Abstract
The purpose of this study was to investigate the effects of perioperative administration of oral meloxicam prior to and following the application of caustic paste to disbud neonatal dairy calves. Sixty-one 3–4-d-old Holstein heifer calves were randomly assigned to one of four treatment groups of 15–16 calves. The treatment groups were: 1) M1, caustic paste disbudding and oral meloxicam (45 mg) with a placebo 24 h later; 2) M2, treatment M1 followed by a second 45-mg dose of meloxicam 24 h later instead of placebo; 3) CONTROL, treatment M1 with placebo in place of meloxicam; and 4) SHAM, sham disbudding with placebo in place of meloxicam. Infrared thermography was used to quantify eye and horn bud temperatures. Pressure algometry was used to measure Mechanical nociceptive threshold (MNT) surrounding the horn bud. Average daily gain and body weight (BW) were obtained by weighing each animal throughout the study and calculating the changes over time. Plasma was collected and analyzed for cortisol and substance P concentrations. Substance P and cortisol decreased in all animals over time, regardless of treatment. Mean plasma substance P concentration across all time points was greater (P < 0.05) in the SHAM group than M1 or M2 but not different (P > 0.05) than the CONTROL group. The MNT and ocular temperatures decreased over time across all treatments (P < 0.05). Mean BW increased over time across all treatments (P < 0.05). A significant interaction (P < 0.05) between treatment and sampling time was observed at 12 h following treatment application for both mean horn bud temperature and the ratio between horn bud and ocular temperature. Overall, the results of this study suggest that meloxicam administration at a dose of 45 mg per animal may have limited influence as the primary modulator of pain and inflammatory response in calves that have been disbudded with caustic paste at 3 d of age.
Keywords: calf, dehorn, disbud, horn, meloxicam, pain
INTRODUCTION
One of the most common painful procedures for North American dairy cattle is removal of the horns or horn buds. In 2014, 94.3% of North American dairy operations reported routine removal of the horns of heifer calves on their premises (National Animal Health Monitoring System [NAHMS], 2014). Of those dairy operations, 16.4% utilized caustic paste for disbudding at 2.3 wk of age (NAHMS, 2014). In the same survey, 5.6% of the dairy operations that used caustic paste for routine disbudding of dairy calves utilized analgesic and/or anesthetic pain mitigation (NAHMS, 2014). Across all dairy operations that performed routine horn removal, pain mitigation use was dependent on the person that performed the procedure as 62.7% of veterinarians, 29.2% of employees, and 14.9% of owner/operators reported that they used anesthetic and/or analgesic pain mitigation for any routine horn removal procedures (NAHMS, 2014). Caustic paste disbudding has been suggested to cause less acute pain response than cautery disbudding (Vickers et al., 2005), and the procedure itself has a more innocuous appearance than other horn removal methods (Vickers et al., 2005). However, more recent work has suggested that caustic paste may be more noxious than cautery disbudding but deceiving to assess due to the differing methods of application and action (Braz et al., 2012; Hempstead et al., 2018).
Based on behavioral and cortisol responses, peak pain appears to occur within the first 4 h following caustic paste application, with the potential for chronic pain after that time period (Morisse et al., 1995; Stilwell et al., 2009). Winder et al. (2017) reported that acute pain persisted for a minimum of 180 min following the application of caustic paste to disbud 3–5-d-old Holstein calves that were treated with 0.5 mg/kg subcutaneous meloxicam 15 min before caustic paste application. In the same study, regional anesthesia in the form of a lidocaine cornual nerve block (5 mL per side) effectively reduced acute pain indication by algometry for approximately 180 min in calves that were treated with the same dose of meloxicam 15 min before caustic paste application. Braz et al. (2012) indicated that caustic paste disbudding caused severe pain for the first 30 min following paste application and found tramadol to be ineffective in controlling acute pain associated with the procedure. Stilwell et al. (2008, 2009) reported that flunixin meglumine alone was also ineffective in controlling a postapplication cortisol increase in dairy calves following caustic paste application.
One potential option for pain mitigation for caustic paste disbudding is meloxicam. Meloxicam is a long-acting NSAID that is similarly available both parenterally and orally, has peak availability at approximately 12 h, and has a half-life of 28 h (Coetzee et al., 2009). This makes meloxicam uniquely suited for administration up to 8 h prior to dehorning or shortly before the application of caustic paste and may make a second dose unnecessary until days after the initial dose was administered as meloxicam may suppress the concentrations of prostaglandin E2 (PGE2), an important prostaglandin associated with the Cyclooxygenase-2 (COX-2) inflammatory pathway, for as long as 3 d (Allen et al., 2013). To this point in time, no previous work had been published regarding the impact of meloxicam as the primary pain mitigation therapy for caustic paste disbudding, and the research on caustic paste disbudding in general is limited. As a result, this study was focused on exploring the impact of practical meloxicam administration techniques on dairy calves. The objective of this study was to investigate the effects of perioperative administration of oral meloxicam prior to and following the application of caustic paste to disbud neonatal dairy calves.
MATERIALS AND METHODS
Institutional Animal Care and Use Committee Approval
Prior to the commencement of this study, animal use and associated procedures were approved by the University of Wisconsin—River Falls Institutional Animal Care and Use Committee. The protocol number associated with this study was A15-16–7.
Animals and Housing
Sixty-one female Holstein calves (41.2 ± 0.1 kg body weight [BW]) were housed in individual outdoor hutches on a sand base with a layer of wood shavings and straw bedding inside each hutch (approximate area: 2.4 m2) and in an individual outdoor exercise space accompanying each hutch (approximate area: 2 m2). Each calf was fitted with a thermal jacket that was worn for the duration of the study at the time of separation from her dam. All calves were born and housed on the same commercial dairy farm in Western Wisconsin between February and April 2016. Each calf was separated from her dam within an hour of birth and fed 3.8 liters of colostrum by gastroesophageal tube within 2 h of birth. A second feeding of colostrum was administered by bottle 12–24 h after the initial colostrum feeding. Following the second colostrum feeding, each calf was fed 1.9 liters of pasteurized whole milk three times daily. All calves were enrolled in the study and received their assigned treatments at exactly 3 d of age. The number of calves that were enrolled in the study on each day varied depending on the number of calves that were born 3 d earlier. The treatments were assigned in rolling order such that each treatment was represented once in each group of four calves that were consecutively enrolled in the study. The specific treatment that each calf received was dependent on the chronological birth order and next treatment assignment in the repeating treatment application order. Farm staff were blind to all treatments. Some project personnel were not blind to the treatment groups if they were involved in treatment application. However, the specific outcomes that were assessed were unlikely to be affected by the treatment knowledge of those personnel.
Experimental Design and Treatments
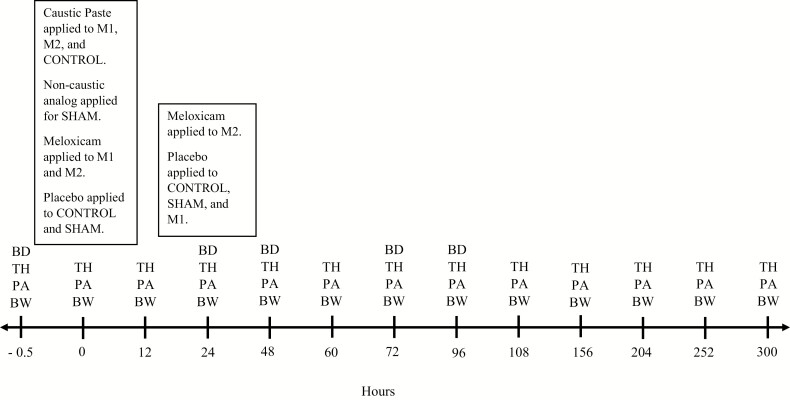
Each calf was assigned to one of four treatment groups. Treatment assignments were provided in rolling order as calves were enrolled in the study at 3 d of age. The treatment groups were: 1) M1, caustic paste disbudding and oral meloxicam (45 mg) with a placebo 24 h later; 2) M2, treatment M1 followed by a second 45-mg dose of meloxicam 24 h later instead of placebo; 3) CONTROL, treatment M1 with placebo in place of meloxicam; and 4) SHAM, sham disbudding with placebo in place of meloxicam. The treatment application and sample collection timeline may be observed in Figure 1. At each time point, ocular and horn bud thermographs were collected followed by algometry data for the tissue surrounding the horn buds. On odd-numbered evenings beginning on day 1, BW was also recorded in addition to the thermographs and algometry readings. Thermographs and algometry readings were collected during the morning feedings on days 1–5 and on odd-numbered evenings for each calf, resulting in measurements for time points −5 min, 0, 12, 24, 48, 60, 72, 96, 108, 156, 204, 252, and 300 h from disbudding. For all treatments and measurements except blood collection, calves were restrained in sternal recumbency with one student straddling the calf while applying one hand to the base of the head and one to the nose to limit movement of the calf’s head.
Figure 1.
This timeline graphic outlines the treatment administration times and sample collection times over the course of the study. The treatment groups were: 1) M1: caustic paste disbudding and oral meloxicam (45 mg) with a placebo 24 h later; 2) M2: treatment M1 followed by a second 45-mg dose of meloxicam 24 h later instead of placebo; 3) CONTROL: treatment M1 with placebo in place of meloxicam; and 4) SHAM: sham disbudding with placebo in place of meloxicam. Sample collections are defined as: BD = blood draw, TH = ocular and horn bud infrared thermography, PA = pressure algometry, WT = body weight. Time point 0 marks the measurements recorded immediately after treatment application.
Administration of Oral Meloxicam
All calves in this experiment were fed 1.9 liters of pasteurized milk after the administration of meloxicam and collection of measurements at each time point. The experimental dose of meloxicam or placebo was administered via balling gun. Three—15 mg meloxicam tablets (Unichem Pharmaceuticals (USA) Inc., Rochelle Park, NJ) were loaded into empty gel capsules (size 00, Torpac Inc., Fairfield, NJ) and two capsules at a time were administered to calves in the M1 and M2 treatment groups at the scheduled administration times. Of the two capsules, one capsule was always empty and served to assist in holding the meloxicam or placebo capsule in the balling gun during passage into the esophagus. The second capsule contained a total of 45 mg of meloxicam. Each dose of meloxicam provided approximately 1.0 mg meloxicam per kg of calf BW. Meloxicam or placebo delivery occurred approximately 5 min before the disbudding procedure and just before the feeding 24 h postdisbudding. Calves that received placebo at either or both time points received empty gel capsules via balling gun instead of meloxicam in gel capsules.
Disbudding and Sham Disbudding Procedure
Prior to disbudding treatment application, a battery-operated hair clipper was used to trim the hair to a length of approximately 1 mm within a 3-cm radius of the horn buds. A commercially available caustic paste product that contained a mixture of sodium and calcium hydroxide (Dr Naylor Dehorning Paste, H. W. Naylor Company Inc, Morris, NY) or a noncaustic analog (Up and Up creamy diaper rash ointment, Target Stores, Minneapolis, MN) was administered to the horn bud. The noncaustic analog was similar in consistency to the caustic paste but differed in color. The caustic paste was light red in color and the noncaustic analog was white. Paste, either caustic or noncaustic, was evenly applied with a gloved hand at a thickness of approximately 1 mm to an area on the horn bud that was approximately 18 mm in diameter
BW and Average Daily Gain
The BW of each animal involved in this study was collected from a digital hanging scale (Product #34626, Roughneck Digital Hanging Scale, Northern Tool + Equipment, Burnsville, MN) equipped with a commercially available sling (Item # 17123, Calf Weighing Sling, Valley Vet Supply, Marysville, KS) to safely suspend each calf during weight collection. BW was recorded on odd-numbered evenings over a 13-d period beginning on the day of disbudding and meloxicam treatment application. To calculate ADG, the total amount of BW change between BW collection events was divided by the total number of experimental days that elapsed since the previous BW collection event.
Blood Collection
Whole blood was collected via jugular venipuncture with 21-g × 2.54-cm blood collection needles (REF 450042, Greiner Bio-One GmbH, Kremsmünster, Austria) from all animals in each of the four treatment groups. Blood was collected prior to the administration of meloxicam and caustic paste (time −5 min) to obtain baseline substance P and cortisol concentrations, in addition to times 24, 48, 72, and 96 h. At each collection time, a single 10-mL nonadditive blood collection tube (BD, Franklin Lakes, NJ) and a single 6-mL blood collection tube with lithium heparin additive (BD, Franklin Lakes, NJ) were collected from each calf. Immediately after collection, 6 mL of whole blood from the nonadditive tube was transferred to a 6-mL blood collection tube that contained sodium ethylenediaminetetraacetic acid (EDTA) additive plus 300 μL of benzamidine solution. Within 30 min of collection, all whole blood was centrifuged in the original collection tube for cortisol and the sodium EDTA plus benzamidine tubes for substance P at 2,500 × g for 15 min. Plasma was transferred to microcentrifuge tubes with disposable transfer pipettes and then frozen in liquid nitrogen before transferring to storage at −80 °C until analysis via radioimmunoassay at the Iowa State Veterinary diagnostic laboratory.
Cortisol and Substance P Quantification
Plasma substance P and cortisol concentrations were quantified at the Iowa State University Veterinary Diagnostic Laboratory following procedures that were described by Kleinhenz et al. (2018). A commercially available kit (catalog # 06B-256440, Corti-Cote Cortisol RIA Kit, MP Biomedicals Inc., Santa Ana, CA) was used to estimate plasma cortisol concentrations for each sample in duplicate following the manufacturer’s procedure. The duplicate values were averaged for each sample prior to statistical analysis. The coefficient of variation for intra-assay variability was 10.31%. The coefficient of variation for interassay variability was 5.59%.
Substance P was quantified in nonextracted plasma following procedures that were outlined by Kleinhenz et al. (2018), Van Engen et al. (2014), and Liu et al. (2008). A two-antibody radioimmunoassay (RIA) was used to estimate plasma substance P concentrations for each sample in duplicate. The duplicate values were averaged for each sample prior to statistical analysis. The coefficient of variation for intra-assay variability was 10.55%. The coefficient of variation for interassay variability was 27.64%.
Meloxicam Quantification
Plasma meloxicam concentrations were quantified for calves in the M1 and M2 treatment groups at the Iowa State University Veterinary Diagnostic Laboratory following procedures similar to those described by Repenning et al. (2013). High-pressure liquid chromatography tandem–mass spectrometry (HPLC–MS) was performed with a LTQ ion trap (Thermo Scientific, San Jose, CA) coupled with an Agilent 1100 series pump and autosampler (Agilent Technologies, Santa Clara, CA) to analyze each sample in single. The instrument injection volume was set to 20 μL. The mobile phases were A: 0.1% formic acid in water; and B: 0.1% formic acid in acetonitrile. The gradient curve began at 25% B with a linear gradient to 95% B in 5 min, maintained for 1.75 min, then re-equilibration to 25% B. The flow rate was 0.25 mL/min and increased to 0.325 mL/min at 5.25 min. Separation was performed with an UltraCore C18 100- × 2.1-mm column (Kinetex XB-C18, Phenomenex, Totrrence, CA). Meloxicam eluted at 5.4 min and piroxicam eluted at 4.7 min. Four selected reaction monitoring transitions each were monitored for meloxicam (115, 141, 184, and 352 m/z) and piroxicam (227, 270, 282, and 332 m/z). Sequences were batch processed with a method developed in Xcalibur software (Thermo Scientific, San Jose, CA). The method automatically identified and integrated each peak in each sample and calculated the calibration curve based on a weighted (1/X) quadratic fit. Plasma concentrations of meloxicam in unknown samples were calculated by the Xcalibur software based on the calibration curve. The correlation coefficient (r2) was > 0.98 across the concentration range of 5–5,000 ng/mL. QC samples were within tolerance of ±15% of the nominal value.
Infrared Thermography
Infrared thermography has previously been used to observe the change in temperature of the eye during painful procedures in several studies (Stewart et al., 2008; Allen et al., 2013; Stock et al., 2015; Kleinhenz et al., 2018). An infrared camera (FLIR E8 Thermal Camera, FLIR Systems, Boston, MA) was used to collect three thermal images of each eye and each horn bud for each animal at the time points identified in Figure 1. Thermographs were collected from a distance of approximately 30.5 cm with the camera focal point in perpendicular alignment with the target location. A laser tape measure (Bosch GLM 10 Compact Laser Measure, Robert Bosch GmbH, Stuttgart, Germany) was attached to the thermal camera to facilitate rapid, accurate, and consistent distance determination.
Ocular thermographs were collected first and then each calf was blindfolded and allowed to acclimate to the blindfold while horn bud thermographs were collected prior to pressure algometry. Stock et al. (2015) also used a blindfold to assist in maintaining stable body and head position during algometry. Thermographs were analyzed with FLIR Tools software (FLIR Systems, Boston, MA). For each image, the area of interest (either eye or horn bud) was selected via a function in FLIR Tools, and the maximum temperature of the selected field was recorded and entered into a Microsoft Excel spreadsheet and used for statistical analysis. The mean of the three maximum temperatures recorded from the thermal images for each eye and each horn bud was ultimately included in the statistical analyses.
Pressure Algometry
Pressure algometry has been used to assess the mechanical nociceptive threshold (MNT) of animals in previous experiments investigating the pain response to disbudding (Heinrich et al., 2010; Allen et al., 2013; Glynn et al., 2013; Winder et al., 2017; Adcock and Tucker, 2018). MNT has been used as a measure of sensitivity to physical stimulus; a greater MNT value suggests a greater tolerance to force and decreased sensitivity. A pressure algometer (Wagner Force Ten FDX 25 Compact Digital Force Gage, Wagner Instruments, CT) was used to apply pressure to three areas anterior to, lateral to, and posterior to each horn bud at the edge of the paste that was applied. A 1-cm2 flat rubber tip accessory was affixed to the algometer. Force was applied at an approximate rate of 1.0 kgf/s until the animal attempted to move its head away from the applied pressure, similar to the procedure in Winder et al. (2017). Maximum force did not exceed 10 kg force to prevent excessive discomfort for the calf. The maximum force applied prior to movement away from the pressure was recorded for each of the three locations for each of the horn buds at each collection time identified in Figure 1. The mean MNT for each horn bud at each collection time was included in the statistical analyses.
Statistical Analyses
All continuous data for pain mitigation treatment (CONTROL, SHAM, M1, and M2), sampling time, and pain mitigation by sampling time effects were analyzed using models constructed within the MIXED procedure of SAS (SAS Enterprise Guide 4.3, SAS Institute Inc., Cary, NC) in an unpaired-comparison design and mean separation performed with the Tukey’s test designation. The daily ambient temperature, relative humidity, and wind speed was collected with an on-site weather station (Model # 01604DI, AcuRite Pro Color Weather Station, Chaney Instrument Co., Lake Geneva, WI) and included as covariates nested within calf within day since treatment application within the analysis models. We took a backward stepwise approach to analysis output interpretation by observing the interaction effect of pain mitigation treatment and sampling time first. If a significant interaction effect did not exist, individual main effects (pain mitigation treatment and sampling time, individually) were observed. Significant differences in treatment effects were recognized at α ≤ 0.05.
RESULTS AND DISCUSSION
Currently, meloxicam is considered to be a nonsteroidal anti-inflammatory drug (NSAID) belonging to the oxicam class that is only approved for use in Australia, the European Union, and Canada with a labeled use as therapy for acute respiratory disease, diarrhea, mastitis and alleviation of acute pain and inflammation in cattle (Cotter, 2013; Health Canada, 2014; Boehringer Ingelheim, 2016; European Medicines Agency [EMA], 2018). On the contrary, the United States only has one drug, called flunixin meglumine, approved by the Food and Drug Administration (FDA) as an NSAID for cattle with a label referencing pain treatment. Therefore, any administration of meloxicam falls into the category of extra label drug use (ELDU). A valid veterinary patient client relationship (VCPR) must be maintained in order to administer drugs extra label to livestock (U.S. Food and Drug Administration, 1994). Thus, use of meloxicam is ELDU and requires a valid VCPR in order to be administered to livestock in the United States.
The plasma meloxicam concentrations of calves that were treated with meloxicam are reported in Table 1. Plasma from calves that were not treated with meloxicam, namely, the CONTROL and SHAM calves, were not assayed to conserve resources allocated to this study. The concentrations observed in this study were congruous with those reported by Allen et al. (2013). Allen et al. (2013) indicated some analgesic effects of meloxicam on indicators of pain and inflammation in 8–10-wk-old Holstein calves that also received a lidocaine cornual nerve block prior to hot iron dehorning. The M1 and M2 treatments resulted in plasma meloxicam concentrations that were not different at 24 h following the initial meloxicam dose (P > 0.05). However, the plasma meloxicam concentration continued to rise in the M2 treatment group because a second dose was delivered to that treatment group at 24 h. For the remainder of the 96-h plasma sampling period, M2 calves displayed plasma meloxicam concentrations that were approximately twice as elevated as those observed in M1 calves (P < 0.0001).
Table 1.
Least squares means for interaction effect of meloxicam treatment and sampling time on plasma meloxicam concentration (ng/mL) of 3-d-old Holstein calves disbudded with caustic paste (N = 30)
| Sampling time | ||||||
|---|---|---|---|---|---|---|
| Meloxicam treatment1 | 24 h | 48 h | 72 h | 96 h | Pooled SE | P-value (treatment × time) |
| M1, n = 15 | 3348.87de | 3092.07e | 2608.33f | 2182.13g | 156.50 | < 0.0001 |
| M2, n = 15 | 3570.40d | 6622.90a | 5629.86b | 4872.47c |
a–gMeans with like superscripts within all rows and columns do not differ (P > 0.05).
1Treatments: 1) M1, caustic paste disbudding and oral Meloxicam (45 mg) with a placebo 24 h later and 2) M2, treatment M1 followed by a second 45-mg dose of meloxicam 24 h later instead of placebo.
Table 2 reports the impact of meloxicam treatment on growth, algometry, thermography, and select blood constituents of the calves in this study. There were no statistically significant treatment effects on average daily gain (ADG), BW, maximum ocular temperature, or plasma cortisol concentration. Repenning et al. (2013) also reported that oral meloxicam did not impact ADG during an experiment involving band castration of beef bulls. Those results, and ours, concur with Adcock and Tucker (2018), who reported that the ADG of Holstein and Jersey heifer calves was not impacted by cautery disbudding without pain mitigation at 3 or 35 d of age. These results are similar to another study that investigated pain management associated with horn removal in cattle. Fraccaro et al. (2013) found that meloxicam, along with gabapentin (an antiepileptic agent), had no significant influence on blood PGE2 concentrations in 6-mo-old Holstein steers that were provided a lidocaine cornual nerve block and mechanically dehorned with a Barnes dehorner.
Table 2.
Least squares means for the effect of pain treatment options on the growth, algometry, thermography, and specific blood constituent concentrations of 3-d-old Holstein calves disbudded with caustic paste (N = 61)
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Dependent variable | CONTROL (n = 16) | SHAM (n = 15) | M1 (n = 15) | M2 (n = 15) | SE | P-value |
| Growth | ||||||
| ADG, kg | 0.3 | 0.44 | 0.63 | 0.53 | 0.4 | 0.7445 |
| BW, kg | 44.8 | 44.9 | 43.4 | 43.3 | 1.9 | 0.3193 |
| Horn bud algometry | ||||||
| Mean MNT, kgf | 6.57ab | 7.41a | 6.26b | 7.20ab | 0.30 | 0.0212 |
| Ocular thermography | ||||||
| Maximum OT, °C | 36.21 | 36.28 | 35.97 | 36.03 | 0.23 | 0.1281 |
| Blood constituents | ||||||
| Cortisol, ng/mL | 52.60 | 57.25 | 47.59 | 55.91 | 6.25 | 0.2574 |
| Substance P, pg/mL | 164.47ab | 198.53a | 144.50b | 144.74ab | 12.30 | 0.0058 |
a,bMeans with like superscripts within each row do not differ (P > 0.05).
1Treatments: 1) M1, caustic paste disbudding and oral meloxicam (45 mg) with a placebo 24 h later; 2) M2, treatment M1 followed by a second 45-mg dose of meloxicam 24 h later instead of placebo; 3) CONTROL, treatment M1 with placebo in place of meloxicam; and 4) SHAM, sham disbudding with placebo in place of meloxicam.
When substance P is assayed, it is generally recommended to assay cortisol as well to determine if the stressor that impacts substance P release is caused by pain or human presence and restraint stress. Meléndez et al. (2018) explained that substance P and cortisol are associated with different but interrelated mechanisms. Cortisol is a reliable indicator of acute stress that returns to normal concentrations following the stress-inducing event, such as an acutely painful event or human presence and restraint. Substance P is a pain-related neuromodulator that is implicated in the production of prostaglandin E2, an inflammatory marker that has been shown to increase after COX enzyme inhibitors are cleared (Meléndez et al., 2018). In our study, CONTROL and M2 plasma substance P concentrations were intermediate and not different (P > 0.05) than SHAM or M1. However, SHAM resulted in greater plasma substance P concentrations than M1 (P < 0.0001). It is unclear to us why substance P concentration was elevated for the SHAM group as this observation does not concur with the findings of other researchers (Coetzee et al., 2012). Previous investigations of the relationship between substance P concentration and painful procedures have also reported varying results. A study that included oral meloxicam before and after band castration of beef bulls found that plasma concentrations of substance P did not differ between castration treatments, whether with or without meloxicam administration (Repenning et al., 2013). Stock et al. (2015) reported that firocoxib administration to cautery disbudded calves did not influence plasma substance P concentration compared with a placebo.
Other investigators have reported stress and performance responses that differ from the findings in this study. Coetzee et al. (2012) reported that calves treated with meloxicam for scoop dehorning reached a level of ADG that was approximately double the ADG of calves that were not treated. Heinrich et al. (2009) treated 6–12-wk-old Holstein calves that were cautery disbudded with a lidocaine cornual nerve block and intramuscular meloxicam (0.5 mg/kg BW). They reported that meloxicam treatment caused a reduction in serum cortisol for 6 h following the dehorning procedure but no difference in concentration was observed 24-h postdehorning (Heinrich et al., 2009). While we cannot be certain of all causative factors associated with discrepancies between studies, it is very important to note the differences in method of administration (injection vs. oral) and dose (0.5 vs. 1 mg/kg BW) in these studies compared with our study.
Table 3 reports the impact of sampling time on the concentration of plasma cortisol and substance P in all calves in the study, regardless of treatment. The concentration of both molecules in plasma decreased over the course of the sampling period. The change in cortisol concentration over the course of this study is typical of studies where blood sampling occurs at multiple time points over several hours to multiple days. It is generally acknowledged that cortisol concentration typically increases rapidly following exposure to acute stressors and then declines toward normal concentrations that vary rhythmically throughout the day, even with the prolonged presence of the same acute stressor (Apple et al., 2005). Stock et al. (2015) identified a similar time effect on cortisol concentrations in preweaned dairy calves that were cautery disbudded. Our data suggest that the change in substance P and cortisol concentration across all calves over time may have been resultant of human presence and restraint stress. Investigation of this concept is warranted and necessary. In addition, specific guidance regarding the optimal time of day to measure substance P is needed for cattle. In our study, we consistently collected all blood for assay during the same time frame (0500–0700 hours) each day. It is possible that the 24-h time lapse between substance P sample collections in our study could have missed changes in concentration that occurred during the first 24 h in particular. However, as the study progressed and pain status likely shifted from acute to chronic, our sampling regimen should have been sufficient to identify differences in chronic substance P production in response to the disbudding lesions.
Table 3.
Least squares means for the effect of sampling time on specific blood constituent concentrations of 3-d-old Holstein calves disbudded with caustic paste (N = 61)
| Sampling time | |||||||
|---|---|---|---|---|---|---|---|
| Dependent variable | −5 min | 24 h | 48 h | 72 h | 96 h | SE | P-value |
| Blood constituents | |||||||
| Cortisol, ng/mL | 68.25a | 58.52a | 62.68a | 44.31b | 32.93c | 5.89 | <0.0001 |
| Substance P, pg/mL | 194.82a | 172.80b | 159.60bc | 150.09bc | 137.98c | 8.36 | <0.0001 |
a–dMeans with like superscripts within each row do not differ (P > 0.05).
Table 4 shows the impact of sampling time on ADG, BW, MNT, and ocular temperature (OT). ADG was not significantly different between sampling times (P = 0.74). However, BW increased across sampling times (P < 0.0001). Both MNT and OT declined over the sampling period. It is possible that this may have been a result of the adjustment of homeostatic mechanisms in the neonatal calves following parturition. In a review, Kirovski (2015) described the dependence of neonatal calves on nonshivering thermogenesis, a process that involves the uncoupling of adenosine triphosphate (ATP) synthase from the electron transport chain, resulting in heat production in brown adipose tissue. Brown adipose tissue recedes during the first month of life for bovine calves and other neonatal ruminants. It is possible that the decline in OT that we observed was an indication of the progressive reduction in nonshivering thermogenesis through the first month of life. Glynn et al. (2013) reported a similar time effect on MNT values proximal to the horns of Holstein steers.
Table 4.
Least squares means for the effect of sampling time on growth, algometry, and thermography of 3-d-old Holstein calves disbudded with caustic paste (N = 61)
| Sampling Time | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | −5 min | 0 h | 12 h | 24 h | 48 h | 60 h | 72 h | 96 h | 108 h | 156 h | 204 h | 252 h | 300 h | SE | P-value |
| Growth | |||||||||||||||
| ADG, kg | N/A | N/A | N/A | N/A | N/A | 0.40 | N/A | N/A | 0.60 | 0.49 | 0.45 | 0.46 | 0.46 | 0.1 | 0.0690 |
| BW, kg | N/A | N/A | 41.2d | N/A | N/A | 42.0d | N/A | N/A | 43.6c | 44.2c | 45.1b | 46.0a | 46.8e | 0.1 | <0.0001 |
| Algometry | |||||||||||||||
| Mean MNT, kgf | 8.32ab | 7.91ab | 8.56a | 7.50abcd | 7.72abc | 6.45abcd | 6.73abcd | 7.03abcd | 5.68cd | 6.55abcd | 5.99bcd | 5.60d | 5.16d | 0.50 | <0.0001 |
| Thermography | |||||||||||||||
| Maximum OT, °C | 36.97a | 36.97a | 36.70ab | 36.54abc | 36.12bcd | 36.04cd | 35.75d | 36.07bcd | 35.95cd | 35.99cd | 35.93cd | 35.56de | 34.99e | 0.25 | <0.0001 |
a–eMeans with like superscripts within each row do not differ (P > 0.05).
Table 5 reports the interaction effects of meloxicam treatment and sampling time on mean horn bud temperature by thermography and mean horn bud to ocular temperature ratio. It is important to note that the values reported at −5 min for both variables occurred before the horn buds were shaved for paste application. Longer and thicker hair provided greater insulation and cooler temperatures at the surface of the hair coat that was captured in the thermal image. Both dependent variables exhibited the treatment by sampling time interaction effect at time 0 h, which was immediately after the application of caustic paste for CONTROL, M1, and M2 calves or the noncaustic analog for SHAM. Horn bud temperature was greater in the CONTROL and M1 groups than SHAM but not different than M2. Mean horn bud to ocular temperature ratio was less in the SHAM group than any treatment that received caustic paste. No other difference was observed between treatments at any other sampling time. The data for both horn bud temperature and horn bud to ocular temperature ratio suggest that the heat production associated with apparent tissue inflammation from caustic paste application did not last longer than 12 h, regardless of meloxicam treatment. It is important to note that the absence of heat is not sufficient to conclude that pain has subsided. Adcock and Tucker (2018) found that the surface temperature of cautery disbudding wounds did not differ from the surface temperature of nondisbudded tissue during a 3-wk period following disbudding. However, the same project found that calves remained sensitive to touch near the disbudding site for 9 wk. Overall, our findings align with the conclusions of Coetzee et al. (2012) that administration of meloxicam as the only pain mitigation therapy for horn tissue removal is insufficient in the control of acute pain but may be valuable in the control of chronic pain associated with postprocedure healing. It is important to note that the discussion of the results in this study included reference to an array of studies that focused on the responses of cattle to horn removal and the impact of multiple pain mitigation strategies on those responses. Due to the limited amount of data regarding caustic paste disbudding that was available at the time of this publication, reference to other disbudding methods was necessary. However, the specific mechanisms of action are vastly different between cautery, caustic, and mechanical horn removal and different mechanisms and magnitudes of pain response must be expected and acknowledged.
Table 5.
Least squares means for the interaction effect of meloxicam treatment1 and sampling time on mean horn bud temperature and mean horn bud to ocular temperature ratio of 3-d-old Holstein calves disbudded with caustic paste (N = 61)
| Sampling time | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | −5 min | 0 h | 12 h | 24 h | 48 h | 60 h | 72 h | 96 h | 108 h | 156 h | 204 h | 252 h | 300 h | SE | P-value |
| Mean horn bud temperature, °C | |||||||||||||||
| CONTROL | 25.46 | 36.75a | 33.55 | 32.49 | 32.29 | 32.86 | 32.93 | 33.60 | 33.66 | 33.93 | 34.48 | 34.02 | 33.46 | 0.46 | <0.0001 |
| SHAM | 25.99 | 34.57b | 33.46 | 33.46 | 32.13 | 33.20 | 32.19 | 32.94 | 33.30 | 33.28 | 33.40 | 33.28 | 33.22 | ||
| M1 | 25.19 | 36.73a | 33.01 | 32.76 | 33.14 | 33.20 | 32.79 | 32.87 | 33.25 | 33.60 | 33.69 | 33.43 | 32.93 | ||
| M2 | 23.96 | 36.64ab | 33.01 | 32.14 | 33.41 | 33.29 | 32.60 | 33.15 | 33.00 | 33.45 | 33.09 | 33.93 | 33.21 | ||
| Mean horn bud: ocular temperature | |||||||||||||||
| CONTROL | 0.68 | 1.00a | 0.91 | 0.89 | 0.90 | 0.91 | 0.92 | 0.93 | 0.93 | 0.94 | 0.95 | 0.96 | 0.96 | 0.01 | <0.0001 |
| SHAM | 0.69 | 0.93b | 0.91 | 0.92 | 0.89 | 0.92 | 0.90 | 0.91 | 0.92 | 0.91 | 0.92 | 0.93 | 0.93 | ||
| M1 | 0.67 | 1.00a | 0.90 | 0.89 | 0.91 | 0.93 | 0.92 | 0.91 | 0.93 | 0.94 | 0.95 | 0.94 | 0.96 | ||
| M2 | 0.65 | 1.00a | 0.90 | 0.88 | 0.92 | 0.92 | 0.92 | 0.92 | 0.92 | 0.94 | 0.93 | 0.96 | 0.95 |
a,bMeans within dependent variable with like superscripts within column do not differ (P > 0.05).
1Treatments: 1) M1, caustic paste disbudding and oral meloxicam (45 mg) with a placebo 24 h later; 2) M2, treatment M1 followed by a second 45-mg dose of meloxicam 24 h later instead of placebo; 3) CONTROL, treatment M1 with placebo in place of meloxicam; and 4) SHAM, sham disbudding with placebo in place of meloxicam.
Our data suggest that meloxicam, as applied in our study, is not sufficient as the primary therapy to control pain and inflammation associated with caustic paste disbudding. Further study regarding the impact of cornual nerve block in conjunction with meloxicam administration on caustic paste disbudded calves is warranted as other previously cited work has identified the value of regional anesthesia to controlling the acute pain associated with caustic paste disbudding and the need for effective chronic pain mitigation. The variables that we utilized in this study may not have been completely effective in quantifying the magnitude of chronic pain associated with caustic paste administration as no overall effect was observed between the CONTROL, M1, and M2 treatment groups over time. This study would have benefitted from inclusion of assays of behavioral indicators of pain and discomfort. Preliminary work in our lab has suggested that behaviors associated with chronic pain and discomfort may be subtle in young calves and considerable care must be taken to quantify behaviors that effectively represent changes in pain status. A behavioral assay with capability to detect pain-induced pessimism and anhedonia has recently been suggested by other researchers for the assessment of mood changes in calves that were cautery disbudded (Lecorps et al., 2019). We recommend that future work to investigate the impact of pain mitigation strategies on pain response in calves, specifically, and animals, in general, include variables that effectively quantify both physiological and behavioral indicators of changes to affective states. We agree with the suggestions of previous researchers in the value of utilizing regional anesthesia in conjunction with caustic paste disbudding to control acute pain as meloxicam alone was not effective in this study.
LITERATURE CITED
- Adcock, S. J. J., and Tucker C. B.. . 2018. Painful procedures: when and what should we be measuring in cattle? In: C. B. Tucker, editor, Food science, technology and nutrition. Advances in cattle welfare. Cambridge (UK): Woodhead Publishing. p. 157–198. http://dx.doi.org/10.1016/B978-0-08-100938-3.00008-5 [Google Scholar]
- Allen, K. A., Coetzee J. F., Edwards-Callaway L. N., Glynn H., Dockweiler J., KuKanich B., Lin H., Wang C., Fraccaro E., Jones M., . et al. 2013. The effect of timing of oral meloxicam administration on physiological responses in calves after cautery dehorning with local anesthesia. J. Dairy Sci. 96:5194–5205. doi: 10.3168/jds.2012-6251 [DOI] [PubMed] [Google Scholar]
- Apple, J. K., Kegley E. B., Galloway D. L., Wistuba T. J., and Rakes L. K.. . 2005. Duration of restraint and isolation stress as a model to study the dark-cutting condition in cattle. J. Anim. Sci. 83:1202–1214. doi: 10.2527/2005.8351202x [DOI] [PubMed] [Google Scholar]
- Boehringer Ingelheim . 2016. Metacam® 20 mg/mL Solution for Injection. Available from http://files.boehringer.com.au/files/CMI/Metacam%2020%20NZ.pdf. Accessed May 31, 2019.
- Braz, M., Carreira M., Carolino N., Rodrigues T., and Stilwell G.. . 2012. Effect of rectal or intravenous tramadol on the incidence of pain-related behavior after disbudding calves with caustic paste. Appl. Anim. Behav. Sci. 136:20–25. doi:10.1016/j.applanim.2011.11.011 [Google Scholar]
- Coetzee, J. F., KuKanich B., Mosher R., and Allen P. S.. . 2009. Pharmacokinetics of intravenous and oral meloxicam in ruminant calves. Vet. Ther. 10:E1–E8. [PubMed] [Google Scholar]
- Coetzee, J. F., Mosher R. A., KuKanich B., Gehring R., Robert B., Reinbold J. B., and White B. J.. . 2012. Pharmacokinetics and effect of intravenous meloxicam in weaned Holstein calves following scoop dehorning without local anesthesia. BMC Vet. Res. 8:153. doi: 10.1186/1746-6148-8-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter, F. 2013. A GLP tissue residue depletion study in calves following oral / buccal administration of meloxicam. Available from https://www.mla.com.au/research-and-development/search-rd-reports/final-report-details/Animal-Welfare/A-GLP-tissue-residue-depletion-in-calves-following-oral-buccal-administration-of-meloxicam/2072#. Accessed May 31, 2019.
- European Medicines Agency (EMA) . 2018. Metacam (meloxicam): An overview of Metacam and why it is authorized in the EU. Available from https://www.ema.europa.eu/documents/overview/metacam-epar-summary-public_en.pdf. Accessed February 25, 2019.
- Fraccaro, E., Coetzee J. F., Odore R., Edwards-Callaway L. N., Kukanich B., Badino P., Bertolotti L., Glynn H., Dockweiler J., Allen K., . et al. 2013. A study to compare circulating flunixin, meloxicam and gabapentin concentrations with prostaglandin E₂ levels in calves undergoing dehorning. Res. Vet. Sci. 95:204–211. doi: 10.1016/j.rvsc.2013.01.018 [DOI] [PubMed] [Google Scholar]
- Glynn, H. D., Coetzee J. F., Edwards-Callaway L. N., Dockweiler J. C., Allen K. A., Lubbers B., Jones M., Fraccaro E., Bergamasco L. L., and KuKanich B.. . 2013. The pharmacokinetics and effects of meloxicam, gabapentin, and flunixin in postweaning dairy calves following dehorning with local anesthesia. J. Vet. Pharmacol. Ther. 36:550–561. doi: 10.1111/jvp.12042 [DOI] [PubMed] [Google Scholar]
- Health Canada . 2014. Drug Product Database. Available from https://health-products.canada.ca/dpd-bdpp/info.do?code=92558&lang=en. Accessed February 25, 2019.
- Heinrich, A., Duffield T. F., Lissemore K. D., and Millman S. T.. . 2010. The effect of meloxicam on behavior and pain sensitivity of dairy calves following cautery dehorning with a local anesthetic. J. Dairy Sci. 93:2450–2457. doi: 10.3168/jds.2009-2813 [DOI] [PubMed] [Google Scholar]
- Heinrich, A., Duffield T. F., Lissemore K. D., Squires E. J., and Millman S. T.. . 2009. The impact of meloxicam on postsurgical stress associated with cautery dehorning. J. Dairy Sci. 92:540–547. doi: 10.3168/jds.2008-1424 [DOI] [PubMed] [Google Scholar]
- Hempstead, M. N., Waas J. R., Stewart M., Cave V. M., and Sutherland M. A.. . 2018. Evaluation of alternatives to cautery disbudding of dairy goat kids using physiological measures of immediate and longer-term pain. J. Dairy Sci. 101:5374–5387. doi: 10.3168/jds.2017-13814 [DOI] [PubMed] [Google Scholar]
- Kirovski, D. 2015. Endocrine and metabolic adaptations of calves to extra-uterine life. Acta Vet-Beograd. 65:297–318. http://dx.doi.org/10.1515/acve-2015-0025 [Google Scholar]
- Kleinhenz, M. D., Van Engen N. K., Smith J. S., Gorden P. J., Ji J., Wang C., Perkins S. C. B., Coetzee J. F.. . 2018. The impact of transdermal flunixin meglumine on biomarkers of pain in calves when administered at the time of surgical castration without local anesthesia. Livest. Sci. 212:1–6. doi:10.1016/j.livsci.2018.03.016 [Google Scholar]
- Lecorps, B., Ludwig B. R., von Keyserlingk M. A. G., and Weary D. M.. . 2019. Pain-induced pessimism and anhedonia: evidence from a novel probability-based judgment bias test. Front. Behav. Neurosci. 13:54. doi: 10.3389/fnbeh.2019.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., Liu H., and Li Z.. . 2008. Formation of neuromuscular junctions and synthesis of sensory neuropeptides in the co-cultures of dorsal root ganglion and cardiac myocytes. Cell Mol. Neurobiol. 28:939–947. doi:10.1007/s10571-008-9268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez, D. M., Marti S., Pajor E. A., Moya D., Gellatly D., Janzen E. D., and Schwartzkopf-Genswein K. S.. . 2018. Effect of a single dose of meloxicam prior to band or knife castration in 1-wk-old beef calves: I. Acute pain. J. Anim. Sci. 96:1268–1280. doi: 10.1093/jas/sky034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisse, J. P., Cotte J. P., and Huonnic D.. . 1995. Effect of dehorning on behaviour and plasma cortisol responses in young calves. J Applied Behavioral Sci. 43: 239–237. doi:10.1016/0168-1591(95)00569-E [Google Scholar]
- National Animal Health Monitoring System (NAHMS) . 2014. Dairy 2014: health and management practices on U.S. dairy operations. Available from https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_PartIII.pdf. Accessed February 25, 2019.
- Repenning, P. E., Ahola J. K., Callan R. J., French J. T., Giles R. L., Bigler B. J., Coetzee J. F., Wulf L. W., Peel R. K., Whittier J. C., . et al. 2013. Impact of oral meloxicam administration before and after band castration on feedlot performance and behavioral response in weanling beef bulls. J. Anim. Sci. 91:4965–4974. http://dx.doi.org/10.2527/jas2012-6070 [DOI] [PubMed] [Google Scholar]
- Stewart, M., Stafford K. J., Dowling S. K., Schaefer A. L., and Webster J. R.. . 2008. Eye temperature and heart rate variability of calves disbudded with or without local anaesthetic. Physiol. Behav. 93:789–797. doi: 10.1016/j.physbeh.2007.11.044 [DOI] [PubMed] [Google Scholar]
- Stilwell, G., Lima M. S., and Broom D. M.. . 2008. Comparing plasma cortisol and behaviour of calves dehorned with caustic paste after non-steroidal-anti-inflammatory analgesia. J. Livestock Sci. 119:63–69. doi:10.1016/j.livsci.2008.02.013 [Google Scholar]
- Stilwell, G., Campos de Carvalho R., Lima M. S., and Broom D. M.. . 2009. Effect of caustic paste disbudding, using local anaesthesia with and without analgesia, on behaviour and cortisol of calves. Appl. Anim. Behav. Sci. 116:35–44. [Google Scholar]
- Stock, M. L., Millman S. T., Barth L. A., Van Engen N. K., Hsu W. H., Wang C., Gehring R., Parsons R. L., and Coetzee J. F.. . 2015. The effects of firocoxib on cautery disbudding pain and stress responses in preweaned dairy calves. J. Dairy Sci. 98:6058–6069. doi: 10.3168/jds.2014-8877 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. 1994. Conditions for permitted extralabel animal and human drug use in food-producing animals. Washington, DC: U.S. Food and Drug Administration. 530 CFR part 20. 61 FR 57743. [Google Scholar]
- Van Engen, N. K., Stock M. L., Engelken T., Vann R. C., Wulf L. W., Karriker L. A., Busby W. D., Lakritz J., Carpenter A. J., Bradford B. J., . et al. 2014. Impact of oral meloxicam on circulating physiological biomarkers of stress and inflammation in beef steers after long-distance transportation. J. Anim. Sci. 92:498–510. http://dx.doi.org/10.2527/jas2013-6857 [DOI] [PubMed] [Google Scholar]
- Vickers, K. J., Niel L., Kiehlbauch L. M., and Weary D. M.. . 2005. Calf response to caustic paste and hot-iron dehorning using sedation with and without local anesthetic. J. Dairy Sci. 88:1454–1459. doi:10.3168/jds.S0022-0302(05)72813-7 [DOI] [PubMed] [Google Scholar]
- Winder, C. B., LeBlanc S. J., Haley D. B., Lissemore K. D., Godkin M. A., and Duffield T. F.. . 2017. Clinical trial of local anesthetic protocols for acute pain associated with caustic pain disbudding in dairy calves. J. Dairy Sci. 100:6429–6441. doi:10.3168/jds.2017-12724 [DOI] [PubMed] [Google Scholar]