Pre-exposure prophylaxis (PrEP) for HIV prevention is highly effective and well-tolerated among groups at high risk for HIV infection, including young men who have sex with men (YMSM; Centers for Disease Control and Prevention, 2017; Hosek et al., 2017; Volk et al., 2015). However, uptake of PrEP for HIV prevention has been limited, and increasing attention has been placed on addressing barriers to PrEP utilization by target populations (Hosek et al., 2016, 2017). Recent work has relied on the PrEP care continuum, a schematic developed by Kelly et al. (2015) to conceptualize and assess PrEP initiation and adherence across five sequential benchmarks: (a) at-risk for HIV, (b) aware/willing to use PrEP, (c) have access to health care, (d) likely to receive a PrEP prescription, and (e) adherent to an effective PrEP regimen.
Studies and interventions have largely focused on the latter steps of the PrEP continuum (e.g., PrEP delivery, adherence; Krishnaratne, Hensen, Cordes, Enstone, & Hargreaves, 2016; Marcus et al., 2014). However, some scholars have suggested revisions to the continuum that place greater emphasis on early stages related to being at-risk for HIV and aware/willing to use PrEP (Nunn et al., 2017; Parsons et al., 2017). Nunn et al. (2017) developed a nine-step model, which included identifying individuals at high risk for HIV, increasing HIV risk awareness, enhancing PrEP awareness, facilitating PrEP access, linking to PrEP care, prescribing PrEP, initiating PrEP, adhering to PrEP, and retaining PrEP users in care. Additionally, Parsons et al. (2017) developed a five-step continuum that was more firmly grounded in a motivational stage of change framework and considered factors that may influence HIV prevention behavior change. The five stages in this model were PrEP precontemplation (unwilling to take PrEP or does not identify as a good candidate), PrEP contemplation (willing to take PrEP and identifies as a good candidate), PrEParation (has a provider and intends to initiate PrEP), PrEP action and initiation (spoke to a provider about PrEP and initiated PrEP), and PrEP maintenance (adherent to PrEP and routine HIV testing). These continua present patient-centered models that consider individual self-perceived HIV risk, self-identification as a PrEP candidate, and motivations to initiate PrEP. Diagrams of the three proposed PrEP continuum models are presented in Figure 1.
Figure 1.
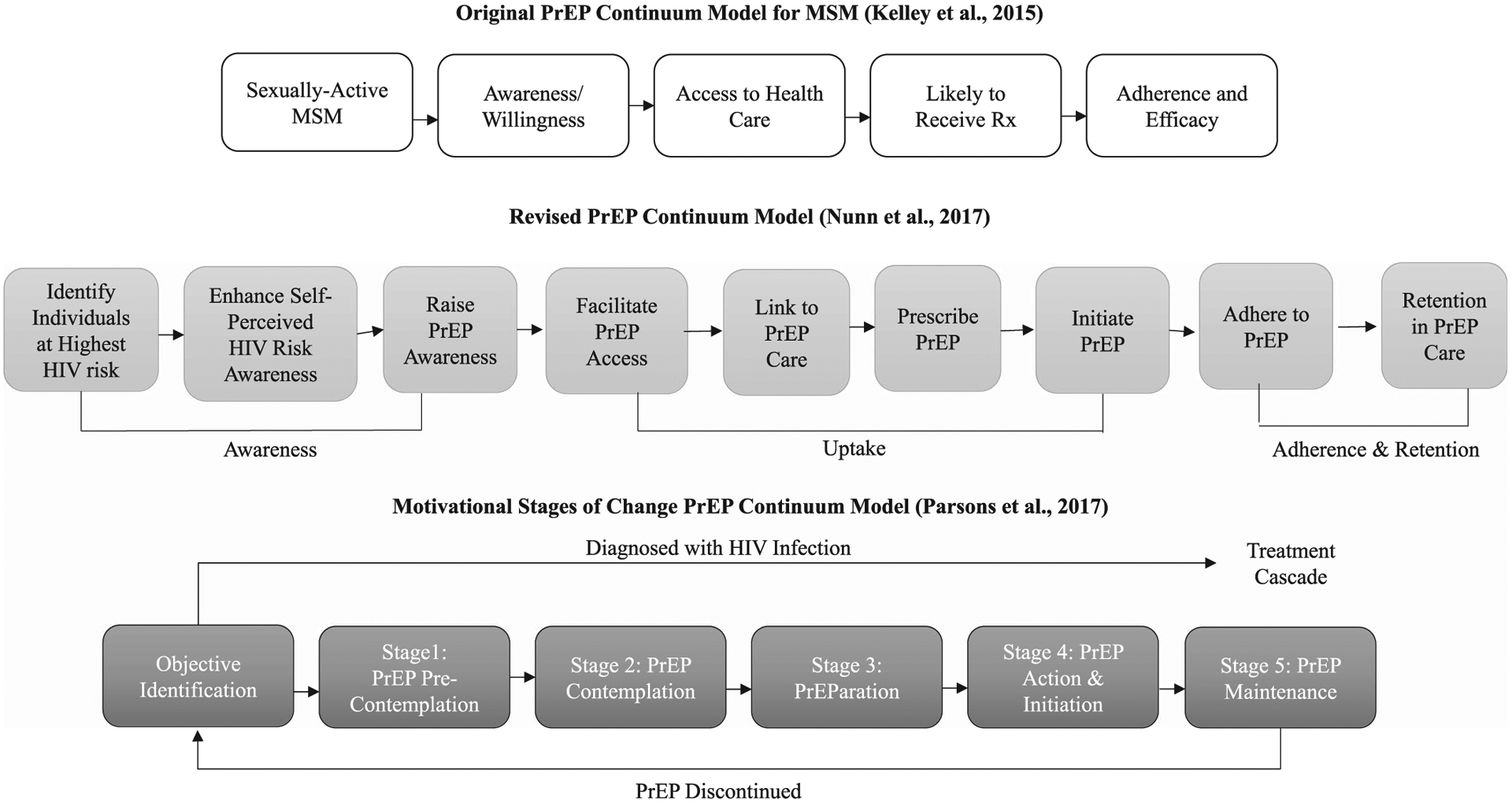
Three proposed models of the PrEP continuum. Note. MSM = men who have sex with men; PrEP = pre-exposure prophylaxis; Rx = prescription.
Overall, revisions to the original continuum suggest that psychosocial factors, including patient perceptions of their own HIV risk and candidacy for PrEP, warrant investigation as potential barriers to PrEP uptake. Thus, the purpose of our study was to explore the relationship between objective HIV risk (PrEP indication as informed by Centers for Disease Control and Prevention [CDC] guidance; Centers for Disease Control and Prevention, 2017) and self-perceived HIV risk, PrEP awareness, PrEP interest, and PrEP use in a diverse sample of YMSM.
Method
We used a secondary analysis of data from a baseline survey for an experimental study that assessed the impact of race on HIV and sexually transmitted infection (STI) risk perceptions among YMSM and was conducted at three Midwestern universities in the United States (Hill, Rosentel, & Hebert, 2019). YMSM were recruited from July 2015 to June 2016 using flyers and electronic advertisements. Inclusion criteria for the parent study included: (a) self-identifying as gay, bisexual, or a man who has had sex with men; (b) being ages 18 to 24 years; and (c) being able to read and write in English. Participants were prescreened prior to survey administration to ensure eligibility. Because the analysis was focused on HIV prevention perceptions and behaviors, participants who reported that they were living with HIV were excluded from the analytic sample. All participants provided informed consent and received $30 for participation. The Biological Sciences Division/University of Chicago Medical Center Institutional Review Board approved all study procedures.
Measures
The baseline survey included measures assessing participant characteristics (e.g., age, race/ethnicity) and sexual behaviors/history, including ever trading sex for money or drugs (transactional sex; yes/no); number of male partners for penile–anal intercourse (PAI) in the past 3 months (measured continuously); condom use during most recent PAI with a male partner (yes/no); intoxication during most recent sex (yes/no); and previous diagnosis of an STI (yes/no/never tested).
Participants were categorized as PrEP indicated or not PrEP indicated based on criteria informed by the Centers for Disease Control and Prevention, 2017. The CDC offers guidance for health care providers to assess risk of sexual HIV acquisition in men who have sex with men and recommended behavioral indicators for PrEP candidacy, including inconsistent condom use, history of commercial sex work, high number of sexual partners, and previous STIs (Centers for Disease Control and Prevention, 2017). The guidance was designed as a decision aid for providers rather than a decision calculator or eligibility criteria. Although the CDC guidance also includes a recommended assessment of sexual behaviors as a tool for providers, the questions in our sample assessment did not fully encompass all CDC recommended indicators for PrEP candidacy. We categorized participants as PrEP-indicated based on measures the investigators determined most indicative of the CDC outlined criteria rather than only using the measures in the CDC sample behavioral assessment. The measures we used included: (a) no condom use during most recent PAI, (b) transactional sex (ever), (c) five or more male PAI partners in the past 3 months, and (d) previously tested positive for an STI (ever). If a participant met any of these criteria, he was classified as PrEP-indicated and categorized as having objective HIV risk.
Self-perceived HIV risk was assessed by asking participants how likely they were to become infected with HIV from current sexual practices (very unlikely, unlikely, somewhat likely, likely, very likely). Consistent with other studies, participants who indicated somewhat likely or higher were categorized as perceiving themselves to be at moderate to high risk for HIV (Kesler et al., 2016; MacKellar et al.,2007). PrEP awareness and use was assessed by asking whether participants had ever heard of PrEP as a way to prevent HIV infection (yes/no) and whether they were currently taking PrEP (yes/no). PrEP initiation interest was assessed by asking participants how likely they were to start taking PrEP in the next 6 months (very unlikely, unlikely, somewhat likely, likely, very likely, taking PrEP); participants were categorized as interested in initiating PrEP if they indicated somewhat likely to very likely.
Data Analysis
Descriptive statistics were used to describe the sample. Chi-square tests, Fisher exact tests, and t-tests were used to assess associations between PrEP-indication (objective risk) and sociodemographic characteristics, such as ethnicity, college attainment, monthly income, and sexual orientation, as well as PrEP continuum variables, including self-perceived risk, PrEP awareness, PrEP initiation interest, and PrEP use. T-tests were used for pairwise comparisons of continuous variables including age. Chi-square tests were used for pairwise comparisons of categorical variables when crosstab expected cell counts were all greater than or equal to five. Fisher exact tests were used for pairwise comparisons of categorical variables when any expected cell counts were less than five. The tests used for each statistical comparison are delineated in Tables 1 and 2.
Table 1.
Sociodemographic and PrEP Continuum Variables by PrEP-Indication Status (N = 130)
| All Participants (n = 130) | PrEP-Indicated (n = 71) | Not PrEP-Indicated (n = 59) | p | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||
| Sociodemographic variables | ||||
| Agea | 20.7 (1.76) | 20.9 (1.68) | 20.4 (1.83) | .122 |
| Race/ethnicity | % (n) | % (n) | % (n) | |
| Non-Hispanic/Latino Whiteb | 56.2 (73) | 50.7 (36) | 62.7 (37) | .170 |
| Black/African Americanb | 20.0 (26) | 21.1 (15) | 18.6 (11) | .725 |
| Hispanic/Latinob | 16.2 (21) | 23.9 (17) | 6.8 (4) | .008* |
| Asianb | 15.4 (20) | 12.7 (9) | 18.6 (11) | .348 |
| At least some college educationb | 87.7 (114) | 85.9 (61) | 89.8 (53) | .499 |
| Monthly income <$1,000b | 52.3 (68) | 46.5 (33) | 59.3 (35) | .144 |
| Sexual orientation | ||||
| Gay/homosexualb | 69.2 (90) | 64.8 (46) | 74.6 (44) | .229 |
| Bisexualb | 30.0 (39) | 33.8 (24) | 25.4 (15) | .299 |
| Straight/heterosexualc | 0.8 (1) | 1.4 (1) | 0.0 (0) | 1.000 |
| PrEP continuum variables | ||||
| Self-perceived to be at moderate to high risk for HIVb | 10.8 (14) | 11.3 (8) | 10.2 (6) | .841 |
| PrEP-awareb | 58.5 (76) | 63.4 (45) | 52.5 (31) | .212 |
| PrEP initiation interestb | 27.7 (36) | 26.8 (19) | 28.8 (17) | .795 |
| PrEP-usingc | 1.5 (2) | 2.8 (2) | 0.0 (0) | .500 |
Note. PrEP = pre-exposure prophylaxis.
t-test.
Chi-square test.
Fisher exact test.
p < .05.
Table 2.
PrEP Continuum Variables by Centers for Disease Control and Prevention Behavioral Indicators for PrEP (N = 130)
| PrEP Continuum Variables | Condom Use at Last PAI (n = 83) | No Condom Use at Last PAI (n = 47) | p |
|---|---|---|---|
| % (n) | % (n) | ||
| Self-perceived to be at moderate to high risk for HIVa | 12.0 (10) | 8.5 (4) | .532 |
| PrEP-awarea | 54.2 (45) | 66.0 (31) | .192 |
| PrEP initiation interestb | 27.7 (23) | 27.7 (13) | .995 |
| PrEP-usingb | 0.0 (0) | 4.3 (2) | .129 |
| No previous STI (n = 101) | Previous STI (n = 29) | p | |
| % (n) | % (n) | ||
| Self-perceived to be at moderate to high risk for HIVb | 10.9 (11) | 10.3 (3) | 1.000 |
| PrEP-awarea | 57.4 (58) | 62.1 (18) | .655 |
| PrEP initiation interesta | 27.7 (28) | 27.6 (8) | .988 |
| PrEP-usingb | 0.0 (0) | 6.9 (2) | .048* |
| No history of transactional sex (n = 107) | History of transactional sex (n = 23) | p | |
| % (n) | % (n) | ||
| Self-perceived to be at moderate to high risk for HIVb | 10.3 (11) | 13.0 (3) | .713 |
| PrEP-awarea | 58.9 (63) | 56.5 (13) | .835 |
| PrEP initiation interesta | 26.2 (28) | 34.8 (8) | .402 |
| PrEP-usingb | 0.0 (0) | 8.7 (2) | .030* |
| Less than 5 PAI partners, past 3 months (n = 123) | 5 or more PAI partners, past 3 months (n = 7) | p | |
| % (n) | % (n) | ||
| Self-perceived to be at moderate to high risk for HIVb | 11.4 (14) | 0.0 (0) | 1.000 |
| PrEP-awareb | 57.7 (71) | 71.4 (5) | .699 |
| PrEP initiation interestb | 26.8 (33) | 42.9 (3) | .395 |
| PrEP-usingb | 0.8 (1) | 14.3 (1) | .105 |
Note. PAI = penile–anal intercourse; PrEP = pre-exposure prophylaxis; STI = sexually transmitted infection.
Chi-square test.
Fisher exact test.
p < .05.
Results
Overall, 135 YMSM participated in the study; 130 were included in the analytic subset after people living with HIV were excluded (Table 1). The mean age of the sample was 20.7 years (SD = 1.76). The sample was racially diverse: 56.2% non-Hispanic/Latino White, 20% Black/African American, 16.2% Hispanic/Latino, and 15.4% Asian. Most participants (87.7%) reported having some college education or higher. More than half (52.3%) reported earning less than $1,000 per month. A majority (69.2%) described themselves as gay/homosexual and 30.0% as bisexual.
A majority of the sample (54.6%) met indications for PrEP based on our CDC-informed criteria. Overall, 36.2% reported not using a condom during last PAI, 22.3% reported being diagnosed with an STI, 17.7% reported engaging in transactional sex, 5.4% reported having five or more PAI partners in the past 3 months, and 29.2% reported being intoxicated during last PAI. Self-perceived HIV risk was low, with 10.8% reporting a somewhat likely to very likely chance of becoming infected with HIV. PrEP awareness was moderate at 58.5%. PrEP initiation interest and use were low: 27.7% and 1.5%, respectively. Chi-square, Fisher exact, and t-tests comparing PrEP-indicated and non-indicated participants only revealed one significant association: a higher proportion of PrEP-indicated participants were Hispanic/Latino (23.9% vs. 6.8%; p = .008). No significant differences between PrEP-indicated and non-indicated participants were observed in self-perceived HIV risk (11.3% vs. 10.2%; p = .841), PrEP awareness (63.4% vs. 52.5%; p = .212), PrEP initiation interest (26.8% vs. 28.8%; p = .795), and PrEP use (2.8% vs. 0.0%; p = .194). Chi-square and Fisher exact tests assessing the relationship between PrEP continuum variables and individual PrEP-indication variables revealed only two significant associations (Table 2): Participants were more likely to be using PrEP if they reported a previous STI (6.9% vs. 0.0%; p = .048) or transactional sex (8.7% vs. 0.0%; p = .030).
Discussion
Consistent with other studies, our findings highlighted a stark discordance between objective HIV risk (PrEP-indication) and self-perceived HIV-risk among YMSM (Blumenthal et al., 2019; MacKellar et al., 2007). A majority of the sample was engaged in high-risk behaviors, indicating they were candidates for PrEP. However, only 11.3% of PrEP-indicated men reported being at least somewhat likely to become infected with HIV, making them no more likely to report a high risk for HIV than non-indicated participants. As the inclusion of HIV risk self-awareness and PrEP contemplation in recent PrEP care continuum models suggest, low HIV risk perception may present a barrier to PrEP uptake by YMSM (Nunn et al., 2017; Parsons et al., 2017). This was supported by findings from Blumenthal et al., (2019), which found that low perceived HIV risk was the most frequently cited reason for not initiating PrEP in their sample of YMSM. Accordingly, PrEP interventions may benefit from integrating patient education tools such as validated HIV risk calculators, visual aids to increase health literacy, and mobile health applications focused on HIV prevention, all of which may help YMSM more accurately understand risks associated with sexual behaviors (Blumenthal et al., 2019; Chen & Dowdy, 2014; Cho et al., 2018; Garcia-Retamero & Cokely, 2013; Milam et al., 2015).
We also found that PrEP awareness, initiation interest, and use were relatively low, and PrEP-indicated participants were no more likely to be aware of, interested in initiating, or using PrEP than non-indicated participants. Thus, interventions aimed at moving participants along the PrEP continuum, particularly those focused on linkage and initiation, may need to better target patients based on behavioral risk (e.g., condomless PAI) rather than solely relying on population membership (e.g., YMSM). Furthermore, intoxication during PAI was relatively common in our sample (29.2%) and may warrant consideration as a criterion for PrEP-indication, given how it may impair sexual decision-making (Allen, Myers, & Ray, 2015; Morgan et al., 2015).
Although no significant associations were observed between PrEP indication and PrEP awareness, initiation interest, and use, there were statistically significant associations between individual PrEP indication criteria and PrEP use. Participants who reported a previous STI and participants who reported transactional sex were significantly more likely to report using PrEP. This may be in part due to local efforts that aim to link individuals with a positive STI to PrEP as well as public health programs targeted to individuals involved in transactional sex work. Nevertheless, it should be noted that PrEP use was still low for individuals with a history of STI and those engaged in transactional sex work, at 6.9% and 8.7%, respectively.
Although our study offers insight into the relationship between objective PrEP-indication and self-perceived HIV risk, it was not without limitations. First, we used a secondary analysis of data from an experimental study in Chicago and mainly relied on descriptive statistics.
Additionally, although our sample was racially and socioeconomically diverse, it was small and non-representative. Thus, our sample cannot be generalized to all YMSM, and research is needed to assess how discordance between objective and self-perceived HIV risk may affect PrEP initiation. Our classification of PrEP-indication was informed by CDC guidance, which recommends assessing a number of behavioral criteria including inconsistent condom use, history of commercial sex work, high number of sexual partners, and previous STIs. However, our measures for assessing these factors may have differed from those used by some providers given that the CDC has not provided standardized measures for assessing the suggested criteria. Additionally, our classification of PrEP-indication did not incorporate one CDC-suggested criterion: having a sexual partner living with HIV (Centers for Disease Control and Prevention, 2017). Our survey did not assess the HIV status of participant sexual partners because previous studies have suggested that participant reports of partner HIV status may be unreliable (Grey, Rothenberg, Sullivan, & Rosenberg, 2014; MacKeller et al., 2006).
Conclusion
Overall, our findings suggest that interventions focused on improving accurate HIV risk perceptions in YMSM may be needed. Research should assess whether the incorporation of risk assessment and patient-centered education tools into clinical protocols increases the number of YMSM continuing along the PrEP continuum. Further, we have highlighted that additional factors may warrant consideration when assessing behavioral indicators for PrEP in YMSM, including substance use during PAI. Research exploring such factors may help to strengthen clinical guidance for providers who offer HIV education and PrEP consultation to YMSM.
Acknowledgements
This work received funding from the University of Chicago Center for Health Administration Studies (CHAS) and Institute for Translational Medicine (ITM).
Footnotes
Disclosures
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
References
- Allen VC, Myers HF, & Ray L (2015). The association between alcohol consumption and condom use: Considering correlates of HIV risk among black men who have sex with men. AIDS and Behavior, 19(9), 1689–1700. doi: 10.1007/s10461-015-1075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal J, Jain S, Mulvihill E, Sun S, Hanashiro M, Ellorin E, & Morris S (2019). Perceived versus calculated HIV risk. Journal of Acquired Immune Deficiency Syndromes, 80(2), e23–e29. doi: 10.1097/QAI.0000000000001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). Pre-exposure prophylaxis for the prevention of HIV Infection in the United States—2017 update: A clinical practice guideline. Retrieved from https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
- Chen A, & Dowdy D (2014). Clinical effectiveness and cost-effectiveness of HIV pre-exposure prophylaxis in men who have sex with men: Risk calculators for real-world decision-making. PLoS One, 9(10), e108742. doi: 10.1371/journal.pone.0108742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Powell D, Pichon A, Thai J, Bruce J, Kuhns LM, … Schnall R (2018). A mobile health intervention for HIV prevention among racially and ethnically diverse young men: Usability evaluation. JMIR MHealth and UHealth, 6(9), e11450. doi: 10.2196/11450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Retamero R,&Cokely ET (2013). Theinfluenceofskills, message frame, and visual aids on prevention of sexually transmitted diseases. Behavioral Decision Making, 27(2), 179–189. doi: 10.1002/bdm.1797 [DOI] [Google Scholar]
- Grey JA, Rothenberg R, Sullivan PS, & Rosenberg ES (2014). Racial differences in the accuracy of perceived partner HIV status among men who have sex with men (MSM) in Atlanta, Georgia. Journal of the International Association of Providers of AIDS Care, 14(1), 26–32. doi: 10.1177/2325957414555226 [DOI] [PubMed] [Google Scholar]
- Hill BJ, Rosentel K, & Hebert L (2019). Assessing the impact of race on HIV/STI risk perceptions among young men who have sex with men using an experimental approach. Journal of Acquired Immune Deficiency Syndromes, 81(2), 153–157. doi: 10.1097/QAI.0000000000002004 [DOI] [PubMed] [Google Scholar]
- Hosek S, Celum C, Wilson C, Kapogiannis B, Delany-Moretlwe S, & Bekker LG (2016). Preventing HIV among adolescents with oral PrEP: Observations and challenges in the United States and South Africa. Journal of the International AIDS Society, 19(7 Suppl 6), 21107. doi: 10.7448/IAS.19.7.21107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosek S, Rudy B, Landovitz R, Kapogiannis B, Siberry G, Rutledge B, … Wilson CM (2017). An HIV pre-exposure prophylaxis demonstration project and safety study for young MSM. Journal of Acquired Immune Deficiency Syndromes, 74(1), 21–29. doi: 10.1097/QAI.0000000000001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley C, Kahle E, Siegler A, Sanchez T, Del Rio C, Sullivan PS, & Rosenberg ES (2015). Applying a PrEP continuum of care for men who have sex with men in Atlanta, Georgia. Clinical Infectious Diseases, 61(10), 1590–1597. doi: 10.1093/cid/civ664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler MA, Kaul R, Myers T, Liu J, Loutfy M, Remis RS, & Gesnick D (2016). Perceived HIV risk, actual sexual HIV risk and willingness to take pre-exposure prophylaxis among men who have sex with men in Toronto, Canada. AIDS Care, 28(11), 1378–1385. doi: 10.1080/09540121.2016.1178703 [DOI] [PubMed] [Google Scholar]
- Krishnaratne S, Hensen B, Cordes J, Enstone J, & Hargreaves JR (2016). Interventions to strengthen the HIV prevention cascade: A systematic review of reviews. Laboratory Hematology, 3(7), e307–317. doi: 10.1016/S2352-3018(16)30038-8 [DOI] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Behel S, Secura GM, Bingham T, Celentano DD, … Torian LV (2006). HIV exposures from young men who have sex with men who disclose being HIV-negative. AIDS, 20(12), 1637–1644. doi: 10.1097/01.aids.0000238410.67700.d1 [DOI] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Secura GM, Behel S, Bingham T, Celentano DD, … Torian LV (2007). Perceptions of lifetime risk and actual risk for acquiring HIV among young men who have sex with men. AIDS and Behavior, 11(2), 263–270. doi: 10.1007/s10461-006-9136-0 [DOI] [PubMed] [Google Scholar]
- Milam J, Morris S, Jain S, Sun X, Dubé MP, Daar ES, … Haubrich R (2015). Randomized controlled trial of an internet application to reduce HIV transmission behavior among HIV infected men who have sex with men. AIDS and Behavior, 20(6), 1173–1181. doi: 10.1007/s10461-015-1215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E, Skaathun B, Michaels S, Young L, Khanna A, Friedman SR, … Schneider J (2015). Marijuana use as a sex-drug is associated with HIV risk among black MSM and their network. AIDS and Behavior, 20(3), 600–607. doi: 10.1007/s10461-015-1195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn A, Brinkley-Rubinstein L, Oldenburg C, Mayer KH, Mimiaga M, Patel R, & Chan PA (2017) Defining the HIV pre-exposure prophylaxis care continuum. AIDS, 31(5), 731–734. doi: 10.1097/QAD.0000000000001385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J, Rendina H, Lassiter JM, Whitfield TH, Starks TJ, & Grov C (2017). Uptake of HIV pre-exposure prophylaxis (PrEP) in a national cohort of gay and bisexual men in the United States. Journal of Acquired Immune Deficiency Syndromes, 74(3), 285–292. doi: 10.1097/QAI.0000000000001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehan JL, Buisker T, Horvath T, Amico KR, Fuchs JD, Buchbinder SP, & Liu AY (2014). Helping our patients take HIV pre-exposure prophylaxis (PrEP): A systematic review of adherence interventions. HIV Medicine, 15(7), 385–395. doi: 10.1111/hiv.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk JE, Marcus JL, Phengrasamy T, Blechinger D, Nguyen DP, Follansbee S, & Hare CB (2015). No new HIV infections with increasing use of HIV pre-exposure prophylaxis in a clinical practice setting. Clinical Infectious Diseases, 61(10), 1601–1603. doi: 10.1093/cid/civ778 [DOI] [PMC free article] [PubMed] [Google Scholar]


