Abstract
The epidermal mucus covering the surface of a snail represents an important barrier to trematode larvae attempting to penetrate the snail and may play a role in mediating snail-trematode compatibility. In this study, Facioloides magna miracidia were exposed to mucus harvested from a compatible snail host, Lymnaea elodes (palustris), and from an incompatible snail, Helisoma trivolvis. In vitro treatment of freshly hatched miracidia with snail-derived mucus exerted dramatically different effects on larvae depending on snail species. At the lowest dilution of mucus tested (1:3) mean damage rates (tegumental damage and/or larval lysis and death) were as high as 100% for miracidia exposed to H. trivolvis mucus, while none of F. magna miracidia were damaged in L. elodes mucus. A dilution series for each snail species, and treatments with heat and proteinase K were performed to characterize the component(s) of mucus inducing the observed morphological changes. The damaging effects of H. trivolvis mucus were concentration dependent and completely abrogated by heat (65 C, 30 min) and proteinase treatment, strongly implicating a heat-labile protein(s) in mucus as the active cytotoxic agent(s). In contrast to our prediction that miracidial contact with mucus of compatible L. elodes would trigger larval transformation, mucus from either snail species tested exhibited little to no activity. Overall these data demonstrate the presence of a potent cytotoxic protein-like factor in the mucus of F. magna–incompatible H. trivolvis, and its absence in the mucus of the compatible snail, L. elodes. This finding supports the notion that the epidermal mucus layer may be serving as an important determinant of larval trematode-snail compatibility.
Mucus, generally defined as a viscous extracellular secretion comprising mucopolysaccharides, glycoproteins, and lipids, is commonly used across many invertebrate taxa to coat epithelial surfaces, functioning to prevent water loss/desiccation, provide structural support, or navigate the environment (Denny, 1989). In addition to its function as a physical barrier to environmental threats or conditions, mucus also has been shown to serve a number of physiologic functions, and various chemical components of mucus that facilitate these functions have been identified. Some examples include the use of mucus lectins as bacterial agglutinins or in food gathering (Espinosa et al., 2009; 2010; Ito et al., 2011), the presence of toxins as anti-predator mechanisms (Kelley et al., 2003; Greenwood et al., 2004), regulation of internal cadmium levels via excretion through mucus (Notten et al., 2006), the selective maintenance of bacterial communities in corals (Brown and Bythell, 2005; Ritchie, 2006), and in anti-pathogen defense (Iguchi et al., 1982; Kubota et al., 1985; Otsuka-Fuchino et al., 1992; Ijima et al., 2003; Espinosa et al., 2013; Zhong et al., 2013). Although the variety of invertebrate mucus functions studied thus far suggests that mucus is a very complex substance able to accomplish a range of important tasks, its role in protection appears to be a common theme, whether against invading or attacking organisms or from environmental, abiotic threats.
Larval trematodes employ several mechanisms for the successful infection of snail intermediate hosts. These include physical and chemical environmental factors (e.g., water conditions, light, temperature) and chemo-attractants that place miracidia in the proximity of the host (Basch, 1991). The first snail tissue a miracidium typically encounters is the layer of mucus produced by muciferous epidermal cells of the mantle. This is essentially the first opportunity for the snail to directly subvert attachment and penetration by the miracidial stage and therefore represents a potentially important barrier to infection by larval trematodes. However, in contrast to other functions assigned to molluscan mucus, there has been little previous work on the role of epidermal mucus in determining snail-trematode compatibility, specifically that focusing on its potential anti-parasitic activity. Previous studies have focused mainly on the larval chemo-attractant characteristics of mucus or other potential mucus sources using compatible snail host-parasite systems. For example, Wilson (1968) discovered several potential attractants in Lymnaea truncatula mucus for compatible Fasciola hepatica miracidia, including several free amino acids, glucose, Na+, K+, and a number of free lipids. Other investigators have reported miracidial chemo-attractant activity in snail-conditioned water that was attributed to a diversity of compounds including free amino acids, sialic acid, Mg++, or glycoproteins (Chernin, 1970; Prechel et al., 1976; Roberts et al., 1980; Haberl and Haas, 1992; Kalbe et al., 1997).
Because previous work suggests that mucus may play an important role in influencing miracidial infectivity by serving as a source of chemical attractants or anti-parasite factors, the present study was designed to investigate the possible role of mucus in counteracting infection by parasites confronting incompatible snail species. In this study we compared the in vitro effects of isolated mucus from the headfeet of an incompatible snail Helisoma trivolvis and a compatible snail host, Lymnaea elodes (L. palustris) (Clarke, 1973) on freshly hatched Fascioloides magna miracidia. As an added dimension to this study, because mucus and other body secretions are commonly comprised of glycoproteins with cytolytic or other defense-related function (Yamasaki et al., 1989; Ehara et al., 2002; Li and Graham, 2007; Zhong et al., 2013), we also sought to assess whether mucus larvicidal activity may be protein based. Finally, the geographic distribution of H. trivolvis is nearly ubiquitous in the continental United States and naturally overlaps F. magna in parts of its range. Similarly, L. elodes was collected from a region where F. magna is endemic and has been shown to be a natural intermediate host for this fluke species (Foreyt and Todd, 1978). Using this natural parasite-host system, we hypothesized that significantly greater miracidial damage rates would be caused by H. trivolvis mucus than by L. elodes mucus. We also hypothesized that in vitro miracidial transformation into sporocysts (normal larval development) would be supported by L. elodes mucus, as demonstrated by significantly higher transformation rates in L. elodes mucus than in H. trivolvis mucus or control buffer.
MATERIALS AND METHODS
Snail collection and laboratory maintenance
Pond snails, L. elodes, were maintained on a 12:12 hr light-dark cycle in 10-gal glass aquaria containing aerated artificial pond water (Noland and Carriker, 1946) supplemented with chalk serving as a Ca++ source. They were fed green leaf lettuce and Tetramin™ fish food ad libitum. The laboratory population of L. elodes was established from wild-caught adults collected from small marshes on the University of Wisconsin–Madison campus during late summer and fall of 2009 and maintained in culture for ~10 mo before use in experiments. Helisoma trivolvis snails were obtained from Dr. Jane Huffman (East Stroudsburg University, East Stroudsburg, Pennsylvania) in June 2010, who acquired them from Dean Pond near East Stroudsburg, Pennsylvania. They were maintained in the laboratory under identical conditions as L. elodes for ~3 mo prior to use in experiments. In establishing laboratory colonies of wild caught snails of either species, their shells were thoroughly cleaned with 70% EtOH and screened for pre-existing patent trematode infections by placing individual snails into wells of 12-well TC plates containing artificial pond water, exposing them to a bright light source for 1–2 hr, followed by a 3 hr to overnight recovery period in the dark and finally microscopic examination of wells for cercariae and/or encysted metacercariae. In initial screenings few snails were found to be shedding cercariae, and those snails were isolated from the general colony and not used in experiments.
Harvesting and preparation of F. magna eggs
Eggs of F. magna were obtained from the infected livers of hunter-killed white-tailed deer in Saint Croix State Park in Minnesota on 14 November 2009. Within hours of the death of the deer, adult flukes and the dark brown, egg-rich fluid from fibrous liver cysts were collected. Once removed from the cysts, the adult flukes were placed in well water to facilitate the expulsion of eggs from the fluke uterus. These eggs were combined with cyst fluids then cleaned and concentrated by repeated gravity sedimentation and resuspension in well water, followed by a sterile distilled water wash, and finally passage of the suspension through a 180-μm soil sieve for final collection and concentration of eggs by centrifugation. Following cleaning, eggs were placed in covered glass petri dishes, each containing sterile distilled water of ~5 mm depth, and allowed to embryonate at room temperature for approximately 3–4 wk. When a few hatched, and free-swimming miracidia were observed in a dish, the eggs were then transferred to 50-ml plastic conical centrifuge tubes in sterile distilled water and stored at 4 C. When miracidia were needed, eggs were stimulated to hatch with an initial incubation in sterile artificial pond water (Noland and Carriker, 1946) for 20 min at 33 C, followed by incubation at 22 C for 1–2 hr. After hatching, miracidia were transferred by micropipette to sterile Chernin’s balanced salt solution (CBSS; Chernin, 1963) containing penicillin and streptomycin, followed by transfer to wells of a sterile 96-well tissue culture plate containing test media dissolved in CBSS.
Preparation of mucus samples
Mucus was collected from snails using a modified headfoot retraction method (Sminia and Barendsen, 1980). Typically this method is used for collecting hemolymph and involves abruptly chasing the snail’s headfoot back into its shell by a sharp jab to the muscle with an implement, resulting in the forcefully ejection of hemolymph from the hemal pore as it retracts into its shell. We used a similar protocol with the exception that the headfoot surface is gently stroked with a plastic pipette tip, encouraging secretion of excess mucus while minimizing retraction and any expulsion of hemolymph. Mucus was collected from the headfeet of Helisoma (.12 mm shell diameter) and Lymnaea (>20 mm shell length) using a P100 micropipette tip that had been cut at a bevel to increase the area of suction and better allow passage of mucus into the tip. Mucus from 5–8 individual snails of the same species was pooled in a sterile 1.5 ml microcentrifuge tube and examined visually for signs of hemolymph contamination. Pinkish- or bluish-tinged samples were discarded. Mucus sample volumes varied with each collection (range: 100–250 μl) depending on the number of treatments and controls to be run in a given experiment. Immediately after collection, samples were initially diluted to 1/3 with CBSS to decrease viscosity, followed by passage through a sterile 53-μm sieve to remove any extraneous material prior to testing. Mean protein concentrations of the 1/3 diluted mucus for L. elodes and H. trivolvis were 0.78 ± 0.55 and 0.19 ± 0.02 mg/ml, respectively. Because we wanted to compare the endogenous effects of naturally occurring mucus present in both snails, no attempt was made to normalize mucus samples based on protein concentration. Isolated mucus samples were kept on ice during and between all preparation steps and used within several hours of harvest and preparation.
A single collection of mucus pooled from multiple snails tested against freshly hatched batches of F. magna miracidia constituted one independent replicate for the serial dilution experiment (1/3, 1/30, 1/300, and 1/3,000 dilution of mucus in CBSS), as well as experiments testing for the effects of heat and proteinase treatments on activity. In total, the mucus dilution experiment was conducted 8 times using new mucus samples and miracidia, and the heat and PK treatment experiments were replicated 3 and 5 times, respectively. Appropriate controls were performed in each experimental replicate as described below. Typically 10–12 miracidia were scored in each treatment/control group within a single experiment. All reactions were conducted in wells of a 96-well tissue culture plate as detailed in the “Assay procedures” section.
Heat and proteinase treatments of mucus
In order to test for the possibility that active cytotoxic or transformation-inducing factors may be proteinaceous in nature, mucus samples were subjected to heat and protease treatments prior to testing. Heat treatments were conducted on 1/3 dilutions of mucus from both snail species and involved heating the mucus in a water bath at 65 C for 30 min, followed by centrifugation at 14,000 g for 4 min and retrieval of the supernatant for use in assays.
Proteinase K-conjugated agarose beads were prepared using Tritirachium album proteinase K (PK) (sp. activity ≥ 30 units/mg protein; Sigma-Aldrich, St. Louis, Missouri) and Affi-gel 10 active ester agarose beads (Bio-Rad, Richmond, California). Affi-gel beads (1.5 ml suspension) were separated from the carrier solvent (isopropanol) by centrifugation, followed by 3 washes in 0.1 M Mops buffer, pH 7.5, at 4 C. A packed-bead volume of ~0.75 ml of beads was then incubated in 1.2 ml of PK (15 mg/ml Mops) for 2 hr at 4 C under constant agitation. Conjugated beads were washed 3 times in Mops buffer by centrifugation, followed by 2 washes in ice-cold 50% glycerol in Mops, and finally resuspended in 2 volumes of 50% glycerol for stable storage at −20 C. Before and after each treatment of snail mucus, beads were rinsed 3 times in Mops buffer as described above. Because PK-conjugated beads were reused after mucus treatments, washed beads were again rinsed and stored in ice-cold 50% glycerol/Mops. The same batches of beads were always used to treat mucus from the same snail species in subsequent experiments. Finally, to control for possible nonspecific adsorption of potentially cytotoxic mucus proteins by affi-gel beads, bovine serum albumin (BSA) -conjugated beads were produced as described above, substituting BSA (Sigma-Aldrich) for PK.
PK digestion of 1/3-diluted mucus samples from both snail species was conducted by incubating mucus with PK-conjugated beads for 3 hr at 33 C at a mucus:bead volume ratio of 2:3. After treatment, mucus samples were separated from the beads by brief spins with a microcentrifuge at 4 C followed by retrieval of the digested mucus supernatants. Control mucus preparations for these assays included an identical incubation of 1/3-diluted mucus samples with BSA-coated beads (bead control) in place of PK beads as well as an untreated aliquot of 1/3-diluted mucus that was incubated for the same duration (3 hr) and at the same temperature (33 C) as the PK-digested sample (temperature control).
Assay procedures
After 90 μl of test/control media were added to wells of a 96-well plate, miracidia in ~10 μl of pond water were introduced to each well. Miracidia were then examined under an inverted compound microscope at 1, 4, and 24 hr and scored as “swimming” (Fig. 1A), “settled” (settled to the bottom of the well, no longer swimming), “transforming” (having lost some or all ciliated epidermal plates) (Fig. 1B, C), or “damaged” (completely lysed/ruptured or exhibiting tegumental damage/blebbling with lysed epidermal plates) (Fig. 1D, E). These defined categories were used to quantify the effects of mucus preparations on miracidia in test and control media. Since natural morbidity leading to varying degrees of degradation was observed in miracidia at extended assay lengths (24 hr) and because consistent mucus-associated miracidial damage was observed within a few hours, only data collected at 4 hr post-treatment were used for statistical analysis of damage rates. However, since consistent miracidial transformation (that could visually be distinguished from any other morphological changes) was often observed to take longer than 4 hr, data taken at 24 hr were used for statistical analysis of transformation rates. In our analysis, when assessing the damaging effects of mucus we pooled the number of living (i.e., motile) miracidia and those undergoing transformation into one category (representing undamaged larvae) and grouped lysed, immobile miracidia into our “damaged” category for each replicate experiment. Similarly, transformation data were determined based on the number of transforming miracidia within a given treatment population. In each experimental replicate data were expressed as a percentage of larvae that were damaged or transforming.
Figure 1.
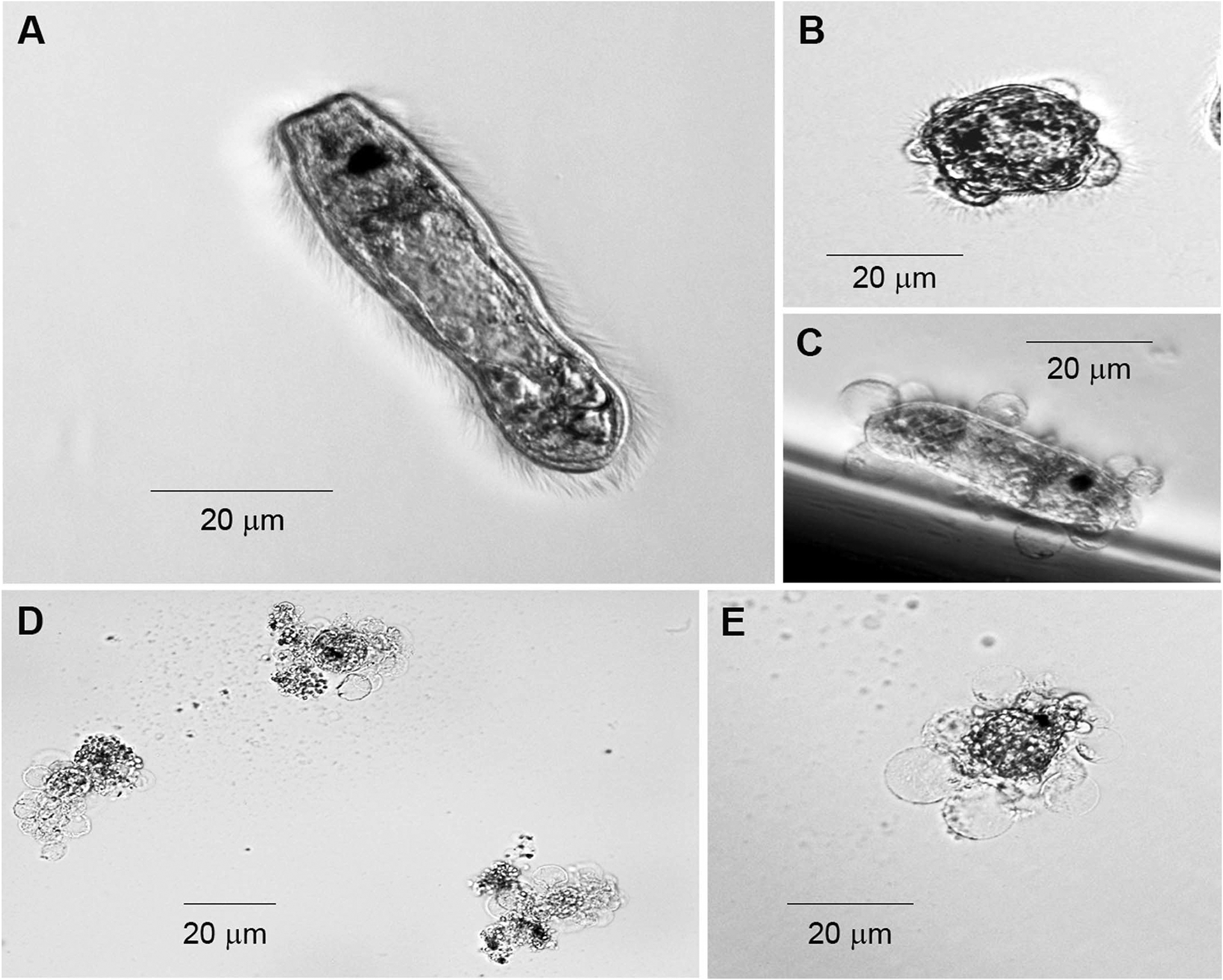
Morphological features of Fascioloides magna miracidia under in vitro experimental conditions. (A) Fascioloides magna miracidium exhibiting normal morphology. (B, C) Miracidia undergoing in vitro transformation to the primary sporocyst stage at 24 hr in culture. (D, E) Miracidia displaying damaging effects of mucus exposure at 4 hr. Note extensive loss of tegumental integrity with accompanying release of internal contents in larvae undergoing cytolysis.
Statistical Analyses
Descriptive statistics (mean, standard error of the mean, percentage damage, or percentage transformation) were determined using Excel 2008 for Mac (version 12.3.6). As the outcome variable was a proportion, based on unequal numbers of miracidia in a given trial, data were arc sine square root transformed (Arc Sine (Y)1/2) using Excel with the following conditions: Outcome proportions of 0% and 100% were substituted by (1/4n) and (100−1/4n) respectively, where n = number of individuals in the trial. A repeated measures 2-factor ANOVA with sphericity correction was used to analyze the effect of mucus dilution (1/3, 1/30. 1/300, 1/3,000, CBSS) on larval damage and transformation (with snail species and dilution representing independent and dependent variables, respectively) (MedCalc; http://www.medcalc.org/statistics.php). Follow-up tests to a significant 2-factor ANOVA result included repeated measures 1-factor ANOVA and Tukey’s post hoc means comparisons. The effects of heat or proteinase K treatment were analyzed using a 2-factor ANOVA with replication with snail species (H. trivolvis and L. elodes) and mucus treatment (heat, PK) as variables. One-factor ANOVAs with replication and Tukey’s means comparisons were used as follow-up tests to significant 2-factor analyses (Excel and Statistix for Windows). Significance was set at P < 0.05. All data are graphically represented as untransformed percentages.
RESULTS
Exposure of F. magna miracidia (Fig. 1A) to H. trivolvis muscus resulted in rapid immobilization of larvae followed by lysis of the tegumental surface and rupturing of internal contents (Fig. 1D, E). These morphological changes were in contrast to transforming miracidia in which the sporocyst tegument remained fully intact during detachment (Fig. 1B) and after release (Fig. 1C) of the ciliated epidermal plates.
Repeated measures 2-factor ANOVA showed a significant snail and dilution effect (F1,14 = 302.1, P < 0.001 and F4,56 = 191.3, P < 0.001, respectively) on miracidial killing, as well as a significant snail × dilution interaction (P < 0.001). Helisoma trivolvis mucus exerted a strong damaging effect on F. magna miracidia after 4 hr of exposure to various mucus dilutions (1-factor ANOVA, F4,28 = 201.9, P < 0.001) (Figs. 1D, E, 2). Followup Tukey’s means comparisons further showed that miracidial damage was dose dependent with significant larval damage rates at 1/3 and 1/30 dilutions followed by nonsignificant rates at higher dilutions and the CBSS control. No significant differences were detected between the buffer control and L. elodes mucus treatment at all dilutions tested (F4,28 = 1.17, P = 0.344; Fig. 2).
Figure 2.
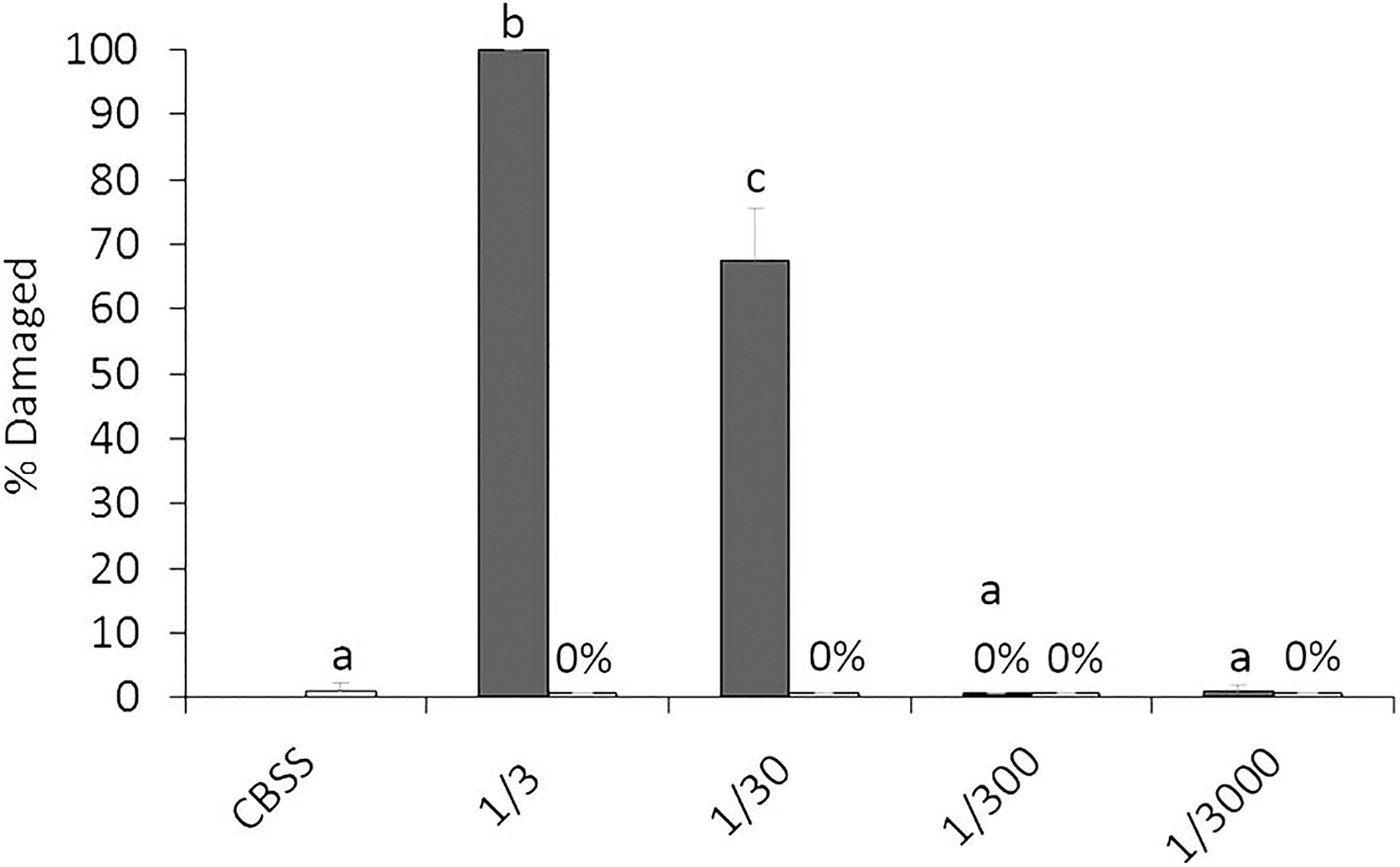
Mean miracidial damage rates after 4 hr of incubation in mucus from Helisoma trivolvis (dark-shaded columns) and Lymnaea elodes (light-shaded columns) at various dilutions in CBSS (1/3, 1/30, 1/300, 1/3,000) or in CBSS alone (CBSS). For H. trivolvis means with different letters are significantly different at the P < 0.05 level by Tukey’s means comparison tests. Repeated measures 1-factor ANOVA revealed no significant effect of E. elodes mucus dilutions on rates of miracidial damage; n = 8. Error bars denote standard error.
Mucus of both snails was subjected to heat (65 C for 30 min) proteinase K (PK) treatments in order to test whether or not mucus cytolytic activity might be protein-mediated. There was significant effect of heat treatment on lytic activity of mucus by 2-factor ANOVA (F2,17 = 271.0, P = 1.03E−10). This activity was again solely attributed to H. trivolvis mucus, as subjecting 1/3-diluted H. trivolvis mucus to a heat treatment completely abrogated miracidial damage at 4 hr when compared to the assay temperature control (Fig. 3A; F2,8 = 564, P = 1.48E−07). Similarly, there was a significant effect of PK treatment of snail mucus (F4,49 = 49.8, P = 6.32E−15) on miracidial damage that was associated exclusively with H. trivolvis samples. Proteinase treatment of H. trivolvis mucus resulted in 70–90% reductions in miracidial damage rates when compared to the temperature control (mucus 33 C, 3 hr) and agarose bead control (mucus exposed to the BSA-coated agarose beads) (Fig. 3B; F4,24 = 49.9, P = 3.97E−10). Tukey’s comparison groupings showed that lytic rates for untreated H. trivolvis mucus, 33 C control mucus and BSA-bead control mucus treatments were not different from each other, but were greater than PK-treated H. trivolvis mucus and the CBSS-only control. In contrast to H. trivolvis, treatment of L. elodes mucus with heat (F2,8 = 0.939, P = 0.441) or PK (F4,24 = 0.284, P = 0.884) had no effect on miracidial damage (Fig. 3A, B).
Figure 3.
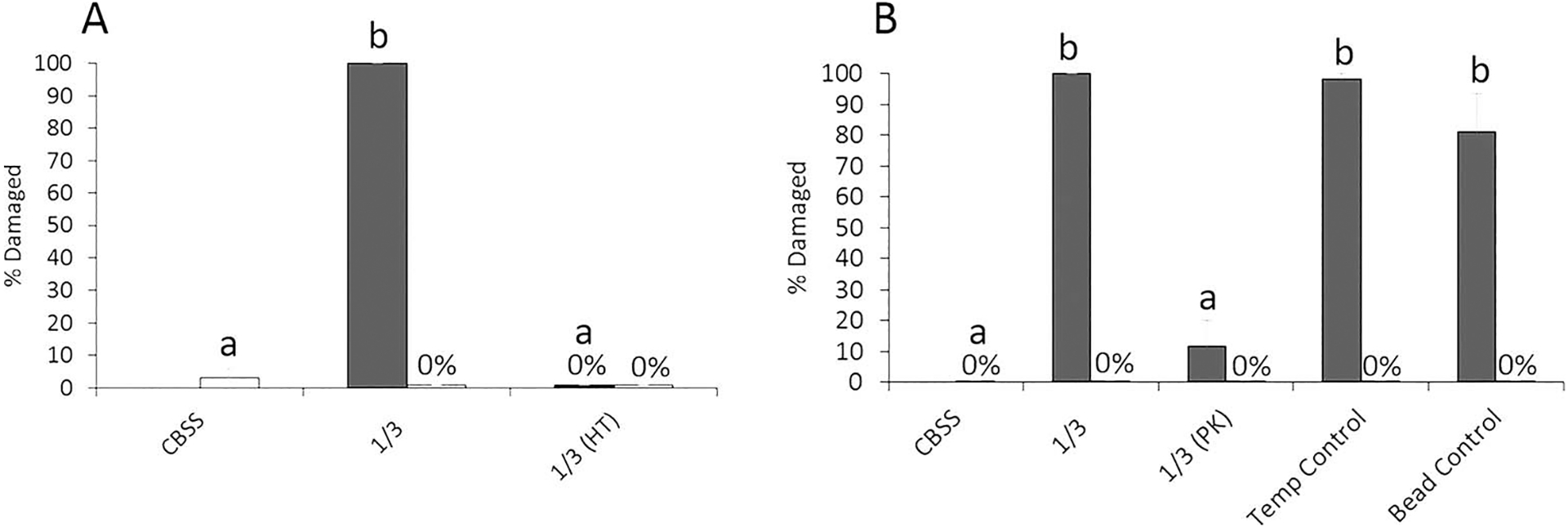
Mean miracidial damage rates after 4 hr of incubation in 1/3-diluted Helisoma trivolvis (dark-shaded columns) and Lymnaea elodes (light-shaded columns) mucus after heat-treatment (HT) (A) or proteinase K (PK) digestion (B). PK treatment controls include those for incubation temperature (Temp Control) and bead specificity (Bead Control) (see Materials and Methods for details). Untreated mucus (1/3 dilution) and buffer only (CBSS) served as positive and negative assay controls, respectively. For H. trivolvis means with different letters are significantly different at the P < 0.05 level by Tukey’s means comparison tests. No significant effect of HT or PK digestion of L. elodes mucus on larval damage rates was seen by 1-factor ANOVA with replication. Here n = 3 for HT and n = 5 for PK experiments. Error bars denote standard errors.
A repeated measures 2-factor ANOVA of the between-snails effect of mucus dilutions on miracidial transformation rates was significant (F1,14 = 2.36, P = 0.147). However, there was a significant within-subjects treatment effect (F4,56 = 5.30, P = 0.001) that appears to be attributed to H. trivolvis mucus at a 1/300 dilution (Fig. 4). There also was a significant within-snail × dilution interaction effect (F4,56 = 3.92, P = 0.007).
Figure 4.
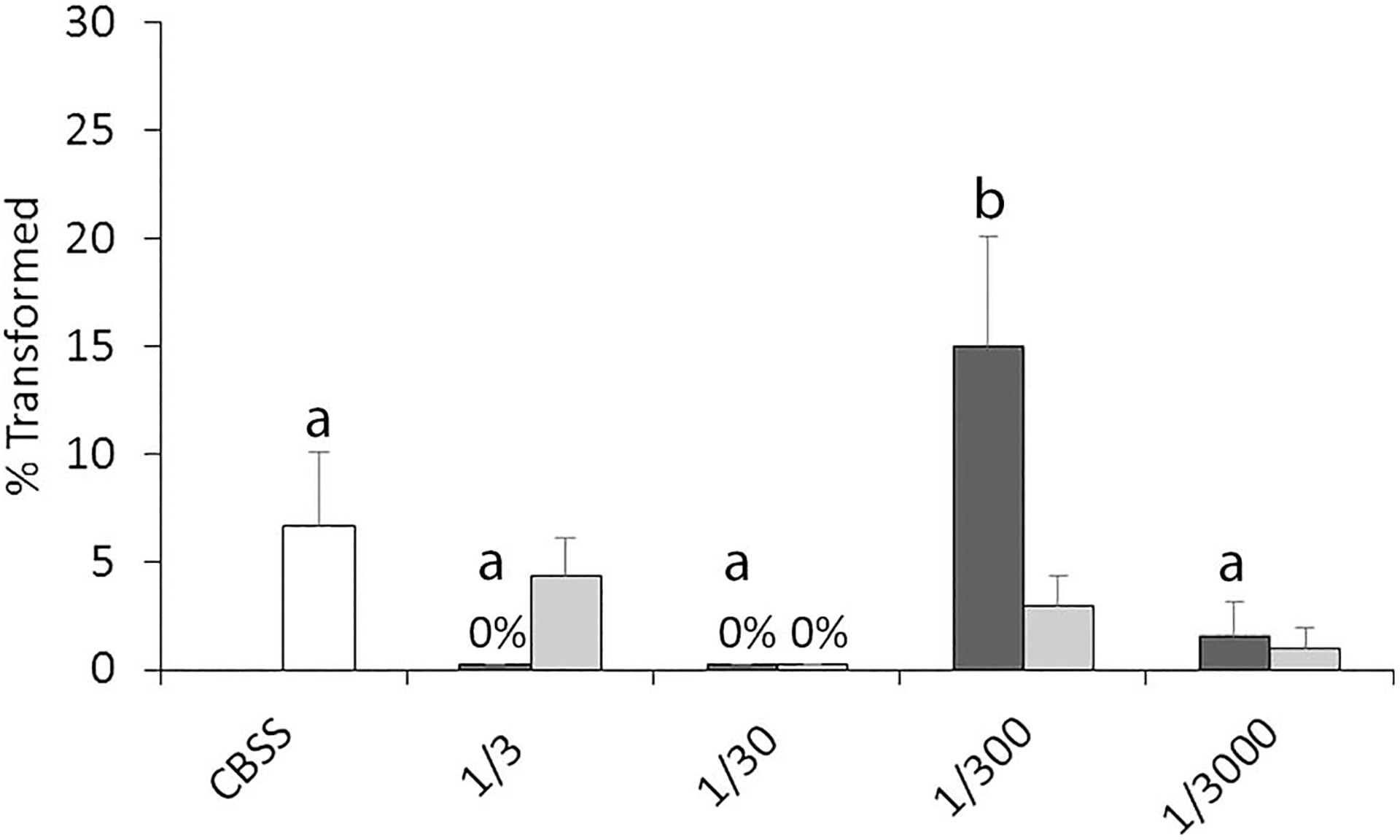
Mean miracidial transformation rates after 24 hr in vitro cultivation in mucus of Helisoma trivolvis (dark-shaded columns) and Lymnaea. elodes (light-shaded columns) at various dilutions in CBSS (1/3, 1/30, 1/300, 1/3,000) or in CBSS alone (CBSS). For H. trivolvis means with different letters are significantly different at the P < 0.05 level by Tukey’s means comparison tests. The effect of E. elodes mucus dilution on transformation rates was not significant by repeated measures one-factor ANOVA; n = 8. Error bars denote standard errors.
Finally, results of 2-factor ANOVA revealed there was no significant effect of heat treatment (F2,17 = 0.518, P = 0.608) on miracidial transformation rates after 24 hr in culture (Fig. 5A). By contrast, PK treatment of mucus had a significant effect on miracidial transformation rates (F4,49 = 2.738, P = 0.04) (Fig. 5B), with PK-treated Helisoma mucus stimulating higher transformation rates than untreated mucus or assay controls (BSA-bead and 33 C). However, transformation rates of PK-treated miracidia did not group differently from CBSS controls by Tukey’s test.
Figure 5.
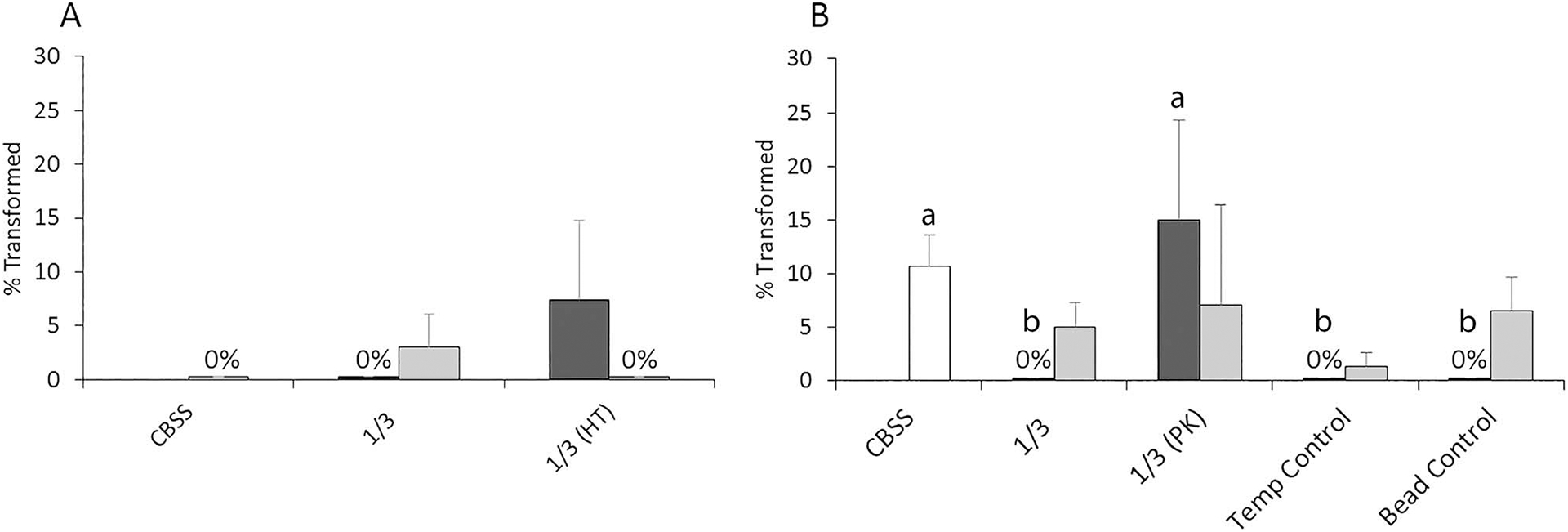
Mean miracidial transformation rates after 24 hr in 1/3-diluted Helisoma trivolvis (dark-shaded columns) and Lymnaea elodes (light-shaded columns) mucus after heat-treatment (HT) (A) or proteinase K (PK) digestion (B). PK treatment controls include those for incubation temperature (Temp Control) and bead specificity (Bead Control) (see Materials and Methods for details). Untreated mucus (1/3) and buffer only (CBSS) served as positive and negative assay controls, respectively. HT had no significant effect on larval transformation rates in either snail species by 2-factor ANOVA with replication, while a significant PK treatment effect was detected for H. trivolvis mucus only. In this case, means with different letters are significantly different at the P < 0.05 level by Tukey’s means comparison tests. Here n = 3 for HT and n = 5 for PK experiments. Error bars denote standard errors.
DISCUSSION
Our results demonstrate the presence of a water-soluble component(s) in H. trivolvis mucus that is cytotoxic to F. magna miracidia. The cytotoxic activity can be titrated to control levels by dilution with CBSS and is heat- and proteinase-sensitive. A similar toxic effect was not observed with identically prepared mucus of the compatible snail L. elodes and suggests that the mucus layer covering the mantle surface may constitute a potentially important defensive barrier to infection by aborting miracidial attachment to and/or entry into incompatible snail species. Although physiological host-parasite incompatibility may be associated with geographic separation of trematode and snail species (Sapp and Loker, 2000), given that planorbid snails generally, including Helisoma spp., are not known to serve as a natural intermediate hosts for F. magna, our findings are consistent with the prediction that H. trivolvis mucus would cause significantly more damage to F. magna miracidia than that of L. elodes (palustris), a known competent host of F. magna in Wisconsin (Foreyt and Todd, 1978). A similar damaging effect was demonstrated upon contact and attempted attachment of Schistosoma mansoni miracidia with the incompatible snail Helisoma caribaeum (Chernin and Perlstein, 1969). Interestingly, however, even within compatible trematode-snail systems, behavior-altering activity of snail-secreted mucus may depend on the parasite and snail species. For example, mucus from the compatible lymnaeid snail, L. truncatula, served to stimulate swimming activity of Fasciola hepatica miracidia (Wilson, 1968; Wilson and Denison, 1970), whereas mucus had no behavior-altering effect on miracidia when tested in a compatible S. mansoni–Biomphalaria glabrata system (Chernin, 1970). Therefore it is important that cross-species comparisons of factors believed to influence trematode-snail compatibility are well defined and methods consistently followed to allow for general conclusions to be drawn.
Based on results of the present study and earlier findings (Wilson, 1968), mucus-associated negative (e.g., cytotoxic) or positive (e.g., activity stimulants or attractants) effects correlate quite well with F. magna compatibility with the snail host. This is generally consistent with results of a multi-snail/multi-trematode study (Sapp and Loker, 2000), in which significant toxic effects of lymnaeid snail plasma were observed in trematode larvae that normally utilize planorbids as intermediate hosts. However, inconsistent correlations, for example, the benign effects of incompatible lymnaeid and Helix aspersa plasma on S. mansoni sporocysts indicate that internal regulators of infection success (e.g., physiological environment or innate immune system) are not completely reliable for predicting compatibility. For F. magna, mucus cytotoxicity correlates well with compatibility when applied to snails related at the family and/or genus level. This is contrary to findings by Sapp and Loker (2000) who reported similar miracidial attachment/penetration in 4 incompatible trematode-snail combinations when compared to compatible pairings. Attachment and attempted penetration requires direct contact with epidermal mucus, and if such contact posed a cytotoxic barrier, one might have predicted lower attachment scores for incompatible matches. Explanations for these contrary results include the possibility that attached miracidia may have been killed in situ and were not detected in the assay, or as pointed out earlier, different parasite-host species combinations may differ in their response to external factors, in this case susceptibility to mucus cytotoxicity. Whether this type of infection barrier exists for fasciolids in general or specifically in F. magna–snail interactions requires further investigation. In addition, a question still remains as to whether a mucus barrier exists for F. magna, not only in incompatible snails differing at the family or genus level, but also between snail species within a genus that differ in their compatibility to larval infection. Recent studies with the protistan parasite Perkinsus marinus demonstrated that mucus from the mantle and gills of a compatible oyster species, Crassostrea virginica, strongly enhanced P. marinus growth and viability in culture, whereas mucus from an incompatible species, C. gigas, had the opposite effect (Espinosa et al., 2013), suggesting a role of mucus in the “fine-tuning” of host specificity among closely related species. This question is currently under investigation using various species of Lymnaea.
Very little is known about the biochemical composition of snail mucus that may be mediating its cytotoxic effect. The complete abrogation of cytotoxic activity in F. magna mucus by heat and proteinase K digestion in this study strongly supports the toxic factor(s) being a protein(s), either wholly or in part. Previous studies have implicated free amino acids, fatty acids, divalent cations such as Mg++, and other chemicals in snail conditioned water (SCW) as miracidial attractants (reviewed by Sukhdeo and Mettrick, 1987). The finding of 16 amino acids in L. truncatula mucus (Wilson, 1968), as well as comparable numbers in L. palustris and H. trivolvis SCW (Wright and Ronald, 1972; Prechel et al., 1976), suggests that mucus may be serving as one of the sources of secreted attractants. It is unlikely, however, that any of these chemicals at the concentrations tested carry significant toxicity to miracidia. More recently, a macromolecular glycoconjugate (>300 kDa) was identified in SCW from B. glabrata (Haberl and Haas, 1992) that exhibited chemotactic activity for S. mansoni miracidia. Chemical treatments of this molecule suggested it was a mucopolysaccharide and implicated its carbohydrate moieties as mediating larval attraction. Although mucus may have been a source of this molecule, its polysaccharide structure and insensitivity to proteinase K treatment indicate that the chemo-attractive activity in SCW and cytotoxic activity of mucus in planorbid snails are mediated by different molecules.
One of the predictions of our original hypothesis was not supported, namely, that mucus from the compatible L. elodes snail would stimulate F. magna miracidial transformation to a greater degree than H. trivolvis mucus. This hypothesis was based on previous observations that in vivo transformation of F. magna miracidia is triggered by miracidial attachment to mantle epithelium and introduction of the primary sporocyst into a compatible snail host (Coil, 1977, 1981). In the present study, instead we observed that contact with mucus from either snail species tested had little to no effect on transformation rates compared to controls. If anything, Helisoma mucus supported higher levels of transformation, although the effect is very small. These results suggest that, under our experimental conditions, mucus from the compatible L. elodes snail lacks sufficient concentrations of stimulatory molecules involved in initiating this process or that stimulating molecules located at the snail surface require contact with specific anatomical or sensory structures of the miracidium, perhaps associated with the apical papillae. In addition, the finding that PK treatment of H. trivolvis muscus exerts an enhancing effect on transformation suggests that the triggers for miracidial transformation for F. magna may be quite complex, involving multiple factors not accounted for in our in vitro assay system.
For future studies the next steps in learning more about potential anti-parasitic factors present in mantle mucus secretions are to isolate and characterize the cytotoxic proteins in H. trivolvis mucus. Because of the relatively close taxonomic and phylogenetic relationship between Helisoma and Biomphalaria (both Fam. Planorbidae members), the completion of the draft sequence of the B. glabrata genome (https://www.vectorbase.org/organisms/biomphalaria-glabrata) with ongoing annotation and assembly, and growing transcriptomic/proteomic databases, there are currently opportunities for conducting comparative proteomics-based homology investigations on mucus proteins of H. trivolvis and other molluscan species. Studies focused on the specificity of mucus cytotoxins to discriminate between compatible and incompatible trematodes at the level of snail species or strains will provide valuable insights into the critical factors underlying host specificity between larval flukes and their snail intermediate hosts.
ACKNOWLEDGMENTS
We thank Dr. Jane Huffman for supplying the H. trivolvis snails, and Marilia Cavalcante, Mary Schulz, and Nathalie Dinguirard for assistance in manuscript preparation. K.C. was supported by NIH training grant T32 RR17503.
LITERATURE CITED
- Basch PF 1991. Schistosomes: Development, reproduction, and host relations. Oxford University Press, New York, New York, 248 p. [Google Scholar]
- Brown BE, and Bythell JC. 2005. Perspectives on mucus secretion in coral reefs. Marine Ecology Progress Series 296: 291–309. [Google Scholar]
- Chernin E 1963. Observations on hearts explanted in vitro from the snail Australorbis glabratus. Journal of Parasitology 49: 353–364. [PubMed] [Google Scholar]
- ———. 1970. Behavioral responses of miracidia of Schistosoma mansoni and other trematodes to substances emitted by snails. Journal of Parasitology 56: 287–296. [PubMed] [Google Scholar]
- ———, AND Perlstein JM. 1969. Further studies on interference with the host-finding capacity of Schistosoma mansoni miracidia. Journal of Parasitology 55: 500–508. [PubMed] [Google Scholar]
- Clarke AH 1973. Canadian freshwater molluscs. Malacologia 13–14: 1–492. [PubMed] [Google Scholar]
- Coil WH 1977. The penetration of Fascioloides magna miracidia into the snail host Fossaria bulimoides. Zeitschrift für Parasitenkunde 52: 53–59. [DOI] [PubMed] [Google Scholar]
- ———. 1981. Miracidial penetration in Fascioloides magna (Trematoda). Zeitschrift für Parasitenkunde 65: 299–307. [DOI] [PubMed] [Google Scholar]
- Denny MW 1989. Invertebrate mucous secretions: Functional alternatives to vertebrate paradigms. Symposia of the Society for Experimental Biology 43: 337–366. [PubMed] [Google Scholar]
- Ehara T, Kitajima S, Kanzawa N, Tamiya T, and Tsuchiya T. 2002. Antimicrobial action of achacin is mediated by L-amino acid oxidase activity. Federation of European Biochemical Societies Letters. 531: 509–512. [DOI] [PubMed] [Google Scholar]
- Espinosa EP, Perrigault M, Ward JE, Shumway SE, and Allam B. 2009. Lectins associated with the feeding organs of the oyster Crassostrea virginica can mediate particle selection. Biological Bulletin 217: 130–141. [DOI] [PubMed] [Google Scholar]
- ———, ———, ———, ———, and ———. 2010. Microalgal cell surface carbohydrates as recognition sites for particle sorting in suspension-feeding bivalves. Biological Bulletin 218: 75–86. [DOI] [PubMed] [Google Scholar]
- ———, Winnicki S, and Allam B. 2013. Early host-pathogen interactions in a marine bivalve: Crassostrea virginica pallial mucus modulates Perkinsus marinus growth and virulence. Diseases of Aquatic Organisms 104: 237–247. [DOI] [PubMed] [Google Scholar]
- Foreyt WJ, and Todd AC. 1978. Experimental infection of lymnaeid snails in Wisconsin with miracidia of Fascioloides magna and Fasciola hepatica. Journal of Parasitology 64: 1132–1134 [PubMed] [Google Scholar]
- Greenwood PG, Garry K, Hunter A, and Jennings M. 2004. Adaptable defense: A nudibranch mucus inhibits nematocyst discharge and changes with prey type. Biological Bulletin 206: 113–120. [DOI] [PubMed] [Google Scholar]
- Haberl B, and Haas W. 1992. Miracidium of Schistosoma mansoni: A macromolecular glycoconjugate as signal for the behaviour after contact with the snail host. Comparative Biochemistry and Physiology Part A: Physiology 101: 329–333. [DOI] [PubMed] [Google Scholar]
- Iguchi SMM, Aikawa T, and Matsumoto JJ. 1982. Antibacterial activity of snail mucus mucin. Comparative Biochemistry and Physiology Part A: Physiology 72: 571–574. [DOI] [PubMed] [Google Scholar]
- Ijima R, Kisugi J, and Yamazaki M. 2003. A novel antimicrobial peptide from the sea hare Dolabella auricularia. Developmental and Comparative Immunology 27: 305–311. [DOI] [PubMed] [Google Scholar]
- Ito S, Shimizu M, Nagatsuka M, Kitajima S, Honda M, Tsuchiya T, and Kanzawa N. 2011. High molecular weight lectin isolated from the mucus of the giant African snail Achatina fulica. Biosciences, Biotechnology, and Biochemistry 75: 20–25. [DOI] [PubMed] [Google Scholar]
- Kalbe M, Haberl B, and Haas W. 1997. Miracidial host-finding in Fasciola hepatica and Trichobilharzia ocellata is stimulated by species-specific glycoconjugates released from the snail host. Parasitology Research 83: 806–812. [DOI] [PubMed] [Google Scholar]
- Kelly WP, Wolters AM, Sack JT, Jockusch RA, Jurchen JC, Williams ER, Sweedler JV, and Gilly WF. 2003. Characterization of a novel gastropod toxin (6-bromo-2-mercapto-tryptamine) that inhibits shaker K channel activity. Journal of Biological Chemistry 278: 34935–34942. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Watanabe Y, Otsuka H, Tamiya T, Tsuchiya T, and Matsumoto JJ. 1985. Purification and characterization of an antibacterial factor from snail mucus. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology 82: 345–348. [DOI] [PubMed] [Google Scholar]
- Li D, and Graham LD. 2007. Epiphragmin, the major protein of epiphragm mucus from the vineyard snail, Cernuella virgata. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 148: 192–200. [DOI] [PubMed] [Google Scholar]
- Noland LE, and Carriker MR. 1946. Observations on the biology of the snail Lymnaea stagnalis appressa during twenty generations in laboratory culture. American Midland Naturalist 36: 467–493. [Google Scholar]
- Notten MJM, Oosthoek AJP, Rozema J, AND Aerts R. 2006. The land snail Cepaea nemoralis regulates internal Cd levels when fed on Cd-enriched stinging nettle (Urtica dioica) leaves at low, field-relevant concentrations. Environmental Pollution 139: 296–305. [DOI] [PubMed] [Google Scholar]
- Otsuka-Fuchino H, Watanabe Y, Hirakawa C, Tamiya T, Matsumoto JJ, and Tsuchiya T. 1992. Bactericidal action of a glycoprotein from the body surface mucus of giant African snail. Comparative Biochemistry and Physiology Part C. Comparative Pharmacology and Toxicology 101: 607–613. [DOI] [PubMed] [Google Scholar]
- Prechel DP, Cain GD, and M Nollen P. 1976. Responses of Megalodiscus temperatus miracidia to amino and sialic acids found in snail-conditioned water. Journal of Parasitology 62: 693–697. [PubMed] [Google Scholar]
- Richie KB 2006. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Marine Ecology Progress Series 322: 1–14. [Google Scholar]
- Roberts TM, Linck RW, AND Chernin E. 1980. Effector mechanism in the response of Schistosoma mansoni miracidia to snail-conditioned water. Canadian Journal of Zoology 50: 855–860. [DOI] [PubMed] [Google Scholar]
- Sapp KK, and Loker ES. 2000. Mechanisms underlying digeneansnail specificity: Role of miracidial attachment and host plasma factors. Journal of Parasitology 86: 1012–1019. [DOI] [PubMed] [Google Scholar]
- Sminia T, and Barendsen L. 1980. A comparative morphological and enzyme histochemical study on blood cells of the freshwater snails Lymnaea stagnalis, Biomphalaria glabrata, and Bulinus truncatus. Journal of Morphology 165: 31–39. [DOI] [PubMed] [Google Scholar]
- Sukhdeo MVK, and Mettrick DF 1987. Parasite behaviour: Understanding platyhelminth responses. Advances in Parasitology 26: 74–144. [DOI] [PubMed] [Google Scholar]
- Wilson RA 1968. An investigation into the mucus produced by Lymnaea truncatula, the snail host of Fasciola hepatica. Comparative Biochemistry and Physiology 24: 629–633. [DOI] [PubMed] [Google Scholar]
- ———, and Denison J. 1970. Studies on the activity of the miracidium of the common liver fluke, Fasciola hepatica. Comparative Biochemistry and Physiology 32: 301–313. [DOI] [PubMed] [Google Scholar]
- Wright DGS, and Ronald K. 1972. Effects of amino acids and light on the behavior of miracidia of Schistosomatium douthtti. Canadian Journal of Zoology 50: 855–860. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Kimura K, Kissuge J, Muramoto K, and Kamiya H. 1989. Isolation and characterization of a novel cytolytic factor in purple fluid of the sea hare, Aplysia kurodai. Cancer Research 49: 3834–3838. [PubMed] [Google Scholar]
- Zhong J, Wand W, Yang X, Yan X, and Liu R. 2013. A novel cysteine-rich antimicrobial peptide from the mucus of the snail of Achatina fulica. Peptides 39: 1–5. [DOI] [PubMed] [Google Scholar]


