Abstract
Purpose: We assessed the clinical feasibility of C-reactive protein to lymphocyte ratio (CLR) as a determinant of survival in patients with non-small cell lung cancer (NSCLC) undergoing curative surgical resection.
Methods: A retrospective study was conducted on patients with stage I and II NSCLC undergoing curative resection. Demographic and clinical variables, including CLR, were collected and analyzed. The Cox proportional hazards model was used to calculate hazard ratios for overall survival (OS) and cancer-specific survival (CSS). The Mann-Whitney U test was used to compare differences between two independent groups.
Results: The median age of the patients was 69.0 years, and male patients comprised 63.9% of all patients. A total of 164 (75.9%) patients were categorized as having stage I disease and 52 (24.1%) as having stage II disease. Using the multivariate Cox model, age (hazard ratio [HR] 1.08, p<0.001), lymphatic invasion (HR 3.12, p=0.004), stage (HR 5.10, p<0.001), and CLR (HR 1.01, p=0.003) were significant determinants of OS. In addition, age (HR 1.11, p=0.002), lymphatic invasion (HR 3.16, p=0.010), stage (HR 6.89, p<0.001), and CLR (HR 1.05, p=0.002) were significant determinants of CSS.
Conclusions: Our findings show that CLR could be a determinant of survival in NSCLC patients undergoing curative surgical resection.
Keywords: Carcinoma, Non-Small Cell Lung; Pulmonary Surgical Procedures; Prognosis; Lymphocyte Count; C-Reactive Protein
Introduction
In patients with stage I or II Non-small cell lung cancer (NSCLC), surgical resection is widely accepted as the optimal therapeutic option 1. However, despite substantial advances in surgical techniques, adjuvant treatment (such as chemotherapy, epidermal growth factor receptor tyrosine kinase inhibitor, radiation therapy, or chemoradiation), and the development of new prognostic models, the prognosis remains far from satisfactory 2. Therefore, the search for crucial prognostic factors that can assist thoracic surgeons in identifying high-risk patients who may benefit from perioperative treatment or clinical trials is required.
The demographic and clinical variables that have been considered important determinants of survival of NSCLC are as follows: age, sex, body mass index (BMI), performance status, smoking, reduced forced expiratory volume in one second (FEV1), histology, tumor-node-metastasis (TNM) stage, T stage, N stage, tumor size, pleural invasion, lymphatic invasion, vascular invasion, type of surgery, and R0 resection (i.e., a microscopically margin-negative resection) 2-5. In addition, the impact of nutritional variables such as sarcopenia on surgical outcomes has been reported in NSCLC 6.
Moreover, the hematological and biochemical markers that have been reported in patients with NSCLC are as follows: C-reactive protein (CRP), peripheral blood lymphocyte count, the Glasgow prognostic score, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and monocyte-to-lymphocyte ratio 3, 7-9.
Recently, the clinical significance of the combination of peripheral blood lymphocyte count and CRP as prognosticators for gastrointestinal cancers (including gastric, colorectal, and pancreatic cancers) has been studied by some researchers 10-15. In a study by Kono et al., the CRP to lymphocyte ratio (CLR) was a determinant of the overall survival (OS) and disease-specific survival (DSS) in patients with gastric cancer 13. In addition, CLR is a feasible determinant of OS in pancreatic cancer 14. However, the clinical value of CLR has not been evaluated in tumors outside the gastrointestinal tract. Therefore, in this study, we assessed the clinical value of CLR in patients with NSCLC undergoing curative surgical resection.
Methods
Patients
The medical records of consecutive patients with NSCLC undergoing surgical resection from July 2006 to December 2019 at the Kyung Hee University Hospital at Gangdong were reviewed. The chest-abdomen-pelvis computed tomography (CT) scans and the combination of CT with positron emission tomography was a regular part of standard cancer staging. In addition, CT and magnetic resonance imaging were the main imaging techniques used to diagnose brain metastases.
The inclusion criteria were as follows: (i) primary NSCLC according to the 2015 World Health Organization classification of lung tumors 16; (ii) stage I or II, according to the 8th edition of the TNM classification (Union for International Cancer Control) 17; (iii) underwent pneumonectomy, sleeve lobectomy, bilobectomy, lobectomy, or segmentectomy; and (iv) underwent R0 resection. The exclusion criteria were: (i) underwent preoperative chemotherapy, radiotherapy, or another anti-cancer treatment prior to surgery; (ii) presence of small cell lung cancer, sarcomatoid carcinoma, or neuroendocrine tumor; (iii) previous malignancies within the last 5 years, or concurrent second malignancies; (iv) history of liver cirrhosis, stage 4 or 5 chronic kidney disease, (v) stage III or IV disease, (vi) positive test for human immunodeficiency virus, severe infections within 4 weeks prior to surgery, or active autoimmune diseases that required systemic or immunosuppressive agents at the time of surgery.
Demographic and clinical variables
The variables collected in this study were as follows: age at surgery, sex, smoking history, BMI, Eastern Cooperative Oncology Group scale of Performance Status, percent predicted FEV1, types of surgery, histology, size and extent of the primary tumor, lymph node involvement, TNM stage, pleural invasion, lymphatic invasion, vascular invasion, and residual disease. A BMI of less than 18.5 kg/m2 was considered underweight. Classification of pleural invasion was as follows: PL0, tumor within the subpleural lung parenchyma or superficial invasion into the pleural connective tissue; PL1, tumor invasion beyond the elastic layer; PL2, tumor invasion to the pleural surface; and PL3, tumor invasion into any component of the parietal pleura 18. The classification of residual disease was as follows: R0, resection for cure or complete remission; R1, microscopic residual tumor; and R2, macroscopic residual tumor 19.
The laboratory variables collected were as follows: white blood cell count, differential count, hemoglobin concentration, serum creatinine level, serum albumin level, and CRP level. The CLR was calculated as follows: [CRP level (mg/dL) × 100]/peripheral blood lymphocyte count (number/μL), as reported previously 13, 14. Blood test results were analyzed through tests performed within 7 days before surgery. If more than one test result was available, the test result closest to the date of the surgery was selected for further analysis. The diagnosis of anemia was based on a hemoglobin concentration below 13 g/dL in men and 12 g/dL in women.
Statistical analyses
Summaries of the data are expressed as the median with interquartile range, or number with percent. OS was measured from the date of surgical resection to the date of death from any cause or last follow-up. Cancer-specific survival (CSS) was measured from the date of surgical resection to the date of death due to cancer in the absence of other causes of death. Individuals who died of causes other than cancer were treated as censored.
The Cox proportional hazards model was used to calculate hazard ratios, which were performed only on variables that met the proportional hazards assumption on the basis of graphic plots of Schoenfeld residuals. Only variables with p <0.05 in the univariate analysis were included in the multivariate Cox model. Harrell's concordance probability (C-index) for the Cox model was performed to measure the discriminative power of a risk prediction model. The variance inflation factor (VIF) was calculated for the diagnosis of multicollinearity.
The decision curve analysis (DCA) displays estimates of the (standardized) net benefit over a range of probability thresholds used to categorize observations as 'high risk'. Bootstrap analysis with 500 resamples was used for the analysis.
In addition, a bootstrap cross-validation estimate of the C-index at different time points was applied to assess and compare the discriminative power of two regression models (i.e., baseline vs. full model). The number of bootstrap samples was 1000, and sampling with replacement from the original data set was accomplished.
The Mann-Whitney U test was used to compare differences between two independent groups when the dependent variable was continuous. All p-values presented were 2-sided, and statistical significance was declared at p<0.05. Data were analyzed using the R package.
Results
Demographic and clinical characteristics of the patients
A total of 331 patients were initially enrolled, and 117 patients were excluded for the following reasons: (i) 19 patients had concurrent malignant tumors or had other types of malignant tumors within 5 years; (ii) 4 patients had been diagnosed with neuroendocrine tumors, and 4 patients with sarcomas; (iii) 7 patients had been treated with chemotherapy or radiotherapy for NSCLC before surgery; (iv) 56 patients had stage III or stage IV disease; (v) 2 patients had no R0 resection; (vi) 9 patients had no available CRP data; (vii) 16 patients had undergone pulmonary metastasectomy for tumors other than lung cancer; and (viii) 4 patients had benign tumors of the lung. Finally, 214 patients with NSCLC were analyzed.
The most common surgical procedure was lobectomy, which was performed on 197 patients (77.3%), followed by segmentectomy (21.3%), and pneumonectomy (3%). The most common histological subtype was adenocarcinoma (69.9%), followed by squamous cell carcinoma (26.4%), adenosquamous cell carcinoma (1.4%), pleomorphic carcinoma (1.4%), and large cell carcinoma (0.9%). A total of 164 (75.9%) patients were categorized as having stage I disease, and 52 (24.1%) as stage II (Table 1).
Table 1.
Demographic and clinical characteristics
| Variables | Median (IQR), or n (%) |
|---|---|
| Age, years | 69.0 (62.0-75.0) |
| Sex | |
| Male | 136 (63.6) |
| Female | 78 (36.4) |
| Smoker | |
| Current | 42 (19.6) |
| Former | 86 (40.2) |
| Never | 86 (40.2) |
| ECOG PS | |
| 0/1 | 206 (96.3) |
| 2 | 8 (3.7) |
| Percent predicted FEV1 | 102.3 (90.0-115.0) |
| BMI, kg/m2 | 23.7 (21.8-25.8) |
| Types of surgery | |
| Pneumonectomy/lobectomy | 168 (78.5) |
| Segmentectomy | 46 (21.5) |
| Histology | |
| Adenocarcinoma | 151 (70.6) |
| Squamous cell carcinoma | 55 (25.7) |
| Others† | 8 (3.7) |
| Size of tumor, cm | 2.7 (1.9-3.5) |
| T-stage | |
| pT1 | 100 (46.8) |
| pT2 | 97 (45.3) |
| pT3 | 17 (7.9) |
| N-stage | |
| pN0 | 197 (92.1) |
| pN1 | 17 (7.9) |
| TNM Stage‡ | |
| IA/IB | 163 (76.2) |
| IIA/IIB | 51 (23.8) |
| Pleural invasion | |
| PL0 | 157 (73.4) |
| PL1/2/3 | 57 (26.6) |
| Lymphatic invasion | |
| No | 193 (90.2) |
| Yes | 21 (9.8) |
| Vascular invasion | |
| No | 204 (95.3) |
| Yes | 10 (4.7) |
| Albumin, g/dL | 4.1 (3.9-4.3) |
| Anemia§ | |
| No | 143 (66.8) |
| Yes | 71 (33.2) |
| CLR | 0.8 (0.4-2.6) |
† Adenosquamous cell carcinoma (3 cases), pleomorphic carcinoma (3 cases), and large cell carcinoma (2 cases).
‡ AJCC 8th edition.
§ The cutoff point is 12 g/dL for female patients and 13 g/dL for male patients.
IQR, interquartile range; ECOG PS, Eastern Cooperative Oncology Group performance status; FEV1, forced expiratory volume in one second; BMI, body mass index; PL, pleural invasion; TNM, tumor-node-metastasis; CLR, CRP to lymphocyte ratio.
Determinants of survival
The median follow-up period was 34 months (range, 1-151 months). Using the univariate Cox model, age, sex, smoker, tumor size, nodal invasion, TNM stage, lymphatic invasion, vascular invasion, albumin level, and CLR were identified as significant determinants of OS. Using the multivariate Cox model, age (HR 1.08, 95% CI 1.04-1.14, p<0.001), lymphatic invasion (HR 3.12, 95% CI 1.43-6.81, p=0.004), stage (HR 5.10, 95% CI 2.45-10.59, p<0.001), and CLR (HR 1.01, 95% CI 1.00-1.02, p=0.003) were identified as significant determinants of OS. Harrell's C-index for the Cox model was 0.8337, indicating excellent discrimination. VIFs for age, lymphatic invasion, stage, and CLR were 1.10, 1.02, 1.01, and 1.19, respectively, indicating no significant collinearity between the covariates (Table 2).
Table 2.
Univariate and multivariate Cox proportional hazards regression analysis of overall survival and cancer-specific survival
| Covariates | Overall Survival | Cancer-specific Survival | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| (A) Univariate analysis | ||||
| Age, years† | 1.09 (1.04-1.15) | <0.001 | 1.10 (1.04-1.17) | <0.001 |
| Sex (female vs. male) | 0.33 (0.14-0.79) | 0.013 | 0.29 (0.10-0.85) | 0.024 |
| Smoker (current/former vs. never) | 2.23 (1.05-4.74) | 0.038 | 2.52 (1.01-6.30) | 0.048 |
| ECOG PS (2 vs. 0/1) | 2.13 (0.51-8.93) | 0.300 | 0.79 (0.34-1.81) | 0.574 |
| Underweight (yes vs. no) | 1.95 (0.68-5.52) | 0.212 | 1.47 (0.35-6.24) | 0.601 |
| Resection (Segmentectomy vs. PN/L) | 0.62 (0.22-1.78) | 0.374 | 0.56 (0.17-1.91) | 0.359 |
| Histology (SQC vs. non-SQC) | 1.51 (0.99-2.29) | 0.053 | 1.32 (0.81-2.13) | 0.261 |
| Size of tumor, cm† | 1.68 (1.37-2.05) | <0.001 | 1.62 (1.30-2.23) | <0.001 |
| Nodal invasion (yes vs. no) | 2.41 (1.00-5.80) | 0.050 | 3.64 (1.45-9.09) | 0.006 |
| TNM stage (II vs. I) | 6.64 (3.35-13.16) | <0.001 | 8.77 (3.80-20.22) | <0.001 |
| Pleural invasion (PL0 vs. PL1/2/3) | 0.90 (0.41-1.99) | 0.794 | 0.91 (0.37-2.27) | 0.844 |
| Lymphatic invasion (yes vs. no) | 5.15 (2.45-10.87) | <0.001 | 5.71 (2.44-13.47) | <0.001 |
| Vascular invasion (yes vs. no) | 4.25 (1.48-12.17) | 0.007 | 4.12 (1.22-13.93) | 0.023 |
| Albumin, g/dL† | 0.20 (0.08-0.48) | <0.001 | 0.24 (0.09-0.70) | 0.009 |
| Anemia (yes vs. no) ‡ | 1.92 (0.99-3.69) | 0.052 | 1.71 (0.79-3.72) | 0.173 |
| CLR† | 1.03 (1.01-1.06) | 0.004 | 1.04 (1.01-1.06) | 0.002 |
| (B) Multivariate analysis | ||||
| Age, years† | 1.09 (1.04-1.15) | <0.001 | 1.10 (1.04-1.16) | 0.002 |
| TNM stage (II vs. I) | 5.10 (2.45-10.59) | <0.001 | 6.89 (2.84-16.69) | <0.001 |
| Lymphatic invasion (yes vs. no) | 3.15 (1.45-6.86) | 0.004 | 3.16 (1.31-7.60) | 0.010 |
| CLR† | 1.04 (1.02-1.07) | 0.001 | 1.05 (1.02-1.07) | 0.002 |
† Continuous variables.
‡ The cutoff point is 12 g/dL in female patients and 13 g/dL in male patients.
HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PFP, percent FEV1 predicted; PN/L, pneumonectomy or lobectomy; PL0, tumor within the subpleural lung parenchyma or invading superficially into the pleural connective tissue; PL1, tumor invaded beyond the elastic layer; PL2, tumor invaded the pleural surface; PL3, tumor invaded any component of the parietal pleura; TNM, tumor-node-metastasis; SQC, squamous cell; CLR, CRP to lymphocyte ratio.
Using the univariate Cox model, the same variables as those of OS were identified as significant determinants of CSS. Using multivariate analysis, age (HR 1.10, 95% CI 1.04-1.16, p=0.002), lymphatic invasion (HR 6.89, 95% CI 2.84-16.69, p<0.001), stage (HR 3.16, 95% CI 1.31-7.60, p=0.010), and CLR (HR 1.05, 95% CI 1.02-1.07, p=0.002) were identified as determinants of CSS. Harrell's C-index for the Cox model was 0.8571, indicating excellent discrimination. VIFs for age, lymphatic invasion, stage, and CLR were 1.10, 1.02, 1.01, and 1.10, respectively, indicating no significant collinearity between the covariates (Table 2).
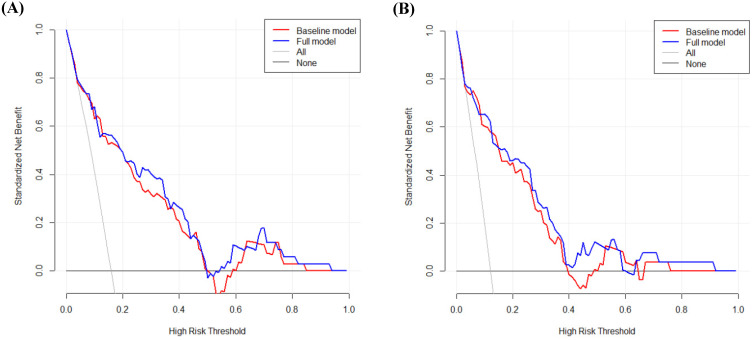
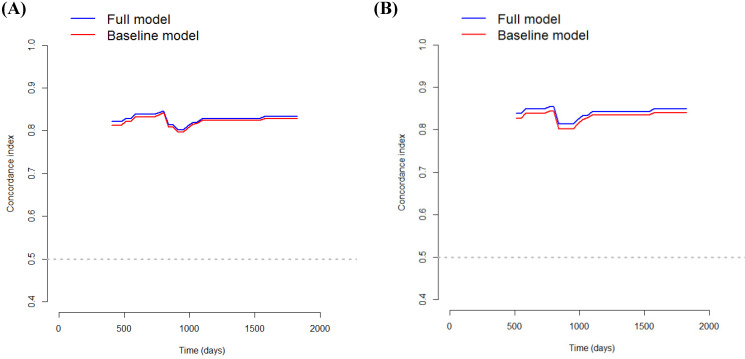
DCA was performed to calculate the clinical net benefit of the baseline model (i.e., three covariates of age, lymphatic invasion, and stage) and full model (i.e., four covariates of age, lymphatic invasion, stage, and CLR) at a certain range of threshold probability in predicting OS and CSS. The DCA revealed that the net benefit of the full model was superior to that of the baseline model at most of the threshold probabilities in terms of OS and CSS (Fig. 1A and Fig. 1B). In addition, bootstrap cross-validation also revealed higher C-indices for the full model (i.e., age, lymphatic invasion, stage, and CLR) than the baseline model (i.e., age, lymphatic invasion, and stage) in terms of OS and CSS (Fig. 2A and Fig. 2B).
Figure 1.
Decision curve analysis to calculate the clinical net benefit of each model for overall survival (A) and cancer-specific survival (B). The baseline model includes three covariates (i.e., age, lymphatic invasion, and stage), and the full model includes four covariates (i.e., age, lymphatic invasion, stage, and CLR). All, a line that all patients are surviving; None, a line that no patient is surviving.
Figure 2.
The bootstrap cross-validation estimates of the C-index at different time points to assess and compare the discriminative power of two regression models for overall survival (A) and cancer-specific survival (B). The baseline model includes three covariates (i.e., age, lymphatic invasion, and stage), and the full model includes four covariates (i.e., age, lymphatic invasion, stage, and CLR).
Correlation between basal patient characteristics and CLR
There were significant differences in CLR according to age (p=0.003), sex (p<0.001), smoking history (p<0.001), BMI (p=0.020), histology (p<0.001), tumor size (p<0.001), TNM stage (p=0.006), serum albumin level (p=0.001), and anemia (p<0.001) when Mann-Whitney U test was applied (Table 3).
Table 3.
C-reactive protein to lymphocyte ratios according to the covariates
| Covariates | CLR | |
|---|---|---|
| Median (IQR) | p-value | |
| Age, years | ||
| <65 | 0.51 (0.23-1.76) | 0.003 |
| ≥65 | 1.00 (0.51-3.00) | |
| Sex | ||
| Male | 1.19 (0.47-4.31) | <0.001 |
| Female | 0.57 (0.25-1.20) | |
| Smoking | ||
| Never | 0.57 (0.31-1.19) | <0.001 |
| Former/current | 1.38 (0.47-4.46) | |
| ECOG PS | ||
| 0/1 | 0.81 (0.43-2.57) | 0.356 |
| 2 | 1.55 (0.97-6.15) | |
| BMI, kg/m2 | ||
| <18.5 | 2.41 (0.96-15.71) | 0.020 |
| ≥18.5 | 0.81 (0.42-2.37) | |
| Histology | ||
| SQC | 2.93 (1.22-8.14) | <0.001 |
| Non-SQC | 0.62 (0.35-1.54) | |
| Size of tumor, cm† | ||
| <4.0 | 0.72 (0.41-1.82) | <0.001 |
| ≥4.0 | 2.70 (0.81-5.65) | |
| Nodal invasion | ||
| No | 0.87 (0.44-2.93) | 0.129 |
| Yes | 0.53 (0.30-1.13) | |
| TNM stage‡ | ||
| IA/IB | 0.73 (0.41-1.81) | 0.006 |
| IIA/IIB | 1.78 (0.55-5.11) | |
| Pleural invasion | ||
| PL0 | 0.82 (0.43-2.46) | 0.639 |
| PL1/2/3 | 0.87 (0.43-2.46) | |
| Lymphatic invasion | ||
| No | 0.85 (0.43-2.48) | 0.657 |
| Yes | 0.81 (0.30-2.93) | |
| Vascular invasion | ||
| No | 0.83 (0.43-2.51) | 0.570 |
| Yes | 1.26 (0.54-4.78) | |
| Albumin, g/dL | ||
| <3.5 | 9.90 (3.24-18.28) | 0.001 |
| ≥3.5 | 0.81 (0.42-2.26) | |
| Anemia§ | ||
| No | 0.73 (0.39-1.61) | <0.001 |
| Yes | 1.71 (0.58-6.75) | |
† Determined by a receiver operating characteristic curve analysis.
‡ AJCC 8th edition.
§ The cutoff point is 12 g/dL in female patients and 13 g/dL in male patients.
CLR, C-reactive protein to lymphocyte ratio; IQR, interquartile range; ECOG PS, Eastern Cooperative Oncology Group performance status; BMI, body mass index; SQC, squamous cell; TNM, tumor-node-metastasis; PL, pleural invasion.
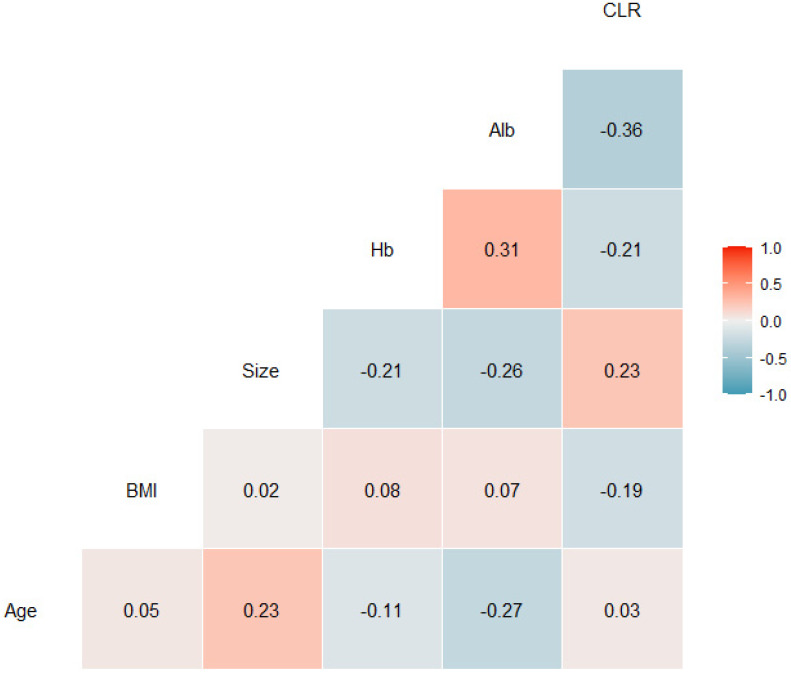
In addition, there was a significant correlation between CLR and serum albumin level (r=-0.36). However, there were only weak correlations between CLR and age (r=0.03), BMI (r=-0.19), tumor size (r=0.23), and hemoglobin concentration (r=-0.21) using tests for Pearson correlation (Fig. 3).
Figure 3.
Correlation coefficients between patient characteristic and C-reactive protein to lymphocyte ratios. Alb, serum albumin level; BMI, body mass index; CLR, C-reactive protein to lymphocyte ratio; Hb, hemoglobin concentration.
Discussion
The purpose of the present study was to assess the clinical feasibility of CLR in a cohort of NSCLC patients undergoing curative surgical resection. In this study, we found that preoperative CLR was a significant determinant of OS and CSS.
As CLR is a relatively new concept, there are only a few available studies, irrespective of cancer type. In a study by Kono et al., using a multivariate Cox model, CLR was found to be a significant determinant of OS and DSS in patients with gastric cancer undergoing curative surgery 13. In addition, in a study by Fan et al., pre-treatment CLR was found to be a feasible biomarker for the prediction of OS in patients with pancreatic cancer 14.
In our study, using the multivariate Cox model, CLR, along with three covariates (such as age, lymphatic invasion, and stages), was a significant determinant of both OS and CSS. Regarding OS, Harrell's C-index for the Cox model was 0.8337, indicating excellent discrimination. Moreover, the VIFs for age, lymphatic invasion, stage, and CLR showed no significant collinearity between these covariates. As for CSS, Harrell's C-index for the Cox model was 0.8571, indicating excellent discrimination. Furthermore, the VIFs for age, lymphatic invasion, stage, and CLR showed no significant collinearity between these covariates.
When excluding CLR from the model, the C-index for OS decreased to 0.8269, and that for CSS decreased to 0.8345, highlighting the significance of CLR as a determinant of survival. Bootstrap cross-validation also revealed a higher C-index for both OS and CSS in the full model (i.e., age, lymphatic invasion, stage, and CLR) than in the baseline model (i.e., age, lymphatic invasion, and stage), therefore, the full model including CLR seems to have wider clinical applicability than the baseline model (Fig. 2A and Fig. 2B). In addition, when CLR was replaced with CRP, the C-index for OS decreased to 0.8336, and that for CSS decreased to 0.8440. As such, the models including CLR rather than CRP are more predictive.
However, the mechanisms underlying the clinical significance of CLR remain unclear. CRP, an acute-phase reactant, is one of the most frequently used markers that reflects the body's systemic inflammatory response 20, 21. In patients with malignant tumors, CRP levels are reportedly modulated by cytokines, particularly interleukin 6, which is produced by the tumor cells themselves or by the surrounding cells 22. The role of CRP in tumorigenesis has also been elucidated in malignant tumors 23. Furthermore, in NSCLC, preoperative CRP has been suggested as a significant determinant of oncological outcome 3, 5, 24, 25. The peripheral blood absolute lymphocyte count (ALC) has been suggested as an important nutritional index in patients with various diseases 9, 26. In addition, its role as a marker for host cell-mediated cytotoxic immunity against infection and tumors has also been suggested 27. In NSCLC, preoperative ALC has been considered a good indicator of oncological outcome 9, 28, 29. As CRP reflects the level of the systemic inflammatory response 21 and ALC reflects the level of the nutritional and immunologic status 30, CLR could reflect the balance between inflammation, immunity, and nutrition in the body 10-15. In this study, relative increases in CLR levels were demonstrated in the elderly, male gender, former/current smoker, underweight, squamous cell carcinoma, increased tumor size, stage II, hypoalbuminemia, and anemia (Table 3). These findings may, at least in part, give an answer to why the CLR is an independent prognostic factor for OS and CSS in our study.
In the present study, age was associated with OS and CSS in a multivariate Cox model. The prognostic significance of age has been reported in previous studies on patients with NSCLC undergoing curative surgical resection 2-4, 7, 31, 32. In addition, lymphatic invasion was associated with poor OS and CSS rates in the multivariate Cox model. The prognostic significance of lymphatic invasion has been reported in previous studies on patients with NSCLC undergoing curative surgical resection 2, 8, 31.
The strengths of this study were as follows. First, the present study was the first to demonstrate the role of preoperative CLR as a determinant of survival in patients with NSCLC undergoing surgical resection. Using a multivariate Cox model, we found that CLR was a significant determinant of long-term outcomes (i.e., OS and CSS) along with age, lymphatic invasion, and stage. In this study, CLR was treated as a continuous variable, instead of being dichotomized according to the cutoffs. Optimizing cutoffs by minimizing the p-value can lead to bias, such as an overestimation of the prognostic impact, since cutoffs cannot be applied in different cohorts. In addition, dichotomization based on cutoffs falsely suggests that there are two qualitatively different subgroups when the prognostic impact is in fact linear 33. As such, our findings may help thoracic surgeons better differentiate patients with poor long-term survival using CLR with traditional stratification tools prior to therapeutic resection for NSCLC. Second, CLR is a combination of test items that are routinely tested before surgery, and it has the advantage of being able to obtain the test results quickly without the need for expensive test equipment.
This study had some limitations, therefore, the research results must be interpreted carefully. First, this study was conducted retrospectively. Therefore, missing data was inevitable and may have affected the results. Second, while both potential biases and random errors were controlled from the study plan to the implementation, our study had a limitation of single-center data analysis without validation through independent cohorts. However, based on the results of this study, it is possible to conduct a prospective study with an independent external verification group as the next step of this study.
In conclusion, our study demonstrated that preoperative CLR is an important determinant of OS and CSS in stage I and II NSCLC patients undergoing curative resection. However, external validation of our results is a prerequisite before they are subjected to clinical applications.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics Committee Approval
This study protocol was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (IRB2021-04-009). Written consent was waived because this study was a retrospective study and waiver does not negatively affect the rights and well-being of the subject. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki for medical research.
Author Contributions
Study design: Jae-Joon Hwang, Sookyung Lee; Data collection: Joon Young Hur, Dae Hyun Kim; Statistical analysis: Wankyu Eo; Manuscript: Wankyu Eo, Soomin An.
References
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Annals of thoracic surgery. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-u. discussion 22-3. [DOI] [PubMed] [Google Scholar]
- 2.Balata H, Foden P, Edwards T, Chaturvedi A, Elshafi M, Tempowski A. et al. Predicting survival following surgical resection of lung cancer using clinical and pathological variables: The development and validation of the LNC-PATH score. Lung cancer (Amsterdam, Netherlands) 2018;125:29–34. doi: 10.1016/j.lungcan.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Lim JU, Yeo CD, Kang HS, Park CK, Kim JS, Kim JW. et al. Prognostic value of platelet count and lymphocyte to monocyte ratio combination in stage IV non-small cell lung cancer with malignant pleural effusion. PloS one. 2018;13:e0200341. doi: 10.1371/journal.pone.0200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onaitis MW, Furnary AP, Kosinski AS, Kim S, Boffa D, Tong BC. et al. Prediction of Long-Term Survival After Lung Cancer Surgery for Elderly Patients in The Society of Thoracic Surgeons General Thoracic Surgery Database. The Annals of thoracic surgery. 2018;105:309–16. doi: 10.1016/j.athoracsur.2017.06.071. [DOI] [PubMed] [Google Scholar]
- 5.Hara M, Matsuzaki Y, Shimuzu T, Tomita M, Ayabe T, Enomoto Y. et al. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer research. 2007;27:3001–4. [PubMed] [Google Scholar]
- 6.Shinohara S, Otsuki R, Kobayashi K, Sugaya M, Matsuo M, Nakagawa M. Impact of Sarcopenia on Surgical Outcomes in Non-small Cell Lung Cancer. Annals of surgical oncology. 2020;27:2427–35. doi: 10.1245/s10434-020-08224-z. [DOI] [PubMed] [Google Scholar]
- 7.Tomita M, Shimizu T, Ayabe T, Yonei A, Onitsuka T. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer research. 2011;31:2995–8. [PubMed] [Google Scholar]
- 8.Xia H, Sun Z, Deng L, Zhu D, Wang D. Prognostic Significance of the Preoperative Lymphocyte to Monocyte Ratio in Patients with Stage I Non-Small Cell Lung Cancer Undergoing Complete Resection. Cancer investigation. 2016;34:378–84. doi: 10.1080/07357907.2016.1213276. [DOI] [PubMed] [Google Scholar]
- 9.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2009;137:425–8. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 10.Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ichikawa T, Yin C. et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clinical nutrition (Edinburgh, Scotland) 2020;39:1209–17. doi: 10.1016/j.clnu.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, Lymphocyte-C-reactive Protein Ratio as Promising New Marker for Predicting Surgical and Oncological Outcomes in Colorectal Cancer. Annals of surgery. 2019. [DOI] [PubMed]
- 12.Okugawa Y, Toiyama Y, Fujikawa H, Ide S, Yamamoto A, Omura Y, Prognostic Potential of Lymphocyte-C-Reactive Protein Ratio in Patients with Rectal Cancer Receiving Preoperative Chemoradiotherapy. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2020. [DOI] [PubMed]
- 13.Kono Y, Saito H, Murakami Y, Shishido Y, Kuroda H, Matsunaga T. et al. Postoperative ratio of the maximum C-reactive protein level to the minimum peripheral lymphocyte count as a prognostic indicator for gastric cancer patients. Surgery today. 2019;49:206–13. doi: 10.1007/s00595-018-1724-x. [DOI] [PubMed] [Google Scholar]
- 14.Fan Z, Luo G, Gong Y, Xu H, Qian Y, Deng S, Prognostic Value of the C-Reactive Protein/Lymphocyte Ratio in Pancreatic Cancer. Annals of surgical oncology. 2020. [DOI] [PubMed]
- 15.Nozoe T, Kono M, Kuma S, Tsujita E, Ohga T. New scoring system to create a prognostic criteria in colorectal carcinoma based on serum elevation of C-reactive protein and decrease in lymphocyte in peripheral blood. The journal of medical investigation: JMI. 2019;66:264–8. doi: 10.2152/jmi.66.264. [DOI] [PubMed] [Google Scholar]
- 16.Travis WD. The 2015 WHO classification of lung tumors. Der Pathologe. 2014;35(Suppl 2):188. doi: 10.1007/s00292-014-1974-3. [DOI] [PubMed] [Google Scholar]
- 17.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Kawase A, Yoshida J, Miyaoka E, Asamura H, Fujii Y, Nakanishi Y. et al. Visceral pleural invasion classification in non-small-cell lung cancer in the 7th edition of the tumor, node, metastasis classification for lung cancer: validation analysis based on a large-scale nationwide database. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8:606–11. doi: 10.1097/JTO.0b013e31828632b8. [DOI] [PubMed] [Google Scholar]
- 19.Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathology, research and practice. 1994;190:115–23. doi: 10.1016/S0344-0338(11)80700-4. [DOI] [PubMed] [Google Scholar]
- 20.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. The Journal of experimental medicine. 2000;192:1353–64. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Z, Zhang Y, Wu H. Regulation of C-reactive protein conformation in inflammation. Inflammation research: official journal of the European Histamine Research Society [et al] 2019;68:815–23. doi: 10.1007/s00011-019-01269-1. [DOI] [PubMed] [Google Scholar]
- 22.Morris-Stiff G, Gomez D, Prasad KR. C-reactive protein in liver cancer surgery. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2008;34:727–9. doi: 10.1016/j.ejso.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Liu Z, Liu H, He J, Yang J, Lin P, C-reactive protein promotes bone destruction in human myeloma through the CD32-p38 MAPK-Twist axis. Science signaling. 2017. 10. [DOI] [PMC free article] [PubMed]
- 24.Alifano M, Falcoz PE, Seegers V, Roche N, Schussler O, Younes M. et al. Preresection serum C-reactive protein measurement and survival among patients with resectable non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. 2011;142:1161–7. doi: 10.1016/j.jtcvs.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Hara M, Yonei A, Ayabe T, Tomita M, Nakamura K, Onitsuka T. Postoperative serum C-reactive protein levels in non-small cell lung cancer patients. Annals of thoracic and cardiovascular surgery: official journal of the Association of Thoracic and Cardiovascular Surgeons of Asia. 2010;16:85–90. [PubMed] [Google Scholar]
- 26.Kanda M, Mizuno A, Tanaka C, Kobayashi D, Fujiwara M, Iwata N. et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine. 2016;95:e3781. doi: 10.1097/MD.0000000000003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karimi S, Chattopadhyay S, Chakraborty NG. Manipulation of regulatory T cells and antigen-specific cytotoxic T lymphocyte-based tumour immunotherapy. Immunology. 2015;144:186–96. doi: 10.1111/imm.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu J. et al. Prognostic Significance of Combination of Preoperative Platelet Count and Neutrophil-Lymphocyte Ratio (COP-NLR) in Patients with Non-Small Cell Lung Cancer: Based on a Large Cohort Study. PloS one. 2015;10:e0126496. doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Wang S, Zhang H, Zhang B, Wang C. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer. Cancer biology & medicine. 2018;15:88–96. doi: 10.20892/j.issn.2095-3941.2017.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast cancer research: BCR. 2017;19:2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Zhao J, Zhang M, Han L, Wang M, Xingde L. The combination of plasma fibrinogen and neutrophil lymphocyte ratio (F-NLR) is a predictive factor in patients with resectable non small cell lung cancer. Journal of cellular physiology. 2018;233:4216–24. doi: 10.1002/jcp.26239. [DOI] [PubMed] [Google Scholar]
- 32.Li SJ, Lv WY, Du H, Li YJ, Zhang WB, Che GW. et al. Albumin-to-alkaline phosphatase ratio as a novel prognostic indicator for patients undergoing minimally invasive lung cancer surgery: Propensity score matching analysis using a prospective database. International journal of surgery (London, England) 2019;69:32–42. doi: 10.1016/j.ijsu.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Hacker UT, Hasenclever D, Linder N, Stocker G, Chung HC, Kang YK. et al. Prognostic role of body composition parameters in gastric/gastroesophageal junction cancer patients from the EXPAND trial. Journal of cachexia, sarcopenia and muscle. 2020;11:135–44. doi: 10.1002/jcsm.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]