Abstract
Background and Purpose:
Previous evidence suggests that anodal transcranial direct current stimulation (A-tDCS) applied to the left hemisphere can improve aphasic participants’ ability to name common objects. The current study further examined this issue in a more tightly controlled experiment in participants with fluent aphasia.
Methods:
We examined the effect of A-tDCS on reaction time (RT) during overt picture naming in eight chronic stroke participants. Anode electrode placement targeted peri-lesional brain regions that showed the greatest activation on a pre-treatment fMRI scan administered during overt picture naming, with the reference cathode electrode placed on the contralateral forehead. A-tDCS (1 mA; 20-min) was compared to sham tDCS (S-tDCS) in a cross-over design. Participants received ten sessions of computerized anomia treatment; five sessions included A-tDCS and five included S-tDCS.
Results:
Coupling A-tDCS with behavioral language treatment reduced RT during naming of trained items immediately post-treatment, Z=1.96, p=.025, and at subsequent testing three weeks later, Z=2.52, p=.006.
Conclusions:
A-tDCS administered during language treatment decreased processing time during picture naming by fluent aphasic participants. Additional studies combining A-tDCS, an inexpensive method with no reported serious side effects, with behavioral language therapy are recommended.
Keywords: anomia, brain stimulation, recovery, stroke, tDCS
Introduction
Recently, our group demonstrated how anodal transcranial direct current stimulation (A-tDCS) can enhance the effect of behavioral aphasia treatment.1 Ten patients, with varying types and severities of chronic aphasia, received computerized aphasia treatment coupled with A-tDCS to the left frontal lobe. In four of these patients, A-tDCS significantly amplified the effect of the aphasia treatment compared to sham-tDCS (S-tDCS). Inspection of the data suggested that good responders primarily had left frontal lobe damage; for those patients, stimulation occurred closer to the peri-lesional rim compared to the remaining patients whose damage was more posterior. Although the effect of A-tDCS varied across patients, this study yielded overall positive results, warranting further research.
The current study improved on the previous study in the following ways: 1) In addition to blinding both participants and clinicians who scored pre and post naming tests, clinicians who administered the tDCS protocol and the computerized treatment were also blinded to stimulation type; 2) Instead of including a broad range of aphasia types and lesion sites, all participants had fluent aphasias with posterior cortical or sub-cortical lesions; and 3) Maintenance testing was extended from one to three weeks post-treatment.
The final difference between the present study and our earlier work was the selection of a posterior rather than anterior focus for stimulation. Although Baker et al1 targeted the left frontal lobe with A-tDCS, recent work2–4 suggests that increased left hemisphere activation in both anterior and posterior regions supports treatment-assisted improvement in naming among aphasic participants. Therefore, anode electrode placement here targeted peri-lesional brain regions showing the greatest activation on a pre-treatment functional MRI (fMRI) scan during overt picture naming.
We maintained the design feature whereby A-tDCS was compared to a placebo (S-tDCS) administered in a crossover design. Each participant received ten sessions of computerized aphasia treatment, five of which included A-tDCS and the other five, S-tDCS.
The participants in this study had relatively good scores on the assessment used to chart naming improvement, limiting naming accuracy as a measurement of treatment-related change. Therefore, RT was chosen as the dependent measure since we expected it would be sensitive to treatment-related changes among participants whose anomia, in most cases, was mild.
Methods
Participants ranged in age from 53–79 (M=68.13, SD=10.40); time post-stroke ranged from 10 to 150 months (M=58.38, SD=44.60). A computerized anomia treatment coupled with tDCS was administered during each of two treatment phases. Each treatment phase lasted one week, with three weeks separating the two treatment phases (A-tDCS vs. S-tDCS). The number of treatment sessions (5 consecutive days for each treatment phase), length of stimulation (20-min per session), and stimulation intensity (1 mA) was modeled after previous research.1 A computerized naming assessment of both trained and untrained words was conducted six times for each treatment phase (A-tDCS & S-tDCS): twice at baseline, twice immediately following the final treatment session for each phase, and twice at three weeks following completion of each treatment phase.
During both A-tDCS and S-tDCS, the anode electrode was placed over the pre-designated area of the scalp overlying left posterior cortex. The reference cathode electrode was placed on the right forehead. To ensure blinding, in-house software switched the tDCS on and off without intervention from the participant or experimenter. For S-tDCS, the stimulator was turned off following 45 s; for A-tDCS, stimulation was maintained for 20 min.
The self-administered computerized treatment consisted of a spoken word-picture matching task occurring concurrently with the application of tDCS. Beginning 5 min before the start of tDCS, this treatment was modeled after tasks used in previous studies that have resulted in improved naming accuracy in participants with aphasia.1,5 Separate word lists were used for each treatment phase.1
Results
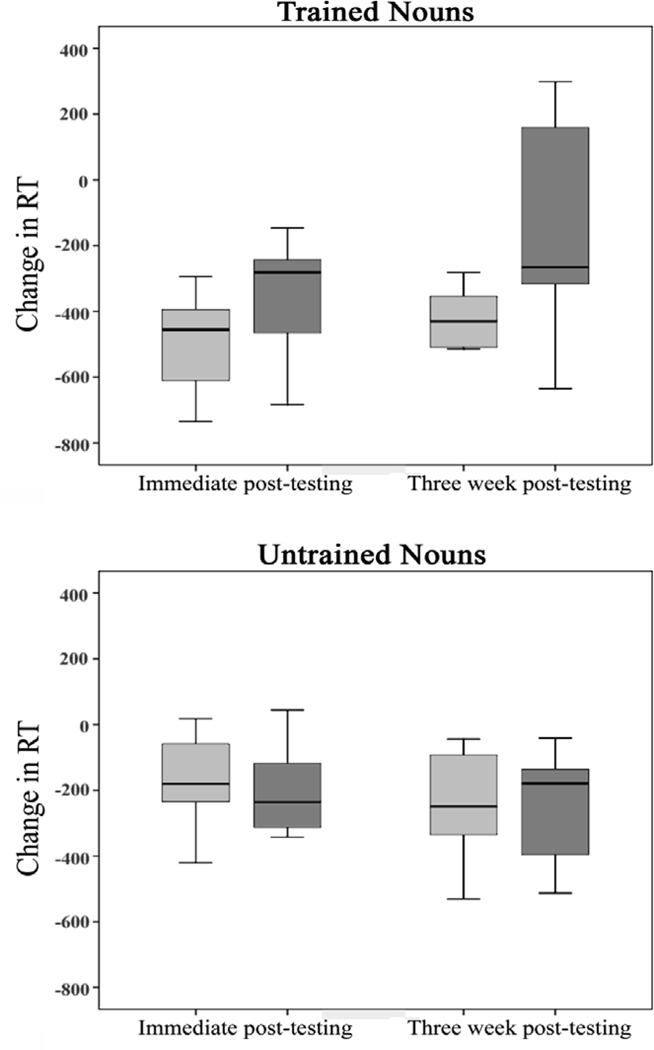
Following A-tDCS, the median group change in RT for trained items was −455.57 ms (Inter-quartile range (IQR)=−672.08 to −393.93) immediately post-treatment and −430.06 ms (IQR=−511.63 to −346.83) at three-weeks post-treatment. Comparable reductions in RT following S-tDCS were −281.17 ms (IQR=−516.54 to −241.77) immediately post-treatment and −265.86 ms (IQR=−328.25 to 228.62) three-weeks post-treatment (Figure 1). To minimize the effect of outliers, all RT values greater than +/− 2 SD from the mean were removed and the mean recalculated. A Wilcoxon signed rank test (1-tailed) revealed greater reduction in RT during correct naming of trained nouns following A-tDCS compared to S-tDCS immediately upon treatment completion, Z=1.96, p=.025, and also at three weeks post-testing, Z=2.52, p=.006. Upon immediate post-testing, seven of eight participants experienced greater reduction in RT following the A-tDCS compared to the S-tDCS. At three-weeks post-testing, all eight participants experienced greater reduction in RT after A-tDCS compared to S-tDCS. The probability that out of 16 comparisons, 15 occurred in a direction that favored A-tDCS (seven for immediate post-testing and eight for three-week follow-up) was calculated: p=.0002 (based on binomial distribution).
Figure 1.
Reduction in RT following A-tDCS (light gray) and S-tDCS (dark gray). The line in the middle of each box represents the median. The top and bottom of each box denotes the inter-quartile range. The error bars represent the data range; RT is measured milliseconds.
Discussion
This study included eight participants with fluent aphasia and revealed greater treatment-related reduction in RT during naming of trained items following A-tDCS compared to S-tDCS immediately after treatment completion as well as at three-week follow-up testing. This treatment effect was not accounted for by unspecific arousal differences (i.e., changes in blood pressure, heart rate recordings, etc.), differences in comfort level (i.e., scalp sensations), or treatment order.
Differences in stimulus generalization were absent as RTs were similar for untrained items following both treatment conditions. However, the receptive treatment task did not include overt naming, suggesting that greater response generalization occurred following A-tDCS than S-tDCS. This relates to the work of Fritsch and colleagues,6 who found that the effect of A-tDCS upon motor learning is stimulus-driven since A-tDCS in the absence of behavioral training did not improve task performance. Specifically, their study revealed A-tDCS induces secretion of brain derived neurotrophic factor (BDNF), a protein crucial for new learning. It is possible that increased BDNF secretion in peri-lesional areas promoted improved naming performance among our participants. Based on Baker et al1 and the current data, it is reasonable to propose that positive treatment effects may be further enhanced and maintained by coupling language stimulation with A-tDCS applied to the left hemisphere.
The current findings warrant further investigation to evaluate the effect of A-tDCS upon aphasia recovery. Clearly, more research is needed to understand factors such as stimulus generalization, brain plasticity associated with A-tDCS, the necessary time course of stimulation, and perhaps most importantly, the ecological validity of this method. Our hope is that in the future, research such as this may aid aphasia recovery.
Supplementary Material
Acknowledgements
Sources of Funding: Grant support – DC008355 (PI: JF) and DC009571 (PI: JF & CR) from the National Institute on Deafness and Other Communication Disorders, and NS054266 (PI: CR) from the National Institute for Neurological Disorders and Stroke.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fridriksson J Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. The Journal of Neuroscience. 2010;30:11558–11564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin PI, Naeser MA, Ho M, Doron KW, Kurland J, Kaplan J, Wang Y, Nicholas M, Baker EH, Alonso M, Fregni F, Pascual-Leone A. Overt naming fMRI pre- and post-TMS: Two nonfluent aphasia patients, with and without improved naming post-TMS. Brain & Language. 2009;111:20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, McArdle J, Braun AR. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience. 2010;22:1299–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridriksson J, Baker JM, Whiteside J, Eoute D, Moser D, Vesselinov R, Rorden, C. Treating visual speech perception to improve speech production in non-fluent aphasia. Stroke. 2009;40:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron. 2010;66:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.