patient from central Australia: a case report. [2020 Oct 15];Transplant Proc. 2018 Dec;50(10):3940–3942. doi: 10.1016/j.transproceed.2018.08.031. [Internet] [DOI] [PubMed] [Google Scholar]
- 76.Tanajura D, Castro N, Oliveira P, Abraão-Neto MA, Carvalho NB, et al. Neurological manifestations in human T-cell lymphotropic virus type 1 (HTLV-1)-infected individuals without HTLV-1-associated myelopathy/tropical spastic paraparesis: a longitudinal cohort study. [2020 Oct 15];Clin Infect Dis. 2015 Jul;61(1):49–56. doi: 10.1093/cid/civ229. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haziot ME, Gascon MR, Assone T, Fonseca LAM, Luiz OC, Smid J, et al. Detection of clinical and neurological signs in apparently asymptomatic HTLV-1 infected carriers: Association with high proviral load. [2020 Oct 15];PLoS Negl Trop Dis. 2019 May;13:e0006967. doi: 10.1371/journal.pntd.0006967. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamano Y, Nagai M, Brennan M, Mora CA, Soldan SS, Tomaru U, et al. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8+ T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood. 2002 Jan;99(1):88–94. doi: 10.1182/blood.v99.1.88. [Internet] [DOI] [PubMed] [Google Scholar]
- 79.Montanheiro PA, Oliveira ACP, Posada-Vergara MP, Milagres AC, Tauil C, et al. Human T-cell lymphotropic virus type I (HTLV-I) proviral DNA viral load among asymptomatic patients and patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. [2020 Oct 15];Brazilian J Med Biol Res. 2005 Nov;38(11):1643–1647. doi: 10.1590/S0100-879X2005001100011. [Internet] [DOI] [PubMed] [Google Scholar]
- 80.Champs APS, Passos VMA, Barreto SM, Vaz LS, Ribas JGR. HTLV-1 associated myelopathy: clinical and epidemiological profile in a 10-year case series study. [2020 Oct 15];Rev Soc Bras Med Trop. 2010 43(6):668–672. doi: 10.1590/S0037-86822010000600013. [Internet] [DOI] [PubMed] [Google Scholar]
- 81.Okajima R, Casseb J, Sanches JA. Co-presentation of human T-cell lymphotropic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis and adult-onset infective dermatitis associated with HTLV-1 infection. [2020 Oct 15];Int J Dermatol. 2013 Jan;52(1):63–68. doi: 10.1111/j.1365-4632.2012.05606.x. [Internet] [DOI] [PubMed] [Google Scholar]
- 82.Okajima R, Oliveira ACP, Smid J, Casseb J, Sanches JA. High prevalence of skin disorders among HTLV-1 infected individuals independent of clinical status. [2020 Oct 15];PLoS Negl Trop Dis. 2013 Nov;7(11):e2546. doi: 10.1371/journal.pntd.0002546. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Araujo AQC, Wedemann D. HTLV-1 Associated neurological complex. What is hidden below the water? [2020 Oct 15];AIDS Rev. 2019 21(4):211–217. doi: 10.24875/aidsrev.19000108. [Internet] [DOI] [PubMed] [Google Scholar]
- 84.Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Arimura K, Kubota H, et al. HTLV-I proviral load correlates with progression of motor disability in HAM/TSP: analysis of 239 HAM/TSP patients including 64 patients followed up for 10 years. [2020 Oct 15];J Neurovirol. 2001 Jun;7(3):228–234. doi: 10.1080/13550280152403272. [Internet] [DOI] [PubMed] [Google Scholar]
- 85.Rosadas C, Puccioni-Sohler M. Relevance of retrovirus quantification in cerebrospinal fluid for neurologic diagnosis. [2020 Oct 15];J Biomed Sci. 2015 Aug;22(1):66–66. doi: 10.1186/s12929-015-0170-y. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayashi D, Kubota R, Takenouchi N, Nakamura T, Umehara F, Arimura K, et al. Accumulation of human T-lymphotropic virus type I (HTLV-I)-infected cells in the cerebrospinal fluid during the exacerbation of HTLV-I-associated myelopathy. [2020 Oct 15];J Neurovirol. 2008 Oct;14(5):459–463. doi: 10.1080/13550280802178538. [Internet] [DOI] [PubMed] [Google Scholar]
- 87.Lezin A, Olindo S, Oliere S, Varrin-Doyer M, Martin R, Cabre P, et al. Human T lymphotropic virus type I (HTLV-I) proviral load in cerebrospinal fluid: a new criterion for the diagnosis of HTLV-I-associated myelopathy/tropical spastic paraparesis? [2020 Oct 15];J Infect Dis. 2005 Jun;191(11):1830–1834. doi: 10.1086/429962. [Internet] [DOI] [PubMed] [Google Scholar]
- 88.Starling ALB, Coelho-dos-Reis JGA, Peruhype-Magalhães V, Pascoal-Xavier MA, Gonçalves DU, Béia SR, et al. Immunological signature of the different clinical stages of the HTLV-1 infection: establishing serum biomarkers for HTLV-1-associated disease morbidity. [2020 Oct 15];Biomarkers. 2015 20(6-7):502–512. doi: 10.3109/1354750x.2015.1094141. [Internet] [DOI] [PubMed] [Google Scholar]
- 89.Yamauchi J, Araya N, Yagishita N, Sato T, Yamano Y. An update on human T-cell leukemia virus type I (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) focusing on clinical and laboratory biomarkers. [2020 Oct 15];Pharmacol Ther. 2020 Aug;:107669. doi: 10.1016/j.pharmthera.2020.107669. [Internet] [DOI] [PubMed] [Google Scholar]
- 90.Apoliano CF, Assone T, Maciel da Silva BC, Corral MA, Oliveira ACP, Fonseca LAM, et al. Interferon-γ secretion enzyme-linked immunospot assay determined among human T cell lymphotropic virus type 1-infected subjects: a potential laboratory marker for early HTLV-1-associated myelopathy/tropical spastic paraparesis diagnosis. [2020 Oct 15];AIDS Res Hum Retroviruses. 2020 Jan;36(1):6–7. doi: 10.1089/aid.2018.0290. [Internet] [DOI] [PubMed] [Google Scholar]
- 91.Tajima K, Cartier L. Epidemiological features of HTLV-I and adult T cell leukemia. [2020 Oct 15];Intervirology. 1995 38(3-4):238–246. doi: 10.1159/000150438. [Internet] [DOI] [PubMed] [Google Scholar]
- 92.Kondo T, Kono H, Miyamoto N, Yoshida R, Toki H, Matsumoto I, et al. Age- and sex-specific cumulative rate and risk of ATLL for HTLV-I carriers. [2020 Oct 15];Int J cancer. 1989 Jun;43(6):1061–1064. doi: 10.1002/ijc.2910430618. [Internet] [DOI] [PubMed] [Google Scholar]
- 93.Nosaka K, Iwanaga M, Imaizumi Y, Ishitsuka K, Ishizawa K, Ishida Y, et al. Epidemiological and clinical features of adult T-cell leukemia-lymphoma in Japan, 2010-2011: a nationwide survey. [2020 Oct 15];Cancer Sci. 2017 Dec;108(12):2478–2486. doi: 10.1111/cas.13398. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwanaga M, Watanabe T, Utsunomiya A, Okayama A, Uchimaru K, Koh KR, et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. [2020 Oct 15];Blood. 2010 Aug;116(8):1211–1219. doi: 10.1182/blood-2009-12-257410. [Internet] [DOI] [PubMed] [Google Scholar]
- 95.Phillips AA, Shapira I, Willim RD, Sanmugarajah J, Solomon WB, Horwitz SM, et al. A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a multicenter clinicopathologic experience and new prognostic score. [2020 Oct 15];Cancer. 2010 Jul;116(14):3438–3446. doi: 10.1002/cncr.25147. [Internet] [DOI] [PubMed] [Google Scholar]
- 96.Rosadas C, Puccioni-Sohler M, Oliveira ACP, Casseb J, Sousa M, Taylor GP. Adult T-cell leukaemia/lymphoma in Brazil: a rare disease or rarely diagnosed? [2020 Oct 15];Br J Haematol. 2020 Feb;188(4):e46–e49. doi: 10.1111/bjh.16318. [Internet] [DOI] [PubMed] [Google Scholar]
- 97.van Tienen C, Visser O, Lugtenburg P, Taylor G, Cook L. Overrepresentation of patients from HTLV-1 endemic countries among T cell Non-Hodgkin lymphomas in the Netherlands: an indication of under-diagnosis of Adult T cell leukaemia/lymphoma. [2020 Oct 15];Br J Haematol. 2018 Feb;184(4):688–689. doi: 10.1111/bjh.15160. [Internet] [DOI] [PubMed] [Google Scholar]
- 98.Lymphoma Study Group Major prognostic factors of patients with adult T-cell leukemia-lymphoma: a cooperative study. [2020 Oct 15];Leuk Res. 1991 15(2-3):81–90. doi: 10.1016/0145-2126(91)90087-A. [Internet] [DOI] [PubMed] [Google Scholar]
- 99.Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W, Jr, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. [2020 Oct 15];J Clin Oncol. 2009 Jan;27(3):453–459. doi: 10.1200/jco.2008.18.2428. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87) [2020 Oct 15];Br J Haematol. 1991 Nov;79(3):428–437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [Internet] [DOI] [PubMed] [Google Scholar]
- 101.Yared JA, Kimball AS. Optimizing management of patients with adult T cell leukemia-lymphoma. [2020 Oct 15];Cancers (Basel) 2015 Dec;7(4):2318–2329. doi: 10.3390/cancers7040893. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brites C, Weyll M, Pedroso C, Badaró R. Severe and Norwegian scabies are strongly associated with retroviral (HIV-1/HTLV-1) infection in Bahia, Brazil. [2020 Oct 15];AIDS. 2002 Jun;16(9):1292–1293. doi: 10.1097/00002030-200206140-00015. [Internet] [DOI] [PubMed] [Google Scholar]
- 103.Dantas L, Netto E, Glesby MJ, Carvalho EM, Machado P. Dermatological manifestations of individuals infected with human T cell lymphotropic virus type I (HTLV-I) [2020 Oct 15];Int J Dermatol. 2014 Sep;53(9):1098–1102. doi: 10.1111/ijd.12170. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.LaGrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. [2020 Oct 15];Lancet (London, England) 1990 Dec;336(8727):1345–1347. doi: 10.1016/0140-6736(90)92896-p. [Internet] [DOI] [PubMed] [Google Scholar]
- 105.Bittencourt AL, Primo J, Oliveira MFP. Manifestations of the human T-cell lymphotropic virus type I infection in childhood and adolescence. [2020 Oct 15];J Pediatr (Rio J) 2006 82(6):411–420. doi: 10.2223/JPED.1573. [Internet] [DOI] [PubMed] [Google Scholar]
- 106.Oliveira MFSP, Fatal PL, Primo JRL, Silva JLS, Batista ES, Ferré L, et al. Infective dermatitis associated with human T-cell lymphotropic virus type 1: evaluation of 42 cases observed in Bahia, Brazil. [2020 Oct 15];Clin Infect Dis. 2012 Jun;54(12):1714–1719. doi: 10.1093/cid/cis273. [Internet] [DOI] [PubMed] [Google Scholar]
- 107.Bittencourt AL, Oliveira MFP. Cutaneous manifestations associated with HTLV-1 infection. [2020 Oct 15];Int J Dermatol. 2010 Oct;49(10):1099–1110. doi: 10.1111/j.1365-4632.2010.04568.x. [Internet] [DOI] [PubMed] [Google Scholar]
- 108.Ohba N, Matsumoto M, Sameshima M, Kabayama Y, Nakao K, Unoki K, et al. Ocular manifestations in patients infected with human T-lymphotropic virus type I. Jpn J Ophthalmol. 1989;33(1):1–12. [PubMed] [Google Scholar]
- 109.Mochizuki M, Tajima K, Watanabe T, Yamaguchi K. Human T lymphotropic virus type 1 uveitis. [2020 Oct 15];Br J Ophthalmol. 1994 Feb;78(2):149–154. doi: 10.1136/bjo.78.2.149. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brites C, Sampalo J, Oliveira A. HIV/human T-cell lymphotropic virus coinfection revisited: impact on AIDS progression. [2020 Oct 15];AIDS Rev. 2009 Jan-Mar;11(1):8–16. [Internet] Available from: https://pubmed.ncbi.nlm.nih.gov/19290030. [PubMed] [Google Scholar]
- 111.Pedroso C, Netto EM, Weyll N, Brites C. Coinfection by HIV-1 and human lymphotropic virus type 1 in Brazilian children is strongly associated with a shorter survival time. [2020 Oct 15];J Acquir Immune Defic Syndr. 2011 Aug;57(Suppl 3):S208–S211. doi: 10.1097/qai.0b013e31821e9baf. [Internet] [DOI] [PubMed] [Google Scholar]
- 112.Brites C, Miranda F, Luz E, Netto EM. Early and successful combination antiretroviral therapy normalizes survival time in patients coinfected with human immunodeficiency virus and human T-cell lymphotrophic virus type 1. [2020 Oct 15];Clin Infect Dis. 2020 Jun;71(1):196–200. doi: 10.1093/cid/ciz756. [Internet] [DOI] [PubMed] [Google Scholar]
- 113.Boschi-Pinto C, Stuver S, Okayama A, Trichopoulod D, Orav EJ, Tsubouchi H, et al. A followu-p study of morbidity and mortality associated with hepatitis C virus infection and its interaction with human T lymphotropic virus type I in Miyazaki, Japan. [2020 Oct 15];J Infect Dis. 2000 Jan;181(1):35–41. doi: 10.1086/315177. [Internet] [DOI] [PubMed] [Google Scholar]
- 114.Brites C, Abrahão M, Bozza P, Netto EM, Lyra A, Bahia F. Infection by HTLV-1 Is associated with high levels of proinflammatory cytokines in HIV-HCV-coinfected patients. [2020 Oct 15];J Acquir Immune Defic Syndr. 2018 Feb;77(2):230–234. doi: 10.1097/qai.0000000000001576. [Internet] [DOI] [PubMed] [Google Scholar]
- 115.Bahia F, Novais V, Evans J, Marchand CL, Netto E, Page K, et al. The impact of human T-cell lymphotropic virus i infection on clinical and immunologic outcomes in patients coinfected with HIV and hepatitis C virus. [2020 Oct 15];J Acquir Immune Defic Syndr. 2011 Aug;57(3):S202–S207. doi: 10.1097/QAI.0b013e31821e9a1e. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marchand CL, Bahia F, Page K, Brites C. Hepatitis C virus infection and spontaneous clearance in HTLV-1 and HIV co-infected patients in Salvador, Bahia, Brazil. [2020 Oct 15];Braz J Infect Dis. 2015 19(5):486–491. doi: 10.1016/j.bjid.2015.06.007. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gillet NA, Cook L, Laydon DJ, Hlela C, Verdonck K, Alvarez C, et al. Strongyloidiasis and infective dermatitis alter human T lymphotropic virus-1 clonality in vivo. [2020 Oct 15];PLoS Pathog. 2013 Apr;9(4):e1003263. doi: 10.1371/journal.ppat.1003263. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakada K, Yamaguchi K, Furugen S, Nakasone K, Oshiro Y, Kohakura M, et al. Monoclonal integration of HTLV-I proviral DNA in patients with strongyloidiasis. [2020 Oct 15];Int J Cancer. 1987 Aug;40(2):145–148. doi: 10.1002/ijc.2910400203. [Internet] [DOI] [PubMed] [Google Scholar]
- 119.Newton RC, Limpuangthip P, Greenberg S, Gam A, Neva FA. Strongyloides stercoralis hyperinfection in a carrier of HTLV-I virus with evidence of selective immunosuppression. [2020 Oct 15];Am J Med. 1992 Feb;92(2):202–208. doi: 10.1016/0002-9343(92)90113-p. [Internet] [DOI] [PubMed] [Google Scholar]
- 120.Terashima A, Alvarez H, Tello R, Infante R, Freedman DO, Gotuzzo E. Treatment failure in intestinal strongyloidiasis: an indicator of HTLV-I infection. [2020 Oct 15];Int J Infect Dis. 2002 Mar;6(1):28–30. doi: 10.1016/s1201-9712(02)90132-3. [Internet] [DOI] [PubMed] [Google Scholar]
- 121.Gotuzzo E, Moody J, Verdonck K, Cabada MM, González E, van Dooren S, et al. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1 - associated myelopathy / tropical spastic paraparesis or strongyloidiasis. [2020 Oct 15];Rev Panam Salud Publica. 2007 Oct;22(4):223–230. doi: 10.1590/s1020-49892007000900001. [Internet] [DOI] [PubMed] [Google Scholar]
- 122.Porto MAF, Muniz A, Oliveira J, Jr, Carvalho EM. Implicações clinicas e imunológicas da associação entre o HTLV-1 e a estrongiloidíase. Rev Soc Bras Med Trop. 2002;35(6):641–649. doi: 10.1590/S0037-86822002000600016. [Internet] [DOI] [PubMed] [Google Scholar]
- 123.Sato Y, Shiroma Y. Concurrent infections with Strongyloides and T-cell leukemia virus and their possible effect on immune responses of host. [2020 Oct 15];Clin Immunol Immunopathol. 1989 Aug;52(2):214–224. doi: 10.1016/0090-1229(89)90173-6. [Internet] [DOI] [PubMed] [Google Scholar]
- 124.Salles F, Bacellar A, Amorim M, Orge G, Sundberg M, Lima M, et al. Treatment of strongyloidiasis in HTLV-1 and Strongyloides stercoralis coinfected patients is associated with increased tnfα and decreased soluble IL2 receptor levels. [2020 Oct 15];Trans R Soc Trop Med Hyg. 2013 Aug;107(8):526–529. doi: 10.1093/trstmh/trt052. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gabet A-S, Mortreux F, Talarmin A, Plumelle Y, Leclercq I, Leroy A, et al. High circulating proviral load with oligoclonal expansion of HTLV-1 bearing T cells in HTLV-1 carriers with strongyloidiasis. [2020 Oct 15];Oncogene. 2000 Oct;19(43):4954–4960. doi: 10.1038/sj.onc.1203870. [Internet] [DOI] [PubMed] [Google Scholar]
- 126.Plumelle Y, Gonin C, Edouard A, Bucher BJ, Thomas L, Brebion A, et al. Effect of Strongyloides stercoralis infection and eosinophilia on age at onset and prognosis of adult T-cell leukemia. [2020 Oct 15];Am J Clin Pathol. 1997 Jan;107(1):81–87. doi: 10.1093/ajcp/107.1.81. [Internet] [DOI] [PubMed] [Google Scholar]
- 127.Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. [2020 Oct 15];Lancet Infect Dis. 2019 Apr;20(4):407–408. doi: 10.1016/S1473-3099(20)30133-X. [Internet] [DOI] [PubMed] [Google Scholar]
- 128.Marinho J, Galvao-Castro B, Rodrigues LC, Barreto ML. Increased risk of tuberculosis with human T-lymphotropic virus-1 infection a case-control study. [2020 Oct 15];J Acquir Immune Defic Syndr. 2005 40(5):625–628. doi: 10.1097/01.qai.0000174252.73516.7a. [Internet] Available from: https://www.arca.fiocruz.br/handle/icict/8131. [DOI] [PubMed] [Google Scholar]
- 129.Norrgren HR, Bamba S, Larsen O, Silva Z, Aaby P, Koivula T, et al. Increased prevalence of HTLV-1 in patients with pulmonary tuberculosis coinfected with HIV, but not in HIV-negative patients with tuberculosis. [2020 Oct 15];J Acquir Immune Defic Syndr. 2008 Aug;48(5):607–610. doi: 10.1097/qai.0b013e31817efb83. [Internet] [DOI] [PubMed] [Google Scholar]
- 130.Moreira ED, Ribeiro TT, Swanson P, Sampoio C, Filho, Melo A, Brites C, et al. Seroepidemiology of human T-cell lymphotropic virus type I/II in northeastern Brazil. J Acquir Immune Defic Syndr. 1993 Aug;6(8):959–963. [PubMed] [Google Scholar]
- 131.Hanada S, Uematsu T, Iwahashi M, Nomura K, Utsunomiya A, Kodama M, et al. The prevalence of human T-cell leukemia virus type I infection in patients with hematologic and nonhematologic diseases in an adult T-cell leukemia-endemic area of Japan. [2020 Oct 15];Cancer. 1989 Sep;64(6):1290–1295. doi: 10.1002/1097-0142(19890915)64:6<1290::aid-cncr2820640620>3.0.co;2-z. [Internet] [DOI] [PubMed] [Google Scholar]
- 132.Verdonck K, Gonzalez E, Schrooten W, Vanham G, Gotuzzo E. HTLV-1 infection is associated with a history of active tuberculosis among family members of HTLV-1-infected patients in Peru. [2020 Oct 15];Epidemiol Infect. 2008 Aug;136(8):1076–1083. doi: 10.1017/S0950268807009521. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brasil. Ministério da Saúde . Diário Oficial da União. Brasília (DF): Oct, 2009. [2020 jun 14]. Portaria n. 2.600, de 21 de outubro de 2009. Aprova o Regulamento Técnico do Sistema Nacional de Transplantes. [Internet] Seção I:77. Available from: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2009/prt2600_21_10_2009.html. [Google Scholar]
- 134.Brasil. Ministério da Saúde . Diário Oficial da União. Brasília (DF): Jun, 2016. [2020 jun 14]. Portaria n. 23, de 31 de maio de 2016. Torna pública a decisão de incorporar os procedimentos laboratoriais por técnicas de Western Blot e PCR em tempo real no diagnóstico de leucemia/linfoma de células T do adulto associado ao HTLV-1, no âmbito do Sistema Único de Saúde - SUS. [Internet] Seção I:45. Available from: http://bvsms.saude.gov.br/bvs/saudelegis/sctie/2016/prt0023_31_05_2016.html%09%09%09%09%09%09. [Google Scholar]
- 135.Itabashi K, Miyazawa T, Sekizawa A, Tokita A, Saito S, Moriuchi H, et al. A nationwide antenatal human T-cell leukemia virus type-1 antibody screening in Japan. [2020 Oct 15];Front Microbiol. 2020 Apr;11:595–595. doi: 10.3389/fmicb.2020.00595. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cassar O, Gessain A. Serological and molecular methods to study epidemiological aspects of human T-cell lymphotropic virus type 1 infection. [2020 Oct 15];Methods Mol Biol. 2017 1582:3–24. doi: 10.1007/978-1-4939-6872-5_1. [Internet] [DOI] [PubMed] [Google Scholar]
- 137.Puccioni-Sohler M, Grassi MFR, Galvão-Castro B, Caterino A, Proietti ABFC, Vicente ACP, et al. Increasing awareness of human T-lymphotropic virus type-1 infection: a serious, invisible, and neglected health problem in Brazil. [2020 Oct 15];Rev Soc Bras Med Trop. 2019 Oct;52:e20190343. doi: 10.1590/0037-8682-0343-2019. [Internet] [DOI] [PubMed] [Google Scholar]
- 138.Silva Brito V, Santos FLN, Gonçalves NLS, Araújo THA, Nascimento DSV, Pereira FM, et al. Performance of commercially available serological screening tests for human T-cell lymphotropic virus infection in Brazil. [2020 Oct 15];J Clin Microbiol. 2018 Nov;56(12):e00961. doi: 10.1128/jcm.00961-18. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cánepa C, Salido J, Ruggieri M, Fraile S, Pataccini G, Berinii C, et al. Low Proviral load is associated with indeterminate western blot patterns in human T-cell lymphotropic virus type 1 infected individuals: could punctual mutations be related? [2020 Oct 15];Viruses. 2015 Nov;7(11):5643–5658. doi: 10.3390/v7112897. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tebourski F, Slim A, Elgaaied A. The significance of combining World Health Organization and Center for Disease Control criteria to resolve indeterminate human immunodeficiency virus type-1 Western blot results. [2020 Oct 15];Diagn Microbiol Infect Dis. 2004 Jan;48(1):59–61. doi: 10.1016/j.diagmicrobio.2003.08.004. [Internet] [DOI] [PubMed] [Google Scholar]
- 141.Ishak R, Vallinoto ACR, Azevedo VN, Vicente ACP, Hall WW, Ishak MO. Molecular evidence for infection by HTLV-2 among individuals with negative serological screening tests for HTLV antibodies. [2020 Oct 15];Epidemiol Infect. 2007 May;135(4):604–609. doi: 10.1017/s0950268806006984. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuramitsu M, Sekizuka T, Yamochi T, Firouzi S, Sato T, Umeki K, et al. Proviral features of human T cell leukemia virus type 1 in carriers with indeterminate western blot analysis results. [2020 Oct 15];J Clin Microbiol. 2017 Sep;55(9):2838–2849. doi: 10.1128/jcm.00659-17. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Araujo A C, Casseb J S, Neitzert E, Souza ML, Mammano F, Mistro AD, et al. HTLV-I and HTLV-II infections among HIV-1 seropositive patients in Sao Paulo, Brazil. [2020 Oct 15];Eur J Epidemiol. 1994 Apr;10(2):165–171. doi: 10.1007/bf01730366. [Internet] [DOI] [PubMed] [Google Scholar]
- 144.Campos KR, Gonçalves MG, Costa NA, Caterino-de-Araujo A. Comparative performances of serologic and molecular assays for detecting human T lymphotropic virus type 1 and type 2 (HTLV-1 and HTLV-2) in patients infected with human immunodeficiency virus type 1 (HIV-1) [2020 Oct 15];Brazilian J Infect Dis. 2017 21(3):297–305. doi: 10.1016/j.bjid.2017.02.005. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jacob F, Santos-Fortuna E, Azevedo RS, Caterino-de-Araujo A. Serological patterns and temporal trends of HTLV-1/2 infection in high-risk populations attending Public Health Units in São Paulo, Brazil. [2020 Oct 15];J Clin Virol. 2008 Jun;42(2):149–155. doi: 10.1016/j.jcv.2008.01.017. [Internet] [DOI] [PubMed] [Google Scholar]
- 146.Morimoto HK, Morimoto AA, Reiche EMV, Ueda LT, Matsuo T, Reiche FV, et al. Difficulties in the diagnosis of HTLV-2 infection in HIV/AIDS patients from Brazil: comparative performances of serologic and molecular assays, and detection of HTLV-2b subtype. [2020 Oct 15];Rev Inst Med Trop São Paulo. 2007 Jul-Aug;49(4):225–230. doi: 10.1590/S0036-46652007000400006. [Internet] [DOI] [PubMed] [Google Scholar]
- 147.Mangano AM, Remesar M, del Pozo A, Sen L. Human T lymphotropic virus types I and II proviral sequences in Argentinian blood donors with indeterminate Western blot patterns. J Med Virol. 2004 Oct;74(2):323–327. doi: 10.1002/jmv.20172. [Internet] [DOI] [PubMed] [Google Scholar]
- 148.Martins ML, Santos ACS, Namen-Lopes MS, Barbosa-Stancioli EF, Utsch DG, Carneiro-Proietti ABF. Long-term serological follow-up of blood donors with an HTLV-Indeterminate Western Blot: Antibody Profile of Seroconverters and Individuals With False Reactions. [2020 Oct 15];J Med Virol. 2010 Oct;82(10):1746–1753. doi: 10.1002/jmv.21881. [Internet] [DOI] [PubMed] [Google Scholar]
- 149.Abrams A, Akahata Y, Jacobson S. The prevalence and significance of HTLV-I/II seroindeterminate western blot patterns. [2020 Oct 15];Viruses. 2011 Aug;3(8):1320–1331. doi: 10.3390/v3081320. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Campos KR, Santos FLN, Silva Brito V, Gonçalves NLS, Araújo THA, Galvão-Castro B, et al. Line immunoassay for confirmation and discrimination of human T-cell lymphotropic virus infections in inconclusive western blot serum samples from Brazil. [2020 Oct 15];J Clin Microbiol. 2019 Dec;58(1):e01384-19. doi: 10.1128/jcm.01384-19. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Umeki K, Umekita K, Hashikura Y, Yamamoto I, Kubo K, Nagatomo Y, et al. Evaluation of line immunoassay to detect HTLV-1 infection in an endemic area, southwestern Japan; comparison with polymerase chain reaction and western blot. [2020 Oct 15];Clin Lab. 2017 Feb;63(2):227–233. doi: 10.7754/clin.lab.2016.160501. [Internet] [DOI] [PubMed] [Google Scholar]
- 152.Okuma K, Kuramitsu M, Niwa T, Taniguchi T, Masaki Y, Ueda G, et al. Establishment of a novel diagnostic test algorithm for human T-cell leukemia virus type 1 infection with line immunoassay replacement of western blotting: a collaborative study for performance evaluation of diagnostic assays in Japan. [2020 Oct 15];Retrovirology. 2020 Aug;17(1):26–26. doi: 10.1186/s12977-020-00534-0. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Primo J, Siqueira I, Nascimento MCF, Oliveira MF, Farre L, Carvalho EM. High HTLV-1 proviral load, a marker for HTLV-1 associated myelopathy/tropical spastic paraparesis, is also detected in patients with infective dermatitis associated with HTLV-1. [2020 Oct 15];Brazilian J Med Biol Res. 2009 Jul;42(8):761–764. doi: 10.1590/S0100-879X2009005000008. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hisada M, Okayama A, Shioiri S, Spiegelman DL, Stuver SO, Mueller NE. Risk factors for adult T-cell leukemia among carriers of human T-lymphotropic virus type I. Blood. 1998 Nov;92(10):3557–3561. [PubMed] [Google Scholar]
- 155.Rosadas C, Cabral-Castro MJ, Vicente ACP, Peralta JM, Puccioni-Sohler M. Validation of a quantitative real-time PCR assay for HTLV-1 proviral load in peripheral blood mononuclear cells. [2020 Oct 15];J Virol Methods. 2013 Nov;193(2):536–541. doi: 10.1016/j.jviromet.2013.07.040. [Internet] [DOI] [PubMed] [Google Scholar]
- 156.Cabral F, Arruda LB, Araújo ML, Montanheiro P, Smid J, Oliveira ACP. Detection of human T-cell lymphotropic virus type 1 in plasma samples. [2020 Oct 15];Virus Res. 2012 Jan;163(1):87–90. doi: 10.1016/j.virusres.2011.08.014. [Internet] [DOI] [PubMed] [Google Scholar]
- 157.Demontis MA, Sadiq MT, Golz S, Taylor GP. HTLV-1 viral RNA is detected rarely in plasma of HTLV-1 infected subjects. [2020 Oct 15];J Med Virol. 2015 Dec;87(12):2130–2134. doi: 10.1002/jmv.24264. [Internet] [DOI] [PubMed] [Google Scholar]
- 158.Tamegão-lopes BP, Rezende PR, Cunha LM. Carga proviral do HTLV-1 e HTLV-2: um método simples através da PCR quantitativa em tempo real. Rev Soc Bras Med Trop. 2006 Nov-Dez;39(6):548–552. doi: 10.1590/S0037-86822006000600007. [Internet] [DOI] [PubMed] [Google Scholar]
- 159.Medeiros ACM, Vidal LRR, Von Linsingen R, Ferin AN, Strapasson TB, Almeida SM, et al. Confirmatory molecular method for HTLV-1/2 infection in high-risk pregnant women. [2020 Oct 15];J Med Virol. 2018 May;90(5):998–1001. doi: 10.1002/jmv.25014. [DOI] [PubMed] [Google Scholar]
- 160.Kamihira S, Yamano Y, Iwanaga M, Sasaki D, Satake M, Okayama A, et al. Intra- and inter-laboratory variability in human T-cell leukemia virus type-1 proviral load quantification using real-time polymerase chain reaction assays: a multi-center study. [2020 Oct 15];Cancer Sci. 2010 Nov;101(11):2361–2367. doi: 10.1111/j.1349-7006.2010.01720.x. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hayden RT, Gu Z, Ingersoll J, Abdul-Ali D, Pounds S, Caliendo AM, et al. Comparison of droplet digital PCR to real-time PCR for quantitative detection of cytomegalovirus. [2020 Oct 15];J Clin Microbiol. 2013 Feb;51(2):540–546. doi: 10.1128/jcm.02620-12. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grassi MFR, Olavarria VN, Kruschewsky RA, Yamano Y, Jacobson S, Taylor GP, et al. Utility of HTLV proviral load quantification in diagnosis of HTLV-1-associated myelopathy requires international standardization. [2020 Oct 15];J Clin Virol. 2013 Nov;58(3):584–586. doi: 10.1016/j.jcv.2013.09.003. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kuramitsu M, Okuma K, Yamochi T, Sato T, Sasaki D, Hasegawa H, et al. Standardization of quantitative PCR for human T-cell leukemia virus type 1 in Japan: a collaborative study. [2020 Oct 15];J Clin Microbiol. 2015 Nov;53(11):3485–3491. doi: 10.1128/jcm.01628-15. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee T-H, Chafets DM, Busch MP, Murphy EL. Quantitation of HTLV-I and II proviral load using real-time quantitative PCR with SYBR Green chemistry. [2020 Oct 15];J Clin Virol. 2004 Dec;31(4):275–282. doi: 10.1016/j.jcv.2004.05.016. [Internet] [DOI] [PubMed] [Google Scholar]
- 165.Rosadas C, Tosswill JH, Tedder R, Taylor GP. Pregnancy does not adversely impact diagnostic tests for HTLV-1/2 infection. [2020 Oct 15];PLoS Negl Trop Dis. 2019 Sep;13(9):e0007736. doi: 10.1371/journal.pntd.0007736. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Montanheiro P, Olah I, Fukumori LMI, Smid J, Oliveira ACP, Kankaki LIB, et al. Low DNA HTLV-2 proviral load among women in São Paulo City. [2020 Oct 15];Virus Res. 2008 Jul;135(1):22–25. doi: 10.1016/j.virusres.2008.01.015. [Internet] [DOI] [PubMed] [Google Scholar]
- 167.Cook LBM, Melamed A, Demontis MA, Laydon DJ, Fox JM, Tosswill JHC, et al. Rapid dissemination of human T-lymphotropic virus type 1 during primary infection in transplant recipients. [2020 Oct 15];Retrovirology. 2016 Jan;13:3–3. doi: 10.1186/s12977-015-0236-7. [Internet]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Murphy EL. Infection with human T-lymphotropic virus types-1 and -2 (HTLV-1 and -2): implications for blood transfusion safety. [2020 Oct 15];Transfus Clin Biol. 2016 Feb;23(1):13–19. doi: 10.1016/j.tracli.2015.12.001. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ministério da Saúde (BR). Secretaria de Vigilância em Saúde . Guia de manejo clínico da infecção pelo HTLV. Brasilia: Ministério da Saúde; 2013. [2019 fev 4]. [Internet] Available from: http://www.sierj.org.br/artigos/htlv_manual_final_pdf_25082.pdf. [Google Scholar]
- 170.Ministério da Saúde (BR) Protocolo de uso zidovudina para tratamento do adulto com leucemia/linfoma associado ao Vírus HTLV-1. Brasília: Ministério da Saúde; 2016. [2020 out 15]. [Internet] Available from: http://www.aids.gov.br/pt-br/pub/2016/protocolo-de-uso-da-zidovudina-para-tratamento-do-adulto-com-leucemialinfoma-associado-ao. [Google Scholar]
- 171.Cook L B, Fuji S, Hermine O, Bazarbachi A, Ramos JC, Ratner L, et al. Revised adult T-cell leukemia-lymphoma international consensus meeting report. [2020 Oct 15];J Clin Oncol. 2019 Mar;37(8):677–687. doi: 10.1200/jco.18.00501. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Araujo A, Bangham CRM, Casseb J, Gotuzzo E, Jacobson S, Martin F, et al. Management of HAM/TSP. [2020 Oct 15];Neurol Clin Pract. 2020 Mar; doi: 10.1212/CPJ.0000000000000832. [Internet] [DOI] [Google Scholar]
- 173.Zihlmann KF, Alvarenga AT, Casseb J. Living invisible: HTLV-1-Infected persons and the lack of care in public health. [2020 Oct 15];PLoS Negl Trop Dis. 2012 6(6):e1705. doi: 10.1371/journal.pntd.0001705. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Centers for Disease Control and Prevention - CDC Recommendations for counseling persons infected with human T-lymphotrophic virus, types I and II. [2020 Oct 15];MMWR. 1993 Jun;42(RR-9):1–13. [Internet] Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/00021234.htm. [PubMed] [Google Scholar]
- 175.Coler-Reilly ALG, Yagishita N, Suzuki H, Sato T, Araya N, Inoue E, et al. Nation-wide epidemiological study of Japanese patients with rare viral myelopathy using novel registration system (HAM-net) [2020 Oct 15];Orphanet J Rare Dis. 2016 May;11(1):69–69. doi: 10.1186/s13023-016-0451-x. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Olindo S, Jeannin S, Saint-Vil M, Signate A, Edjmonana-Kaptue M, Joux J, Merle H, et al. Temporal trends in human T-lymphotropic virus 1 (HTLV-1) associated myelopathy/tropical spastic paraparesis (HAM/TSP) incidence in Martinique over 25 years (1986-2010) [2020 Oct 15];PLoS Negl Trop Dis. 2018 Mar;12(3):e0006304. doi: 10.1371/journal.pntd.0006304. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brant LJ, Cawley C, Davison KL, Taylor GP, the HTLV National Register Steering C Recruiting individuals into the HTLV cohort study in the United Kingdom: clinical findings and challenges in the first six years, 2003 to 2009. [2020 Oct 15];Euro Surveill. 2011 Nov;16(46):20017–20017. doi: 10.2807/ese.16.46.20017-en. [Internet] [DOI] [PubMed] [Google Scholar]
- 178.Mendoza C, Pirón M, Gonzalez R, Jiménez A, Caballero E, Roc L, et al. Clinical presentation of individuals with human T-cell leukemia virus type-1 infection in Spain. [2020 Oct 15];Open Forum Infect Dis. 2019 Jan;6(2):ofz036–ofz036. doi: 10.1093/ofid/ofz036. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moura AA, Mello MJG, Correia JB. Prevalence of syphilis, human immunodeficiency virus, hepatitis B virus, and human T-lymphotropic virus infections and coinfections during prenatal screening in an urban Northeastern Brazilian population. [2020 Oct 15];Int J Infect Dis. 2015 Oct;39:10–15. doi: 10.1016/j.ijid.2015.07.022. [Internet] [DOI] [PubMed] [Google Scholar]
- 180.Figueiró-Filho EA, Senefonte FRA, Lopes AHA, Morais OO, Souza VG, Júnior, Maia TL, et al. Freqüência das infecções pelo HIV-1, rubéola, sífilis, toxoplasmose, citomegalovírus, herpes simples, hepatite B, hepatite C, doença de Chagas e HTLV I/II em gestantes, do Estado de Mato Grosso do Sul. [2020 out 15];Rev Soc Bras Med Trop. 2007 Mar-Abr;40(2):181–187. doi: 10.1590/S0037-86822007000200007. [Internet] [DOI] [PubMed] [Google Scholar]
- 181.Oliveira SR, Avelino MM. Soroprevalência do vírus linfotrópico-T humano tipo I entre gestantes em Goiânia, GO, Brasil. [2020 out 15];Rev Bras Ginecol Obstet. 2006 28(8):467–472. doi: 10.1590/S0100-72032006000800005. [Internet] [DOI] [Google Scholar]
- 182.Olbrich-Neto J, Meira DA. Soroprevalence of HTLV-I/II, HIV, siphylis and toxoplasmosis among pregnant women seen at Botucatu - São Paulo - Brazil: risk factors for HTLV-I/II infection. [2020 Oct 15];Rev Soc Bras Med Trop. 2004 37(1):28–32. doi: 10.1590/S0037-86822004000100008. [Internet] [DOI] [PubMed] [Google Scholar]
- 183.Bittencourt AL, Dourado I, Filho PB, Santos M, Valadão E, Alcantara LC, et al. Human T-cell lymphotropic virus type 1 infection among pregnant women in northeastern Brazil. [2020 Oct 15];J Acquir Immune Defic Syndr. 2001 Mar;26(5):490–494. doi: 10.1097/00126334-200104150-00016. [Internet] [DOI] [PubMed] [Google Scholar]
- 184.Broutet N, Queiroz Sousa A, Basilio FP, Sa HL, Simon F, Dabis F. Prevalence of HIV-1, HIV-2 and HTLV antibody, in Fortaleza, Ceara, Brazil, 1993-1994. [2020 Oct 15];Int J STD AIDS. 1996 Aug-Sep;7(5):365–369. doi: 10.1258/0956462961918103. [Internet] [DOI] [PubMed] [Google Scholar]
- 185.Santos JI, Lopes MA, Deliège-Vasconcelos E, Couto-Fernandez JC, Patel BN, Barreto ML. Seroprevalence of HIV, HTLV-I/II and other perinatally-transmitted pathogens in Salvador, Bahia. [2020 Oct 15];Rev Inst Med Trop São Paulo. 1995 Jul-Aug;37(4):343–348. doi: 10.1590/S0036-46651995000400010. [Internet] [DOI] [PubMed] [Google Scholar]
- 186.Mendes FCM, Lima JRO, Melo BO, Pinto CMFS, Maia HS, Ferro TAF, et al. Molecular detection of human T cell lymphotropic virus type 1 in pregnant women from Maranhão state, Brazil. [2020 Oct 15];Braz J Microbiol. 2020 Jun;51(2):637–645. doi: 10.1007/s42770-020-00233-0. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sodré Barmpas DB, Monteiro DLM, Taquette SR, Rodrigues NCP, Trajano AJB, Cunha JC, et al. Pregnancy outcomes and mother-to-child transmission rate in HTLV-1/2 infected women attending two public hospitals in the metropolitan area of Rio de Janeiro. [2020 Oct 15];PLoS Negl Trop Dis. 2019 Jun;13(6):e0007404. doi: 10.1371/journal.pntd.0007404. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Guerra AB, Siravenha LQ, Laurentino RV, Feitosa RNM, Azecedo VN, Vallinoto ACR, et al. Seroprevalence of HIV, HTLV, CMV, HBV and rubella virus infections in pregnant adolescents who received care in the city of Belém, Pará, Northern Brazil. [2020 Oct 15];BMC Pregnancy Childbirth. 2018 May;18(1):169–169. doi: 10.1186/s12884-018-1753-x. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Monteiro DLM, Taquette SR, Sodré Barmpas DB, Rodrigues NCP, Teixeira SAM, Villela LHC, et al. Prevalence of HTLV-1/2 in pregnant women living in the metropolitan area of Rio de Janeiro. [2020 Oct 15];PLoS Negl Trop Dis. 2014 Sep;8:e3146. doi: 10.1371/journal.pntd.0003146. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boa-Sorte N, Purificação A, Amorim T, Assunção L, Reis A, Galvão-Castro B. Dried blood spot testing for the antenatal screening of HTLV, HIV, syphilis, toxoplasmosis and hepatitis B and C: prevalence, accuracy and operational aspects. [2020 Oct 15];Brazilian J Infect Dis. 2014 Nov-Dec;18(6):618–624. doi: 10.1016/j.bjid.2014.05.009. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mello MAG, Conceição AF, Sousa SMB, Alcântara LC, Marin LJ, Raiol MRS, et al. HTLV-1 in pregnant women from the Southern Bahia, Brazil: a neglected condition despite the high prevalence. [2020 Oct 15];Virol J. 2014 Feb;11:28–28. doi: 10.1186/1743-422X-11-28. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sequeira CG, Tamegão-Lopes BP, Santos EJM, Ventura AMR, Moraes-Pinto MI, Succi RCM. Estudo descritivo da infecção pelo HTLV em uma população de gestantes do Estado do Pará, norte do Brasil. [2020 out 15];Rev Soc Bras Med Trop. 2012 45(4):453–456. doi: 10.1590/S0037-86822012005000007. [Internet] [DOI] [PubMed] [Google Scholar]
- 193.Souza VG, Martins ML, Carneiro-Proietti ABF, Januário JN, Ladeira RVP, Silva CMS, et al. High prevalence of HTLV-1 and 2 viruses in pregnant women in São Luis, state of Maranhão, Brazil. [2020 Oct 15];Rev Soc Bras Med Trop. 2012 Mar-Apr;45(2):159–162. doi: 10.1590/S0037-86822012000200004. [Internet] [DOI] [PubMed] [Google Scholar]
- 194.Machado-Filho AC, Sardinha JFJ, Ponte RL, Costa EP, da Silva SS, Martinez-Espinosa FE. Prevalence of infection for HIV, HTLV, HBV and of syphilis and chlamydia in pregnant women in a tertiary health unit in the western Brazilian Amazon region. [2020 Oct 15];Rev Bras Ginecol Obstet. 2010 Apr;32(4):176–183. doi: 10.1590/S0100-72032010000400005. [Internet] [DOI] [PubMed] [Google Scholar]
- 195.Magalhães T, Mota-Miranda AC, Alcantara LCJ, Galvão-Castro B, Rios-Grassi MF. Phylogenetic and molecular analysis of HTLV-1 isolates from a medium sized town in Northern of Brazil: Tracing a common origin of the virus from the most endemic city in the country. [2020 Oct 15];J Med Virol. 2008 Nov;80(11):2040–2045. doi: 10.1002/jmv.21278. [Internet] [DOI] [PubMed] [Google Scholar]
- 196.Dal Fabbro MMFJ, Cunha RV, Bóia MN, Portela P, Botelho CA, Freitas GMB, et al. Infecção pelo HTLV 1/2: atuação no pré-natal como estratégia de controle da doença no Estado de Mato Grosso do Sul. [2020 Oct 15];Rev Soc Bras Med Tro. 2008 Mar-Apr;41(2):148–151. doi: 10.1590/S0037-86822008000200003. [DOI] [PubMed] [Google Scholar]
- 197.The T and B-cell malignancy study group The third nation-wide study on adult T-cell leukemia/lymphoma (ATL) in Japan: characteristic patterns of HLA antigen and HTLV-I infection in ATL patients and their relatives. The T- and B-cell Malignancy Study Group. [2020 Oct 15];Int J Cancer. 1988 Apr;41(4):505–512. doi: 10.1002/ijc.2910410406. [Internet] [DOI] [PubMed] [Google Scholar]
- 198.Bartholomew C, Jack N, Edwards J, Charles W, Corbin D, Cleghorn FR, et al. HTLV-I serostatus of mothers of patients with adult T-cell leukemia and HTLV-I-associated myelopathy/tropical spastic paraparesis. J Hum Virol. 1998 May-Jun;1(4):302–305. [PubMed] [Google Scholar]
- 199.Hino S. Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): the ATL Prevention Program Nagasaki. [2020 Oct 15];Proc Jpn Acad Ser B Phys Biol Sci. 2011 87(4):152–166. doi: 10.2183/pjab.87.152. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ureta-Vidal A, Angelin-Duclos C, Tortevoye P, Murphy E, Lepere JF, Buigues RP, et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: Implication of high antiviral antibody titer and high proviral load in carrier mothers. [2020 Oct 15];Int J Cancer. 1999 Sep;82(6):832–836. doi: 10.1002/(sici)1097-0215(19990909)82:6<832::aid-ijc11>3.0.co;2-p. [Internet] [DOI] [PubMed] [Google Scholar]
- 201.Oki T, Yoshinaga M, Otsuka H, Miyata K, Sonoda S, Nagata Y. A sero-epidemiological study on mother-to-child transmission of HTLV-I in southern Kyushu, Japan. [2020 Oct 15];Asia-Oceania J Obstet Gynaecol. 1992 Dec;18(4):371–377. doi: 10.1111/j.1447-0756.1992.tb00333.x. [Internet] [DOI] [PubMed] [Google Scholar]
- 202.Takahashi K, Takezaki T, Oki T, Kawakami K, Yashiki S, Fujiyoshi T, et al. Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. [2020 Oct 15];Int J Cancer. 1991 Nov;49(5):673–677. doi: 10.1002/ijc.2910490508. [Internet]. [DOI] [PubMed] [Google Scholar]
- 203.Ando Y, Matsumoto Y, Nakano S, Saito K, Kakimoto K, Tanigawa T, et al. Long-term follow-up study of HTLV-I infection in bottle-fed children born to seropositive mothers. [2020 Oct 15];J Infect. 2003 Jan;46(1):9–11. doi: 10.1053/jinf.2002.1081. [Internet] [DOI] [PubMed] [Google Scholar]
- 204.Nishijima T, Shimada S, Noda H, Miyake K. Towards the elimination of HTLV-1 infection in Japan. [2020 Oct 15];Lancet Infect Dis. 2019 Jan;19(1):15–16. doi: 10.1016/S1473-3099(18)30735-7. [Internet] [DOI] [PubMed] [Google Scholar]
- 205.Ishak R, Vallinoto AC, Azevedo VN, Lewis M, Hall WW, Ishak MO. Molecular evidence of mother-to-child transmission of HTLV-IIc in the Kararao Village (Kayapo) in the Amazon region of Brazil. [2020 Oct 5];Rev Soc Bras Med Trop. 2001 Nov-Dec;34(6):519–525. doi: 10.1590/S0037-86822001000600004. [Internet] [DOI] [PubMed] [Google Scholar]
- 206.Silva EA, Otsuki K, Leite ACB, Alamy AH, Sa D, Vicente ACP. HTLV-II Infection associated with a chronic neurodegenerative disease: clinical and molecular analysis. [2020 Oct 15];J Med Virol. 2002 Feb;66(2):253–257. doi: 10.1002/jmv.2138. [Internet] [DOI] [PubMed] [Google Scholar]
- 207.Catalan-Soares B, Barbosa-Stancioli EF, Alcantara LCJ, et al. HTLV-2 Horizontal and vertical transmission in a family from a Brazilian urban area: seroepidemiological, clinical and molecular study. [2020 Oct 15];AIDS Res Hum Retroviruses. 2005 Jun;21(6):521–526. doi: 10.1089/aid.2005.21.521. [Internet] [DOI] [PubMed] [Google Scholar]
- 208.Renner JDP, Laurino JP, Menna-Barreto M, Schmitt VM. Molecular evidence of HTLV-II subtype B among an urban population living in South Brazil. [2020 Oct 15];AIDS Res Hum Retroviruses. 2006 Apr;22(4):301–306. doi: 10.1089/aid.2006.22.301. [Internet] [DOI] [PubMed] [Google Scholar]
- 209.Ishak R, Ishak MO, Azevedo VN, Santos DEM, Vallinoto ACR, Saraiva JCP, et al. Detection of HTLV-IIa blood donors in an urban area of the Amazon Region of Brazil (Belém, PA) [2020 Oct 15];Rev Soc Bras Med Trop. 1998 Mar-Apr;31(2):193–197. doi: 10.1590/S0037-86821998000200005. [Internet] [DOI] [PubMed] [Google Scholar]