Abstract
Rationale
Cocaine use disorder (CUD) is associated with dysregulation of the hypothalamic-pituitary-adrenal axis, which plays a critical role in the human stress response. Men and women with CUD differ in reactivity to social stressors. The hypothalamic neuropeptide oxytocin is involved in anxiolytic and natural reward processes, and has shown therapeutic potential for addictive disorders and stress reduction.
Objectives
To examine the impact of oxytocin (oxytocin (OXY) vs. placebo (PBO)) and gender (female (F) vs. male (M)) on response to a social stress task in individuals with CUD. To explore whether ovarian hormones moderate this stress response.
Methods
One hundred twelve adults with CUD were randomized to receive 40 IU intranasal oxytocin (n = 56) or matching placebo (n = 56). Forty minutes after drug administration, participants were exposed to a social stressor. Generalized linear mixed models were used to examine neuroendocrine (cortisol) and subjective (craving, stress) response at pre-stressor, stressor + 0, + 10, + 30, + 60 min.
Results
Gender moderated the effect of oxytocin on neuroendocrine response (p = 0.048); women receiving oxytocin (F + OXY) showed blunted cortisol response compared to the other three groups (F + PBO; M + OXY; M + PBO). There was a main effect of gender on subjective stress response; women reported greater stress following the stressor compared to men (p = 0.016). Oxytocin had no significant effect on craving or stress, and gender did not moderate the effect of oxytocin on either measure. Higher endogenous progesterone was associated with lower craving response in women (p = 0.033).
Conclusions
Oxytocin may have differential effects in men and women with CUD. Women may be at greater risk for relapse in response to social stressors, but ovarian hormones may attenuate this effect.
Keywords: Oxytocin, Cocaine, Cocaine use disorder, Stress, Cue-reactivity, Cortisol, Gender, Sex
Background
Stress has been consistently implicated in the development and maintenance of addictive disorders (Brady and Sinha 2005; Higgins and Marlatt 1975; Koob and Le Moal 2001). Negative reinforcement models of addiction posit that drug abuse is a coping strategy to reduce stress and alleviate withdrawal-related negative affect (Baker et al. 2004), while neurobiological models emphasize incentive sensitization and stress allostasis which are associated with neuroadaptations in reward, learning, and stress pathways, and, subsequently, to compulsive drug taking despite adverse consequences (Kalivas and Volkow 2005; Koob and Le Moal 1997; Robinson and Berridge 2003). Over the past several years, the role of stress in addiction has been well-studied, yet translational research leading to effective therapeutic interventions is limited. The neuropeptide oxytocin (OXY) has shown promise in animal models, with more mixed results in human studies (see Lee et al. 2016 for review). However, important gender differences in CUD, stress responding, and OXY expression (discussed below) suggest potential gender-based efficacy of OXY and underscore the importance of a priori gender-informed study design. The current study investigated the impact of OXY in men and women with cocaine use disorder (CUD). Primary outcomes included subjective stress and craving (informed by psychological models) and cortisol (informed by biological models).
Cocaine use is associated with dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which plays a key role in the autonomic stress response system. Laboratory studies of stress have reliably used cue-reactivity paradigms to elicit stress- and cue-induced physiological arousal and craving (Fox et al. 2008; McRae-Clark et al. 2011; Sinha et al. 2000), which is associated with relapse to cocaine (Paliwal et al. 2008; Weiss et al. 2003). For example, in human laboratory studies, stress-induced cocaine craving and cortisol response are associated with shorter time to relapse and greater quantity consumed during follow-up (Back et al. 2010; Sinha et al. 2006). Important gender differences in response to cue-reactivity paradigms have also been demonstrated. In one study of individuals with CUD, corticostriatal-limbic hyperactivity was associated differentially in response to stress cues in women and drug cues in men (Potenza et al. 2012). Likewise, in response to drug cues, CUD men and women both demonstrated a correlation between craving and peak stress, but only women demonstrated this association in response to a social stressor (Waldrop et al. 2010). In this same study, women showed a blunted cortisol response to a social stressor compared to men. These data suggest that women may be more susceptible to stress cues, while men may be more susceptible to drug cues.
Gender differences in the biological, behavioral, and clinical correlates of CUD are myriad. The most recent epidemiological data indicate that approximately five million adults have used cocaine in the past year, of which the majority (64%) are men (CBHSQ 2017). However, women display greater vulnerability to addiction, report more negative consequences, demonstrate worse treatment outcomes, and exhibit a telescoping effect where they progress more rapidly from initiation of cocaine use to CUD and treatment entry (Cotto et al. 2010; Hernandez-Avila et al. 2004; Kay et al. 2010; Lynch et al. 2002; Ridenour et al. 2005). Evidence from preclinical studies also show gender differences in CUD. Compared to males, female rats exhibit greater motivated cocaine seeking across all stages of addiction (Anker and Carroll 2010a, b, 2011) and show greater cocaine- and stress-primed reinstatement, while males show greater cued reinstatement (Anker and Carroll 2010a, b; Buffalari et al. 2012; Feltenstein et al. 2011). Despite a growing evidence base on gender differences in CUD and stress, research on gender-based interventions that target the stress system are scarce.
OXY is a hypothalamic neuropeptide that plays a critical role in complex social cognition and behavior. OXY is involved in anxiolytic and natural reward processes and plays an important role in neuroadaptations in stress, learning, and memory pathways due to chronic drug abuse (Lee et al. 2016). As such, OXY may demonstrate therapeutic effects through regulation of HPA and neuroendocrine function (de Oliveira et al. 2012; Heinrichs et al. 2003; Kosfeld et al. 2005). In preclinical studies, OXY has shown promise as a therapeutic agent for addiction and stress-related disorders. For example, OXY has been shown to attenuate the development of opioid tolerance and inhibit cocaine- and methamphetamine-induced drug-seeking behaviors, reward, and reinstatement in rodents (Sarnyai and Kovacs 2014). Clincial studies offer more mixed support. OXY has been shown to attenuate anticipatory anxiety, stress, and HPA axis activation (de Oliveira et al. 2012; Heinrichs et al. 2003; Kosfeld et al. 2005), attenuate stress-induced craving in cannabis users (McRae-Clark et al. 2011), reduce drug-induced craving in cigarette smokers (Miller et al. 2016), and attenuate craving and withdrawal symptoms in opioid-dependent men (Moeini et al. 2019). Yet in other human laboratory studies, OXY did not attenuate stress-induced craving, stress reactivity, smoking behavior, or subjective smoking response (e.g., satisfaction) in cigarette smokers (McClure et al. 2019; Van Hedger et al. 2020), and produced mixed gender effects in response to a social stress task in cannabis users (Reed et al. 2019). Additional evidence suggests sex differences in OXY expression in reward circuitry (Dumais et al. 2013), and sex hormones have been found to increase plasma OXY levels and OXY receptor binding (Borrow and Handa 2017). Together, these findings suggest that OXY may differentially affect stress responding in cocaine-dependent men and women.
The primary aim of the current study was to examine the effect of OXY on subjective and neuroendrocrine response to a social stress task in men and and women with CUD. We hypothesized that in individuals with CUD, OXY would have a greater attenuating effect on the stress response than placebo. Secondarily, we investigated whether gender moderated the effect of OXY on stress responding. Given evidence that women may be more susceptible to stress cues, we hypothesized that women given OXY would demonstrate an attenuated stress response compared to women receiving placebo and men. Exploratory analyses investigated how ovarian hormones, which have been shown to affect drug reward and stress response (Fox et al. 2013; Sofuoglu et al. 1999), and trauma, which is associated with dysregulated stress response (DeSantis et al. 2011; Newport et al. 2000), impact stress responding in individuals with CUD.
Methods
Participants
Participants took part in a three-day human laboratory study investigating the efficacy of OXY to reduce subjective and neuroendocrine response to stressors. The current analysis included data only from the social stress task component of the study (day 1); data from a neuroimaging paradigm conducted on days 2 and 3 have been reported elsewhere (Joseph et al. 2019). Individuals with CUD were recruited via local media advertisements over a 54-month period. Written informed consent was obtained prior to the baseline assessment. General exclusion criteria included (1) pregnancy, nursing, or plan to become pregnant during the course of the study; (2) women who had a complete hysterectomy, were post-menopausal, or receiving hormone replacement or hormonal contraceptive therapy; (3) history of or current significant hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal, or neurological diseases; (4) history of or current psychotic, panic, eating, or bipolar affective disorders; (5) current major depressive disorder and PTSD; (6) history of or current medical conditions that might affect HPA axis activity; (7) synthetic glucocorticoid or exogenous steroid therapy within 1 month of testing; (8) psychotropic medications (with the exception of selective serotonin reuptake inhibitors), opiates or opiate antagonists, benzodiazepines, antipsychotics, beta-blockers, and other medications that might interfere with HPA axis activity or physiologic measurements; (9) acute illness or fever; (10) Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for substance dependence except alcohol, nicotine, or marijuana within the past 60 days; or (11) unwillingness or inability to maintain abstinence from cocaine and other drugs of abuse (except nicotine) for 3 days prior to the cue-reactivity sessions. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki and received Institutional Review Board (IRB) approval.
Assessment
Participants who met pre-screening criteria were evaluated for study eligibility. The Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998) was used to assess current and lifetime psychiatric disorders, and the substance use module of the Structured Clinical Interview for DSM-IV (SCID-IV) was used to assess current and lifetime substance use disorders (First et al. 2002). The Time-Line Follow-Back (Sobell and Sobell 1992) assessed substance use in the 90 days prior to the study. A physical examination was conducted to assess for medical exclusions. Participants who met all inclusion criteria and no exclusion criteria were scheduled to complete the study procedures. They were instructed to abstain from cocaine and other drugs for at least 3 days prior to the test session.
Measures collected
Hormonal measures
Unstimulated passive saliva samples (passive drool method) were collected to measure cortisol, progesterone, and estradiol.
Cortisol
Cortisol was measured using the Salimetrics expanded range, high sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics®). This kit has a lower sensitivity level of < 0.003 μg/dl. The correlation between salivary and serum cortisol has been shown to be high (r = 0.91, p < 0.0001; Salimetrics® package insert) which is supported by existing literature (Dorn et al. 2007). Cortisol is commonly measured as an indicator of stress response in human laboratory studies.
Progesterone
Progesterone was measured using the Salimetrics salivary progesterone enzyme immunoassay kit. The lower limit of sensitivity is 5 pg/ml, and the correlation between serum and saliva is highly significant (r = 0.80, p < 0.0001).
Estradiol
Estradiol was measured using the Salimetrics 17β-estradiol enzyme immunoassay kit collected in EDTA tubes and processed using a chemiluminescent immunoassay. The lower limit of sensitivity is 0.1 pg/ml, and correlation with serum is high (r = 0.80, p < 0.001).
Subjective measures
A modified version of the Within Session Rating Scale was used to assess subjective ratings of craving and stress (Childress et al. 1986). This 0–10 visual analogue scale is anchored with the adjectival modifiers (“not at all,” “extremely”). The Childhood Trauma Questionnaire (Bernstein and Fink 1998) is a 25-item self-report measure used to assess the extent to which participants experienced childhood abuse and neglect. Responses are coded on a 5-point Likert scale ranging from 1 (never true) to 5 (very often true) and a total score of 37 or greater is used as a cut point to indicate the presence of childhood trauma.
Study procedures
General procedures
Participants were expected to remain abstinent from cocaine and other drugs for the 3-day period prior to the test session in order to minimize the impact of recent drug/alcohol use on stress reactivity. Participants were instructed to arrive at the Addiction Sciences Division (ASD) on the test day and to avoid caffeinated beverages. If the individual was nicotine-dependent, (s)he was provided with nicotine patches throughout their clinic stay. Upon arriving at the ASD, participants provided a urine sample and were breathalyzed. The urine sample was tested for the presence of amphetamines, d-methamphetamine, cocaine, opioids, THC, and benzodiazepines; if female, a urine pregnancy test was also administered. If the pregnancy and urine drug tests were negative (with the exception of THC), the session proceeded. A blood pressure cuff was then placed on the subject’s arm, and baseline subjective measures, physiologic measures (blood pressure and heart rate), and salivary progesterone, estradiol, and cortisol were collected. In the event of a positive drug screen, the laboratory visit was rescheduled.
Medication administration
Participants were administered 40 IUs of OXY nasal spray or matching placebo (saline spray) 40 min prior to study tasks. This dose was selected based on previous studies that have used similar doses of OXY (Ditzen et al. 2009; Heinrichs et al. 2003; Kubzansky et al. 2009), as well as our own previous pilot work (McRae-Clark et al. 2013; Sherman et al. 2017). Timing of administration was also based on our pilot work and previous studies showing central activity of OXY 40 min after intranasal administration (Heinrichs et al. 2009; McRae-Clark et al. 2013; Sherman et al. 2017). Intranasal OXY and matching placebo were compounded by the MUSC Investigational Drug Service. Randomization was done by the MUSC Investigational Drug Service, who kept a record of the blind. To achieve balance in sample size with respect to gender, a block randomized design with randomly varying block sizes was used.
Trier Social Stress Test
The Trier Social Stress Test (TSST) is a standardized psychological stress challenge which has been used extensively in research studies. A meta-analysis supports its utility in evoking an HPA axis stress response in a laboratory setting (Dickerson and Kemeny 2004). It has induced a robust and reliable physiological stress response in cocaine, marijuana, and alcohol-dependent individuals in our previous studies (McRae-Clark et al. 2011; McRae et al. 2006; Waldrop et al. 2010). When the TSST begins, the participant is told that (s)he will give a speech and perform an arithmetic task. The topic of the speech will be why (s)he should be hired for a particular job (the individual’s “dream job”) and the participant is given 5 min to prepare the speech. After 5 min, three individuals unfamiliar to the participant (the audience) enter the room and are seated; the individual is then instructed by one audience member to stand and begin his/her prepared speech (without notes). The speech is delivered for 5 min. If the individual pauses, (s)he will be instructed to continue. At the end of the speech task, the individual is instructed to serially subtract 13 from 1022 as quickly and accurately as possible. If they are incorrect, they are instructed to begin again. The mental math recitation continues for 5 min, at the end of which time the spokesperson instructs the individual to stop and be seated, and the audience leaves the room.
After initial study day procedures (see “General procedures”), participants were seated in the ASD testing room where they were allowed to read until the TSST procedures began. Participants were randomly assigned to receive study medication (intranasal OXY or placebo; see “Medication administration”) 40 min prior to the TSST. Additional baseline assessments of subjective stress and craving, and salivary cortisol were collected at 20 min pre-TSST and 5 min pre-TSST. Immediately following the TSST, these measures were repeated. Measurements were also obtained 10-, 30-, and 60-min post-TSST (see Table 1 for a detailed experimental timeline).
Table 1.
Experimental timeline. 11:30 am assessment is the true baseline prior to medication administration; all analyses were adjusted for this time point. 1:40 pm and 1:55 pm assessments were combined to create the pre-TSST time point used in all analyses. UDS urine drug screen, BP/HR blood pressure/heart rate, TSST Trier Social Stress Task
| 11:00 am | UDS, nicotine patch |
| 11:30 am | Saliva no. 1: progesterone/estradiol/cortisol, craving, stress |
| 12:00–12:30 pm | Lunch (record amount consumed) |
| 1:20 pm | Oxytocin or placebo administered |
| 1:40 pm | Saliva no. 2: cortisol, craving, stress |
| 1:55 pm | Saliva no. 3: cortisol, craving, stress |
| 2:00–2:15 pm | TSST |
| 2:15 pm | Saliva no. 4: cortisol, craving, stress |
| 2:25 pm | Saliva no. 5: cortisol, craving, stress |
| 2:45 pm | Saliva no. 6: cortisol, craving, stress |
| 3:15 pm | Saliva no. 7: cortisol, craving, stress |
| Assess for AEs, discharge |
Statistical analysis
Study participants were randomized to receive either active OXY or matching placebo prior to the test session. Demographic, clinical, and use history characteristics summarized by randomized treatment assignment were compared using chi-square test statistics for categorical data and Wilcoxon rank-sum test for continuous data. Baseline characteristics were assessed for bivariate associations with planned study outcomes as well as any modification of treatment effects. When evidence of a relationship existed (p < 0.1), these variables were included in the model building process. Longitudinal neuroendocrine and subjective responses to the TSST across treatment assignment were assessed using generalized linear mixed effect models adjusted for pre-medication levels of model outcomes. All post medication time points were included in the models (pre-TSST, TSST + 0, + 10, + 30, + 60) and comparisons of interest were assessed using treatment group level means and associated standard errors. Correlation structures were independently compared (first-order autoregressive, banded, and unstructured) and the final model structure was chosen using minimization of Akaike’s information criterion (AIC; Bozdogan 1987). Normality of model residuals was assessed using Q-Q plots and data were transformed to address residual skewness when necessary (cortisol values were log10 transformed prior to longitudinal anlaysis).
Further, to investigate the effects of ovarian hormones on response to the TSST in females, progesterone and estradiol levels were added to primary analytic models. Due to lack of linearity with both neuroendocrine and subjective responses, the relationships were assessed using categorization at the median values of each ovarian hormone (progesterone ≤ 106.8 pg/ml and estradiol ≤ 1.79 pg/ml are in the low group). Models investigating the relationship of hormones with study outcomes additionally controlled for participant age. All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc. Cary, NC, USA). Model results are noted as model-based means and associated standard errors. Non-significant interactions were removed from the final models. Significance was set at a two-sided p value of 0.05.
Results
Study participants
Demographic and clinical characteristics of the OXY and placebo randomized participants are shown in Table 2. One hundred twelve participants were randomized to receive either active OXY (n = 56) or matched placebo (n = 56). Participants were, on average, 42 years old (SD = 9.8), were primarily male (57%), and mostly non-white (72%). Of the 112 participants randomized, 75% were cigarette smokers (n = 84) who smoked an average of 11 cigarettes per day (SD = 6.6). Study participants averaged greater than 16 years of cocaine use (SD = 8.9) at study entry and used an average of 17.6 days per month (SD = 7.7). There were no baseline differences in demographics of cocaine use characteristics between participants randomized to OXY and placebo (p’s > 0.20). However, participants in the placebo group had a numerically higher rate of reported alcohol abuse or dependence as compared to the OXY group (30.4% vs. 19.6%; p = 0.19). Reported alcohol abuse or dependence was tested for confounding/effect modification in each outcome model and found to not significantly impact treatment or gender estimates. Subjective measures of craving and stress were similar across treatment groups at the pre-medication study baseline (p > 0.40); however, cortisol was numerically greater in the placebo group as compared to OXY (p = 0.051).
Table 2.
Demographics and clinical characteristics
| Overall | Oxytocin | Placebo | p valuea | |
|---|---|---|---|---|
| Demographics | N = 112 | n = 56 | n = 56 | |
| Age | 42.3 (9.8) | 42.3 (10.3) | 42.3 (9.3) | 0.972 |
| Male | 57.1 (64) | 58.9 (33) | 55.4 (31) | 0.703 |
| Caucasian | 26.8 (30) | 21.4 (12) | 32.1 (18) | 0.201 |
| Smoker | 75.0 (84) | 73.2 (41) | 80.4 (45) | 0.496 |
| CPD | 11.0 (6.6) | 11.2 (7.1) | 10.9 (6.2) | 0.872 |
| Alcohol abuse or dependence | 25.0 (28) | 19.6 (11) | 30.4 (17) | 0.190 |
| Cocaine use characteristics | ||||
| Age of first use | 21.4 (6.1) | 21.1 (5.5) | 21.7 (6.7) | 0.727 |
| Age of dependence onset | 29.2 (8.5) | 29.8 (8.0) | 28.7 (9.0) | 0.351 |
| Total years of use | 16.6 (8.9) | 16.5 (8.8) | 16.8 (9.0) | 0.784 |
| Using days per month | 17.6 (7.7) | 17.7 (7.4) | 17.5 (7.9) | 0.958 |
| Quantity per day ($) | 85.3 (83.3) | 82.7 (77.2) | 87.9 (89.7) | 0.942 |
| Past treatment | 46.0 (51) | 44.6 (25) | 47.3 (26) | 0.781 |
| Physiologic and subjective variables | ||||
| Pre-medication | ||||
| Craving | 3.5 (3.0) | 3.6 (2.8) | 3.4 (3.1) | 0.463 |
| Stress | 3.1 (3.2) | 3.0 (3.1) | 3.1 (3.4) | 0.959 |
| Anxiety | 3.3 (3.2) | 3.3 (3.2) | 3.3 (3.2) | 0.979 |
| Cortisol | 0.34 (0.53) | 0.29 (0.21) | 0.38 (0.72) | 0.051 |
| Post-medication | ||||
| Craving | 2.8 (2.9) | 2.9 (2.9) | 2.8 (3.0) | 0.929 |
| Stress | 2.3 (2.7) | 2.1 (2.6) | 2.4 (2.9) | 0.773 |
| Anxiety | 2.7 (2.7) | 2.8 (2.8) | 2.5 (2.7) | 0.465 |
| Cortisol | 0.24 (0.31) | 0.21 (0.11) | 0.28 (0.42) | 0.133 |
CPD cigarettes per day
Demographic and clinical characteristics were compared across gender using a t test statistic for continuous data and a chi-squared test statistic for categorical data
Effects of OXY and gender on stress response to the TSST
Cortisol
Prior to study drug administration, cortisol levels in the group randomized to OXY were numerically greater than those randomized to placebo (see Table 2: p = 0.051) and persisted following study drug administration. A significant increase in the initial cortisol response to the TSST was noted from the pre-TSST time point to TSST + 10 time point (t95 = 2.6; p = 0.012). This was primarily driven by a positive response in the placebo-treated participants (β = 0.10, SEM = 0.04; t95 = 2.45; p = 0.016) that was attenuated in the OXY-treated participants (β =0.05, SEM = 0.04; t95 = 1.20; p = 0.23). There was no differential effect of treatment on the complete response profile over time (pre-TSST to TSST + 60; treatment × time interaction; F4,95 = 0.22; p = 0.92). However, the effect of OXY on the complete cortisol response profile was moderated by gender with female OXY participants having a blunted cortisol response to the TSST as compared to the other groups (treatment × time × gender interaction; F4,93 = 2.50; p = 0.048). All cortisol models included randomized treatment assignment (treatment), measurement time point (time), baseline cortisol values (pre-medication), gender, and the interaction between gender and study treatment (see Fig. 1a for results).
Fig. 1.
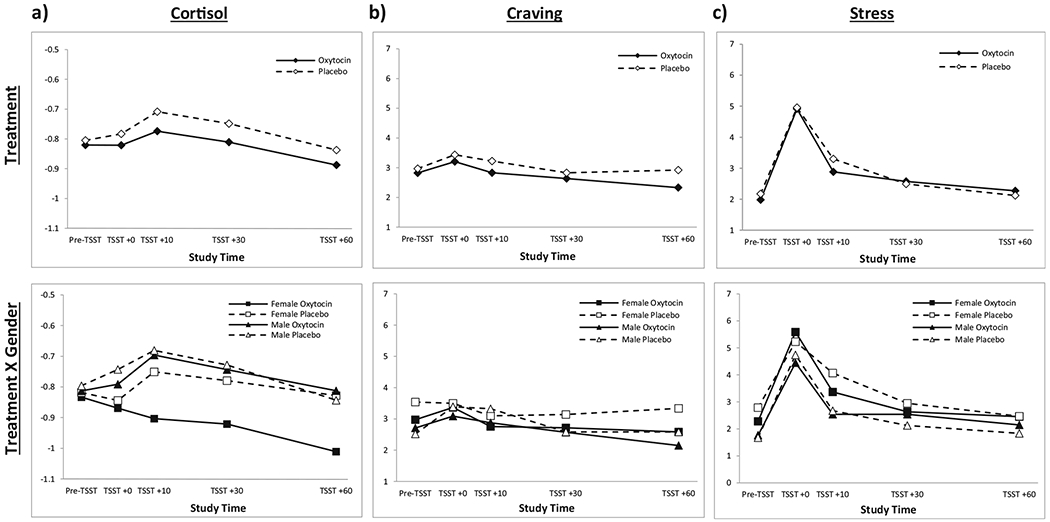
Physiological and subjective response to Trier Social Stress Task (TSST). a Significant increase in initial cortisol response from pre-TSST to TSST + 10 (t95 = 2.6; p = 0.012), driven by elevated response in placebo group (β = 0.10, SEM = 0.04; t95 = 2.45; p = 0.016) and attenuated response in OXY group (β = 0.05, SEM = 0.04; t95 = 1.20; p = 0.23). No significant treatment × time interaction for the complete response profile (F4,95 = 0.22; p = 0.92). Treatment × time × gender interaction was significant (F4,93 = 2.50; p = 0.048) with female OXY participants having a blunted cortisol response compared to the other groups. b No significant effect of OXY on initial craving response to the TSST (OXY: β = 0.38, SEM = 0.33; t109 = 1.14; p = 0.26; placebo: β = 0.46, SEM = 0.33; t109 = 1.39; p = 0.17) or on post-TSST response profile (F4,109 = 0.79; p = 0.53). Treatment × gender interaction was also not significant (F4,107 = 0.39; p = 0.82). c Initial stress response to the TSST was significant in both the OXY- and placebo-treated participants (oxytocin: β = 2.9, SEM = 0.0.5; t109 = 6.35; p < 0.001; placebo: β =2.8, SEM = 0.5; t109 = 6.04; p < 0.001). No significant effect of OXY on the post-TSST stress response profile (treatment × time, F4,109 = 0.69; p = 0.60). Significant main effect of gender on stress response (F1,108 = 6.05; p = 0.016) such that women had greater stress response, but no interaction of gender × treatment (F4,107 = 0.54; p = 0.70)
Craving
Initial craving response to the TSST was insignificant in both the OXY- and placebo-treated participants (OXY: β = 0.38, SEM = 0.33; t109 = 1.14; p = 0.26; placebo: β = 0.46, SEM = 0.33; t109 = 1.39; p = 0.17) and there was no significant effect of OXY on the post TSST craving response profile (F4,109 = 0.79; p = 0.53). Further, the OXY effect on craving response to the TSST was not modified by gender (F4,107 = 0.39; p = 0.82) (see Fig. 1b for results).
Subjective stress
Initial stress response to the TSST was significant in both the OXY- and placebo-treated participants (oxytocin: β = 2.9, SEM = 0.0.5; t109 = 6.35; p < 0.001; placebo: β = 2.8, SEM = 0.5; t109 = 6.04; p < 0.001). However, there was no significant effect of OXY on the post TSST stress response profile (F4,109 = 0.69; p = 0.60). Although female participants had a greater stress response to the TSST as compared to men (F1,108 = 6.05; p = 0.016), gender did not modify the effect of OXY on the stress response profile (F4,107 = 0.54; p = 0.70) (see Fig. 1c for results).
Effect of ovarian hormones on stress response to TSST
Cortisol
There was no significant effect of progesterone on mean cortisol response following the TSST (F1,33 = 0.86; p = 0.36), and progesterone did not moderate cortisol response across treatment assignment over time (treatment × progesterone × time; F4,133 = 0.79; p = 0.54). Similarly, there was no significant effect of estradiol on cortisol response following the TSST (F1,22 = 0.6; p = 0.45), nor did estradiol moderate cortisol response across treatment assignment over time (treatment × estradiol × time; F4,92 = 1.1; p = 0.38) (see Fig. 2a for results).
Fig. 2.
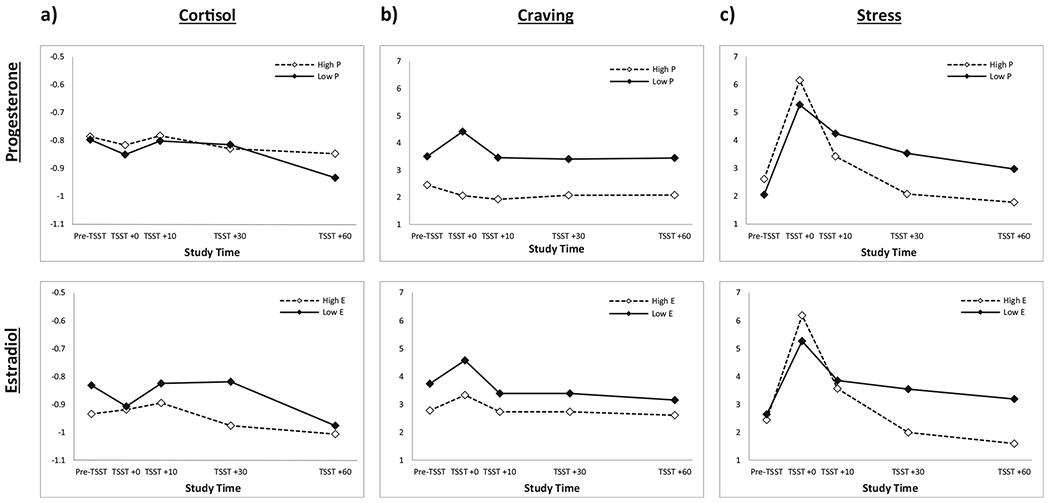
a No main effect of progesterone on cortisol response following the TSST (F1,133 = 0.86; p = 0.36), and treatment × progesterone × time interaction was also not significant (F4,133 = 0.79; p = 0.54). No main effect of estradiol on cortisol response (F1,22 = 0.6; p = 0.45), and treatment × estradiol × time interaction was not significant (F4,92 = 1.1; p = 0.38). b Significant main effect of progesterone on craving response to the TSST (F1,37 = 15.1; p <0.001); women with high progesterone showed blunted subjective craving compared to women with low progesterone. Treatment × progesterone × time interaction was not significant (F4,152 = 0.88; p = 0.48). No significant effect of estradiol on craving response (F1,28 = 4.0, p = 0.055) and treatment × estradiol × time interaction was also not significant (F4,116 = 0.47; p = 0.76). c No significant effect of progesterone (F1,37 = 0.69, p = 0.41) or estradiol (F1,28 = 1.36, p = 0.25) on subjective stress response during the TSST. Neither treatment × progesterone × time interaction (F4,152 = 2.3; p = 0.06) nor treatment × estradiol × time interaction (F4,116 = 2.1; p = 0.09) were significant
Craving
There was a significant effect of progesterone on craving response to the TSST (F1,37 = 15.1; p < 0.001); women with high progesterone showed blunted subjective craving following the TSST compared to women with low progesterone. Progesterone did not moderate the craving response across treatment assignment over time (treatment × progesterone × time; F4,152 = 0.88; p = 0.48). Although not statistically significant, estradiol showed a trend-level effect on craving response to the TSST whereby high estradiol was associated with blunted craving response (F1,28 = 4.0, p = 0.055). Estradiol did not moderate craving response to the TSST over time (treatment × estradiol × time; F4,116 = 0.47; p = 0.76) (see Fig. 2b for results).
Subjective stress
There was no significant effect of progesterone (F1,37 = 0.69, p = 0.41) or estradiol (F1,28 = 1.36, p = 0.25) on subjective stress response during the TSST (Fig. 2c). Although not reaching the level of statistical significance, progesterone levels may moderate the effect of OXY on stress reponse to the TSST (treatment × progesterone × time; F4,152 = 2.3; p = 0.06). Stratified models show that stress response to the TSST in particpants receiving placebo were similar over time (progesterone × time; F4,76 = 1.4; p = 0.24) while initial stress response to the TSST is elevated in OXY participants with high progesterone (progesterone × time; F4,76 = 2.6; p = 0.045). Similarly, estradiol levels may moderate the effect of OXY on stress response to the TSST (treatment × progesterone × time; F4,116 = 2.1; p = 0.09). Stratified models show that stress response to the TSST in particpants receiving placebo were similar over time (estradiol × time; F4,56 = 0.9; p = 0.49) while initial stress response to the TSST is elevated in oxytocin participants with high estradiol (estradiol × time; F4,60 = 2.9; p = 0.029).
Effects of childhood trauma on stress response to the TSST
Cortisol
Immediately following study drug administration and prior to the TSST, there was a significant difference in cortisol levels across treatment assignment and trauma level (pre-TSST: treatment × trauma level; t80 = 2.0; p = 0.048) whereby the placebo-treated, trauma-positive group had depressed cortisol levels as compared to the other three groups (oxytocin/trauma-positive, oxytocin/trauma-negative, and placebo/trauma-negative). These differences persisted throughout the post medication/TSST response (treatment × trauma group: f1,83 = 4.1, p = 0.048). There was no effect of gender on the trauma response differential across treatment assignment (treatment × trauma group × gender: f1,79 = 0.58, p = 0.45).
Craving and stress
The presence of childhood trauma did not modify the relationship between OXY and craving (treatment × trauma group: F1,87 = 0.40; p = 0.53) and stress response to the TSST (treatment × trauma group: F1,87 = 0.05; p = 0.82). Similarly, participant gender did not further modify the effect of OXY/trauma on craving (treatment × trauma group × gender: F4,84 = 0.27; p = 0.90) or stress (treatment × trauma group × gender: F4,84 = 1.1; p = 0.37).
Discussion
The current study investigated the effect of OXY on stress responding in individuals with CUD. Specifically, this human laboratory study tested whether OXY would attenuate neuroendrocine and subjective stress response to a social stress task, and whether participant gender, childhood trauma, and ovarian hormone levels in women would modulate this effect. Findings partially supported study hypotheses. Compared to placebo, OXY attenuated the initial peak cortisol response from pre-TSST to 10-min post-TSST. This effect was modulated over the whole 60-min profile by participant gender such that women in the OXY group showed blunted cortisol response compared to placebo women and both male groups. Subjective stress and craving were not impacted by OXY; however, on average, women showed a greater stress response compared to men. Lastly, ovarian hormone levels in women were not associated with cortisol or subjective stress response profiles, but high progesterone was associated with lower craving response to the TSST.
Gender differences in stress responding and the potential utility of OXY as a pharmacotherapy for CUD have been investigated independently, but to our knowledge, this is the first study to investigate, a priori, gender effects of OXY on stress responding. Gender moderated the effect of OXY on cortisol response such that women on OXY showed an attenuated cortisol response compared to the other three groups. In contrast, women demonstrated a greater overall subjective stress response compared to men. These findings replicate and extend existing evidence suggesting that women may be more vulnerable to stress cues (Potenza et al. 2012; Waldrop et al. 2010), and that OXY may differentially affect stress and drug-cued responding in a sex-specific manner (Leong et al. 2016; for review see Love 2018). Results from the present study extend these bodies of evidence and illustrate two critical points. First, there appears to be a decoupling of neuroendrocrine and subjective stress responding in cocaine-dependent women, and second, that OXY may be more effective at targeting underlying biomarkers of addiction (i.e., HPA axis dysfunction) rather than subjective experiences of stress. Previous studies have demonstrated a decoupling of subjective and biologic response in alcohol-dependent women (Brady et al. 2006); this inconsistency between biologic and subjective response was associated with shorter time to relapse. The decoupling of subjective versus biological stress responding has also been found in healthy men who showed no effect of OXY on cortisol in response to a social stress task, but did show an initial increase in subjective response (Eckstein et al. 2014). Second, in regard to biomarkers, OXY has been shown to modulate HPA axis function (Heinrichs et al. 2003) and to differentially affect amygdala reactivity to fearful faces in PTSD (Flanagan et al. 2019). Together these data underscore the importance of developing not just gender-specific, but stress response-specific treatments. That is, while OXY may hold therapeutic potential for reducing HPA axis reactivity, alternate approaches (e.g., psychosocial interventions) may be needed to effectively target subjective reactivity (i.e., subjective stress and craving).
While OXY did not impact the full stress response profile in our paradigm, it did attenuate initial cortisol response. This initial effect was not found on subjective measures. As discussed above, this may reflect greater efficacy of OXY on biological stress response (i.e., cortisol), particularly during the immediate aftermath of a stressor. While there is evidence showing an association between OXY and subjective reactivity, these studies vary in design and population making it difficult to draw firm conclusions. For example, some studies administered 40 IUs of exogenous OXY to cannabis users 40 min prior to laboratory procedures (McRae-Clark et al. 2011), while other studies administered 20 IUs to cigarette smokers 20 min prior to lab procedures (Miller et al. 2016). The effect and subsequent utility of OXY may vary depending on administration timing and dosage, substance of abuse, and stress-response type (i.e., biological vs. subjective).
Trauma history has been implicated in maladaptive stress responding and CUD, and was therefore included in our analyses. Although trauma did not emerge as a significant predictor of stress response in the current study, early trauma exposure did appear to modulate tonic HPA function. Among trauma-positive participants, those receiving OXY showed higher cortisol levels prior to the stress task compared to those on placebo. As childhood trauma has been linked with blunted tonic cortisol levels in cocaine-dependent adults (Roy 2002), our findings suggest that OXY may in fact restore hypothalamic tone in cocaine-dependent individuals with early-life trauma history. It appears OXY may differentially affect tonic vs. cue-induced HPA function in trauma-exposed individuals. One of our earlier studies showed that childhood adversity modulated the effect of OXY on cortisol levels following the same TSST paradigm (Flanagan et al. 2015), while others have found that OXY attenuated amygdala activity in response to emotional stimuli in adults with PTSD (Flanagan et al. 2019; Koch et al. 2016; Labuschagne et al. 2010) and in response to drug stimuli in men with CUD and a history of childhood trauma (Joseph et al. 2019). In aggregate, evidence suggests an important relationship between trauma, cocaine use, and the role of OXY in modulating HPA function underscoring a need for additional research in this area.
Finally, there is a line of evidence suggesting that women may be more susceptible to stress cues, while men may be more susceptible to drug cues (Potenza et al. 2012; Waldrop et al. 2010). Findings from the current study, as well as previously published results from days 2–3 of the parent study (Joseph et al. 2019), further support this finding and suggest OXY may differentially benefit men and women depending on cue type. In the current study (day 1), the ameliorative effects of OXY on cortisol response to a social stressor were seen only in women, while in the drug cue exposure paradigm (days 2–3), OXY only demonstrated ameliorative effects in men as evidenced by attenuated amygdala activity. Other studies have found no effect of OXY on cortisol response in men, although they did report elevated subjective response (Eckstein et al. 2014); however, there was no female comparison group in this study. When considering OXY as a therapeutic agent for substance use disorders, it is important to consider gender, cue type (i.e., stress vs. drug), and stress response target (i.e., biological vs. subjective).
Exploratory analysis also revealed an association between ovarian hormones and subjective response to the TSST. Women with higher progesterone levels demonstrated lower craving during the TSST paradigm compared to women with lower progesterone levels. Estradiol, while not statistically significant, showed a similar pattern whereby elevated levels were associated with trend-level reductions in craving compared to lower levels. OXY did not moderate the associations between ovarian hormones and craving or cortisol, and while OXY showed trend-level moderation of both progesterone and estradiol on subjective stress response, these effects were not statistically significant. These findings suggest that progesterone, and possibly estradiol, may mitigate stress-induced cocaine craving and relapse in CUD women. While these findings are preliminary given the study was not powered to test hormone effects, they are supported by existing literature illustrating protective effects of progesterone in cocaine- and nicotine-dependent individuals (Fox et al. 2013; Sofuoglu et al. 2001, 2011). Investigations that are powered to test hormone effects may further elucidate the interaction of ovarian hormones and OXY on stress responding in cocaine-dependent women.
Study findings should be interpreted in light of certain limitations. Only one measure of HPA function was assessed in the study, thus limiting our ability to identify other potential biotargets. Continuous measurement of heart rate, blood pressure, and skin conductance could provide a deeper understanding of how OXY effects biological stress response. Second, inclusion of additional cue types, for example, drug cues or personalized imagery, would also offer direct comparison of social stress with other types of stimuli that may precipitate a relapse. Third, the study was not powered to test the interaction of ovarian hormones with OXY. Our findings support existing evidence on the protective effects of progestrerone, but in light of the greater overall stress response in women, further investigation on the interaction of ovarian hormones and OXY is warranted. Finally, we did not include a healthy control group which would have allowed for direct comparison of stress responding in adults with CUD versus healthy controls. Despite these limitations, the current study used randomized, placebo-controlled, double-blind procedures and gender-targeted recruitment to achieve approximately a 50:50 gender split to investigate gender differences, which provides grounds for making firm statistical inferences.
Additional potential limitations regarding the experimental and clinical utility of intransal OXY administration should be considered. Variability in dose delivery can be influenced by several factors, including anatomical variations of the nasal cavity, nasal mucosa state, and method of spray application (Guastella et al. 2013). Peripheral OXY levels were not collected in this study. A high degree of heterogeneity exists between peripheral and central OXY concentrations, although a meta-analysis did report a significant association in these measures after intranasal OXY administration and after experimental stress induction (Valstad et al. 2017). Finally, the relatively short duration of intranasally delivered OXY effects may limit clinical applications as a therapeutic agent (Gossen et al. 2012).
Conclusions
CUD is a major pubic health concern. Stress has been identified as an important treatment target as chronic cocaine use results in dysregulated HPA axis function. Our findings suggest that OXY may be useful in targeting the acute stress response in women, specifically by regulating HPA axis function. In addition, as other evidence suggests, women may be at increased risk for stress-induced relapse, although this may be mitigated by ovarian hormone levels. Finally, the decoupling of neuroendrocrine and subjective responding in women underscores the need for a better understanding of the stress system and its component parts in men and women. Enhancing precision medicine may involve targeting specific components of stress response with a combination of pharmacotherapy and psychosocial interventions.
Funding information
This study was sponsored by a National Institute on Drug Abuse grants P50DA016511 (Brady), U54DA016511 (McRae-Clark), K23DA045099 (Sherman), and K24DA038240 (McRae-Clark), with additional support from the National Center for Advancing Translational Sciences grant UL1TR001450 (Brady).
Footnotes
Compliance with ethical standards Written informed consent was obtained prior to the baseline assessment. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki and received Institutional Review Board (IRB) approval.
Conflict of interest The authors declare that they have no conflicts of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anker JJ, Carroll ME (2010a) Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology 208(2):211–222. 10.1007/s00213-009-1721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2010b) The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev 35(2):315–333. 10.1016/j.neubiorev.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96. 10.1007/7854_2010_93 [DOI] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT (2010) Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend 106(1):21–27. 10.1016/j.drugalcdep.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004) Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 111(1):33–51. 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L (1998) Childhood Trauma Questionnaire: a retrospective self-report manual. The Psychological Corporation, San Antonio [Google Scholar]
- Borrow AP, Handa RJ (2017) Estrogen receptors modulation of anxiety-like behavior. Vitam Horm 103:27–52. 10.1016/bs.vh.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdogan H (1987) Model selection and Akaike’s Information Criterion (AIC): The general theory and its analytical extensions. Psychometrika 52:345–370. 10.1007/BF02294361 [DOI] [Google Scholar]
- Brady KT, Sinha R (2005) Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry 162(8):1483–1493. 10.1176/appi.ajp.162.8.1483 [DOI] [PubMed] [Google Scholar]
- Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK (2006) Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clin Exp Res 30(6):938–946. 10.1111/j.1530-0277.2006.00097.x [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE (2012) Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav 105(2):209–214. 10.1016/j.physbeh.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBHSQ (2017) Key substance use and mental health indicators in the United States: results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP (1986) Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict 81(5):655–660 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3466632 [DOI] [PubMed] [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SR (2010) Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gend Med 7(5):402–413. 10.1016/j.genm.2010.09.004 [DOI] [PubMed] [Google Scholar]
- de Oliveira DC, Zuardi AW, Graeff FG, Queiroz RH, Crippa JA (2012) Anxiolytic-like effect of oxytocin in the simulated public speaking test. J Psychopharmacol 26(4):497–504. 10.1177/0269881111400642 [DOI] [PubMed] [Google Scholar]
- DeSantis SM, Baker NL, Back SE, Spratt E, Ciolino JD, Moran-Santa Maria M, Dipankar B, Brady KT (2011) Gender differences in the effect of early life trauma on hypothalamic-pituitary-adrenal axis functioning. Depress Anxiety 28(5):383–392. 10.1002/da.20795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME (2004) Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull 130(3):355–391. 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M (2009) Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry 65(9):728–731. 10.1016/j.biopsych.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Dorn LD, Lucke JF, Loucks TL, Berga SL (2007) Salivary cortisol reflects serum cortisol: analysis of circadian profiles. Ann Clin Biochem 44(Pt 3):281 – 284. 10.1258/000456307780480954 [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013) Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex-specific ways. Horm Behav 64(4):693–701. 10.1016/j.yhbeh.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Eckstein M, Scheele D, Weber K, Stoffel-Wagner B, Maier W, Hurlemann R (2014) Oxytocin facilitates the sensation of social stress. Hum Brain Mapp 35(9):4741–4750. 10.1002/hbm.22508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE (2011) Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology 216(1):53–62. 10.1007/s00213-011-2187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition. Retrieved from [Google Scholar]
- Flanagan JC, Baker NL, McRae-Clark AL, Brady KT, Moran-Santa Maria MM (2015) Effects of adverse childhood experiences on the association between intranasal oxytocin and social stress reactivity among individuals with cocaine dependence. Psychiatry Res 229(1–2):94–100. 10.1016/j.psychres.2015.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Sippel LM, Santa Maria MMM, Hartwell KJ, Brady KT, Joseph JE (2019) Impact of oxytocin on the neural correlates of fearful face processing in PTSD related to childhood trauma. Eur J Psychotraumatol 10(1):1606626. 10.1080/20008198.2019.1606626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R (2008) Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology 33(4):796—805. 10.1038/sj.npp.1301470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R (2013) The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology 38(9):1532–1544. 10.1016/j.psyneuen.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Grander G, Spreckelmeyer KN (2012) Oxytocin plasma concentrations after single intranasal oxytocin administration—a study in healthy men. Neuropeptides 46(5):211–215. 10.1016/j.npep.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB (2013) Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 38(5):612—625. 10.1016/j.psyneuen.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R et al. (2000) Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284(5):592–597 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U (2003) Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry 54(12): 1389–1398 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14675803 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G (2009) Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol 30(4):548–557. 10.1016/j.yfrne.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004) Opioid-, cannabis- and alcohol-dependent women show more rapid progresssion to substance abuse treatment. Drug Alcohol Depend 74(3):265–272. 10.1016/j.drugalcdep.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA (1975) Fear of interpersonal evaluation as a determinant of alcohol consumption in male social drinkers. J Abnorm Psychol 84(6):644–651. 10.1037//0021-843x.84.6.644 [DOI] [PubMed] [Google Scholar]
- Joseph JE, McRae-Clark A, Sherman BJ, Baker NL, Moran-Santa Maria M, Brady KT (2019) Neural correlates of oxytocin and cue reactivity in cocaine-dependent men and women with and without childhood trauma. Psychopharmacology. 10.1007/s00213-019-05360-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162(8):1403–1413. 10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- Kay A, Taylor TE, Barthwell AG, Wichelecki J, Leopold V (2010) Substance use and women’s health. J Addict Dis 29(2):139–163. 10.1080/10550881003684640 [DOI] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2016) Intranasal oxytocin administration dampens amygdala reactivity towards emotional faces in male and female PTSD patients. Neuropsychopharmacology 41(6):1495–1504. 10.1038/npp.2015.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278(5335):52–58. 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24(2):97–129. 10.1016/S0893-133X(00)00195-0 [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E (2005) Oxytocin increases trust in humans. Nature 435(7042):673–676. 10.1038/nature03701 [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton A, Block J, Adler GK (2009) Protocol for an experimental investigation of the roles of oxytocin and social support in neuroendocrine, cardiovascular, and subjective responses to stress across age and gender. BMC Public Health 9: 481. 10.1186/1471-2458-9-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ (2010) Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35(12):2403–2413. 10.1038/npp.2010.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, Leggio L (2016) Targeting the oxytocin system to treat addictive disorders: rationale and progress to date. CNS Drugs 30(2):109–123. 10.1007/s40263-016-0313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM (2018). The impact of oxytocin on stress: the role of sex. Current opinion in behavioral sciences 23:136–142. 10.1016/j.cobeha.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164(2):121–137. 10.1007/s00213-002-1183-2 [DOI] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Gray KM, Hood CO, Tomko RL, Carpenter MJ, Ramakrishnan VR, Buchanan CJ, Saladin ME (2019) The influence of gender and oxytocin on stress reactivity, cigarette craving, and smoking in a randomized, placebo-controlled laboratory relapse paradigm. Psychopharmacology 237:543–555. 10.1007/s00213-019-05392-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae AL, Brady KT, Carter RE (2006) Buspirone for treatment of marijuana dependence: a pilot study. Am J Addict 15(5):404. 10.1080/10550490600860635 [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, Nicholas K, Brady KT (2011) Stress- and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology 218(1):49–58. 10.1007/s00213-011-2376-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, Brady KT (2013) Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology 228(4):623–631. 10.1007/s00213-013-3062-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Bershad A, King AC, Lee R, de Wit H (2016) Intranasal oxytocin dampens cue-elicited cigarette craving in daily smokers: a pilot study. Behav Pharmacol 27(8):697–703. 10.1097/FBP.0000000000000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeini M, Omidi A, Sehat M, Banafshe HR (2019) The effects of oxytocin on withdrawal, craving and stress response in heroin-dependent patients: a randomized, double-blind clinical trial. Eur Addict Res 25(1):41–47. 10.1159/000496194 [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R (2008) Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend 93(3):252–259. 10.1016/j.drugalcdep.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R (2012) Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry 169(4):406–414. 10.1176/appi.ajp.2011.11020289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Haney M, Manubay J, Campagna BR, Reed B, Foltin RW, Evans SM (2019) Sex differences in stress reactivity after intranasal oxytocin in recreational cannabis users. Pharmacol Biochem Behav 176:72–82. 10.1016/j.pbb.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Maldonado-Molina M, Compton WM, Spitznagel EL, Cottler LB (2005) Factors associated with the transition from abuse to dependence among substance abusers: implications for a measure of addictive liability. Drug Alcohol Depend 80(1): 1–14. 10.1016/j.drugalcdep.2005.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2003) Addiction. Annu Rev Psychol 54:25–53. 10.1146/annurev.psych.54.101601.145237 [DOI] [PubMed] [Google Scholar]
- Roy A (2002) Urinary free cortisol and childhood trauma in cocaine dependent adults. J Psychiatr Res 36(3):173–177. 10.1016/s0022-3956(02)00002-x [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL (2014) Oxytocin in learning and addiction: from early discoveries to the present. Pharmacol Biochem Behav 119:3–9. 10.1016/j.pbb.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–33 quiz 34-57 [PubMed] [Google Scholar]
- Sherman BJ, Baker NL, McRae-Clark AL (2017) Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervenetion. Psychiatry Res 249:318–320. 10.1016/j.psychres.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS (2000) Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152(2):140–148. 10.1007/s002130000499 [DOI] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ (2006) Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63(3):324–331. 10.1001/archpsyc.63.3.324 [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP (eds) Measuring alcohol consumption: psychosocial and biomedical methods. Humana Press, Totawa, pp 41–72 [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 7(3): 274–283 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK (2001) Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav 69(1–2): 299–304 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11420098 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Mooney M (2011) Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology 36(1):123–132. 10.1016/j.psyneuen.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT, Quintana DS (2017) The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci Biobehav Rev 78:117–124. 10.1016/j.neubiorev.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Van Hedger K, Bershad AK, Lee R, de Wit H (2020) Effects of intranasal oxytocin on stress-induced cigarette craving in daily smokers. Nicotine Tob Res 22(1):89–95. 10.1093/ntr/nty159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop AE, Price KL, Desantis SM, Simpson AN, Back SE, McRae AL, Brady KT (2010) Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology 35(6):798–806. 10.1016/j.psyneuen.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]


