Abstract
X-ray excited persistent luminescence (XEPL) imaging has attracted increasing attention in biomedical imaging due to elimination of autofluorescence, high signal-to-noise ratio and repeatable activation with high penetration. However, optical imaging still suffers from limited for high spatial resolution.
Methods: Herein, we report Mn3+-rich manganese oxide (MnOx)-coated chromium-doped zinc gallogermanate (ZGGO) nanoparticles (Mn-ZGGOs). Enhanced XEPL and magnetic resonance (MR) imaging were investigated by the decomposition of MnOx shell in the environment of tumors. We also evaluated the tumor cell-killing mechanism by detection of reactive oxygen (ROS), lipid peroxidation and mitochondrial membrane potential changes in vitro. Furthermore, the in vivo biodistribution, imaging and therapy were studied by U87MG tumor-bearing mice.
Results: In the tumor region, the MnOx shell is quickly decomposed to produce Mn3+ and oxygen (O2) to directly generate singlet oxygen (1O2). The resulting Mn2+ transforms endogenous H2O2 into highly toxic hydroxyl radical (·OH) via a Fenton-like reaction. The Mn2+ ions and ZGGOs also exhibit excellent T1-weighted magnetic resonance (MR) imaging and ultrasensitive XEPL imaging in tumors.
Conclusion: Both the responsive dual-mode imaging and simultaneous self-supplied O2 for the production of 1O2 and oxygen-independent ·OH in tumors allow for more accurate diagnosis of deep tumors and more efficient inhibition of tumor growth without external activation energy.
Keywords: Mn3+-rich oxide, X-ray excited persistent luminescence, tumor environment, chemodynamic therapy, responsive imaging
Introduction
Versus traditional optical technologies, persistent luminescence efficiently avoids the tissue autofluorescence interference due to lack of external illumination from in situ excitation 1, 2. In particular, near-infrared (NIR) emitting persistent luminescence is a promising candidate for in vivo imaging due to deeper tissue penetration depth and higher signal-to-noise ratio 3, 4. The excitation source is mainly UV light 5, which cannot repeatedly stimulate the persistent luminescence to realize in vivo renewable imaging due to a poor tissue penetration depth 6. Recently, white and red light were used as an excitation source to reactivate persistent luminescence for in vivo imaging 7-9, but the light penetration is still limited for further bio-applications. X-rays have been widely used in deep imaging and therapy in clinic, including as excitation sources for in vivo recharged deep tissue imaging 10-14.
Researches had demostrated that ZGGO persistent luminescence nanocomposite could load therapeutic drug for realizing long-term drug tracking and significant tumor therapeutic effect 15, 16. Moreover, the persistent luminescence materials could be employed as photodynamic therapeutic agents. Rencent research showed the ZnGa1.996O4:Cr0.004 persistent luminescence nanomaterial was an effective excitation source to achieve repeatable photodynamic therapy in vivo and effectively inhibited tumor growth 17. Our previous study also demostrated the Zn3Ga2GeO8:Cr3+,Yb3+,Er3+@mSiO2 persistent luminescence nanomaterials realized X-ray induced ultrasensitive persistent luminescence imaging and effective inhibition of orthotopic hepatic tumors 14.
However, persistent luminescence suffers from poor spatial resolution. In contrast, magnetic resonance (MR) imaging offers detailed three-dimensional anatomical images 18. Thus, multi-functional and/or multi-modal imaging probes can combine these strengths. Manganese oxides have been employed as an excellent choice for cancer diagnostics 19-25. Manganese oxides (MnOx) can react with the environment in tumor regions, i.e. low pH, overexpressed glutathione (GSH), and high hydrogen peroxide (H2O2) level to release Mn2+ and O2 26-32. Our previous study demonstrated that magnetic manganese oxide sweetgum-balls could be used as drug carriers to realize enhanced tumor theranostics 33. Recent studies showed that MnOx could degrade to produce Mn2+ and react with H2O2 by generating limited hydroxyl radical (·OH) for chemodynamic therapy (CDT) 34. CDT is a novel anticancer strategy due to generation of highly toxic reactive oxygen 35, 36. The Mn3+-contained in MnOx has strong catalytic ability to achieve the light-free generation of 1O2 to boost dynamic therapy efficacy 37. Thus, this Mn approach is a promising strategy to develop nanotheranostics that can generate several kinds of ROS synchronously from multiple reactants for good therapeutic effects.
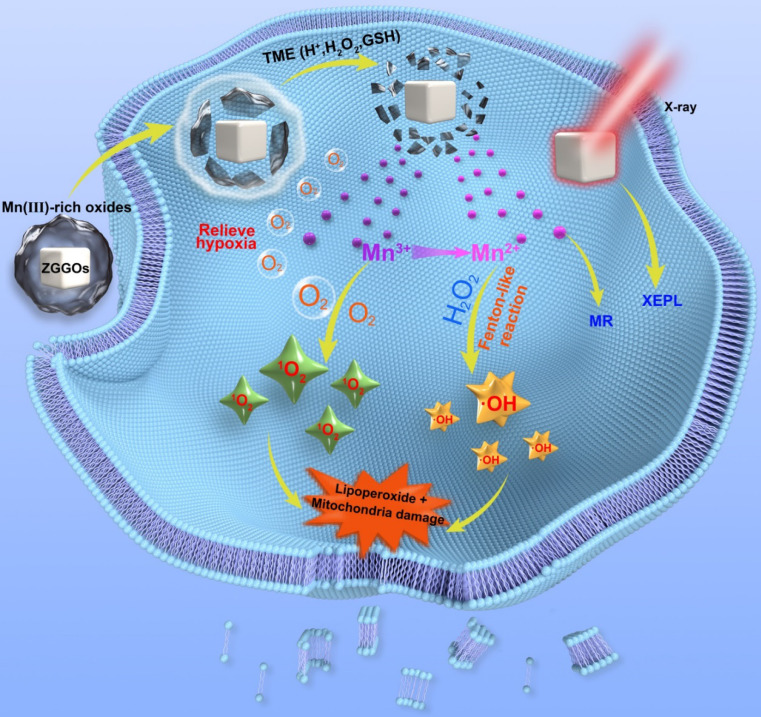
Herein, we present a facile method to prepare Mn3+-rich MnOx coatings on ZGGO nanoparticles (Mn-ZGGOs) (Scheme 1). The environment in tumor regions stimulated the decomposition of MnOx coating to release ions and persistent luminescence nanoparticles. Investigations in vitro and in vivo showed that the MnOx shell could simultaneously release Mn3+ to accelerate endogenous O2 into highly toxic 1O2 and generate more Mn2+. The generated Mn2+ then transforms endogenous H2O2 into the highly toxic ·OH. Thus, effective tumor inhibition in vivo was attributed to the concurrent generation of two different ROS to achieve parallel CDT. At the same time, the released Mn2+ could be used as MR imaging agent with higher spatial resolution. And exposed ZGGOs was also an ultrasensitive XEPL imaging agent with higher tissue penetration depth in tumors.
Scheme 1.
Schematic diagrams of Mn3+-rich oxide/persistent luminescence nanoparticles for light-free generation of singlet oxygen and hydroxyl radicals for responsive imaging and tumor treatment.
Methods
Synthesis of Mn-ZGGOs
10 mg ZGGO nanoparticles were dispersed in 10 mL ethanol by sonication for 1 h, and then centrifuged and washed with deionized water. Next, KMnO4 (20 mg) aqueous solution was dropwise added into the suspension of ZGGO under sonication. After 2 h, the precipitate was obtained by centrifugation at 10000 rpm.
In vitro X-ray recharged persistent luminescence imaging
Mn-ZGGOs solution ([Mn] = 0.2 mg/mL, 200 μL) was excited by X-ray (0.1 Gy). After 60 s, the GSH solution (10 mM, 20 μL) was added into Mn-ZGGOs, and XEPL signals with GSH or without GSH were collected on an IVIS Lumina II imaging system in the BLI mode at different time points, respectively. After 2 h, Mn-ZGGOs solution was re-irradiated by X-rays and the recharged images were acquired.
Measurement of ·OH
25 mM NaHCO3 solution containing Mn-ZGGOs NPs and different concentrations of GSH (0, 0.5, 1.0 and 10 mM) was mixed for 30 min. After centrifugation, 10 μg/mL MB and 10 mM H2O2 were added to the supernatant. Then, the mixture was incubated at 37 °C for 30 min, and the absorbance change of MB at 665 nm was measured.
Measurement of 1O2
Mn-ZGGOs was dissolved in 0.5 mL of PBS with different pH values (7.4, 6.5, and 5.6) and then 5 μL of SOSG (500 μM, dissolved in methanol) was added. After incubation for 30 min, the mixture was centrifuged to remove the unreacted Mn-ZGGOs to avoid the interference of Mn-ZGGOs on SOSG fluorescence.
Generation of O2
Mn-ZGGOs ([Mn] = 0, 0.5 mM) were added into 1 mL PBS solution (pH=5.6) with H2O2 of 10 mM. The produced O2 was detected by the fluorescence change of [Ru(dpp)3]Cl2 probe.
GSH-activated MR imaging performance
The Mn-ZGGOs NPs aqueous solutions with different Mn concentrations were mixed with GSH for 10 min, and then the MRI analysis was performed on 9.4 T clinical MR imaging.
In vitro cytotoxicity
The cytotoxicity of the as-prepared Mn-ZGGOs was investigated using a standard MTT assay. U87MG cells and L02 cells (1 × 104 cells per well) were seeded into 96-well plates and allowed to grow overnight. The cells were incubated with Mn-ZGGOs at different concentrations for 24 h. After that, 10 μL of MTT was added to each well. After incubation for 4 h, the medium was removed, and 150 μL of DMSO was added to dissolve the emerging formazan crystals. The absorbance at 570 nm was measured with a multi-detection microplate reader.
In vitro generation of ROS
U87MG cells (2 × 105) were seeded into glass bottom dishes (35 mm × 10 mm) and allowed to grow overnight. Then cells were replenished with fresh medium containing Mn-ZGGOs ([Mn] = 0, 10 and 20 μg/mL). After incubation for 24 h, the cells were washed with PBS for three times and stained with Hoechst 33342 (10 μg/mL) for 20 min at 37 °C. After washing with PBS for three times, the cells were further stained with SOSG (5 μM) or DCFH-DA (5 μM) for 20 min at 37 °C. Subsequently, those cells were imaged by Olympus FV1200 laser confocal scanning microscope after washing by PBS.
Intracellular lipoperoxide evaluation
U87MG cells (2 × 105) were seeded into glass bottom dishes (35 mm × 10 mm) and allowed to grow overnight. The fresh medium containing Mn-ZGGOs ([Mn] = 0 and 20 μg/mL) was added. After incubation for 24 h, the cells were washed with PBS for three times and stained with Hoechst 33342 (10 μg/mL) for 20 min at 37 °C. After washing with PBS for three times, the cells were stained with a lipoperoxide indicator, DOPIBY C11 (5 μM) for 20 min at 37 °C. The intracellular lipoperoxide was monitored using Olympus FV1200 laser confocal scanning microscope after washing by PBS.
Mitochondrial membrane potential damage evaluation
U87MG cells (2 × 105) were seeded into glass bottom dishes (35 mm × 10 mm) and allowed to grow overnight. The fresh medium containing Mn-ZGGOs ([Mn] = 10, 20 μg/mL) was added. After incubation for 24 h, the cells were washed with PBS for three times and stained with Hoechst 33342 (10 μg/mL) for 20 min at 37 °C. After washing with PBS for three times, the cells were stained with JC-1 dye (5 μM) for 20 min at 37 °C. The damage of mitochondrial membrane potential was observed using Olympus FV1200 laser confocal scanning microscope after washing by PBS.
In vitro O2 generation
U87MG cells (2 × 105) were seeded into glass bottom dishes (35 mm × 10 mm) and allowed to grow overnight. The fresh medium containing Mn-ZGGOs ([Mn] = 20 μg/mL) was added. After incubation for 0, 4, 8 and 24 h, respectively, the cells were washed with PBS for three times and stained with Hoechst 33342 (10 μg/mL) for 20 min at 37 °C. After washing with PBS for three times, the cells were stained with [Ru(dpp)3]Cl2 (10 μg/mL) for 20 min at 37 °C. The production of O2 was evaluated using Olympus FV1200 laser confocal scanning microscope after washing by PBS.
In vivo circulation and biodistribution
Balb/c mice bearing U87MG tumors were intravenously injected with Mn-ZGGOs ([Mn] = 1 mg/kg). The tumors and main organs including hearts, livers, spleens, lungs and kidneys, and blood were collected at varied time (1, 2, 4, 8, 12, 24 and 48 h) after injection and were weighed and digested using HNO3-H2O2 mixture. The Mn content in all samples was measured by ICP-MS.
In vivo XEPL imaging
After X-ray irradiation for 1 min, Mn-ZGGOs ([Mn] = 1 mg/kg) was intravenously injected into U87MG tumor-bearing mice. XEPL images were collected using an IVIS in vivo imaging system and the tumors were activated by X-ray for another 1 min before acquiring XEPL imaging at different times (0, 2, 4, 8, 12 and 24 h) post-injection.
In vivo MR imaging
To confirm the activatable MR imaging effect in tumors, Mn-ZGGOs NPs ([Mn] = 1 mg/kg) were intravenously injected into mice bearing U87MG tumors, and then the MR images were obtained at different time points (0, 2, 4 h, 8, 12 and 24 h) using small animal MR imaging system (9.4T).
In vivo tumor inhibition
When the tumor size reached to ~ 60 mm3, the U87MG tumor-bearing mice were randomized into various groups (n = 5) with different treatments: (1) PBS, (2) ZGGO (6.3 mg/kg), (3) Mn-ZGGOs (8.9 mg/kg, [Mn] = 1 mg/kg), (4) 2×Mn-ZGGOs (17.8 mg/kg, [Mn] = 2 mg/kg). The mice were intravenously injected with different formulations every third day for four times.
Statistical analysis
All data were presented as mean ± standard deviation. Comparison of the data were conducted with a Student's t-test (*: P < 0.05, **: P < 0.01, and ***: P < 0.001).
Results and Discussion
Synthesis and characterization
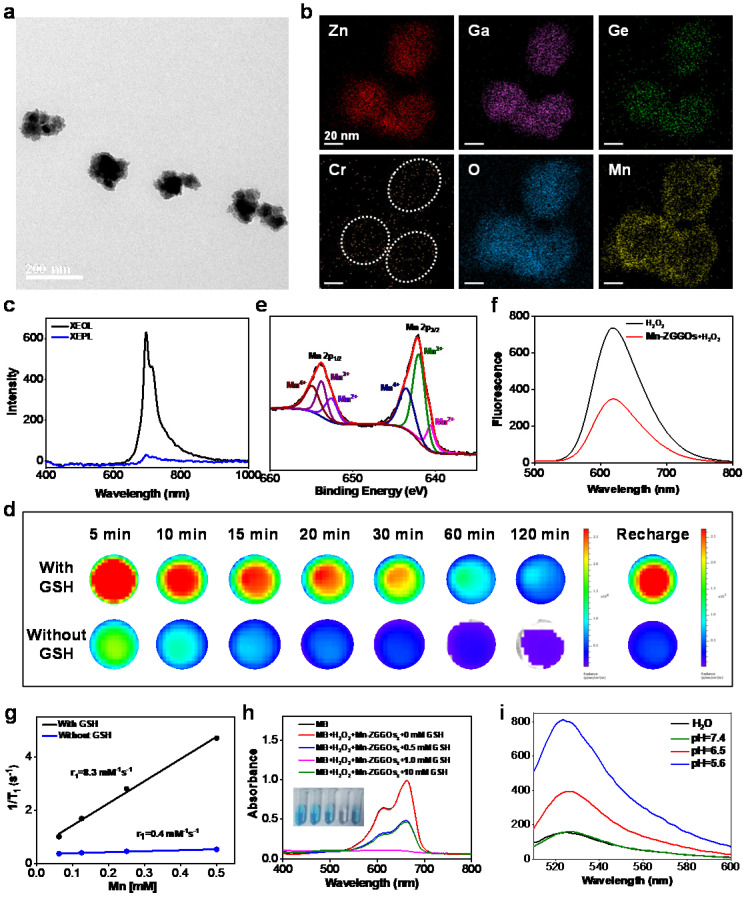
ZGGO nanoparticles (ZGGOs) were prepared following our previous synthesized route 38. The ZGGOs were then stirred in a mixture of KMnO4 and ethanol to coat MnOx layers on the surface of ZGGOs (Mn-ZGGOs). Transmission electron microscopy (TEM) imaging indicated that uniform layers were successfully formed (Figure 1A). Elemental mapping images revealed that the presence of Zn, Ga, Ge, Cr, Mn and O in Mn-ZGGOs as well as Mn circles confirmed the successful coating (Figure 1B). The MnOx layer had very minor impact on ZGGO emission, which still emitted NIR luminescence at ~ 696 nm under/after X-ray irradiation attributed to the typical 2E → 4A2 transition of Cr3+ (Figures 1C, S1) 39. GSH would also be helpful to break-up the MnOx layers 40. Impressively, these XEPL signals could be repeatedly activated by irradiation of X-ray; they recovered the excellent XEPL with GSH treatment (Figures 1D, S2). In addition, to prove the deep tissue-penetration of X-ray excitation and NIR emission, a 1.0 cm pork was placed between solution (Mn-ZGGOs with GSH, Mn-ZGGOs without GSH) and X-ray source (Figure S3). XEPL imaging was performed at 0, 2, and 5 min after ceasing X-ray irradiation. Strong NIR persistent luminescence signals were observed, indicating XEPL possessed deep tissue-penetration depth.
Figure 1.
(A) TEM image of Mn-ZGGOs. (B) Elemental mapping images of Mn-ZGGOs. (C) X-ray excited optical luminescence (XEOL) and X-ray excited persistent luminescence (XEPL) spectra of Mn-ZGGOs. (D) XEPL decay images of the Mn-ZGGOs with GSH or without GSH. (E) XPS of Mn 2p for Mn-ZGGOs. (f) Fluorescence spectra of [Ru(dpp)3]Cl2 of Mn-ZGGOs with or without H2O2. (G) The r1 value of Mn-ZGGOs with or without 1 mM GSH in 9.4 T MR instrument. (H) UV/Vis absorption spectra and photo (inset) of MB after degradation by H2O2 plus GSH-treated Mn-ZGGOs ([HCO3-] = 25 mM, [Mn] = 0.5 mM, [H2O2] = 10 mM). (I) Fluorescence spectra of SOSG (5 μM) incubated with Mn-ZGGOs for 20 min in H2O or different PBS buffer (pH = 7.4, 6.5, 5.6).
In most previous publications, MnOx nanostructures were mainly employed as carriers for cancer treatment 19, 24, 41, 42. Interesting, recent studies found that MnOx containing Mn (III) would generate ROS under the intrinsic acidity within tumor 34, 37. The possible mechanism revealed that the Mn valences (especially Mn(III)) impact the catalytic activity to generate ROS 37. X-ray photoelectron spectroscopy (XPS) analysis showed the presence of Mn (II), Mn (III), and Mn (IV); Mn (III) was as high as 46.5 atom% (Figure 1E). Thus, the Mn-ZGGOs would have a high activity to generate ROS.
Responsive O2 production and MR imaging
We also investigated the decomposition of Mn-ZGGOs under the enviroment in tumor regions. The color changes with Mn-ZGGOs solution and TEM images indicated the rapid breakup of MnOx shell in envioronment within tumor (acidic, GSH) (Figure S4). The decomposition of MnOx would generate Mn2+ and O2 43. O2 was produced in acidic environment (pH=5.6), detecting by the [Ru(dpp)3]Cl2 probe 44. A significant decrease in fluorescence of [Ru(dpp)3]Cl2 was shown in acidic environment (pH=5.6) (Figure 1F), indicating that O2 was generated efficiently under the environment. Obvious brightening signals were observed in T1-weighted MR images with the presence of GSH versus without GSH, indicating the decomposition and release of Mn2+ (Figure S5). The T1 relaxation rate (r1) of Mn-ZGGOs with the presence of GSH was 8.3 mM-1·s-1 (9.4T), which was 23.2-fold higher than that without GSH (Figure 1G).
Responsive ROS generation
Under simulated environment in tumor regions (H2O2, GSH), the absorbance of methylene blue (MB; ·OH probe) dropped sharply in the presence of Mn-ZGGOs, indicating the production of ·OH (Figure 1H). MB degradation efficiency of Mn-ZGGOs reached 51.1% when the GSH concentration was 10 mM, which is higher than that of Mn2+ (34.3%, Figure S6). Moreover, the fluorescence of singlet oxygen sensor green (SOSG) at 525 nm increased sharply under simulated acidic environment when incubated with Mn-ZGGOs, indicating generation of singlet oxygen (Figure 1I). These results were consistent with previous reports, and implied that the high-content of Mn(III) in Mn-ZGGOs could realize oxygen-independent production of ·OH and 1O2 simultaneously and achieve enhanced chemodynamic therapy (CDT) 37. Moreover, ZGGOs and Mn-ZGGOs showed excellent colloidal stability in PBS, DMEM, and fetal bovine serum (FBS) for further bioapplications (Figure S7).
In vitro antitumor effect and mechanism
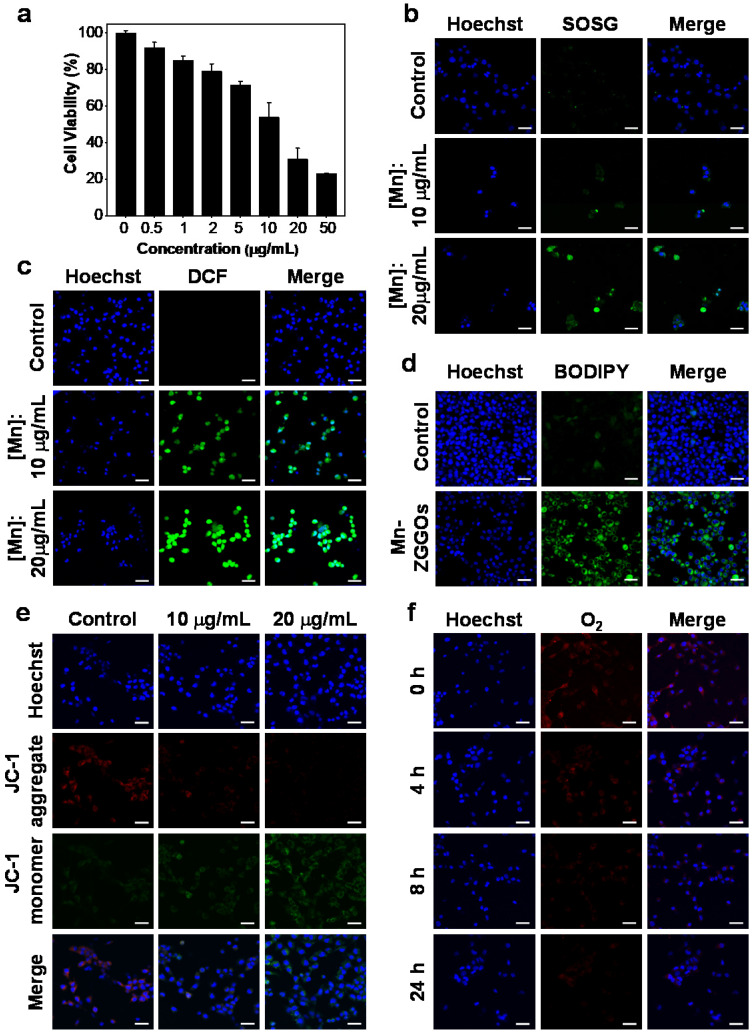
Encouraged by the efficient generation of ROS, we investigated the cellular ROS generation and antitumor effect in vitro. The U87MG cells were incubated with ZGGOs and Mn-ZGGOs, and cell viabilities revealed that the ZGGOs core had no obvious cytotoxicity. The Mn-ZGGOs at low concentrations exhibited excellent cell-killing effect with IC50 of 11.7 μg Mn/mL (Figures 2A, S8). Importantly, Mn-ZGGOs showed low toxicity to normal cells (L02 cells) at the high concentration ([Mn] = 0-50 μg/mL) suggesting less vulnerability to oxidative stress (Figure S9) 45.
Figure 2.
(A) Viability of U87MG cells after 24 h of incubation with Mn-ZGGOs. (B) Confocal images of U87MG cells stained by SOSG after incubating with PBS or Mn-ZGGOs for 24 h. The green fluorescence indicates the presence of 1O2. (C) Confocal images of U87MG cells stained by DCFH-DA after incubating with PBS or Mn-ZGGOs for 24 h. The green fluorescence indicates the presence of ·OH. (D) Confocal images of lipoperoxides in U87MG cells after incubation with PBS or Mn-ZGGOs for 24 h. The green fluorescence is the lipid ROS after the staining with BODIPY C11. (E) Confocal images of the changes in the mitochondrial membrane potential of U87MG cells after incubation with PBS or Mn-ZGGOs for 24 h. The red fluorescence indicates that the membrane potential is positive, and the green fluorescence indicates that the membrane potential decreases. (F) Confocal images of U87MG cells with production of O2 stained by [Ru(dpp)3]Cl2 after incubating with Mn-ZGGOs versus times. The blue fluorescence from Hochest 33342 indicatesthe cell nuclei in (b-e). Scale bar: 40 μm.
We explored the killing mechanism of Mn-ZGGOs. After incubation with Mn-ZGGOs ([Mn] = 0, 10, and 20 μg/mL) for 24 h, U87MG cells were co-stained by Hoechst 33342 (nucleus-staining) and SOSG (1O2 indicator) or DCFH-DA (·OH indicator). The U87MG cells were incubated with Mn-ZGGOs and SOSG exhibited strong green fluorescence, indicating the generation of 1O2 (Figure 2B). More obvious green fluorescence was observed in U87MG cells incubated with Mn-ZGGOs and DCFH-DA (Figure 2C). These results demonstrated efficient production of intracellular ROS including 1O2 from reaction of Mn3+ and O2 and ·OH from the Mn2+-mediated Fenton-like reaction (Figure 2C). More ROS generation induced enhanced cell membrane damage 46. BODIPY C11 showed significant green fluorescence implying high-levels of lipid peroxidation (Figure 2D).
The mitochondrion is vulnerable to the accumulated ROS in the tumor cell resulting in early cellular apoptosis and changes in the mitochondrial membrane potential 47. Therefore, we assessed the cell apoptosis by staining with a mitochondrial membrane potential fluorescence probe, JC-1. The red fluorescence decreased and green increased gradually, indicating increased cell damage by ROS (Figure 2E). Furthermore, the decomposition of MnOx would produce O2 and Mn2+ and expose the ZGGOs. Figure 2F shows that the red fluorescence of [Ru(dpp)3]Cl2 gradually decreased as the incubation time increased, indicating continuous O2 supply of Mn-ZGGOs in U87MG cells, this further relieved the tumor hypoxia. In vitro T1-weighted MR imaging of U87MG cells was evaluated by incubation with different concentrations of Mn-ZGGOs. After 24 h, the U87MG cells precipitated to the bottom of the tubes. Versus the control group, significant MR signals were observed in the U87MG cells incubated with Mn-ZGGOs, which showed the concentration-dependent behavior (Figure S10). Moreover, the exposed ZGGOs could recover the excellent XEPL by decomposition of MnOx. Figure S11 shows that the U87MG cells were incubated with Mn-ZGGOs for 24 h and exhibited significantly more enhanced XEPL signals. These results demonstrated that Mn-ZGGOs would generate ROS (1O2, ·OH) during the decomposition process and release imaging agents (Mn2+, afterglow) as well as release O2 to decrease tumor hypoxia.
In vivo biodistribution and biosafety
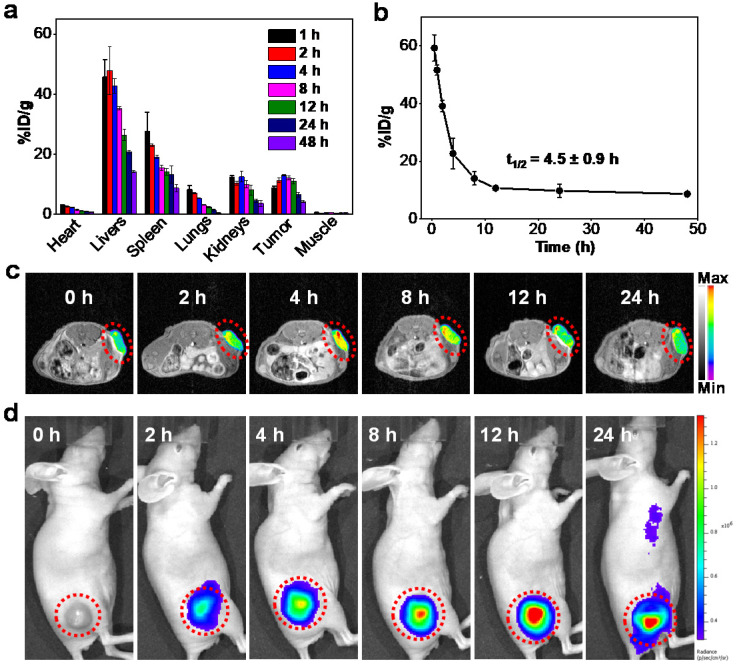
The in vivo blood-clearance behaviors and biodistribution were assessed by measuring the concentrations of Mn in dissolved tissues using inductively coupled plasma mass spectrometry (ICP-MS). The accumulation of Mn-ZGGOs in tumor tissue was as high as 13.2 % of the injected dose of Mn element per gram tissue (%ID g-1) at 4 h post-injection (Figure 3A). The pharmacokinetics showed that the half-life of Mn-ZGGOs was 4.5 ± 0.9 h (Figure 3B), which would benefit effective accumulation of Mn-ZGGOs into tumors. Furthermore, the long-term biocompatibility was evaluated by intravenous injection of Mn-ZGGOs ([Mn] = 1 mg/kg) into healthy mice. Blood biochemistry and hematology analysis showed no significant difference in hepatic function, kidney function, and blood indexes before and after injection of Mn-ZGGOs, indicating that Mn-ZGGOs had no systematic toxicity (Figures S12, S13).
Figure 3.
(A) Biodistribution of Mn-ZGGOs in major organs and tumors after intravenous administration at various time intervals (1, 2, 4, 8, 12, 24, and 48 h). The Mn-ZGGOs concentrations were normalized as the percentage of the injected dose of Mn element per gram of each organ (%ID g-1). (B) Time course of blood levels of Mn-ZGGOs levels following intravenous injection. The half-life time (t1/2) was calculated to be 4.5 ± 0.9 h. (C) In vivo T1-weighted MR images of mice injected intravenously with Mn-ZGGOs. (D) In vivo XEPL imaging of tumor-bearing mice after intravenous injection of Mn-ZGGOs.
In vivo imaging of U87MG tumor
The decomposition of MnOx in vivo was first evaluated by injecting Mn-ZGGOs intratumorally in U87MG tumor-bearing mice as well as normal subcutaneous tissue (Figure S14, black circle: tumor, red circle: normal subcutaneous tissue, Figure S15). In tumors, obvious T1-MR signals and US signals were observed confirming the release of Mn2+ for MR imaging and generation of O2 for US imaging.
Due to the effective response of the environment in tumor regions, we further studied the tumor-diagnosis of Mn-ZGGOs via in vivo imaging. The Mn-ZGGOs were intravenously injected in U87MG tumor-bearing mice ([Mn] = 1 mg/kg): Obvious T1-MR signals (red and yellow signals in tumors) were observed and reached a peak at 4 h post-injection, suggesting effective accumulation and respond decomposition of Mn-ZGGOs in U87MG tumors (Figures 3C, S15A); these findings were confirmed with biodistribution analysis.
As the ZGGOs core of Mn-ZGGOs also had persistent luminescence property, we then irradiated the tumor regions using X-ray to study the in vivo XEPL imaging after 0, 2, 4, 8, 12, and 24 h post-injection. After X-ray excitation (0.1 Gy), the X-ray excited persistent luminescence (XEPL) gradually increased over time and reached a peak at 12 h post-injection (Figures 3D, S17B). The difference was due to the restoration of persistent luminescence via break-up of the MnOx coating in acidic environment in tumor regions.
In vivo U87MG tumor therapy
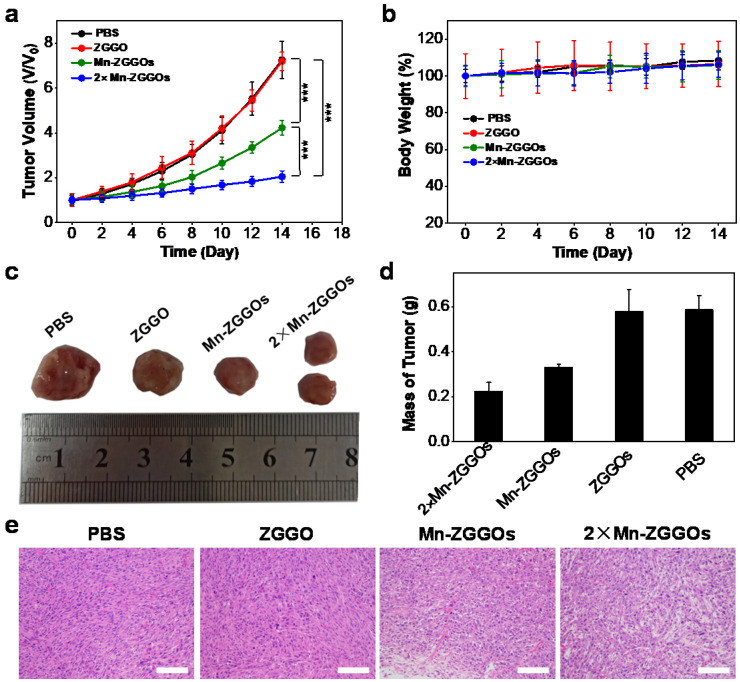
Following the in vivo study of distribution and decomposition of Mn-ZGGOs, the in vivo anticancer efficacies on U87MG tumor-bearing mice were evaluated. When the tumor volumes reached to 60 mm3, the mice were randomly allocated into four groups (n=5 per group): (1) PBS, (2) ZGGO (6.3 mg/kg), (3) Mn-ZGGOs (8.9 mg/kg, [Mn] = 1 mg/kg), and (4) 2×Mn-ZGGOs (17.8 mg/kg, [Mn] = 2 mg/kg). The tumor size and body weight were monitored every two days after injection. Versus controls, the Mn-ZGGOs groups inhibited tumor growth with the inhibition rate of 41.8 % at an Mn dose of 1 mg/kg (Figure 4A). A significantly enhanced tumor inhibition effect (inhibition rate: 71.8 %) was achieved in the 2-fold Mn-ZGGOs. The typical photographs of tumor tissue and the tumor masses at the end of treatment showed a consistent suppressive trend with tumor growth (Figure 4C, 4D). In addition, hematoxylin and eosin (H&E) staining analysis revealed that the tumor suffered more serious damage in CDT groups than those in PBS and ZGGO groups (Figure 4E). Both body weight and H&E staining of the major organs, including hearts, livers, spleens, lungs and kidneys, had no significant change/damage, indicating that Mn-ZGGOs had minor systemic toxicity (Figures 4B, S18). These results demonstrated that the procedure would produce Mn(III)-rich Mn-ZGGOs, and generate abundant ROS for satisfactory enviroment-activated chemodynamic therapeutics and imaging agents to with value in guilding precision cancer therapy.
Figure 4.
(A) Tumor volume curves and (B) Body weight growth curves of four groups of U87MG tumor-bearing mice at 14 days after intravenous injection with different formulations. Error bars are based on mean ± standard deviation (n = 5), ***P<0.001. (C) Typical photographs of excised tumors at day 14 after different treatments. (D) Relative tumor mass after different treatments on day 14. (E) H&E staining of tumor tissues after different treatments on day 14. Scale bar: 50 μm.
Conclusion
In summary, we report an easy and novel method to prepare Mn(III)-rich nanotheranostics of Mn-ZGGOs. Under the environment in tumor regions (acidic pH, high H2O2, and GSH levels), Mn-ZGGOs could react with the GSH/H2O2 to release the agents for imaging and treatment. The released Mn(III)-pool could directly react with O2 to generate 1O2; Mn(II) reacted with H2O2 to generate ·OH. The released in situ Mn(II), O2 and ZGGOs can be employed as MR, US and persistent luminescence diagnostics to guide precision cancer therapy. The tumor cell-killing mechanism in vitro and in vivo was confirmed systematically by detection of ROS, lipid peroxidation and mitochondrial membrane potential changes and tumor inhibition. Overall, this Mn3+-rich Mn-ZGGOs nanotheranostics design promotes the ultrasensitive, X-ray reactivated and high spatial resolution imaging. There is light-free generation of singlet oxygen and hydroxyl radicals for enhanced tumor chemodynamic therapy.
Supplementary Material
Supplementary materials and figures.
Acknowledgments
The work was supported by the National Science Foundation of China (81771977, 82001956), the National Postdoctoral Program for Innovative Talents (BX20200196), Xiamen Science and Technology Plan Project (3502Z20183017), the National University of Singapore Startup Fund (NUHSRO/2020/133/Startup/08), the Fundamental Research Funds for the Central Universities of China (20720180054), and XMU Undergraduate Innovation and Entrepreneurship Training Programs (2020X0567). All animal experiments were approved by the Animal Management and Ethics Committee of the Xiamen University.
Abbreviations
- XEPL
X-ray excited persistent luminescence
- MnOx
manganese oxide
- ZGGO
chromium-doped zinc gallogermanate
- Mn-ZGGOs
manganese oxide-coated chromium-doped zinc gallogermanate nanoparticles
- MR
magnetic resonance
- ROS
reactive oxygen
- O2
oxygen
- 1O2
singlet oxygen
- ·OH
hydroxyl radical
- NIR
near-infrared
- GSH
glutathione
- CDT
chemodynamic therapy
- TEM
transmission electron microscopy
- XPS
X-ray photoelectron spectroscopy
- MB
methylene blue
- SOSG
singlet oxygen sensor green
- FBS
fetal bovine serum
- ICP-MS
inductively coupled plasma mass spectrometry
- H&E
hematoxylin and eosin
References
- 1.Q. le Masne de Chermon, C. Chaneac, J. Seguin, F. Pelle, Maitrejean S, J. Jolivet, et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:9266–71. doi: 10.1073/pnas.0702427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Gecevicius M, Qiu J. Long persistent phosphors-from fundamentals to applications. Chem Soc Rev. 2016;45:2090–136. doi: 10.1039/c5cs00582e. [DOI] [PubMed] [Google Scholar]
- 3.L. Liang, N. Chen, Y. Jia, Q. Ma, J. Wang, Q. Yuan, et al. Recent progress in engineering near-infrared persistent luminescence nanoprobes for time-resolved biosensing/bioimaging. Nano Res. 2019;12:1279–92. [Google Scholar]
- 4.Lecuyer T, Teston E, Ramirez-Garcia G, Maldiney T, Viana B, al e. Chemically engineered persistent luminescence nanoprobes for bioimaging. Theranostics. 2016;6:2488–524. doi: 10.7150/thno.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Lecuyer T, Seguin J, Mignet N, Scherman D, Viana B. et al. Imaging and therapeutic applications of persistent luminescence nanomaterials. Adv Drug Deliv Rev. 2019;138:193–210. doi: 10.1016/j.addr.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Abdukayum A, Chen JT, Zhao Q, Yan XP. Functional near infrared-emitting Cr3+/Pr3+ co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J Am Chem Soc. 2013;135:14125–33. doi: 10.1021/ja404243v. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Ma Q, Wang Y, Shen H, Yuan Q. Recent progress in biomedical applications of persistent luminescence nanoparticles. Nanoscale. 2017;9:6204–18. doi: 10.1039/c7nr01488k. [DOI] [PubMed] [Google Scholar]
- 8.Yan LX, Chen LJ, Zhao X, Yan XP. pH Switchable Nanoplatform for In vivo Persistent Luminescence Imaging and Precise Photothermal Therapy of Bacterial Infection. Adv Funct Mater. 2020;30:190942. [Google Scholar]
- 9.Li Z, Zhang Y, Wu X, Wu X, Maudgal R, Zhang H. et al. In vivo Repeatedly Charging Near-Infrared-Emitting Mesoporous SiO2/ZnGa2O4:Cr3+ Persistent Luminescence Nanocomposites. Adv Sci. 2015;2:1500001. doi: 10.1002/advs.201500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Sun X, Wang GD, Nagata K, Hao Z, Wang A. et al. LiGa5O8:Cr-based theranostic nanoparticles for imaging-guided X-ray induced photodynamic therapy of deep-seated tumors. Mater Horiz. 2017;4:1092–101. doi: 10.1039/C7MH00442G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen ZZ, Wang LC, Manoharan D, Lee CL, Wu LC, Huang WT. et al. Low dose of X-ray-excited long-lasting luminescent concave nanocubes in highly passive targeting deep-seated hepatic tumors. Adv Mater. 2019;31:e1905087. doi: 10.1002/adma.201905087. [DOI] [PubMed] [Google Scholar]
- 12.Sun W, Zhou Z, Pratx G, Chen X, Chen H. Nanoscintillator-mediated X-ray induced photodynamic therapy for deep-seated tumors: from concept to biomedical applications. Theranostics. 2020;10:1296–318. doi: 10.7150/thno.41578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang GD, Nguyen HT, Chen H, Cox PB, Wang L, Nagata K. et al. X-ray induced photodynamic therapy: a combination of radiotherapy and photodynamic therapy. Theranostics. 2016;6:2295–305. doi: 10.7150/thno.16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi T, Sun W, Qin R, Li D, Feng Y, Chen L. et al. X-ray-induced persistent luminescence promotes ultrasensitive imaging and effective inhibition of orthotopic hepatic tumors. Adv Funct Mater. 2020;30:2001166. [Google Scholar]
- 15.Maldiney T, Ballet B, Bessodes M, Scherman D, Richard C. Mesoporous persistent nanophosphors for in vivo optical bioimaging and drug-delivery. Nanoscale. 2014;6:13970–6. doi: 10.1039/c4nr03843f. [DOI] [PubMed] [Google Scholar]
- 16.Chen LJ, Yang CX, Yan XP. Liposome-Coated Persistent Luminescence Nanoparticles as Luminescence Trackable Drug Carrier for Chemotherapy. Anal Chem. 2017;89:6936–9. doi: 10.1021/acs.analchem.7b01397. [DOI] [PubMed] [Google Scholar]
- 17.Fan W, Lu N, Xu C, Liu Y, Lin J, Wang S. et al. Enhanced Afterglow Performance of Persistent Luminescence Implants for Efficient Repeatable Photodynamic Therapy. ACS Nano. 2017;11:5864–72. doi: 10.1021/acsnano.7b01505. [DOI] [PubMed] [Google Scholar]
- 18.Smith BR, Gambhir SS. Nanomaterials for in vivo imaging. Chem Rev. 2017;117:901–86. doi: 10.1021/acs.chemrev.6b00073. [DOI] [PubMed] [Google Scholar]
- 19.Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y. et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang F, Wen L, Liu J, Peng W, Meng Z, Chen Q. et al. Albumin nanocomposites with MnO2/Gd2O3 motifs for precise MR imaging of acute myocardial infarction in rabbit models. Biomaterials. 2020;230:119614. doi: 10.1016/j.biomaterials.2019.119614. [DOI] [PubMed] [Google Scholar]
- 21.Liang K, Li Z, Luo Y, Zhang Q, Yin F, Xu L. et al. Intelligent nanocomposites with Intrinsic blood-brain-barrier crossing ability designed for highly specific MR imaging and sonodynamic therapy of glioblastoma. Small. 2020;16:e1906985. doi: 10.1002/smll.201906985. [DOI] [PubMed] [Google Scholar]
- 22.Zhu J, Xiao T, Zhang J, Che H, Shi Y, Shi X. et al. Surface-charge-switchable nanoclusters for magnetic resonance imaging-guided and glutathione depletion-enhanced photodynamic therapy. ACS Nano. 2020;14:11225–37. doi: 10.1021/acsnano.0c03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu C, Duan X, Cao M, Jiang S, Ban X, Guo N. et al. Targeted magnetic resonance imaging and modulation of hypoxia with multifunctional hyaluronic acid-MnO2 nanoparticles in glioma. Adv Healthc Mater. 2019;8:e1900047. doi: 10.1002/adhm.201900047. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Huang Y, Xue Z, Zeng S. Tumor microenvironment responsive hollow mesoporous Co9S8@MnO2-ICG/DOX intelligent nanoplatform for synergistically enhanced tumor multimodal therapy. Biomaterials. 2020;262:120346. doi: 10.1016/j.biomaterials.2020.120346. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Ye D, Wu M, Chen H, Zhang L, Shi J. et al. Break-up of two-dimensional MnO2 nanosheets promotes ultrasensitive pH-triggered theranostics of cancer. Adv Mater. 2014;26:7019–26. doi: 10.1002/adma.201402572. [DOI] [PubMed] [Google Scholar]
- 26.Fan W, Bu W, Shen B, He Q, Cui Z, Liu Y. et al. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/H2O2-responsive UCL imaging and oxygen-elevated synergetic therapy. Adv Mater. 2015;27:4155–61. doi: 10.1002/adma.201405141. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Dong Z, Fu T, Liu J, Chen Q, Li Y. et al. Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy. Adv Funct Mater. 2016;26:5490–8. [Google Scholar]
- 28.P. Prasad, C. R. Gordijo, A. Z. Abbasi, A. Maeda, A. Ip, A. M. Rauth, et al. Multifunctional albumin MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano. 2014;8:3202–12. doi: 10.1021/nn405773r. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Zhang S, Lin H, Zhao M, Yao H, Zhang L. et al. Theranostic 2D ultrathin MnO2 nanosheets with fast responsibility to endogenous tumor microenvironment and exogenous NIR irradiation. Biomaterials. 2018;155:54–63. doi: 10.1016/j.biomaterials.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Han W, Yang P, Jia T, Dong S, Bi H. et al. Tumor microenvironment-responsive mesoporous MnO2-coated upconversion nanoplatform for self-enhanced tumor theranostics. Adv Funct Mater. 2018;28:1803804. [Google Scholar]
- 31.Chu C, Lin H, Liu H, Wang X, Wang J, Zhang P. et al. Tumor microenvironment-triggered supramolecular system as an in situ nanotheranostic generator for cancer phototherapy. Adv Mater. 2017;29:1605928. doi: 10.1002/adma.201605928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Zhao X, Nie W, Yang Y, Wu C, Liu W. et al. Tumor cell-activated “Sustainable ROS Generator” with homogeneous intratumoral distribution property for improved anti-tumor therapy. Theranostics. 2021;11:379–96. doi: 10.7150/thno.50028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng Y, Ding D, Sun W, Qiu Y, Luo L, Shi T. et al. Magnetic manganese oxide sweetgum-ball nanospheres with large mesopores regulate tumor microenvironments for enhanced tumor nanotheranostics. ACS Appl Mater Interfaces. 2019;11:37461–70. doi: 10.1021/acsami.9b11843. [DOI] [PubMed] [Google Scholar]
- 34.Lin LS, Song J, Song L, Ke K, Liu Y, Zhou Z. et al. Simultaneous fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew Chem Int Ed. 2018;57:4902–6. doi: 10.1002/anie.201712027. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z. et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew Chem Int Ed. 2016;55:2101–6. doi: 10.1002/anie.201510031. [DOI] [PubMed] [Google Scholar]
- 36.Tang Z, Liu Y, He M, Bu W. Chemodynamic therapy: tumour microenvironment-mediated fenton and fenton-like reactions. Angew Chem Int Ed. 2019;58:946–56. doi: 10.1002/anie.201805664. [DOI] [PubMed] [Google Scholar]
- 37.Lu C, Zhang C, Wang P, Zhao Y, Yang Y, Wang Y. et al. Light-free generation of singlet oxygen through manganese-thiophene nanosystems for pH-responsive chemiluminescence imaging and tumor therapy. Chem. 2020;6:2314–34. [Google Scholar]
- 38.Lv Y, Ding D, Zhuang Y, Feng Y, Shi J, Zhang H. et al. Chromium-doped zinc gallogermanate@zeolitic imidazolate framework-8: a multifunctional nnanoplatform for rechargeable in vivo persistent luminescence imaging and pH-responsive drug release. ACS Appl Mater Interfaces. 2019;11:1907–16. doi: 10.1021/acsami.8b19172. [DOI] [PubMed] [Google Scholar]
- 39.Maldiney T, Bessiere A, Seguin J, Teston E, Sharma SK, Viana B. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat Mater. 2014;13:418–26. doi: 10.1038/nmat3908. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Z, Fan H, Zhou G, Bai H, Liang H, Wang R. et al. Activatable fluorescence/MRI bimodal platform for tumor cell imaging via MnO2 nanosheet-aptamer nanoprobe. J Am Chem Soc. 2014;136:11220–3. doi: 10.1021/ja5029364. [DOI] [PubMed] [Google Scholar]
- 41.Wei R, Gong X, Lin H, Zhang K, Li A, Liu K. et al. Versatile octapod-shaped hollow porous manganese(II) oxide nanoplatform for real-time visualization of cargo delivery. Nano Lett. 2019;19:5394–402. doi: 10.1021/acs.nanolett.9b01900. [DOI] [PubMed] [Google Scholar]
- 42.Yang R, Hou M, Gao Y, Lu S, Zhang L, Xu Z. et al. Biomineralization-inspired crystallization of manganese oxide on silk fibroin nanoparticles for in vivo MR/fluorescence imaging-assisted tri-modal therapy of cancer. Theranostics. 2019;9:6314–33. doi: 10.7150/thno.36252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Z, Jia X, Bai J, Ruan Y, Wang C, Li J. et al. MnO2 gatekeeper: An intelligent and O2-evolving shell for preventing premature release of high cargo payload core, overcoming tumor hypoxia, and acidic H2O2-sensitive MRI. Adv Funct Mater. 2017;27:1604258. [Google Scholar]
- 44.F. N. Castellano, Lakowicz JR. A water-soluble luminescence oxygen sensor. Photochem Photobiol. 1998;67:179–83. doi: 10.1562/0031-8655(1998)067<0179:awslos>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 46.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35:830–49. doi: 10.1016/j.ccell.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28:265–80. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and figures.