Summary
Background
In ARIEL3, rucaparib maintenance treatment significantly improved progression-free survival versus placebo. Here, we report prespecified, investigator-assessed, exploratory post-progression endpoints and updated safety data.
Methods
In this ongoing (enrolment complete) randomised, placebo-controlled, phase 3 trial, patients aged 18 years or older who had platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma and an Eastern Cooperative Oncology Group performance status of 0 or 1 who had received at least two previous platinum-based chemotherapy regimens and responded to their last platinum-based regimen were randomly assigned (2:1) to oral rucaparib (600 mg twice daily) or placebo in 28-day cycles using a computer-generated sequence (block size of six with stratification based on homologous recombination repair gene mutation status, progression-free interval following penultimate platinum-based regimen, and best response to most recent platinum-based regimen). Patients, investigators, site staff, assessors, and the funder were masked to assignments. The primary endpoint of investigator-assessed progression-free survival has been previously reported. Prespecified, exploratory outcomes of chemotherapy-free interval (CFI), time to start of first subsequent therapy (TFST), time to disease progression on subsequent therapy or death (PFS2), and time to start of second subsequent therapy (TSST) and updated safety were analysed (visit cutoff Dec 31, 2017). Efficacy analyses were done in all patients randomised to three nested cohorts: patients with BRCA mutations, patients with homologous recombination deficiencies, and the intention-to-treat population. Safety analyses included all patients who received at least one dose of study treatment. This trial is registered with ClinicalTrials.gov, NCT01968213.
Findings
Between April 7, 2014, and July 19, 2016, 564 patients were enrolled and randomly assigned to rucaparib (n=375) or placebo (n=189). Median follow-up was 28·1 months (IQR 22·0–33·6). In the intention-to-treat population, median CFI was 14·3 months (95% CI 13·0–17·4) in the rucaparib group versus 8·8 months (8·0–10·3) in the placebo group (hazard ratio [HR] 0·43 [95% CI 0·35–0·53]; p<0·0001), median TFST was 12·4 months (11·1–15·2) versus 7·2 months (6·4–8·6; HR 0·43 [0·35–0·52]; p<0·0001), median PFS2 was 21·0 months (18·9–23·6) versus 16·5 months (15·2–18·4; HR 0·66 [0·53–0·82]; p=0·0002), and median TSST was 22·4 months (19·1–24·5) versus 17·3 months (14·9–19·4; HR 0·68 [0·54–0·85]; p=0·0007). CFI, TFST, PFS2, and TSST were also significantly longer with rucaparib than placebo in the BRCA-mutant and homologous recombination-deficient cohorts. The most frequent treatment-emergent adverse event of grade 3 or higher was anaemia or decreased haemoglobin (80 [22%] patients in the rucaparib group vs one [1%] patient in the placebo group). Serious treatment-emergent adverse events were reported in 83 (22%) patients in the rucaparib group and 20 (11%) patients in the placebo group. Two treatment-related deaths have been previously reported in this trial; there were no new treatment-related deaths.
Interpretation
In these exploratory analyses over a median follow-up of more than 2 years, rucaparib maintenance treatment led to a clinically meaningful delay in starting subsequent therapy and provided lasting clinical benefits versus placebo in all three analysis cohorts. Updated safety data were consistent with previous reports.
Funding
Clovis Oncology
Introduction
Although most patients with advanced ovarian cancer respond to initial treatment, typically surgery followed by platinum-based or taxane-based chemotherapy, the majority will experience disease recurrence and require subsequent therapies.1 For patients with recurrent ovarian cancer who respond to second-line platinum-based chemotherapy, continuing therapy with bevacizumab as a maintenance therapy or introducing a targeted agent such as a poly(ADP-ribose) polymerase (PARP) inhibitor after chemotherapy has become a standard of care that should be offered to patients.2-7 Maintenance therapy is intended to delay disease progression without negatively affecting patient quality of life.8,9
Rucaparib (formerly known as CO-338, AG-014447, and PF-01367338) is an oral, small molecule inhibitor of PARP1, PARP2, and PARP3.10,11 In the phase 3 ARIEL3 study in patients with advanced, recurrent ovarian cancer, rucaparib maintenance treatment significantly improved investigator-assessed progression-free survival versus placebo in all of the study’s three molecularly defined, nested cohorts: patients with a BRCA1 or BRCA2 (BRCA)-mutated carcinoma (germline, somatic, or unknown origin); patients with a homologous recombination deficient (HRD) carcinoma (BRCA mutation plus wild-type BRCA and high loss of heterozygosity [LOH]); and the intention-to-treat (ITT) population.12 Median progression-free survival was significantly improved in all three cohorts. Based on these results, rucaparib is approved in the USA and the European Union as monotherapy for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who have a complete or partial response to platinum-based chemotherapy.13,14
As new therapies become available and management of ovarian cancer evolves to incorporate strategies such as maintenance, it is important to understand how such treatments influence the post-progression survival of patients. Although overall survival remains the gold standard in oncology trials, including those for ovarian cancer, evaluation can be confounded by subsequent treatments, a long duration of post-progression survival, and crossover to the trial or a similar drug.15 This can be particularly problematic when multiple effective treatments are available. Thus, additional post-progression assessments are needed to help to demonstrate the clinical benefit of an investigative therapy, and organisations such as the Gynecologic Cancer InterGroup (GCIG), Society of Gynecologic Oncology, European Society for Gynaecological Oncology, and European Society for Medical Oncology recommend their incorporation into clinical trials to support observed progression-free survival benefits.16-19
Post-progression assessments include time to start of first subsequent therapy (TFST), time to disease progression on subsequent therapy or death (PFS2), and time to start of second subsequent therapy (TSST). Significant improvements in these endpoints show that clinically meaningful improvements in progression-free survival observed during the study can be maintained beyond the first progression event, can delay the need for subsequent therapy, and can persist throughout the course of subsequent treatments.15 Examination of PFS2 can also provide insight into the influence of an investigative therapy on the efficacy of subsequent therapies and serve as a surrogate for overall survival.20 Additionally, trials of targeted therapy can assess the chemotherapy-free interval (CFI), defined as the time from the last dose of previous chemotherapy to initiation of subsequent chemotherapy, inclusive of the time on targeted therapy or placebo. This endpoint can help to quantify the duration of time that patients avoid the need for chemotherapy and its potentially negative impact on quality of life; side-effects associated with chemotherapy can be more frequent or severe than those associated with targeted therapies. Overall, these endpoints give complementary and comprehensive information on the post-progression benefits of an investigative therapy.
Here, we present results from the prespecified exploratory analyses of CFI, TFST, PFS2, and TSST in ARIEL3 to investigate the durability of clinical benefit with rucaparib maintenance treatment following disease progression and the switch to a subsequent therapy. Additional safety data are also reported (visit cutoff Dec 31, 2017), which represents a more extensive analysis of safety with an additional 8 months of follow-up than reported previously.12
Methods
Study design and patients
Full details of this multicentre, international, double-blind, randomised, phase 3 trial have been published previously.12 This trial was done at 87 hospitals and cancer centres in Australia, Belgium, Canada, France, Germany, Israel, Italy, New Zealand, Spain, the UK, and the USA (appendix pp 1-3). The redacted protocol for the ARIEL3 clinical study is available on ClinicalTrials.gov (NCT01968213).
Eligible patients were aged 18 years or older; had platinum-sensitive, high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube carcinoma; had received at least two previous platinum-based chemotherapy regimens; had Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; had adequate organ function; and must have achieved either a complete response according to Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST), a partial response according to RECIST, or a serological response based on GCIG cancer antigen 125 (CA 125) response criteria to their last platinum-based regimen. Patients must have had documented radiological disease progression more than 6 months after the last dose of the penultimate platinum administered. For entry into the study, CA 125 had to be less than the upper limit of normal. Patients with symptomatic or untreated central nervous system metastases or who had received anticancer therapy fewer than 14 days before starting the study or previous treatment with a PARP inhibitor were excluded. Previous treatment with bevacizumab was permitted, with the exception of bevacizumab maintenance therapy after the most recent platinum-based regimen. On Nov 4, 2014, after 91 patients had been randomised, we made an amendment to the protocol requiring that the most recent platinum-based regimen was to be administered as a chemotherapy doublet and for a minimum of four cycles. Full inclusion and exclusion criteria have been reported previously.12
The study was approved by national or local institutional review boards and was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Conference on Harmonisation. Patients provided written, informed consent before participation.
Randomisation and masking
As reported previously,12 randomisation was computer generated using a block size of six, with the following stratification factors: homologous recombination repair gene mutation status (based on gene mutation only; mutation in BRCA1 or BRCA2, mutation in a non-BRCA gene associated with homologous recombination, or no mutation in BRCA or a homologous recombination gene), progression-free interval following penultimate platinum-based regimen (6 to ≤12 months or >12 months), and best response to most recent platinum-based regimen (complete or partial response). Patients were assigned (2:1) to the rucaparib or placebo group in a masked manner via an interactive web and voice response system. Patients, investigators, site staff, assessors, and the funder were masked to assignments. To ensure masking was maintained, rucaparib and placebo tablets were manufactured to have identical appearances.
Procedures
In the screening phase before randomisation, patient medical history and archival tumour tissue were obtained. Central testing of DNA derived from patient archival tumour tissue samples was done to detect mutations in homologous recombination pathway genes and assess genomic LOH. A cutoff of 16% or greater for ARIEL3 was prespecified as a discriminator for high genomic LOH.12 Full details of the testing protocol have been reported previously.12
In ARIEL3, patients received oral rucaparib 600 mg twice daily or placebo in continuous 28-day cycles until disease progression (as assessed by RECIST), death, or other reason for discontinuation. Dose reductions (in 120 mg decrements to 240 mg twice daily) were permitted if a patient had a grade 3 or higher adverse event or a persistent grade 2 adverse event, as defined by Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Treatment of study drug was to be withheld for any CTCAE grade 3 or 4 toxicities. At the discretion of the investigator, the dose of study drug could be held or reduced for a CTCAE grade 2 toxicity not adequately controlled using concomitant medications or supportive care. Treatment with study drug was to be held until the toxicity resolved to CTCAE grade 2 or less. Twice-daily dosing could then be resumed at either the same dose or a lower dose at the discretion of the investigator. If treatment was resumed at the same dose, and the patient experienced the same toxicity, the dose was to be reduced following resolution of the event to CTCAE grade 2 or less. If the patient continued to experience toxicity, additional dose reduction steps were permitted; however, the investigator was expected to consult with the sponsor’s medical monitor before reducing to 240 mg twice daily. If a patient continued to experience toxicity despite two dose-reduction steps (ie, to a dose of 360 mg twice-daily rucaparib or placebo), or if dosing with study drug was interrupted for more than 14 consecutive days due to toxicity, treatment was to be discontinued, unless otherwise agreed between the investigator and the sponsor.
We did disease assessments including CT scans and CA 125 measurements at screening, every 12 weeks during treatment, following clinical symptoms, and at discontinuation of treatment. Samples were obtained for central laboratory investigations of haematological and clinical chemistry parameters every 2 weeks for the first two cycles and then on day 1 of every subsequent cycle. Patients were monitored for adverse events during study participation, beginning after the first dose of study drug and until 28 days after the last dose of study drug. Following the 28-day window after treatment discontinuation, only serious adverse events assessed as related to study drug and all adverse events of special interest (ie, myelodysplastic syndrome and acute myeloid leukaemia) irrespective of causality, were reported. After the initial treatment phase, long-term follow-up and overall survival data were collected for all patients. Subsequent treatments, secondary malignancy monitoring, and overall survival data will be collected for all patients every 12 weeks (±14 days) until death, loss to follow-up, withdrawal of consent from study, or closure of the study. For patients who discontinued due to disease progression, the schedule and type of subsequent disease assessments were not prespecified by the protocol and were left to the discretion of the investigator.
Outcomes
The primary efficacy endpoint (investigator-assessed progression-free survival) and secondary endpoints (progression-free survival according to blinded, independent, central radiology review, patient-reported outcomes, and safety) in ARIEL3 have been reported previously using the primary efficacy data after unblinding (visit cutoff April 15, 2017).12 Data for the secondary endpoint of overall survival were not yet mature at the time of the present analyses, and the secondary endpoint of population pharmacokinetic modelling will be reported separately.
Here, we report the prespecified, investigator-assessed exploratory endpoints of CFI, TFST, PFS2, and TSST. CFI was defined as the time since the last dose of the most recent chemotherapy regimen to the date of the first dose of a subsequent anticancer therapy after study drug. TFST was defined as the time from randomisation to the date of the first dose of the first subsequent anticancer treatment regimen. PFS2 was defined as the time from randomisation to the second event of disease progression as assessed by the investigator or death due to any cause. This second progression event could be a documented event as defined in the RECIST guidelines or an event of symptomatic or clinical or CA 125 progression. TSST was defined as the time from randomisation to the date of the first dose of the second subsequent anticancer treatment regimen.
An updated safety analysis is presented, which was assessed by monitoring adverse events and vital signs, laboratory testing, and physical examination.
All outcomes and assessments are reported to a visit cutoff of Dec 31, 2017.
Statistical analysis
ARIEL3 was designed to enrol approximately 540 patients and include 180–200 patients with a BRCA mutation in their carcinoma (limited to 150 with a known deleterious germline BRCA mutation) and up to 360 patients without. Subgroup sizes were calculated to result in a 90% power to establish a significant difference between rucaparib and placebo at a one-sided α level of 0·025 given the following assumptions for investigator-assessed median progression-free survival for each analysis cohort: BRCA mutant (12·0 months in the rucaparib group vs 6·0 months in the placebo group; hazard ratio [HR] 0·5), HRD (10·0 months vs 6·0 months; HR 0·6), and ITT population (8·5 months vs 6·0 months; HR 0·7).12 Prespecified and post-hoc exploratory analyses were done for these three molecularly defined, nested cohorts. Post-hoc exploratory analyses were done for subgroups of patients with BRCA wild-type carcinomas based on LOH status (high, low, or indeterminate).
Time-to-event variables (CFI, TFST, PFS2, and TSST) were summarised using Kaplan-Meier methodology. A stratified log-rank test that included the randomisation strata was used to compare treatments. Additionally, a stratified Cox proportional hazard model was used to calculate the HR between the treatment groups for each endpoint. Proportionality of hazards for the Cox proportional hazard assumption (ie, constant relative hazard) was verified graphically using log-log plots for progression-free survival and PFS2 in the ITT population (appendix p 8). As the assumption was met for these analyses (ie, the plot of the log of the cumulative hazard for the rucaparib and placebo groups resulted in parallel curves), the subgroup analyses were deemed appropriate. Per protocol, all endpoints were exploratory and tested at a one-sided 0·025 significance level, without any multiplicity adjustment.
For CFI, TFST, PFS2, and TSST, patients without a documented event (ie, start of a subsequent anticancer therapy after study drug, second progression event, or start of a second subsequent anticancer therapy after study drug) were censored on the date of their last available assessment.
We also report the post-hoc, exploratory endpoint of PFS2–PFS1, defined as the time from investigator-assessed disease progression during ARIEL3 (PFS1) to the second event of investigator-assessed disease progression or death. For this endpoint, patients were censored if they had not experienced a second event of progression or death at the last date known to be alive. Duration of PFS2–PFS1 was set to 1 day for patients who were censored for PFS1 and did not have any further follow-up information. The date of the second event of progression or censoring was used to calculate PFS2–PFS1 for patients who were censored for PFS1 but received subsequent anticancer treatment or had other follow-up data.
The safety population included all patients who received at least one dose of study treatment.
Statistical analyses were done with SAS (version 9.4). This trial is registered with ClinicalTrials.gov, number NCT01968213.
Role of the funding source
ARIEL3 was designed by JAL, EMS, and RLC in collaboration with the funder. This Article was written by the authors, with medical writing and copy-editing support paid for by the funder. Data were collected by the investigators, analysed by the funder, and interpreted by all authors. All authors had full access to all trial data and had final responsibility for the decision to submit these data for publication.
Results
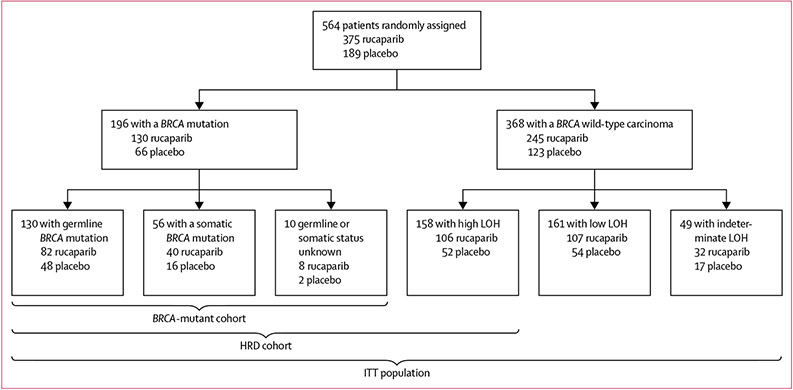
Between April 7, 2014, and July 19, 2016, 564 patients were randomly allocated to the two groups: 375 (66%) to rucaparib and 189 (34%) to placebo and were included in the ITT population; the BRCA-mutant cohort included 130 patients from the rucaparib group and 66 patients from the placebo group, and the HRD cohort included 236 patients from the rucaparib group and 118 patients from the placebo group (figure 1).12 Most patients had an ECOG performance status of 0 and a BRCA wild-type carcinoma (table 1). Most patients in ARIEL3 had received two previous platinum-based chemotherapy regimens, with the remainder having received at least three (table 1). Protocol deviations have previously been reported in full.12 As of the Dec 31, 2017, visit cutoff (median follow-up 28·1 months, IQR 22·0–33·6), 60 (16%) patients in the rucaparib group and five (3%) in the placebo group had not yet progressed and were still receiving treatment.
Figure 1: ARIEL3 populations.
Adapted from Coleman et al12 by permission of Elsevier. HRD=homologous recombination deficient. ITT=intention to treat. LOH=loss of heterozygosity.
Table 1:
Patient demographics and baseline characteristics in intention-to-treat population
| Rucaparib group (n=375) |
Placebo group (n=189) |
|
|---|---|---|
| Age, years | 61·0 (53·0–67·0) |
62·0 (53·0–68·0) |
| ECOG performance status | ||
| 0 | 280 (75%) | 136 (72%) |
| 1 | 95 (25%) | 53 (28%) |
| Diagnosis | ||
| Epithelial ovarian cancer | 312 (83%) | 159 (84%) |
| Fallopian tube cancer | 32 (9%) | 10 (5%) |
| Primary peritoneal cancer | 31 (8%) | 19 (10%) |
| High-grade serous adenocarcinoma | 0 | 1 (1%)* |
| BRCA mutation in the carcinoma | ||
| BRCA mutant | 130 (35%) | 66 (35%) |
| BRCA1 | 80 (21%) | 37 (20%) |
| BRCA2 | 50 (13%) | 29 (15%) |
| Germline | 82 (22%) | 48 (25%) |
| Somatic | 40 (11%) | 16 (8%) |
| Unknown† | 8 (2%) | 2 (1%) |
| BRCA wild type | 245 (65%) | 123 (65%) |
| High LOH | 106 (28%) | 52 (28%) |
| Low LOH | 107 (29%) | 54 (29%) |
| Indeterminate LOH‡ | 32 (9%) | 17 (9%) |
| Number of previous platinum-based regimens | ||
| 2 | 236 (63%) | 126 (67%) |
| 3 | 109 (29%) | 47 (25%) |
| >3 | 30 (8%) | 16 (8%) |
| Time to progression with penultimate platinum-based regimen, months | ||
| 6 to ≤12 | 151 (40%) | 76 (40%) |
| >12 | 224 (60%) | 113 (60%) |
| Response to last platinum-based regimen | ||
| Complete response according to RECIST | 126 (34%) | 64 (34%) |
| Partial response according to RECIST or serological response according to GCIG CA 125 criteria | 249 (66%) | 125 (66%) |
Adapted from Coleman et al12 by permission of Elsevier. Data are median (IQR) or n (%). CA 125=cancer antigen 125. ECOG=Eastern Cooperative Oncology Group. GCIG=Gynecologic Cancer InterGroup. LOH=loss of heterozygosity.
RECIST=Response Evaluation Criteria In Solid Tumors version 1.1.
According to the patient records, origin was fallopian tube or ovary.
Tumour sample was BRCA mutant according to Foundation Medicine’s T5 next-generation sequencing assay, but a blood sample was not available for central germline testing.
Tumour sample was not evaluable for percentage of genomic LOH because of low tumour content or aneuploidy.
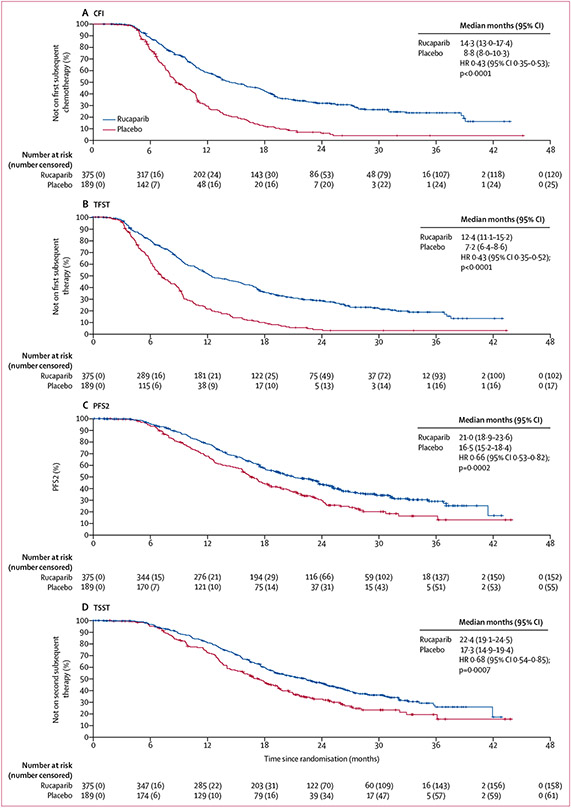
CFI was significantly longer in the rucaparib group than in the placebo group for all three nested cohorts (appendix pp 9-12; figure 2). In the BRCA-mutant cohort, median CFI was 20·8 months (95% CI 17·7–27·8) in the rucaparib group and 8·7 months (7·2–10·9) in the placebo group (HR 0·28 [95% CI 0·19–0·41]; p<0·0001; 75 [58%] events in 130 patients vs 56 [85%] events in 66 patients; appendix p 9). In the HRD cohort, median CFI was 18·0 months (14·3–19·4) in the rucaparib group and 9·1 months (8·0–10·8) in the placebo group (HR 0·40 [0·31–0·53]; p<0·0001; 152 [64%] events in 236 patients vs 101 [86%] events in 118 patients; appendix p 11). In the ITT population, median CFI was 14·3 months (13·0–17·4) in the rucaparib group and 8·8 months (8·0–10·3) in the placebo group (HR 0·43 [0·35–0·53]; p<0·0001; 255 [68%] events in 375 patients vs 164 [87%] events in 189 patients; figure 2A).
Figure 2: Kaplan–Meier estimates of CFI, TFST, PFS2, and TSST in the ITT population.
The ITT population consisted of 375 patients in the rucaparib group and 189 patients in the placebo group. CFI=chemotherapy-free interval. HR=hazard ratio. ITT=intention to treat. PFS2=time to disease progression on subsequent therapy or death. TFST=time to start of first subsequent therapy. TSST=time to start of second subsequent therapy.
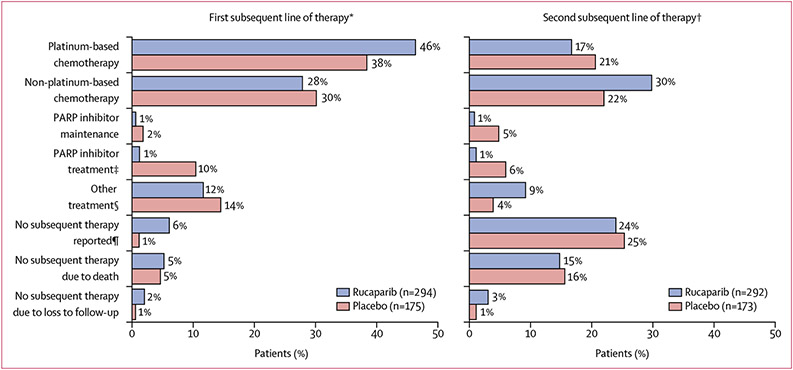
Among patients in the ITT population who discontinued and had not withdrawn consent for follow-up, 134 (46%) of 294 patients from the rucaparib group and 66 (38%) of 175 patients from the placebo group received platinum-based chemotherapy as their first subsequent therapy, with 81 (28%) and 52 (30%) patients receiving non-platinum-based chemotherapy (figure 3). Four (1%) patients from the rucaparib group and 18 (10%) patients from the placebo group received PARP inhibitor treatment as their first subsequent therapy; two (1%) and three (2%), respectively, received PARP inhibitor maintenance as their first subsequent therapy (figure 3). Patients in the rucaparib group had a significantly longer TFST than patients in the placebo group across all the cohorts, with a median TFST of 18·9 months (95% CI 15·9–25·3) versus 7·2 months (5·5–9·1) in the BRCA-mutant cohort (HR 0·28 [95% CI 0·20–0·41]; p<0·0001; 81 [62%] events in 130 patients vs 58 [88%] events in 66 patients; appendix p 9), 16·4 months (12·5–17·9) versus 7·4 months (6·5–9·1) in the HRD cohort (HR 0·39 [0·30–0·51]; p<0·0001; 160 [68%] events in 236 patients vs 106 [90%] events in 118 patients; appendix p 11), and 12·4 months (11·1–15·2) versus 7·2 months (6·4–8·6) in the ITT population (HR 0·43 [0·35–0·52]; p<0·0001; 273 [73%] events in 375 patients vs 172 [91%] events in 189 patients; figure 2B).
Figure 3: First and second subsequent lines of therapy for the ITT population.
Visit cutoff Dec 31, 2017. ITT=intention to treat. PARP=poly(ADP-ribose) polymerase. VEGF=vascular endothelial growth factor. *Eligible patients who discontinued from ARIEL3; excludes 21 patients from the rucaparib group and nine patients from the placebo group who withdrew consent during treatment or follow-up. †Eligible patients who discontinued from ARIEL3; excludes 23 patients from the rucaparib group and 11 patients from the placebo group who withdrew consent during treatment or follow-up. ‡As first subsequent therapy, three patients received olaparib plus cediranib (rucaparib: n=1; placebo: n=2), two received olaparib plus durvalumab (placebo: n=2), and one received olaparib plus radiotherapy (rucaparib: n=1); as second subsequent therapy, one patient received olaparib plus cediranib (rucaparib: n=1) and one received olaparib plus vistusertib (placebo: n=1). §Other treatment includes VEGF inhibitor, hormonal therapy, immunotherapy, investigational treatment (unspecified), radiation, and hyperthermic intraperitoneal chemotherapy. ¶Patient might not have started any subsequent treatment as of the visit cutoff or was transferred to palliative care.
Median investigator-assessed PFS2 was significantly longer in the rucaparib group than in the placebo group in the BRCA-mutant cohort (26·8 months [95% CI 23·4–41·4] vs 18·4 months [15·7–23·6] months; HR 0·56 [95% CI 0·38–0·83]; p=0·0040; 64 [49%] events in 130 patients in the rucaparib group vs 42 [64%] events in 66 patients in the placebo group; appendix p 10), the HRD cohort (25·3 months [21·9–28·5] vs 18·4 months [15·8–22·1]; HR 0·66 [0·49–0·87]; p=0·0042; 125 [53%] events in 236 patients vs 78 [66%] events in 118 patients; appendix p 12), and the ITT population (21·0 months [18·9–23·6] vs 16·5 months [15·2–18·4]; HR 0·66 [0·53–0·82]; p=0·0002; 223 [59%] events in 375 patients vs 134 [71%] events in 189 patients; figure 2C). In a post-hoc analysis, we found no significant difference in PFS2–PFS1 between the rucaparib and placebo groups in all three cohorts (appendix p 13).
In the ITT population, among patients who discontinued and had not withdrawn consent for follow-up, 170 (58%) of 292 patients in the rucaparib group and 100 (58%) of 173 patients in the placebo group had received a second subsequent therapy as of the visit cutoff date. The most common second subsequent therapy was non-platinum-based chemotherapy (87 [30%] patients in the rucaparib group and 38 [22%] patients in the placebo group; figure 3). The proportion of patients receiving a platinum-based chemotherapy as second subsequent therapy (49 [17%] patients in the rucaparib group and 36 [21%] patients in the placebo group) was lower than the proportion of patients who received platinum-based chemotherapy as their first subsequent treatment (figure 3). Four (1%) patients from the rucaparib group and 11 (6%) patients from the placebo group received PARP inhibitor treatment as their second subsequent therapy; three (1%) and eight (5%), respectively, received PARP inhibitor maintenance as their second subsequent therapy (figure 3).
Median TSST was significantly longer for patients in the rucaparib group compared with the placebo group across the three cohorts: 28·8 months (95% CI 24·4–34·2) in the rucaparib group versus 17·7 months (15·1–21·6) in the placebo group in the BRCA-mutant cohort (HR 0·53 [95% CI 0·36–0·80]; p=0·0022; 65 [50%] events in 130 patients vs 41 [62%] events in 66 patients; appendix p 10), 26·2 months (22·9–30·6) versus 19·0 months (15·8–21·7) months in the HRD cohort (HR 0·67 [0·50–0·91]; p=0·010; 123 [52%] events in 236 patients vs 73 [62%] events in 118 patients; appendix p 12), and 22·4 months (19·1–24·5) versus 17·3 months (14·9–19·4) in the ITT population (HR 0·68 [0·54–0·85]; p=0·0007; 217 [58%] events in 375 patients vs 128 [68%] events in 189 patients; figure 2D).
Post-hoc analyses of subgroups of patients with BRCA wild-type carcinomas based on LOH status are shown in the appendix (p 14).
The safety population included 372 (99%) patients who received rucaparib (three [1%] patients withdrew before receiving rucaparib), and 189 (100%) patients who received placebo. At the time of the extended visit cutoff date, median treatment duration for patients in the safety population was 8·3 months (IQR 3·4–18·1) in the rucaparib group and 5·5 months (2·8–8·3) in the placebo group.
Overall, the updated safety profile was comparable to that previously reported,12 with only modest increases in incidences of treatment-emergent adverse events in the rucaparib and placebo groups (table 2; appendix p 4). In the updated safety analysis, a treatment-emergent adverse event of any grade occurred in 372 (100%) of the patients in the rucaparib group, and 182 (96%) in the placebo group. The most common treatment-emergent adverse events of any grade (reported in at least 30% of patients in either group) were nausea, asthenia or fatigue, dysgeusia, anaemia or decreased haemoglobin, constipation, vomiting, increased alanine aminotransferase (ALT) or aspartate aminotransferase (AST), diarrhoea, and abdominal pain (table 2). Treatment-emergent adverse events of grade 3 or higher were reported in 222 (60%) patients in the rucaparib group and 30 (16%) in the placebo group (appendix pp 5-7), the most common of which were anaemia or decreased haemoglobin (80 [22%] patients in the rucaparib group vs one [1%] patient in the placebo group) and increased ALT or AST (38 [10%] vs none). Serious treatment-emergent adverse events were reported in 83 (22%) patients in the rucaparib group and 20 (11%) in the placebo group, most frequently anaemia (16 [4%] in the rucaparib group vs one [1%] in the placebo group), vomiting (seven [2%] vs two [1%]), and pyrexia (six [2%] vs none). Serious treatment-emergent adverse events were considered related to treatment by the investigator for 35 (9%) patients in the rucaparib group and three (2%) in the placebo group, the most frequent of which was anaemia (16 [4%] vs one [1%]). Most treatment-emergent adverse events of anaemia or decreased haemoglobin were managed with dose reduction or treatment interruption and blood transfusions (for grade 2 or 3 events); less than 2% of patients received erythropoietin.
Table 2:
Treatment-emergent adverse events in the safety population (updated data)
| Rucaparib group (n=372) |
Placebo group (n=189) |
|||||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 1–2 | Grade 3 | Grade 4 | |
| Nausea | 268 (72%) | 14 (4%) | 0 | 68 (36%) | 1 (1%) | 0 |
| Asthenia or fatigue | 237 (64%) | 26 (7%) | 0 | 79 (42%) | 5 (3%) | 0 |
| Dysgeusia | 148 (40%) | 0 | 0 | 13 (7%) | 0 | 0 |
| Anaemia or decreased haemoglobin | 65 (17%) | 77 (21%) | 3 (1%) | 9 (5%) | 0 | 1 (1%) |
| Constipation | 134 (36%) | 7 (2%) | 0 | 44 (23%) | 2 (1%) | 0 |
| Vomiting | 123 (33%) | 15 (4%) | 0 | 27 (14%) | 2 (1%) | 0 |
| Increased ALT or AST | 91 (24%) | 38 (10%) | 0 | 8 (4%) | 0 | 0 |
| Diarrhoea | 119 (32%) | 2 (1%) | 0 | 39 (21%) | 2 (1%) | 0 |
| Abdominal pain | 101 (27%) | 11 (3%) | 0 | 48 (25%) | 1 (1%) | 0 |
| Thrombocytopenia or platelet count decreased | 89 (24%) | 13 (3%) | 7 (2%) | 5 (3%) | 0 | 0 |
| Decreased appetite | 85 (23%) | 3 (1%) | 0 | 26 (14%) | 0 | 0 |
| Neutropenia or decreased neutrophil count | 43 (12%) | 22 (6%) | 7 (2%) | 7 (4%) | 1 (1%) | 1 (1%) |
| Headache | 70 (19%) | 1 (<1%) | 0 | 30 (16%) | 1 (1%) | 0 |
| Photosensitivity reaction | 66 (18%) | 2 (1%) | 0 | 1 (1%) | 0 | 0 |
| Blood creatinine increased | 60 (16%) | 1 (<1%) | 0 | 3 (2%) | 0 | 0 |
| Arthralgia | 57 (15%) | 2 (1%) | 0 | 24 (13%) | 0 | 0 |
| Dizziness | 57 (15%) | 0 | 0 | 14 (7%) | 1 (1%) | 0 |
| Cough | 55 (15%) | 0 | 0 | 25 (13%) | 0 | 0 |
| Abdominal pain upper | 52 (14%) | 2 (1%) | 0 | 11 (6%) | 0 | 0 |
| Dyspepsia | 53 (14%) | 1 (<1%) | 0 | 9 (5%) | 0 | 0 |
| Insomnia | 54 (15%) | 0 | 0 | 15 (8%) | 0 | 0 |
| Dyspnoea | 53 (14%) | 0 | 0 | 14 (7%) | 0 | 0 |
| Pruritus | 51 (14%) | 0 | 0 | 20 (11%) | 0 | 0 |
| Back pain | 50 (13%) | 0 | 0 | 28 (15%) | 0 | 0 |
| Rash | 49 (13%) | 1 (<1%) | 0 | 17 (9%) | 0 | 0 |
| Pyrexia | 45 (12%) | 0 | 0 | 9 (5%) | 0 | 0 |
| Upper respiratory tract infection | 44 (12%) | 0 | 0 | 4 (2%) | 2 (1%) | 0 |
| Hypomagnesaemia | 42 (11%) | 1 (<1%) | 0 | 11 (6%) | 0 | 0 |
| Abdominal distension | 42 (11%) | 0 | 0 | 24 (13%) | 0 | 0 |
| Oedema peripheral | 40 (11%) | 1 (<1%) | 0 | 14 (7%) | 0 | 0 |
| Hypertension | 27 (7%) | 9 (2%) | 0 | 12 (6%) | 4 (2%) | 0 |
Data are n (%). Updated data as of Dec 31, 2017 visit cutoff are shown. ALT=alanine aminotransferase. AST=aspartate aminotransferase. Grade 1–2 treatment-emergent adverse events reported in ≥10% of patients and grade 3–4 treatment-emergent adverse events reported in ≥2% of patients are shown; sorted by decreasing incidence in the rucaparib group.
In this updated safety analysis, there were no new treatment-emergent adverse events of myelodysplastic syndrome or acute myeloid leukaemia beyond those previously reported (three [1%] patients in the rucaparib group and none in the placebo group12).
Treatment interruption due to a treatment-emergent adverse event occurred in 243 (65%) patients in the rucaparib group and 19 (10%) in the placebo group (appendix p 4). The most common treatment-emergent adverse events leading to treatment interruption in the rucaparib group were thrombocytopenia or decreased platelets (64 [17%] patients), anaemia or decreased haemoglobin (56 [15%]), increased ALT or AST (38 [10%]), and nausea (38 [10%]), whereas the most common treatment-emergent adverse event associated with treatment interruption in the placebo group was asthenia or fatigue (six [3%] patients).
Dose reduction due to a treatment-emergent adverse event occurred in 206 (55%) patients in the rucaparib group and eight (4%) in the placebo group. The most common treatment-emergent adverse events leading to dose reduction in the rucaparib group were anaemia or decreased haemoglobin (47 [13%] patients), increased ALT or AST (41 [11%]), thrombocytopenia or decreased platelets (40 [11%]), and nausea (37 [10%]), whereas the most common treatment-emergent adverse event leading to dose reduction in the placebo group was asthenia or fatigue (four [2%]).
57 (15%) patients in the rucaparib group and three (2%) in the placebo group discontinued because of a treatment-emergent adverse event (excluding disease progression), of whom 49 (13%) and one (1%) discontinued because of a treatment-emergent adverse event that was considered treatment related. The most common treatment-emergent adverse events leading to discontinuation in the rucaparib group were thrombocytopenia or decreased platelets (11 [3%] patients), anaemia or decreased haemoglobin (ten [3%]), and nausea (ten [3%]). These were also the most common treatment-related adverse events leading to discontinuation in the rucaparib group, with ten (3%) patients discontinuing due to each of the above.
In the previously published analysis (safety visit cutoff April 15, 2017), we reported four deaths in the rucaparib group considered unrelated to treatment by the investigator (two [1%] due to progressive disease, one [<1%] due to cardiac arrest, and one [<1%] due to haematophagic histiocytosis) and two considered related to treatment (one [<1%] due to acute myeloid leukaemia and one [<1%] due to myelodysplastic syndrome); two deaths occurred in the placebo group and were considered unrelated to treatment (one [1%] due to disease progression and one [1%] due to pulmonary embolism).12 At the time of the updated safety visit cutoff date (Dec 31, 2017) there was one (<1%) additional death due to a high-grade B-cell lymphoma in the rucaparib group, which was considered unrelated to rucaparib by the investigator, and none in the placebo group (appendix p 4).
Discussion
The prespecified, exploratory analyses reported here show the durable clinical benefit of rucaparib maintenance treatment in the post-progression period for patients with recurrent ovarian cancer. Median CFI, TFST, PFS2, and TSST were all significantly (1·3–2·6-times) longer for patients who received rucaparib maintenance treatment than those who received placebo, showing a clinically meaningful improvement in these endpoints for all cohorts regardless of mutational status.
The extension of CFI indicated that patients receiving rucaparib were able to delay initiating additional anticancer therapy, potentially allowing them more time to recover from previous negative effects of chemotherapy and postpone further side-effects associated with anticancer therapy. In particular, the side-effects associated with chemotherapy, including neurotoxicity, nausea and vomiting, and hair loss, are of specific concern to patients with ovarian cancer.21,22 The TFST findings were similarly clinically meaningful; in all cohorts, median TFST was approximately twice as long in the rucaparib group than the placebo group, and the significant differences in TFST showed that patients who received rucaparib maintenance treatment were able to delay the start of further therapy for longer than patients receiving placebo. Among first subsequent therapies, the use of platinum-based chemotherapy was higher in the rucaparib group than placebo group, indicating that rucaparib-treated patients had tumours that were still considered platinum sensitive and that these patients remained fit enough to receive additional chemotherapy. Although some patients in the rucaparib group did receive a different PARP inhibitor as their first subsequent treatment, the proportion of subsequent PARP inhibitor use was higher among patients in the placebo group, which is consistent with current understanding regarding the efficacy of PARP inhibitors in patients who have received previous PARP inhibitor therapy. Across both rucaparib and placebo groups, a greater number of patients received a PARP inhibitor as first subsequent therapy in the treatment setting than in the maintenance setting (22 [5%] vs five [1%]).
The lasting benefit of rucaparib treatment was further supported by the PFS2 analyses; across cohorts, the median PFS2 was 1·5–times longer in the rucaparib group than in the placebo group. The PFS2–PFS1 analyses suggest that rucaparib maintenance treatment did not adversely affect the possibility for patients to benefit from subsequent therapy. This is of particular importance as the duration of progression-free survival following relapse has previously been shown to diminish with each line of chemotherapy in women with ovarian cancer.23 Such reductions in progression-free survival are likely to be related to the development of resistance through changes in the tumour, such as mutations or epigenetic modifications, which can accumulate and influence responsiveness to treatment.24 It is possible that differences in the mechanism of action between rucaparib and other drug classes explain why rucaparib had no apparent effect on the efficacy of subsequent therapies. Furthermore, the benefit in PFS2 for patients who received rucaparib and the similarity in PFS2–PFS1 between rucaparib and placebo groups were seen even though 12% of patients in the placebo group received a PARP inhibitor as the first subsequent therapy. Median TSST was also longer across all cohorts for patients who received rucaparib than those who received placebo, supporting the PFS2 analyses and the benefit of previous rucaparib maintenance treatment. As of the visit cutoff, most patients who had a second subsequent therapy received a non-platinum-based chemotherapy, suggesting that fewer of these patients had tumours that were considered platinum sensitive than those who received a first subsequent therapy. More patients received PARP inhibitor maintenance treatment as their second subsequent therapy than as a first subsequent therapy.
Rucaparib maintenance treatment provided durable clinical benefit for patients with recurrent, platinum-sensitive ovarian cancer, with 60 (16%) of 375 patients in the rucaparib group still participating in the study as of Dec 31, 2017, compared with five (3%) of 189 patients in the placebo group. Our exploratory endpoint analyses suggest that rucaparib maintenance does not negatively affect the efficacy of subsequent treatments and further support the progression-free survival benefit observed in patients receiving rucaparib during the study. For each post-progression endpoint, the difference between medians in the rucaparib and placebo groups was consistent with the difference in medians for progression-free survival on study across all cohorts.12 Conversely, if rucaparib maintenance treatment had negatively affected post-progression outcomes, differences between the rucaparib and placebo groups would have been substantially shorter than the initial difference in median progression-free survival.
Similar improvements in post-progression outcomes have been reported from clinical trials of other PARP inhibitors used as second-line maintenance treatment for ovarian cancer. In NOVA, maintenance niraparib significantly improved median CFI and median TFST versus placebo in patients with a germline BRCA mutation and patients without a germline BRCA mutation (this subgroup included patients with a somatic BRCA mutation).4,25 In SOLO2, maintenance olaparib significantly improved median TFST, PFS2, and TSST versus placebo in patients with a BRCA mutation.7 In Study 19, a phase 2 study of maintenance olaparib, median TFST and TSST were significantly longer with olaparib than placebo in patients with and those without a BRCA mutation.26
Safety results as of Dec 31, 2017, were comparable to those reported earlier in terms of their incidence, severity, and nature.12 The safety analysis included an additional 8 months of follow-up, and slight increases in the incidence of treatment-emergent adverse events were not unexpected considering the increased duration of treatment. There was no increase in the incidence of myelodysplastic syndrome or acute myeloid leukaemia with the additional 8 months of follow-up; patients continue to be followed to monitor for these and other adverse events that might develop over time. Treatment-emergent adverse events such as gastrointestinal events, haematological toxicities, and fatigue are considered to be class effects, consistent with those of other PARP inhibitors.3,7,12,27-30 Treatment-emergent adverse events and laboratory abnormalities were managed with treatment interruption, treatment modification, or supportive care, such as antiemetic medications for nausea or vomiting or red blood cell transfusions for anaemia. The low incidence of discontinuations due to adverse events showed that management with supportive care and dose modifications was effective. The extended safety analysis showed that rucaparib had a tolerable safety profile.
Limitations of the current analysis include the fact that the study is ongoing, and long-term follow-up data continue to be collected. Furthermore, overall survival data are not yet mature; these data will be reported when approximately 70% of the events have occurred. Although efforts were made to maintain treatment blinding for the overall survival analysis, treatment unblinding was permitted upon investigator request if a decision regarding subsequent treatment depended on whether or not a patient had received previous PARP inhibitor therapy (eg, previous PARP inhibitor use was an exclusion criterion for a subsequent study); therefore, the process of unblinding might have influenced the final selection of subsequent therapy. Finally, CFI, TFST, PFS2, and TSST were prespecified, exploratory endpoints in ARIEL3; as such, the study was not formally powered to assess differences in these outcomes.
The significant improvement in the clinically meaningful endpoints of CFI, TFST, PFS2, and TSST observed in patients who received rucaparib maintenance treatment compared with those who received placebo provides additional support to the significant improvement in progression-free survival (the primary endpoint) observed with rucaparib versus placebo in ARIEL3. These improvements suggest that when compared with placebo, rucaparib maintenance treatment provided a meaningful delay in starting further therapy and did not affect the possibility of receiving benefit from subsequent therapies after first progression. As with the primary and key secondary efficacy endpoints, improvements in the post-progression endpoints were observed in the BRCA-mutant and HRD cohorts as well as in the ITT population. The updated rucaparib safety profile was consistent with previous reports, and no new safety signals were identified.
Supplementary Material
Research in context.
Evidence before this study
Few data are available on post-progression outcomes for women with recurrent platinum-sensitive ovarian carcinoma who have received poly(ADP-ribose) polymerase (PARP) inhibitor maintenance treatment. Post-progression outcomes can provide clinically meaningful information. Time to start of first subsequent therapy (TFST) can show a difference in the time before further therapy is started between patients who receive a PARP inhibitor and those who received placebo. Time to disease progression on the subsequent line of treatment or death (PFS2) can provide a snapshot of differences in post-progression outcomes to time to second progression, which can be of particular use when overall survival data are unavailable due to trial immaturity or confounded by long post-progression survival or crossover to other treatments.
A search of all PubMed articles published up to Sept 25, 2019, using the search terms (“PARP inhibitor” OR “rucaparib” OR “olaparib” OR “niraparib” OR “veliparib” OR “talazoparib”) AND (“ovarian” AND [“cancer” OR “carcinoma”]) AND “maintenance” with no language restrictions, identified 13 peer-reviewed publications covering trials of PARP inhibitor monotherapy as second-line maintenance treatment, of which only three provide post-progression outcomes data. Patients who received maintenance olaparib in Study 19 or SOLO2 (ie, those with a BRCA1 or BRCA2 [BRCA] mutation) had significantly longer TFST, time to start of second subsequent therapy (TSST), or PFS2 than those in the placebo group. In NOVA, median chemotherapy-free interval (CFI) and TFST were significantly longer with maintenance niraparib than placebo in patients with a germline BRCA mutation and patients without a germline BRCA mutation (this subgroup included patients with a somatic BRCA mutation).
Added value of this study
Our analyses include a comprehensive assessment of CFI, TFST, PFS2, and TSST post-progression outcomes for patients from ARIEL3. To our knowledge, we provide the first report of mature PFS2 data in this setting in an all-comer (ie, intention-to-treat [ITT]) population that includes patients without a BRCA mutation. The significant improvements observed in these post-progression outcomes support the progression-free survival benefit previously reported and the clinical benefit of rucaparib in the second-line maintenance setting.
Implications of all the available evidence
Evaluation of overall survival in clinical trials of ovarian cancer can be challenging given the long duration of post-progression survival, and can be confounded by highly effective subsequent treatments. Therefore, assessment of post-progression outcomes is important to show the clinical benefit of novel therapies, such as whether further anticancer therapies could be delayed and whether patients continue to derive benefit from subsequent therapies. Together, CFI, TFST, PFS2, and TSST provide a complementary and comprehensive assessment of post-progression outcomes following rucaparib maintenance treatment. Our data on these outcomes are consistent with those from other studies, further showing the clinical benefit of PARP inhibitors as second-line maintenance treatment for patients with ovarian cancer.
Acknowledgments
This study was funded by Clovis Oncology. Additional support was provided in part by the Ann Rife Cox Chair in Gynecology and the Judy Reis/Albert Pisani, MD, Ovarian Cancer Research Fund (to RLC) and NIHR Biomedical Research Centre at UCL (to JAL). CA is supported in part by the Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748. Funding was also provided by the US Department of Defense Ovarian Cancer Research Program OC120506, a V Foundation Translational Award, and a Stand Up To Cancer–Ovarian Cancer Research Fund Alliance–National Ovarian Cancer Coalition Dream Team Translational Research Grant (grant number SU2C-AACR-DT16–15; all to EMS). Stand Up To Cancer is a programme of the Entertainment Industry Foundation; research grants are administered by the American Association for Cancer Research, a scientific partner of Stand Up To Cancer. We thank all of the patients and their families and caregivers for their participation in ARIEL3 and the ARIEL3 investigators for their contributions to the administration and execution of the trial. Medical writing and editorial support funded by Clovis Oncology were provided by Nathan Yardley and Shannon Davis of Ashfield Healthcare Communications.
Footnotes
Declaration of interests
JAL has received lecture fees from Clovis Oncology, AstraZeneca, Merck Sharp & Dohme, Pfizer, and Tesaro; served on advisory boards for Clovis Oncology, Artios Pharma, AstraZeneca, Cristal Therapeutics, Merck Sharp & Dohme, Pfizer, Regeneron, Roche, Seattle Genetics, and Tesaro; and received research grants from AstraZeneca and Merck Sharp & Dohme. AMO has served on advisory boards for Clovis Oncology, Amgen, Immunovaccine, and Verastem; received support for travel or accommodation from AstraZeneca; and received honoraria from WebRx. DL has served in a consulting or advisory role for Clovis Oncology, AstraZeneca, ImmunoGen, Merck, PharmaMar, Roche, Takeda, and Tesaro, and received support for travel or accommodation from PharmaMar and Roche. CA has served on a steering committee for Clovis Oncology, AbbVie, Genentech, and Mateon Therapeutics; served on advisory boards for Clovis Oncology, Cerulean Pharma, Eisai/Merck, ImmunoGen, and Tesaro; received research grants from Clovis Oncology, AbbVie, AstraZeneca, and Genentech; and received honoraria from Clovis Oncology, Cerulean Pharma, Eisai/Merck, ImmunoGen, Mateon Therapeutics, and Tesaro. AO has served on advisory boards for Clovis Oncology, AstraZeneca, ImmunoGen, Genmab/Seattle Genetics, PharmaMar, Roche, and Tesaro; received support for travel or accommodation from Clovis Oncology, AstraZeneca, PharmaMar, and Roche; and received research grants from Clovis Oncology, AbbVie Deutschland, Ability Pharmaceuticals, Advaxis, Aeterna Zentaris, Amgen, Aprea Therapeutics, Bristol-Myers Squibb, Eisai, F Hoffmann-La Roche, Regeneron Pharmaceuticals, ImmunoGen, Merck Sharp & Dohme de Espana, Millennium Pharmaceuticals, PharmaMar, and Tesaro. AD has served in a consulting or advisory role for Precision Oncology Australia, Shire Pharmaceuticals, and Specialised Therapeutics Australia. NC has served in a consulting or advisory role for Clovis Oncology, AstraZeneca, BIOCAD, Pfizer, PharmaMar, Roche, and Tesaro. JIW has received research support from AbbVie and AstraZeneca and served on advisory boards for AstraZeneca. ARC has served on advisory boards for AstraZeneca; received research funding from Clovis Oncology and AstraZeneca; and received support for travel and accommodation for congress attendance from Clovis Oncology, AstraZeneca, and Roche. GS has served in a consulting or advisory role for Clovis Oncology, AstraZeneca, PharmaMar, Roche, and Tesaro. AL has served on advisory boards for Clovis Oncology, AstraZeneca, BIOCAD, GamaMabs, Genmab/Seattle Genetics, Merck Sharp & Dohme, Pfizer, PharmaMar, and Tesaro; received support for travel and accommodation from Clovis Oncology, AstraZeneca, Roche, and Tesaro; and reports institutional research grant support from Clovis Oncology, AstraZeneca, GamaMabs, Inivata, Merck Sharp & Dohme, Merus, Sanofi, and Tesaro. RWH has served on speakers bureaus for Clovis Oncology, AstraZeneca, and Tesaro. MAG has served on advisory boards for Clovis Oncology and on speakers bureaus for AstraZeneca, PharmaMar, and Roche. PCF has served on advisory boards for Clovis Oncology and AstraZeneca and received honoraria from AstraZeneca. JCG has served in a consulting or advisory role for AstraZeneca, Bristol-Myers Squibb and Tesaro; served on speakers bureaus for Ipsen and Merck Sharp & Dohme; and received support for travel or accommodation from Astellas, AstraZeneca, and Bristol-Myers Squibb. DMO has served on advisory boards for Clovis Oncology, AbbVie, AstraZeneca, Eisai, Genentech/Roche, Genelux, Iovance Biotherapeutics, Janssen, Novocure, Regeneron, and Tesaro; has served on steering committees for Clovis Oncology, Agenus, Amgen, and Novocure; has served as a consultant for AbbVie, Ambry, AstraZeneca, Genentech/Roche, Gynecologic Oncology Group Foundation, and Tesaro; has given a presentation on ovarian cancer at the National Comprehensive Cancer Network; and his institution has received research support from Clovis Oncology, AbbVie, Agenus, Amgen, Ajinomoto, Array BioPharma, AstraZeneca, Bristol-Myers Squibb, Cerulean Pharma, Eisai, EMD Serono, ERGOMED Clinical Research, Genentech, Gynecologic Oncology Group, INC Research, inVentiv Health Clinical, Iovance Biotherapeutics, Janssen Research and Development, Ludwig Institute for Cancer Research, New Mexico Cancer Care Alliance, Novocure, PRA International, Regeneron Pharmaceuticals, Serono, Stemcentrx, Tesaro, TRACON Pharmaceuticals, VentiRx, Yale University. DKA has served as a scientific advisor for Morphotek and received research funding from Clovis Oncology, Advaxis, AstraZeneca, Pfizer, Syndax, and Tesaro. SB has served on advisory boards and received honoraria from Clovis Oncology, AstraZeneca, PharmaMar, Seattle Genetics, and Tesaro; received honoraria from Merck Serono and Roche; and received support for travel or accommodation from NuCana and Tesaro. JG-D has received research funding from AstraZeneca, Pierre Fabre, and Pfizer; received personal fees from Clovis Oncology, Astellas, Pierre Fabre, and Pfizer; and received nonfinancial support from Astellas, Pierre Fabre, and Pfizer. EMS declares no competing interests. TC, LM, and SG are employees of Clovis Oncology and may own stock or have stock options in that company. RLC reports grants from Clovis Oncology, AstraZeneca, Gateway Foundation, Janssen, Judy Reis/Albert Pisani, MD, Ovarian Cancer Research Fund, Merck, National Institutes of Health, Roche/Genentech, and V-Foundation; has served as an advisor to Clovis Oncology, Agenus, AstraZeneca, GamaMabs, Genmab, Janssen, OncoQuest, Pfizer (Medivation), Regeneron, Roche/Genentech, and Tesaro; and has an endowment as the Ann Rife Cox Chair in Gynecology. All support from Clovis Oncology reported here was received outside of the submitted work or conduct of the study.
Data sharing
Requests for de-identified datasets for the results reported in this publication will be made available to qualified researchers following submission of a methodologically sound proposal to medinfo@clovisoncology.com. Data will be made available for such requests following online publication of this Article and for 1 year thereafter in compliance with applicable privacy laws, data protection, and requirements for consent and anonymisation. Data will be provided by Clovis Oncology. The redacted protocol for the ARIEL3 clinical study is available on ClinicalTrials.gov (NCT01968213). Clovis Oncology does not share identified participant data or a data dictionary.
Contributor Information
Jonathan A Ledermann, Department of Oncology, UCL Cancer Institute, University College London and UCL Hospitals, London, UK.
Amit M Oza, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Domenica Lorusso, Gynecologic Oncology Unit, Fondazione Policlinico Universitario A Gemelli IRCCS, Rome, Italy.
Carol Aghajanian, Gynecologic Medical Oncology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Ana Oaknin, Medical Oncology Department, Vall d’Hebron Institute of Oncology, Barcelona, Spain.
Andrew Dean, Oncology, St John of God Subiaco Hospital, Subiaco, WA, Australia.
Nicoletta Colombo, Gynecologic Cancer Program, University of Milan-Bicocca and European Institute of Oncology, Milan, Italy.
Johanne I Weberpals, Division of Gynecologic Oncology, Ottawa Hospital Research Institute, Ottawa, ON, Canada.
Andrew R Clamp, Department of Medical Oncology, The Christie NHS Foundation Trust and University of Manchester, Manchester, UK.
Giovanni Scambia, Gynecologic Oncology Unit, Fondazione Policlinico Universitario A Gemelli IRCCS, Rome, Italy.
Alexandra Leary, Gynecological Unit, Gustave Roussy Cancer Center, INSERM U981, and Groupe d’Investigateurs Nationaux pour l’Etude des Cancers Ovariens, Villejuif, France.
Robert W Holloway, Gynecologic Oncology, AdventHealth Cancer Institute, Orlando, FL, USA.
Margarita Amenedo Gancedo, Medical Oncology Department, Oncology Center of Galicia, La Coruña, Spain.
Peter C Fong, Medical Oncology Department, Auckland City Hospital, Grafton, Auckland, New Zealand.
Jeffrey C Goh, Department of Oncology, Cancer Care Services, Royal Brisbane and Women’s Hospital, Herston, QLD, Australia; Faculty of Medicine, University of Queensland, St Lucia, QLD, Australia.
David M O’Malley, Gynecologic Oncology, The Ohio State University, James Cancer Center, Columbus, OH, USA.
Deborah K Armstrong, Gynecology and Obstetrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Susana Banerjee, Gynaecology Unit, The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London, UK.
Jesus García-Donas, Division of Medical Oncology, HM Hospitales—Centro Integral Oncológico Hospital de Madrid Clara Campal, Madrid, Spain.
Elizabeth M Swisher, Division of Gynecologic Oncology, University of Washington, Seattle, WA, USA.
Terri Cameron, Clinical Science, Clovis Oncology UK, Cambridge, UK.
Lara Maloney, Clinical Development, Clovis Oncology, Boulder, CO, USA.
Sandra Goble, Biostatistics, Clovis Oncology, Boulder, CO, USA.
Robert L Coleman, Department of Gynecologic Oncology and Reproductive Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
References
- 1.Bouberhan S, Pujade-Lauraine E, Cannistra SA. Advances in the management of platinum-sensitive relapsed ovarian cancer. J Clin Oncol 2019; 37: 242–36. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 2012; 30: 2039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012; 366: 1382–92. [DOI] [PubMed] [Google Scholar]
- 4.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 2016; 375: 215–64. [DOI] [PubMed] [Google Scholar]
- 5.Coleman RL, Brady MF, Herzog TJ, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/ Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017; 18: 779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledermann JA, Embleton AC, Raja F, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 387: 1066–74. [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 1274–84. [DOI] [PubMed] [Google Scholar]
- 8.Markman M Maintenance chemotherapy in the management of epithelial ovarian cancer. Cancer Metastasis Rev 2015; 34: 11–17 [DOI] [PubMed] [Google Scholar]
- 9.DiSilvestro P, Alvarez Secord A. Maintenance treatment of recurrent ovarian cancer: is it ready for prime time? Cancer Treat Rev 2018; 69: 53–65. [DOI] [PubMed] [Google Scholar]
- 10.Wahlberg E, Karlberg T, Kouznetsova E, et al. Family-wide chemical profiling and structural analysis of PARP and tankyrase inhibitors. Nat Biotechnol 2012; 30: 283–88. [DOI] [PubMed] [Google Scholar]
- 11.Thomas HD, Calabrese CR, Batey MA, et al. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther 2007; 6: 945–56. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clovis Oncology. Rubraca (rucaparib) tablets. Prescribing information. Boulder, CO: Clovis Oncology, 2018. [Google Scholar]
- 14.Clovis Oncology. Rubraca (rucaparib) tablets. Summary of product characteristics. Swords, Ireland: Clovis Oncology Ireland, 2019. [Google Scholar]
- 15.Matulonis UA, Oza AM, Ho TW, Ledermann JA. Intermediate clinical endpoints: a bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 2015; 121: 1737–46. [DOI] [PubMed] [Google Scholar]
- 16.Wilson MK, Pujade-Lauraine E, Aoki D, et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recurrent disease. Ann Oncol 2017; 28: 727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joly F, Hilpert F, Okamoto A, Stuart G, Ochiai K, Friedlander M. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer 2017; 78: 133–38. [DOI] [PubMed] [Google Scholar]
- 18.Herzog TJ, Armstrong DK, Brady MF, et al. Ovarian cancer clinical trial endpoints: Society of Gynecologic Oncology white paper. Gynecol Oncol 2014; 132: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombo N, Sessa C, du Bois A, et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol 2019; 30: 672–705. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency. Evaluation of anticancer medicinal products in man. https://www.ema.europa.eu/en/evaluation-anticancer-medicinal-products-man (accessed Sept 25, 2019).
- 21.Rohr I, Keller M, Chekerov R, et al. What are the expectations and preferences of patients with ovarian cancer to a maintenance therapy? A NOGGO/ENGOT-OV22 survey (EXPRESSION IV) in 2101 patients. 2017. https://congress.esgo.org/media/2017/02/ESGO17-Program_in_Word_1.10.17pdf (accessed Sept 25, 2019). [DOI] [PubMed] [Google Scholar]
- 22.Mukamel DB, Wenzel L, Havrilesky LJ, et al. Variations in patient preferences over side effects of treatments for ovarian cancer: baseline results of a randomized controlled clinical trial. Gynecol Oncol 2017; 145 (suppl 1): 47 (abstr). [Google Scholar]
- 23.Hanker LC, Loibl S, Burchardi N, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol 2012; 23: 2605–12. [DOI] [PubMed] [Google Scholar]
- 24.Oronsky B, Carter CA, Reid TR, et al. Confirmatory trials in the evaluation of anticancer medicinal products in man—PFS2: a measure of therapeutic action-at-a-distance. Neoplasia 2015; 17: 716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahner S, Mirza MR, Moore K. ENGOT-OV16/NOVA: results of secondary efficacy endpoints of niraparib maintenance therapy in ovarian cancer. Society of Gynecologic Oncology SGO Annual Meeting on Women’s Cancer; National Harbor, MD, USA; March 12–15, 2017. https://sgo.confex.com/sgo/2017/meetingapp.cgi/Paper/8084 (accessed April 7, 2020). [Google Scholar]
- 26.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014; 15: 852–61. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol 2013; 14: 882–92. [DOI] [PubMed] [Google Scholar]
- 28.Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol 2016; 140: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015; 33: 24–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matulonis UA, Penson RT, Domchek SM, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multistudy analysis of response rates and safety. Ann Oncol 2016; 27: 1013–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.