Summary:
Insomnia is highly prevalent among patients with breast cancer (BC). Although cognitive behavioral therapy for insomnia (CBT-I) is available in integrative oncology settings, it poses unique challenges for BC survivors. Our review aimed to assess the evidence for the therapeutic effects of CBT-I on insomnia in BC. Randomized controlled trials (RCTs) that included patients/survivors with BC andinsomnia, and at least one validated self-report measure of sleep quality were included in the review. Of the 14 included RCTs (total N=1363), the most common components incorporated in CBT-I interventions were sleep hygiene, stimulus control and sleep restriction. Pooled effect sizes favored CBT-I at post-intervention (Hedges’ g=−0.779, 95% CI=−0.949, −0.609), short-term follow-up (within six months, Hedges’ g=−0.653, 95% CI=−0.808, −0.498), and long-term follow-up (12 months, Hedges’ g=−0.335, 95% CI=−0.532, −0.139). In sub-analyses, CBT-I had similar effect sizes regardless of potential modifiers (comparison design, delivery formats, etc.). As an integrative oncology intervention, CBT-I is efficacious for reducing insomnia and improving sleep quality in women treated for BC, with medium-to-large effect sizes that persist after intervention delivery ends. Given the variability in the CBT-I components tested in RCTs, future studies should investigate the optimal integration of CBT-I components for managing insomnia during BC survivorship.
Keywords: Cognitive behavioral therapy for insomnia, breast cancer, meta-analysis, efficacy, randomized controlled trial
INTRODUCTION
Insomnia in patients with cancer is persistent, chronic, and two to three times more prevalent compared to that in the general population (50–60% versus 12–25%) [1–6]. The greater rate of insomnia in the cancer population is attributed to the emotional consequences of cancer diagnosis and to the direct effects of cancer treatments and their side effects. To date, most of the studies on sleep problems in cancer have been conducted in women with breast cancer [6–8]. In fact, prevalence rates of insomnia have shown to be the highest in breast cancer (42% to 69%) compared to other cancer sites (e.g. prostate, gynecologic, head and neck, urinary or GI, etc.) [2]. Notably, women with breast cancer are prone to insomnia for multiple reasons (e.g., discomfort, pain, hot flashes, endocrine therapy and other hormonal changes associated with the breast cancer treatment, fear of recurrence) [9–11]. Patients with breast cancer or survivors with breast cancer history experiencing insomnia are also more likely to endorse high uncertainty about cancer and cancer treatment [12]. Greater insomnia severity is also linked with worsened depression, pain, deregulated circadian rhythm, fatigue, reduced quality of life, disease progression, and even decreased survival [3, 13]. It has also been suggested that cancer-related fatigue provides a false cue for sleep extension and this contributes to insomnia [14]. In addition, breast cancer itself may increase the risk of insomnia and vice versa.
Cognitive behavioral therapy for insomnia (CBT-I) is the gold standard treatment for insomnia, and its efficacy has been well established for primary insomnia and insomnia with a variety of medical or psychiatric comorbid conditions [15–19]. Johnson et al conducted a meta-analysis to investigate the efficacy of CBT-I specifically in cancer survivors [6]. Based on the eight studies included in their analysis, results indicated that CBT-I improved sleep efficiency, sleep onset latency, wake after sleep onset, and insomnia symptom severity. However, only five studies reported on outcomes of women with breast cancer. Therefore, the effect of CBT-I in the particularly affected population with breast cancer history remains unclear.
CBT-I usually consists of multiple weekly sessions as a multi-component treatment that includes sleep hygiene (SH), stimulus control (SC), sleep restriction (SR), cognitive therapy (CT), and relaxation training (RT). In clinical research and practice, there may be variability in whether and how each of these skills is taught. For example, SR is considered as a core component of CBT-I [20], SR alone is not sufficient for sleep improvement [21]. A combined approach is often preferred because that can address several dimensions of insomnia. The objective of CBT-I is to change factors that perpetuate insomnia, including behavioural factors (poor sleep habits, irregular sleep schedules), psychological factors (unrealistic expectations, worry, unhelpful beliefs), and physiological factors (mental and somatic tension, hyperarousal) [22]. CBT-I is typically delivered in the context of four to eight therapy sessions at weekly intervals. The number of follow-up visits can vary as a function of insomnia severity, comorbidity, and patient motivation [22]. Compared to pharmacotherapy, CBT-I may have better long-term beneficial effects that may last well beyond termination of treatment [15], because during CBT-I, patients can learn coping skills to tackle acute insomnia as well as to prevent or mitigate in severity future insomnia episodes [23]. However, the durability of CBT-I in patients with cancer has yet to be determined.
Although CBT is not readily available in most clinical settings, access and delivery can be made easier through use of innovative methods [22]. Currently, CBT-I interventions can be delivered in a wide variety of formats, including individual, group, in person, remote by phone or video, or online. Many of those formats have been proven to be efficacious in studies (e.g., self-help [24], group [25], online [26]), however, are dependent on patients’ engagement. Given that patients with cancer experience physical and psychological challenges, the feasibility of implementing CBT-I in routine clinical oncology practice remains unclear [27]. In Johnson and colleagues’ review published in 2016 [6], they were not able to directly compare the efficacy of CBT-I as delivered via different formats, given the limited number of RCTs published and included at that time in cancer population when they conducted the meta-analysis. Considerable challenges remain to make CBT-I available and accessible to meet population needs, particularly in patients with cancer.
In the present review, we aimed to systematically analyze the available literature to include recent trials and conduct a meta-analysis of randomized controlled trials (RCTs) to determine a more precise estimate of the efficacy of CBT-I on insomnia in patients with breast cancer. In addition, we aimed to compare the effect sizes of CBT-I via different delivery medium (e.g., in-person versus remote technologies) and to evaluate the durability of CBT-I by assessing the short-term (e.g., within 6 months) and long-term (e.g., 12 months) efficacy in this population.
METHODS
Search strategies
A professional medical librarian (P.B.) at Countway Library of Medicine (Harvard) developed and conducted searches in MEDLINE (PubMed), Embase (Elsevier), and PsycINFO (Ebsco), and Web of Science (Clarivate Analytics). Search strategies were customized to each database and included controlled vocabulary and free text synonyms comprising the concepts of insomnia, breast cancer and cognitive behavioral therapy, including individual interventional component (e.g., sleep restriction, stimulus control, etc.). Search strategy for each database is provided in detail (Appendix S1). Bibliographies of included studies and relevant meta-analyses were examined by hand to identify additional studies. Registered clinical trials were also searched on clinicaltrials.gov for completed but not published studies. Search dates were from the year of inception of each database to April 2020.
Study selection
Two of the authors (YM and DH) independently reviewed all identified abstracts for eligibility. We only included RCTs. All RCT studies evaluating CBT-I among patients with breast cancer or survivors with breast cancer history were selected for full text review. Articles were retained if they met the inclusion criteria: (1) Insomnia was clearly diagnosed based on DSM, ICSD, or research diagnostic criteria for insomnia; (2) Measures of insomnia severity or sleep quality were reported; (3) Patients/survivors with breast cancer were the primarily targeted population; (4) CBT-I included, at minimum, three components; (5) CBT-I was used alone, not combined with other active treatment; and (6) Study design was randomized controlled trial. Authors were contacted if their studies were with qualified design but insufficient information. Qualified studies were identified after full-text reading by two authors (YM and QL) independently. Any disagreements were discussed and resolved by consensus with a third author (GY).
Data extraction
All available data from included studies were extracted and double entered into a database for computational analyses. Data extraction was conducted independently by two authors (YM and QL) following Cochrane guidelines and reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [28]. Discrepancies were discussed and reconciled with a third expert reviewer (GY). Data were extracted from all qualified studies using a standardized data extraction form including publication information (title, first author, year, journal), sample characteristics and study design (sample size, age, cancer stage, insomnia duration, diagnostic criteria used, allocation and control condition), CBT-I duration (number, frequency, and length of sessions), modality (individual and group), delivery medium (e.g., in-person or by remote technologies), intervention components (stimulus control, sleep hygiene and/or sleep education, sleep restriction, relaxation training, or relaxation therapy, cognitive therapy, cognitive restructuring), time points for assessments, outcome measure, and compliance/adherence (drop-out). If an included article had missing data on outcomes of interest, authors were contacted by e-mail to obtain data necessary for effect size calculation, otherwise, missing data were calculated and converted from given data following the conduct guidance from Cochrane Handbook for Systematic Reviews of Interventions [29].
Outcome measures
The primary outcome variable of interest was the score from one of the most frequently used self-report sleep questionnaires, the Insomnia Severity Index (ISI). Pittsburgh Sleep Quality Index (PSQI) was used as outcome variable if ISI was not available, or if both ISI and PSQI were included in a study. For both instruments, higher score indicates more severe insomnia (ISI) or worse sleep quality (PSQI).
ISI is a brief, seven-item instrument that assesses insomnia according to the criteria from the Diagnostic and Statistical Manual for Mental Disorders - 4th Edition (DSM-IV) and the International Classification of Sleep Disorders. It is commonly used in clinical as well as research activities [2, 30–32]. Its reliability, validity, and sensitivity to treatment response have been documented in the general population and with patients presenting with primary insomnia and insomnia in cancer settings.
PSQI is a 19-item instrument which assesses sleep quality and disturbances over a one-month time interval. The measure consists of seven “component” scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The sum of scores for these components yields one global score. PSQI can be used as a screening tool for psychiatric practice and research [33], and can evaluate sleep quality, sleep dysfunction in clinical and non-clinical samples [34, 35], and it also has a high test-retest reliability and a good validity for patients with primary insomnia [5].
Because the conduct of CBTI usually includes the use of sleep diaries, more than half of the included studies have total sleep time (TST), sleep onset latency (SOL), wake after sleep onset (WASO) and sleep efficiency (SE, %) data. Some of the studies published sleep diary data in separate papers [36–38], we broadened our search to find sleep diary data and conducted a meta-analysis as the secondary outcomes.
Risk of bias assessment
Two authors (YM and QL) independently assessed risk of bias for all included studies according to Cochrane criteria [29], including selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other sources of bias (baseline imbalance). Each criterion was rated as low, high, or unclear. A global score was assigned to each study (sum of domains with “low risk of bias”), thus the score ranges from 0–7, with higher score indicates lower risk of bias and better quality of study.
Statistical analysis
Statistical analyses were performed using Comprehensive Meta-Analysis Version 3.0 (Biostat, Inc., USA). All measures for pre- and post-intervention, as well as each follow-up time were extracted for analysis. Data entry included common formats from reported results including mean and standard deviation in each group, mean and p (or t) in each group, raw difference and standard error, mean change and standard deviation of difference in each group.
Data conversion followed the conduct guidance from Cochrane Handbook for Systematic Reviews of Interventions [29]. As the pre-test and post-test measure correlations were unavailable in all of the individual study results, correlation level of 0.3 was used with no appreciable differences to the meta-analytic results.
All analyses were weighted to account for variability in sample sizes across studies. Following Cochrane guidelines, for studies that included two CBT-I intervention groups with different delivery format, we split the ‘shared’ control group, conducted two comparisons and adjusted the computed sample size of the control group (n/2) to avoid overestimation of potential intervention effects [29].
Standardized mean differences (SMD) expressed the size of the intervention effect in each study relative to the variability observed. SMD was calculated by the difference in mean outcome between groups divided by the pooled standard deviation of outcome among participants [29].
For common measure of effect across studies, we report Hedges’ (adjusted) g. Forest plots were generated to visually assess the Hedges’g and corresponding 95% confidence intervals (CI) across studies. A negative estimate means the treatment group has a lower or better score. Random effects models were chosen based on the smaller number of studies. The heterogeneity test I2 statistic describes the percentage of the variability in effect estimates that is due to heterogeneity rather than random sampling error. I2 is calculated by ((Q-df)/Q)*100%, where Q is the chi-squared statistic and df is its degrees of freedom. An I2 value > 50% indicates substantial heterogeneity. Evidence of publication bias was assessed through visual inspection of a funnel plot and evaluation of the Egger’s test.
RESULTS
Included studies
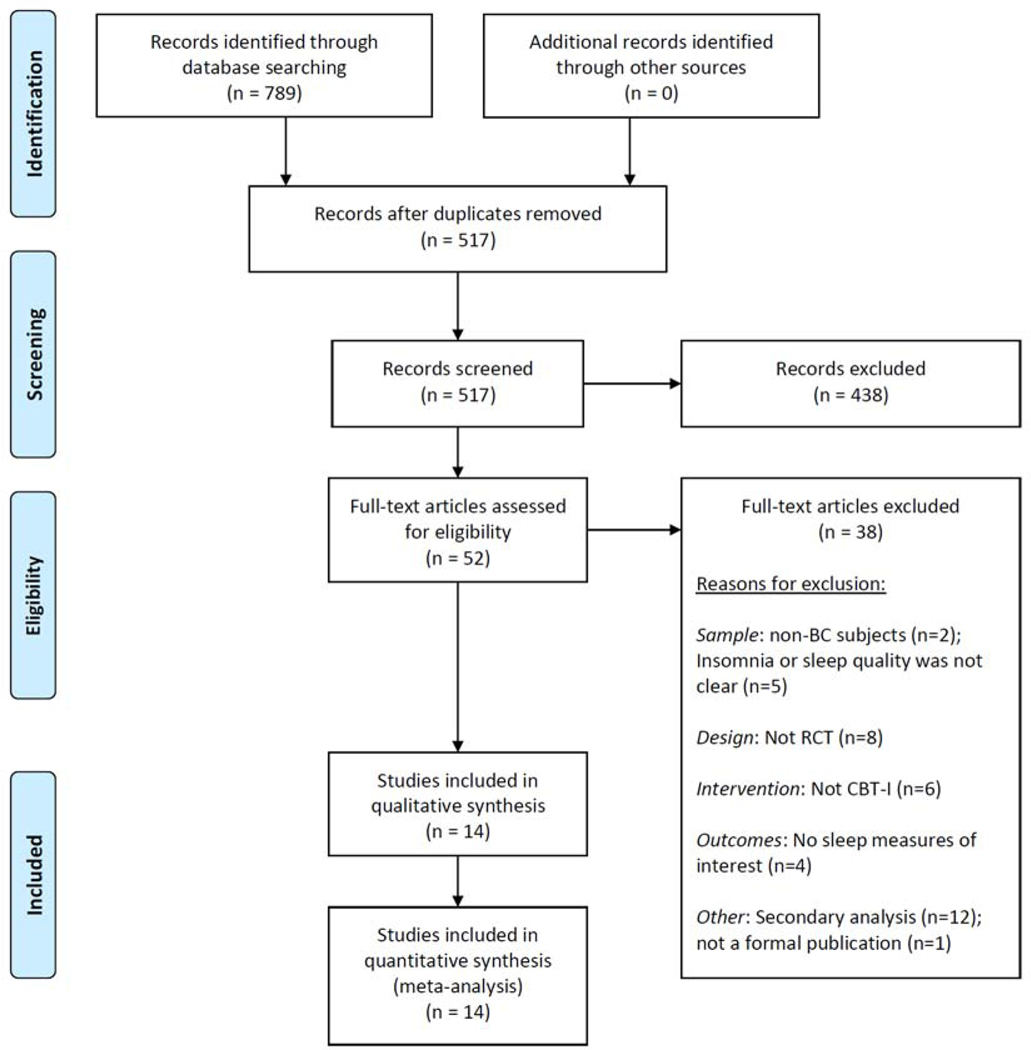
A total of 789 studies were identified through database searching and 272 duplicate records were removed (Figure 1). The 517 unique records were imported to the Covidence platform for abstract screening, then 52 records were identified and full text articles were retrieved and reviewed in detail for eligibility. Of these, thirteen eligible studies were included in the meta-analysis. Reasons for exclusion included non-breast cancer subjects, insomnia diagnosis not clear, not RCT, not CBT-I intervention, and no sleep measures of interest.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Details from the final sample of k=14 RCTs for qualitative synthesis are provided in Table 1, including participants’ characteristics, cancer stages, insomnia diagnostic criteria, insomnia duration, sample size of each group, mean age, CBT-I treatment components, delivery format, length/numbers of CBT-I sessions, follow-up time points, outcome measures, and study quality score. The 14 RCTs [38–53] included 1363 subjects (mean age 52–61 years, 722 CBT-I and 641 controls). Studied subjects include patients/survivors with breast cancer at all stage, and most of them had chronic insomnia. Only the five studies with mixed cancer samples included male subjects, which accounted for a small ratio in the total sample (n=47, 3.4%).
Table 1.
Characteristics of included RCTs of CBT-I.
| Study a | Subjects characteristics | BC Stages | Insomnia defined by | Insomnia duration | CBT-I group, nb | Comparison group, nb | Mean age (yrs) | Treatment components | Length/numbers of CBT-I sessions | Measures and Follow-up | Outcome measures | Sleep diary measures | Fatigue |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Savard J, 2005 [39] | BC survivors, completed radio-/ chemotherapy | I-III | DSM-IV, ICSD, IIS | Chronic | CBT-I (n = 27) | Waiting-list control (n = 30) | 54.1 | SC, SR, CR, SH/SE, fatigue and stress management | 8 weekly 90min sessions, one booster session, in groups of 4–6 patients. | pre, post, 3m, 6m, 12m | ISI | SE (%), TWT, TST, SOL, WASO | MFI |
| Dirksen SR, 2008 [40] | BC survivors, completed cancer treatment≥3m | I-III | DSM-IV-TR, ICSD | Chronic | CBT-I (n = 40) | Component control (n = 41) | 58.3 | CBT-I group: SC, SR, SH/SE Component control group: SH/SE | 6 weeks, in-person classes (~1 or 2 hours) or 15min by-phone sessions | pre, post | ISI | None | POMS-F |
| Berger AM, 2009 [41, 53] | BC patients, enrolled before initial chemotherapy. | I-IIIa | Self-reported history of diagnosis | Chronic | Behavioral therapy (n = 113) | Healthy eating (n = 106) | 52.1 | SC, SR, RT, SH | Plan: 90 min at randomization. Revising BT plan: 30 min, 2 days before each treatment and 30 days after the last treatment. 15 minute, in-person sessions 7 to 9 days after each revision. | pre, post, 1m, 3m, 12m | PSQI | TST, awakenings, WASO, SE (%) | PFS |
| Fiorentino L, 2010 [42] | BC survivors, completed BC treatment | I-IIIa | DSM-IV | Chronic | Individual CBT-I (n = 11) | Delayed treatment control condition (n = 10) | 61 | SR, SC, SH/SE, RT, CT | 6 weekly individual CBT-I sessions | pre, post, 6wk | ISI, PSQI | TST, WASO, awakenings, SE (%) | None |
| Ritterband LM, 2012 [43] | Mixed cancer patients, completed active treatment; 64.3% BC | I-IV + unknown | DSM-IV-TR + symptom criteria | Chronic | Online-CBT-I (n = 14) | Waitlist control (n = 14) | 56.7 | SR, SC, CR, SH, relapse prevention | 6–9 weeks, including 6 online cores (45–60 min each) | pre, post | ISI | SE (%), TST, SOL, WASO, TIB, awakenings | MFSI - Short Form |
| Matthews EE, 2014 [45] | BC survivors, completed BC treatment | I–III | ISI≥8 + IIS | Chronic | P-CBT-I (n=32) | Behavioral placebo treatment (n=28) | 52 | SR, SC, SH, CT | Individual, 6 weekly in-person CBTI sessions (30–60 min each) or by phone (15–20 min each). | pre, post, 3m, 6m | ISI | SE (%), SOL, WASO, TST, awakenings | PFS |
| Garland SN, 2014 [44] | Mixed cancer, 48% BC | nonmetast atic cancer | DSM-IV, ICSD-2 | >1month | CBT-I (n = 47) | MBSR (n = 64) | 58.9 | SC, SR, CT, RT | 8 weekly, 90-minute sessions, for a total of 12 contact hours, in groups of 6–10 patients | pre, post, 3m | ISI, PSQI | SOL, WASO, TST, SE (%) | None |
| Roscoe JA, 2015 [38] | Mixed cancer survivors, 68% BC | N.C. | N.C. | Chronic | CBTI + placebo (n=24) | Placebo (n=25) | 55.4 | SH, SC, SR, (CT, RT) | 7 individual sessions occurring once per week. Sessions conducted in person (30–60 min), and by phone (15–30 min). | pre, post, 3m | ISI, PSQI | SOL, WASO, TST [36] | None |
| Casault L, 2015 [47] | Mixed cancer patients, 79% BC | N.C. | ISI≥8, IIS, regular hypnotic medication usage for more than 6 months | Acute | Self-admini stered minimal CBT-I (n = 20) | No treatment control (n = 18) | 56.9 | SC, SR, CR, SH | Self-help CBT, 6 short booklets and 3 phone consultations with a psychologist, over 6 weeks | pre, post, 3m, 6m | ISI | SOL, WASO, awakenings, TST, SE (%) | MFI |
| Savard J, 2014, 2016 [46, 48] | BC patients, with radiation therapy within the past 18m | 0-III | ISI≥8 or psychotropic medication as a sleep aid≥2 nights in the past 2 wks; | Chronic | PCBT-I (n = 81) VCBT-I (n = 80) | No treatment control (n = 81) | 54.4 | SC, SR, SH | 6 weeks of CBTI P-CBT-I: weekly, 50min individual session V-CBT-I: video segment (5–20min each) + booklets each week | pre, post, 3m, 6m, 12m | ISI | SOL, WASO, EMA, TWT, TST, SE (%) | MFI |
| Irwin MR, 2017 [49] | BC survivor, completed BC treatment | N.C. | DSM-IV-TR, DSM-V, ICSD-2 | N.C | Group CBT-I (n = 45) | TCC (n = 45) | 60.0 | SC, SH, SR, RT, CT | CBT-I teaching 2 months + 1 month of skill consolidation and adherence; Groups of 7 to 10 participants in weekly 120-minute sessions. | pre, mid, post, 3m, 12m | PSQI | TST, SOL, SE (%), WASO | MFSI |
| Palesh O, 2018 [50] | Newly diagnosed BC patients, undergoing chemotherapy | I-III + unknown | ISI≥8 | N.C. | BBT-CI (n = 34) | HEAL (n = 37) | 52.5 | SH, SC, SR, Sleep scheduling, Chronorehabil itation |
Two 60min face-to-face session and four 15min phone call | pre, post, 1m | ISI | None | None |
| Zachariae R, 2018 [51] | BC survivors | 0-III | PSQI>5 + structured screening interview | Chronic | Online-CBT -I (n = 133) |
Waitlist control (n = 122) | 53.1 | SR, SC, SH, CR, relapse prevention. | 9 weeks including 6 successively delivered cores (45–60min each) | pre, post, 6wk | ISI, PSQI | SOL, awakenings, WASO, EMA, TIB, TST, SE (%), Sleep medication | FACIT- F |
| Mercier J, 2018 [52] | Mixed cancer survivors, 53.7% BC | I-III + unknown | ISI≥8 | N.C. | CBT-I (n = 21) | Home-based aerobic exercise (n=20) | 57.1 | SC, SR, CR, SE/SH | 6-weeks: 60min video (5–20min each) + 6 booklets | pre, post, 3m, 6m | ISI, PSQI | SOL, WASO, TST, SE (%) | None |
Abbreviations:
awakenings, the number of awakenings; BBT-CI, brief behavioral therapy for cancer-related insomnia; BC, breast cancer; CBT-I, Cognitive Behavioral Therapy for Insomnia; CR, cognitive restructuring; DSM, Diagnostic and Statistical Manual of Mental disorders; EMA, early morning awakening; FACIT-F, Functional Assessment of Chronic Illness Therapy for Fatigue; ISI, Insomnia Severity Index; m, month; MFI, Multidimensional Fatigue Inventory; MFSI, Multidimensional Fatigue Symptom Inventory; mid, in the middle of intervention period; N.C., not clear; PCBT-I, professionally administered CBT-I; PFS, Piper Fatigue Scale; POMS-F, Profile of Mood States - Fatigue Scale; post, post-intervention; pre, pre-intervention; PSQI, Pittsburgh Sleep Quality Index; RT, relaxation training; SC, stimulus control; SE (%), sleep efficiency, calculated as (TST/TIB)*100; SE, sleep education; SH, sleep hygiene; sleep medication, the proportion of nights on which participants took sleep medication; SOL, sleep onset latency; SR, sleep restriction; TCC, Tai Chi Chih; TIB, time in bed; TST, total sleep time; TWT, total wake time; VCBT-I, video-based CBT-I; WASO, wake after sleep onset; wk, weeks.
Studies are listed in chronological order.
Number reported in the table are for enrolled subjects in each group in each study.
Separate analyses were conducted for all 14 studies and for comparisons between some of the sub-group studies. The studies provided CBT-I intervention in a wide variety of formats, including individual versus group, in person versus by phone, video, or online). The overall quality score was 4.64 out of 7, with 7 being the best.
CBT-I components and sessions
Most CBT-I interventions incorporated at least three of the main components. All studies included stimulus control and sleep restriction; 92.9% of the studies included sleep hygiene/education; fewer studies included cognitive restructuring/therapy (71.4%), relaxation training (35.7%) or other components (28.6%). In general, CBT-I interventions in the included studies were delivered on a weekly basis, but significant variations were found in the length and numbers of sessions. In-person classes can be short (ranged from 15 to 30 minutes) or long (ranged from one to two hours), while by-phone sessions were typically short (ranged from15 to 30 minutes). The total duration of intervention ranged from six weeks to three months. Follow-up length varied with short-term (ranged from one to six months) or long-term (12 months).
Durability of CBT-I
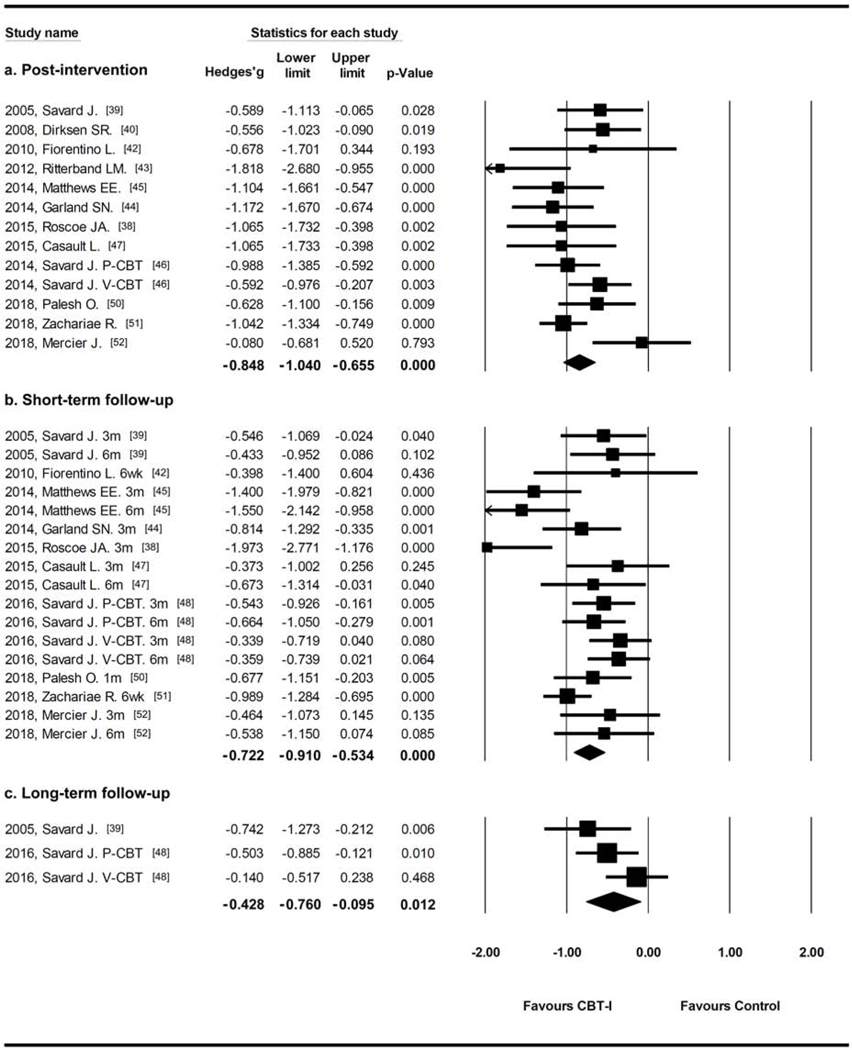
Results from studies using ISI as outcome measure (Figure 2) showed that pooled effect sizes favored CBT-I at post-intervention (Hedges’g=−0.848, 95% CI=−1.040, −0.655, I2=44.5%), short-term follow-up (within six months, Hedges’g=−0.731, 95% CI=−0.922, −0.541, I2=58.0%), and long-term follow-up (after one year, Hedges’g=−0.431, 95% CI=−0.768, −0.095, I2=46.3%).
Figure 2.
Durability analysis from studies with ISI as outcome measures.
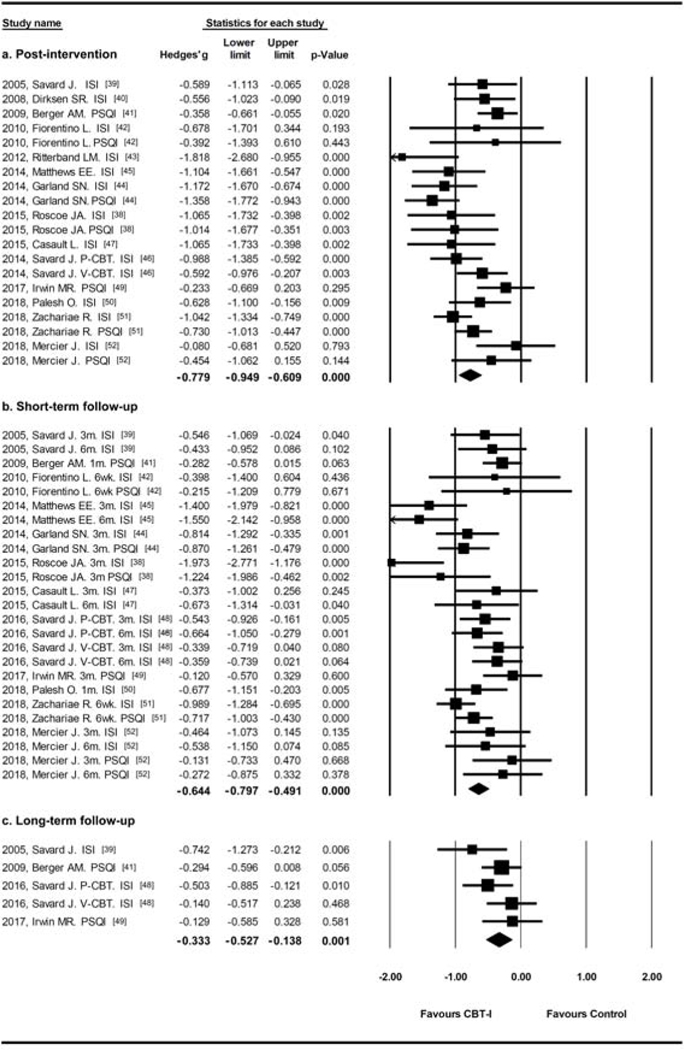
The combination of studies using ISI or PSQI showed similar results (Figure 3), pooled effect sizes favored CBT-I at post-intervention (Hedges’g=−0.779, 95% CI=−0.949, −0.609, I2=58.6%), short-term follow-up (within six months, Hedges’g=−0.653, 95% CI=−0.808, −0.498, I2=59.1%), and long-term follow-up (after one year, Hedges’g=−0.335, 95% CI=−0.532, −0.139, I2=18.5%).
Figure 3.
Durability analysis from studies with ISI or PSQI as outcome measures.
Subgroup analysis
Study population.
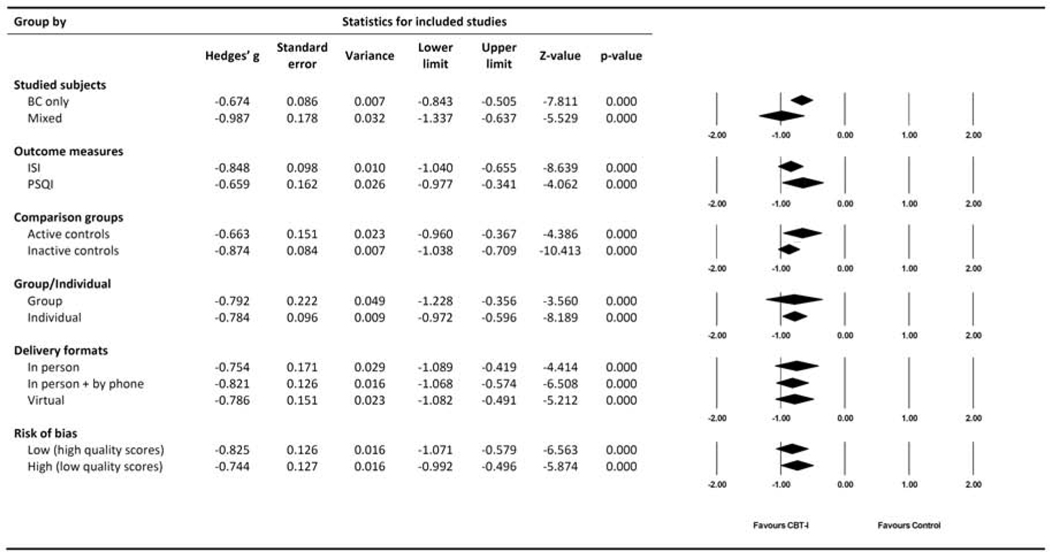
Of the fourteen studies, five studies included mixed cancer types but with breast cancer as the majority (79%, 68%, 64%, 48% and 54% respectively). There was no significant difference between effect size in studies with only breast cancer versus mixed cancers (p=0.115). In the studies with only breast cancer, effect size favored CBT-I at post-intervention (Hedges’g=−0.674, 95% CI=−0.843, −0.505, I2=44.7%), while the effect size was larger in the mixed population (Hedges’g=−0.987, 95% CI=−1.337, −0.637, I2=63.0%).
Outcome measures.
Seven out of the fourteen studies applied PSQI as the main outcome measure, and they showed effect size favored CBT-I at post intervention (Hedges’g=−0.659, 95% CI=−0.977, −0.341, I2=70.6%). The effect size from the twelve studies using ISI also favored CBT-I, but to a larger magnitude (Hedges’g=−0.848, 95% CI=−1.040, −0.655, I2=44.5%). The effect sizes between studies using ISI and PSQI were not significantly different (p=0.320).
Comparison groups.
Among the identified trials, six studies used an inactive control (e.g., waiting list control or usual care) and eight studies used an active control (e.g., healthy eating, Tai Chi Chih, mindfulness-based stress reduction, exercise, etc.). The effect size of CBT-I with inactive controls was Hedges’g =−0.874 (95% CI=−1.038, −0.709, I2=18.1%), and with active controls was Hedges’g =−0.663 (95% CI=−960, −0.367, I2=72.5%). The difference was not significant (p=0.224). To further understand the potential placebo effect, we separately presented the results for both ISI- and PSQI-based outcomes in Table 3. CBT-I has slightly larger effect size when compared to inactive controls, but it shows large effect size regardless of comparison designs.
Table 3.
Efficacy of CBT-I compared with active and inactive controls.
| Subjective sleep outcome measures | Pre-intervention | Post-intervention | Difference between pre- and post-intervention means | Number of Studies | Number of subjects | Weighted overall effect for between group comparasons b | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean a | SD | Mean a | SD | Value | % | Hedges’ g | SE | 95% CI | |||
| ISI | |||||||||||
| CBT-I | 15.73 | 4.03 | 8.04 | 3.89 | −7.69 | −48.89 | 12 | 509 | |||
| Active control | 17.59 | 3.23 | 12.83 | 3.84 | −4.76 | −27.06 | 5 | 153 | −0.72 | 0.19 | (−1.08, −0.35) |
| Inactive control | 14.54 | 4.34 | 11.95 | 4.62 | −2.59 | −17.81 | 7 | 269 | −0.93 | 0.11 | (−1.14, −0.71) |
| PSQI | |||||||||||
| CBT-I | 10.04 | 3.08 | 6.71 | 2.49 | −3.33 | −33.17 | 7 | 326 | |||
| Active control | 9.91 | 3.05 | 8.98 | 3.16 | −0.93 | −9.38 | 4 | 213 | −0.60 | 0.27 | (−1.12, −0.08) |
| Inactive control | 10.78 | 2.86 | 9.55 | 3.07 | −1.23 | −11.41 | 3 | 127 | −0.75 | 0.13 | (−1.00, −0.50) |
The weighted means are calculated by formula (Σ[u*N]/Σ[N]), where u is the mean of the individual study.
The weighted overall effect is calculated by comparing CBT-I with active control or inactive control separately.
Note: Sleep quality ratings were standardized across studies so that lower scores indicate better sleep quality.
Abbreviations: CI, confidence interval; SD, standard deviation; SE, standard error.
Group and individual interventions.
The ten studies using individual interventions showed effect size of Hedges’g =−0.784 (95% CI=−0.972, −0.596, I2=51.1%). The four studies involving group interventions showed a similar effect size, Hedges’g = −0.792 (95% CI=−1.228, −0.356, I2=76.7%), with no significant differences between them (p=0.975).
Delivery format.
Traditional in-person CBT-I showed effect size of Hedges’g = −0.754 (95% CI=−1.089, −0.419, I2=71.8%), CBT-I with combination of in-person and phone sessions showed effect size of Hedges’g = −0.821 (95% CI=−1.068, −0.574, I2=5.5%). Virtually delivered CBT-I showed effect size of Hedges’g =−0.786 (95% CI=−1.082, −0.491, I2=64.0%). Differences were not significant (p=0.950).
The sub-analyses showed that CBT-I remained similarly efficacious regardless of potential modifiers (comparison design, delivery formats, length/numbers of CBT-I sessions).
Sleep dairy outcomes
Results derived from sleep diary outcomes showed that pooled effect sizes favored CBT-I for all the studied measures in this analysis (Table 4), including total sleep time (Hedges’g=0.218), sleep onset latency (Hedges’g=−0.486), wake after sleep onset (Hedges’g=−0.363) and sleep efficieny (Hedges’g=0.531) at post-intervention. The CBT-I effect remained significant during short-term follow-up on all the above sleep measures. For long-term effects, CBT-I effects remained significant on sleep onset latency and sleep efficiency (Table 4).
Table 4.
CBT-I effects and durability on sleep diary outcomes.
| Sleep diary outcomes | Number of studies | Number of subjects | Q | Weighted overall effect | |||
|---|---|---|---|---|---|---|---|
| Hedges’g | SE | 95% CI | p-vale | ||||
| TST | |||||||
| Post-intervention | 10 | 980 | 5.79 | 0.218 | 0.065 | (0.091, 0.345) | 0.001 |
| Short-term follow-up | 6 | 649 | 4.39 | 0.190 | 0.065 | (0.062, 0.318) | 0.004 |
| Long-term follow-up | 3 | 371 | 0.38 | 0.182 | 0.107 | (−0.028, 0.393) | 0.089 |
| SOL | |||||||
| Post-intervention | 9 | 816 | 10.08 | −0.486 | 0.077 | (−0.637, −0.335) | 0.000 |
| Short-term follow-up | 5 | 483 | 5.82 | −0.200 | 0.072 | (−0.341, −0.059) | 0.006 |
| Long-term follow-up | 5 | 371 | 2.22 | −0.295 | 0.108 | (−0.507, −0.084) | 0.006 |
| WASO | |||||||
| Post-intervention | 10 | 950 | 4.05 | −0.363 | 0.065 | (−0.490, −0.236) | 0.000 |
| Short-term follow-up | 6 | 651 | 3.90 | −0.149 | 0.065 | (−0.277, −0.021) | 0.022 |
| Long-term follow-up | 3 | 371 | 0.51 | −0.099 | 0.107 | (−0.309, 0.111) | 0.355 |
| SE (%) | |||||||
| Post-intervention | 9 | 939 | 13.89 | 0.531 | 0.087 | (0.361, 0.702) | 0.000 |
| Short-term follow-up | 6 | 651 | 9.32 | 0.226 | 0.065 | (0.098, 0.354) | 0.001 |
| Long-term follow-up | 3 | 402 | 1.80 | 0.278 | 0.108 | (0.067, 0.489) | 0.010 |
Abbreviations: CI, confidence interval; SD, standard deviation; SE, standard error; SE (%), sleep efficiency; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset.
Study quality and risk of bias
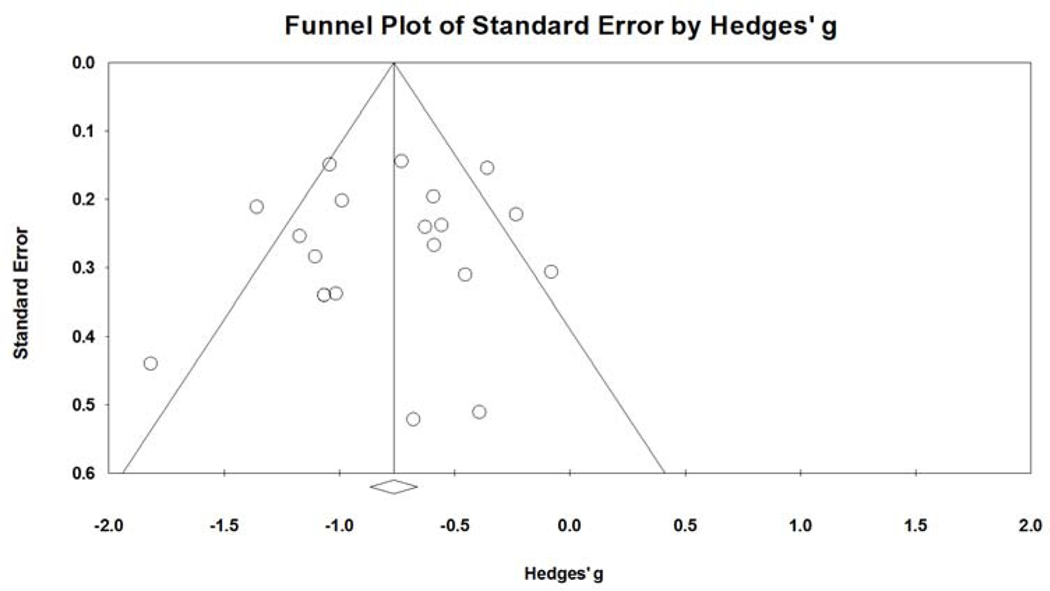
The quality score of included studies ranged from 3–7, where higher score indicates lower risk of bias and better quality of study. Eight studies with score 3 or 4 identified as low quality showed Hedges’g = −0.744 (95% CI=−0.992, −0.496, I2=38.4%); while six studies with score 5, 6 or 7 identified as high quality showed Hedges’g = −0.825 (95% CI=−1.071, −0.579, I2=72.7%), with no difference between groups (p=0.650). No significant patterns were found on the funnel plot of standard error by Hedges’ g, indicating that there was low risk of publication bias.
DISCUSSION
Cognitive behavioral therapy for insomnia (CBT-I) has been supported broadly as a gold standard non-pharmacological treatment for insomnia, including endorsement by the American College of Physicians as first line treatment [54]. Despite the high prevalence and impact of insomnia among adults treated for cancer, insomnia is rarely addressed systematically in the oncology clinic [11], and the efficacy of CBT-I among patients with cancer has to date remained unclear. One recent meta-analysis found that CBT-I has only small-to-moderate sized improvements in several aspects of sleep among cancer survivors [6]; however, findings were not specific to breast cancer survivors, for whom body changes and adjuvant hormone therapies often complicate insomnia treatment. Here, we address this gap and offer recommendations to advance the science and practice of insomnia treatment among patients with breast cancer.
As in other studied populations, CBT-I in patients with breast cancer produced moderate to large treatment effects, and clinically significant effects last up to a year after therapy [55]. Overall, the existing RCTs yield statistically and clinically significant improvements in insomnia and sleep quality by subjective measures, and our findings have critical implications for research and clinical practice.
A unique element of our meta-analysis was identifying components of interventions yielding the greatest effects in sleep quality, including feasibility metrics of duration and modality of delivery. Indeed, 10 years ago, a widely circulated commentary was published: “Despite effectiveness, behavioral therapy for chronic insomnia still underused” [56]. Unfortunately, little has changed to address underutilization of CBT-I among cancer survivors, for whom barriers include time limitations, travel, and illness burden constraints [46, 57]. In the present meta-analysis, sub-analyses revealed CBT-I remained similarly efficacious regardless of potential modifiers (comparison design, delivery formats, length/numbers of CBT-I sessions). The most common CBT-I approach includes behavioral components (SC, SR, RT) combined with a cognitive (CT) and an educational (SH) component. In our analysis, most CBT-I interventions incorporated at least three of the main components (sleep hygiene, stimulus control and sleep restriction). About one third of studies included full components as standard CBT-I, while some studied incorporated other elements (e.g. stress management, relapse prevention, chronorehablitation) with major CBT-I components as a customized package. No significant differences were found between number/length of sessions or modality in the published studies. Since individual, face-to-face therapy with a sleep specialist is not always feasible, alternative treatment delivery models can improve access to CBT [22]. Virtual CBT-I showed slightly more efficacious compared to conventional face-to-face CBT-I or a combination of in-person and by-phone sessions, although differences were not significant. These findings suggest that CBT-I delivered virtually may not only be a pragmatic option for enhancing access to care; it may be comparably efficacious to in-person visits at improving sleep quality. Self-help approaches using printed materials, videos, or internet-based programs are helpful as stand-alone treatments or as additions to professionally administered therapy [22].
Further studies are encouraged to address remaining gaps highlighted by this analysis. First, some subgroups of patients with breast cancer might be under-represented, for example, younger female or breast cancer patients with comorbid psychiatric disorders. Second, if one wants to optimize CBT-I or make it briefer to deliver, our results suggest the need for adaptive trial designs (i.e., sequential multiple assignment randomized trial, SMART; multiphase optimization strategy, MOST) to determine optimal sequence of delivering skills. Recently, Zhou et al.[58] found that a stepped care model for delivering CBT-I to patients with cancer was acceptable and feasible, suggesting that some patients may benefit from a minimal dosage (e.g., one group-delivered session on the topic of sleep hygiene), whereas other patients will benefit from the full CBT-I content. Future studies using adaptive designs could test the optimal sequence and dosage of CBT-I for patients with breast cancer, including virtual vs. in-person sessions, group vs. individual delivery, and number of CBT-I components patients are exposed to. Finally, our findings implicate the potential for virtual delivery of CBT-I. As more insurances offer coverage of eHealth modalities for interfacing with healthcare providers, the acceptability and feasibility of implementing telehealth CBT-I for breast cancer survivors in routine care will be critical future area of research.
Strengths and limitations
Our meta-analysis has focused on a specific population with unique needs, which enhances our confidence in these findings yet limits our ability to generalize to other cancer populations. In existing literatures, CBT-I in cancer population is predominantly targeting breast cancer, possibly because patients with breast cancer have relatively better prognosis, less complications, and longer survivorship. However, we did not see a significantly different effect size in mixed cancer populations. It remains unclear whether sleep disorders differ per cancer type, as we were not able to explore this due to the small number of subjects with cancers other than breast cancer in our analysis. More studies of CBT-I are needed in other cancer population, and future studies are encouraged to explore CBT-I efficacy by comparing subjects with different cancer types. In this review, we did not include objective sleep measures or physiological measures. The reliance on self-reported insomnia and sleep quality may be influenced by recall bias. Due to the study subjects of interest, almost all participants are female, which makes it difficult to explore whether there are sex differences on the CBT-I responses. Insomnia may present prior to or after cancer diagnosis, however information in the pulished studies did no tallow us to differentiate these groups. Due to limited information in the included studies, our analysis was not able to explore the change in daytime function or fatigue levels subsequent to the improvement of sleep quality. We also had heterogeneous population that included patients across the spectrum from new diagnosis to survivorship. Despite these limitations, this meta-analysis provides valuable information on CBT-I treatment in a particularly vulnerable population. It also provides information on durability and the effect of different delivery formats of CBT-I.
CONCLUSIONS
The existing RCTs yield statistically and clinically significant improvements in insomnia and sleep quality by subjective measures. As an integrative oncology intervention, CBT-I is efficacious for reducing insomnia in women treated for BC, with medium-to-large effect sizes that persist up to one year after intervention delivery ends. Given the variability in the CBT-I components tested in RCTs, future studies should investigate the optimal integration of CBT-I components for managing chronic insomnia during BC survivorship.
Supplementary Material
Figure 4.
Sub-group analysis from all pre- and post-intervention comparisons.
Figure 5.
Funnel plot of standard error by Hedges’s g.
Table 2.
Risk of bias assessment and quality score of studiesa.
| Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | Other sources of bias | Overall | ||
|---|---|---|---|---|---|---|---|---|
| Reference | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Baseline imbalance | Score |
| Savard J, 2005 [39] | Unclear | Unclear | High | Unclear | Low | Low | Low | 3 |
| Dirksen SR, 2008 [40] | Low | High | High | Unclear | Low | Low | Low | 4 |
| Berger AM, 2009 [41] | Low | Low | High | Unclear | Low | Low | Low | 5 |
| Fiorentino L, 2010 [42] | Low | Low | High | Unclear | High | Low | Low | 4 |
| Ritterband LM, 2012 [43] | Low | Low | High | Unclear | Low | Low | High | 4 |
| Matthews EE, 2014 [45] | High | High | High | Unclear | Low | Low | Low | 3 |
| Garland SN, 2014 [44] | Low | Low | Low | Low | High | Low | Low | 6 |
| Roscoe JA, 2015 [38] | Low | Low | High | High | High | Low | Low | 4 |
| Casault L, 2015 [47] | Low | Low | High | Unclear | Low | Low | Low | 5 |
| Savard J, 2014 [46] | Low | Low | Low | Low | High | Low | Low | 6 |
| Irwin MR, 2017 [49] | Low | Low | Low | Low | Low | Low | Low | 7 |
| Palesh O, 2018 [50] | Unclear | Unclear | High | Unclear | Low | Low | Low | 3 |
| Zachariae R, 2018 [51] | Low | Low | Low | Low | Low | Low | Low | 7 |
| Mercier J, 2019 [52] | Low | Low | High | Unclear | Low | Low | High | 4 |
Studies are listed in chronological order.
Practice Points.
Most studies on cognitive behavioral therapy for insomnia (CBT-I) in cancer have been conducted in breast cancer.
CBT-I is efficacious for reducing insomnia in women treated for BC, with medium-to-large effect sizes that persist up to one year after intervention delivery ends.
CBT-I had similar effect sizes regardless of comparison design, delivery formats, length/numbers of CBT-I sessions.
Research Agenda.
Given the variability in the CBT-I components tested in RCTs, future studies should investigate the optimal integration of CBT-I components for managing chronic insomnia during BC survivorship.
Optimal design of CBT-I sessions and delivery formats need to be further studied to maximize the efficacy and cost-effectiveness.
Sex differences and the response to CBT-I remains unclear and further studies to address this in a breast cancer population are encouraged.
Further studies are encouraged to investigate the multidimentional correlation of CBT-I, sleep improvement, daytime function and fatigue.
Acknowledgements
This work was supported by the National Institutes of Health (NCCIH T32AT000051, K24 AT009465 and K23AT010157). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Meanwhile, the authors would like to acknowledge Paul Bain, PhD, MLIS, at Countway Library of Medicine of Harvard, for his assistance with searching and retrieving abstracts and publications.
Abbreviations
- BBT-CI
brief behavioral therapy for cancer-related insomnia
- BC
breast cancer
- CBT-I
cognitive behavioral therapy for insomnia
- CI
confidence intervals
- CR
cognitive restructuring
- DSM
Diagnostic and Statistical Manual of Mental disorders
- EMA
early morning awakening
- FACIT-F
Functional Assessment of Chronic Illness Therapy for Fatigue
- ISI
Insomnia Severity Index
- MFI
Multidimensional Fatigue Inventory
- MFSI
Multidimensional Fatigue Symptom Inventory
- PCBT-I
professionally administered CBT-I
- PFS
Piper Fatigue Scale
- POMS-F
Profile of Mood States - Fatigue Scale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSQI
Pittsburgh Sleep Quality Index
- RCT
randomized controlled trial
- RT
relaxation training
- SC
stimulus control
- SE (%)
sleep efficiency
- SE
sleep education
- SH
sleep hygiene
- SMD
standardized mean difference
- SOL
sleep onset latency
- SR
sleep restriction
- TCC
Tai Chi Chih
- TIB
time in bed
- TST
total sleep time
- TWT
total wake time
- VCBT-I
video-based CBT-I
- WASO
wake after sleep onset
Footnotes
Conflict of interest
The authors report no conflict of interest in this review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Howell D, Oliver TK, Keller-Olaman S, Davidson JR, Garland S, Samuels C, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25:791–800. [DOI] [PubMed] [Google Scholar]
- [2].Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3580–6. [DOI] [PubMed] [Google Scholar]
- [3].Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–90. [DOI] [PubMed] [Google Scholar]
- [4].Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of psychosomatic research. 2002;53:737–40. [DOI] [PubMed] [Google Scholar]
- [6].Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep medicine reviews. 2016;27:20–8. [DOI] [PubMed] [Google Scholar]
- [7].Clark J, Cunningham M, McMillan S, Vena C, Parker K. Sleep-wake disturbances in people with cancer part II: evaluating the evidence for clinical decision making. Oncology nursing forum. 2004;31:747–71. [DOI] [PubMed] [Google Scholar]
- [8].Vena C, Parker K, Cunningham M, Clark J, McMillan S. Sleep-wake disturbances in people with cancer part I: an overview of sleep, sleep regulation, and effects of disease and treatment. Oncology nursing forum. 2004;31:735–46. [DOI] [PubMed] [Google Scholar]
- [9].Carpenter JS, Elam JL, Ridner SH, Carney PH, Cherry GJ, Cucullu HL. Sleep, fatigue, and depressive symptoms in breast cancer survivors and matched healthy women experiencing hot flashes. Oncology nursing forum. 2004;31:591–5598. [DOI] [PubMed] [Google Scholar]
- [10].Savard J, Davidson JR, Ivers H, Quesnel C, Rioux D, Dupere V, et al. The association between nocturnal hot flashes and sleep in breast cancer survivors. Journal of pain and symptom management. 2004;27:513–22. [DOI] [PubMed] [Google Scholar]
- [11].Kwak A, Jacobs J, Haggett D, Jimenez R, Peppercorn J. Evaluation and management of insomnia in women with breast cancer. Breast cancer research and treatment. 2020. [DOI] [PubMed] [Google Scholar]
- [12].Hall DL, Mishel MH, Germino BB. Living with cancer-related uncertainty: associations with fatigue, insomnia, and affect in younger breast cancer survivors. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2014;22:2489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:895–908. [DOI] [PubMed] [Google Scholar]
- [14].Garland SN, Irwin MR, Posner D, Perlis ML. Are sleep continuity disturbance and fatigue prodromal symptoms of cancer development? Medical hypotheses. 2018;120:72–5. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep. 2005;28:1049–57. [DOI] [PubMed] [Google Scholar]
- [16].Ballesio A, Aquino M, Feige B, Johann AF, Kyle SD, Spiegelhalder K, et al. The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: A systematic review and network meta-analysis. Sleep medicine reviews. 2018;37:114–29. [DOI] [PubMed] [Google Scholar]
- [17].Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep medicine reviews. 2015;23:54–67. [DOI] [PubMed] [Google Scholar]
- [18].van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: A meta-analysis. Sleep medicine reviews. 2018;38:3–16. [DOI] [PubMed] [Google Scholar]
- [19].Ma Y, Dong M, Mita C, Sun S, Peng CK, Yang AC. Publication analysis on insomnia: how much has been done in the past two decades? Sleep medicine. 2015;16:820–6. * [DOI] [PubMed] [Google Scholar]
- [20].Kyle SD, Aquino MR, Miller CB, Henry AL, Crawford MR, Espie CA, et al. Towards standardisation and improved understanding of sleep restriction therapy for insomnia disorder: A systematic examination of CBT-I trial content. Sleep medicine reviews. 2015;23:83–8. [DOI] [PubMed] [Google Scholar]
- [21].Perlis ML. Cognitive behavioral treatment of insomnia : a session-by-session guide. New York, N.Y.: Springer; 2005. * [Google Scholar]
- [22].Morin CM, Benca R. Chronic insomnia. Lancet (London, England). 2012;379:1129–41. [DOI] [PubMed] [Google Scholar]
- [23].Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep medicine reviews. 2006;10:419–29. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ho FY, Chung KF, Yeung WF, Ng TH, Kwan KS, Yung KP, et al. Self-help cognitive-behavioral therapy for insomnia: a meta-analysis of randomized controlled trials. Sleep medicine reviews. 2015;19:17–28. * [DOI] [PubMed] [Google Scholar]
- [25].Koffel EA, Koffel JB, Gehrman PR. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep medicine reviews. 2015;19:6–16. * [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - A systematic review and meta-analysis of randomized controlled trials. Sleep medicine reviews. 2016;30:1–10. * [DOI] [PubMed] [Google Scholar]
- [27].Morin CM. Cognitive Behavioral Therapy for Chronic Insomnia: State of the Science Versus Current Clinical Practices. Annals of internal medicine. 2015;163:236–7. [DOI] [PubMed] [Google Scholar]
- [28].Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Green JHS. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 ed: The Cochrane Collaboration; 2011. [Google Scholar]
- [30].Wong ML, Lau KNT, Espie CA, Luik AI, Kyle SD, Lau EYY. Psychometric properties of the Sleep Condition Indicator and Insomnia Severity Index in the evaluation of insomnia disorder. Sleep medicine. 2017;33:76–81. [DOI] [PubMed] [Google Scholar]
- [31].Chen PY, Jan YW, Yang CM. Are the Insomnia Severity Index and Pittsburgh Sleep Quality Index valid outcome measures for Cognitive Behavioral Therapy for Insomnia? Inquiry from the perspective of response shifts and longitudinal measurement invariance in their Chinese versions. Sleep medicine. 2017;35:35–40. [DOI] [PubMed] [Google Scholar]
- [32].Gagnon C, Belanger L, Ivers H, Morin CM. Validation of the Insomnia Severity Index in primary care. Journal of the American Board of Family Medicine : JABFM. 2013;26:701–10. [DOI] [PubMed] [Google Scholar]
- [33].Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [34].Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep medicine reviews. 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- [35].Black DS, O’Reilly GA, Olmstead R, Breen EC, Irwin MR. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA internal medicine. 2015;175:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garland SN, Roscoe JA, Heckler CE, Barilla H, Gehrman P, Findley JC, et al. Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors. Sleep medicine. 2016;20:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peoples AR, Garland SN, Perlis ML, Savard J, Heckler CE, Kamen CS, et al. Effects of cognitive behavioral therapy for insomnia and armodafinil on quality of life in cancer survivors: a randomized placebo-controlled trial. Journal of cancer survivorship : research and practice. 2017;11:401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Roscoe JA, Garland SN, Heckler CE, Perlis ML, Peoples AR, Shayne M, et al. Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:6083–96. [DOI] [PubMed] [Google Scholar]
- [40].Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. Journal of advanced nursing. 2008;61:664–75. [DOI] [PubMed] [Google Scholar]
- [41].Berger AM, Kuhn BR, Farr LA, Lynch JC, Agrawal S, Chamberlain J, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psycho-oncology. 2009;18:634–46. [DOI] [PubMed] [Google Scholar]
- [42].Fiorentino L, McQuaid JR, Liu L, Natarajan L, He F, Cornejo M, et al. Individual cognitive behavioral therapy for insomnia in breast cancer survivors: a randomized controlled crossover pilot study. Nature and science of sleep. 2010;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psycho-oncology. 2012;21:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:449–57. [DOI] [PubMed] [Google Scholar]
- [45].Matthews EE, Berger AM, Schmiege SJ, Cook PF, McCarthy MS, Moore CM, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncology nursing forum. 2014;41:241–53. [DOI] [PubMed] [Google Scholar]
- [46].Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37:1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Casault L, Savard J, Ivers H, Savard MH. A randomized-controlled trial of an early minimal cognitive-behavioural therapy for insomnia comorbid with cancer. Behaviour research and therapy. 2015;67:45–54. [DOI] [PubMed] [Google Scholar]
- [48].Savard J, Ivers H, Savard MH, Morin CM. Long-Term Effects of Two Formats of Cognitive Behavioral Therapy for Insomnia Comorbid with Breast Cancer. Sleep. 2016;39:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Nicassio P, Ganz PA, et al. Tai Chi Chih Compared With Cognitive Behavioral Therapy for the Treatment of Insomnia in Survivors of Breast Cancer: A Randomized, Partially Blinded, Noninferiority Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017;35:2656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Palesh O, Scheiber C, Kesler S, Janelsins MC, Guido JJ, Heckler C, et al. Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: effects on insomnia and circadian rhythm during chemotherapy: a phase II randomised multicentre controlled trial. British journal of cancer. 2018;119:274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zachariae R, Amidi A, Damholdt MF, Clausen CDR, Dahlgaard J, Lord H, et al. Internet-Delivered Cognitive-Behavioral Therapy for Insomnia in Breast Cancer Survivors: A Randomized Controlled Trial. Journal of the National Cancer Institute. 2018;110:880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mercier J, Ivers H, Savard J. A non-inferiority randomized controlled trial comparing a home-based aerobic exercise program to a self-administered cognitive-behavioral therapy for insomnia in cancer patients. Sleep. 2018;41. [DOI] [PubMed] [Google Scholar]
- [53].Berger AM, Kuhn BR, Farr LA, Von Essen SG, Chamberlain J, Lynch JC, et al. One-year outcomes of a behavioral therapy intervention trial on sleep quality and cancer-related fatigue. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:6033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Annals of internal medicine. 2016;165:125–33. [DOI] [PubMed] [Google Scholar]
- [55].van der Zweerde T, Bisdounis L, Kyle SD, Lancee J, van Straten A. Cognitive behavioral therapy for insomnia: A meta-analysis of long-term effects in controlled studies. Sleep medicine reviews. 2019;48:101208. * [DOI] [PubMed] [Google Scholar]
- [56].Lamberg L. Despite effectiveness, behavioral therapy for chronic insomnia still underused. Jama. 2008;300:2474–5. [DOI] [PubMed] [Google Scholar]
- [57].Matthews EE, Schmiege SJ, Cook PF, Berger AM, Aloia MS. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: a pilot study. Behavioral sleep medicine. 2012;10:217–29. [DOI] [PubMed] [Google Scholar]
- [58].Zhou ES, Michaud AL, Recklitis CJ. Developing efficient and effective behavioral treatment for insomnia in cancer survivors: Results of a stepped care trial. Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.