Abstract
Chudley–McCullough syndrome (CMS) is an autosomal-recessive disorder characterized by a complex brain malformation and profound congenital sensorineural hearing loss. Postnatal brain imaging findings include ventriculomegaly, partial agenesis of corpus callosum, inferior cerebellar dysplasia, arachnoid cysts, and malformations of cortical development including frontal subcortical heterotopia and polymicrogyria. Prenatal diagnosis of CMS is important due to the markedly less severe neurodevelopmental prognosis compared to disorders with similar brain imaging findings. We report prenatal imaging features that help distinguish CMS from other disorders, including slit-like frontal horns, agenesis of the corpus callosum, frontal subcortical heterotopia, arachnoid cysts, and cerebellar dysplasia. 2016 Wiley Periodicals, Inc.
Keywords: fetal MR, Chudley–McCullough syndrome, ventriculomegaly, prenatal diagnosis, polymicrogyria, agenesis of corpus callosum
INTRODUCTION
We report the prenatal MR imaging of a fetus affected by Chudley–McCullough syndrome (CMS). Both of the parents’ living children had been found to carry a homozygous splice-site mutation (C.1062 + 1G> T) in the G protein-signaling modulator two gene (GPSM2). We review the published literature for other reports of this rare autosomal-recessive syndrome and discuss the relevant fetal brain imaging features that should raise suspicion for this diagnosis. Recognition of CMS will influence prenatal counseling since the disorder has a less severe neurodevelopmental outcome compared to other disorders with similar brain imaging findings.
CLINICAL REPORT
A healthy 30-year-old G4P2 Latin American woman was referred to a regional prenatal care center for counseling at 33 + 4 weeks following second- and third-trimester ultrasound studies at her referring clinic showing stable fetal ventriculomegaly (right lateral ventricle 20 mm, left 13 mm), absent cavum septum pellucidum, and agenesis of the corpus callosum. The remaining anatomy, growth parameters, and amniotic fluid volume were reportedly normal. Retrospectively, following fetal MR imaging (described below), abnormal morphology of the lateral ventricles is appreciable (Fig. 1a). The pregnancy was otherwise unremarkable, with the exception of a small amount of vaginal bleeding at approximately 20 weeks gestation. The patient reported no exposure to tobacco, alcohol, or illicit drugs, and had been taking prenatal vitamins throughout. A maternal serum screen was normal (T21 risk <1/5,000; T18 risk 1/3,163). The patient’s third pregnancy had ended in a first trimester miscarriage.
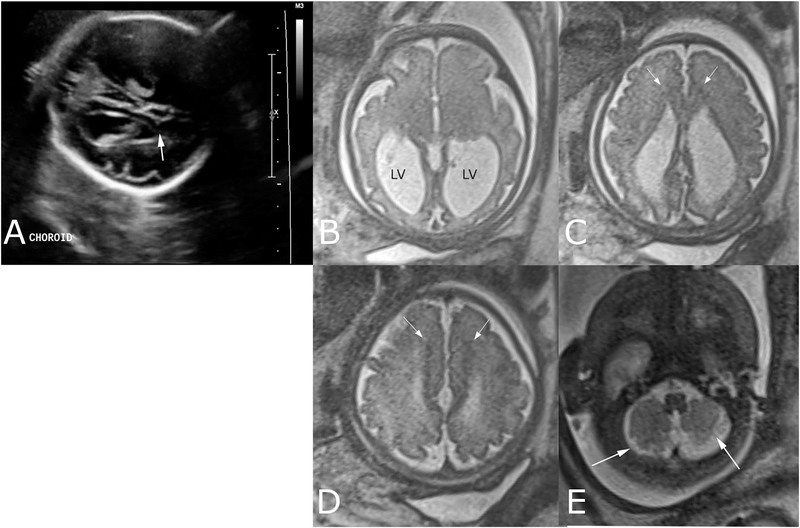
FIG. 1.
Third-trimester prenatal imaging shows findings consistent with Chudley–McCullough syndrome. (A) Single oblique-axial image of fetal head acquired during ultrasound performed at 28 + 1 weeks shows lateral ventriculomegaly and captures abnormally pointed morphology of lateral ventricle frontal horn (arrow), appreciated only in retrospect. Fetal brain MR single-shot fast-spin echo T2-weighted images were later acquired at 33 + 3 weeks; (B) Axial image through the lateral ventricle atria shows abnormal enlargement of the lateral ventricles (LV). Note parallel orientation of the lateral ventricles in axial plane (A and B), typical of dysgenesis of the corpus callosum; (C and D) Axial images through the frontal horns show pointed, small frontal horns of the lateral ventricles and abnormal hypointense signal matching that of gray-matter in the subcortical tissue medially (arrows), consistent with heterotopia; (E) Axial image through the inferior cerebellum shows abnormal organization of the cerebellar folia and fissures (arrows), suggestive of cerebellar dysplasia.
Fetal MR Imaging
Fetal MRI at 33 weeks gestation demonstrated bilateral lateral ventriculomegaly, with disproportionate enlargement of the atria and occipital horns (colpocephaly) and slit-like frontal horns. Additional findings included agenesis of the posterior corpus callosum, small, and pointed frontal horns with an abnormal gyral pattern, a parallel band of gray-matter heterotopia lining the medial aspect of the frontal horns, and dysplasia of the inferior cerebellum (Fig. 1b–e). The remainder of the fetal anatomy on MRI was normal.
Family History
This patient’s two living children (a daughter and a son) had Chudley–McCullough syndrome (CMS) (OMIM 604213), an autosomal recessive condition characterized by sensorineural hearing loss, lateral ventriculomegaly, partial agenesis of the corpus callosum, frontal neuronal migrational anomalies, cerebellar dysplasia, and arachnoid cysts. Mutations in the G protein-signaling modulator two gene(GPSM2) cause this disorder [Doherty et al., 2012], and both of this patient’s children had been found to carry a homozygous splice-site mutation (C.1062 + 1G> T) in GPSM2. The diagnosis of CMS was not made until after the birth of the second child. No abnormalities had been identified prenatally in the first child. In the second child, prenatal ultrasound had revealed severe ventriculomegaly, but colpocephaly was not mentioned in the available records.
Prenatal Counseling
The prenatal imaging findings confirmed the diagnosis and facilitated counseling that the postnatal outcome for this fetus would very likely be similar to the couple’s two living children. Based on the absence of a visualized intraventricular cyst and stable ventriculomegaly at the time of the MRI, the risk for hydrocephalus requiring ventricular shunting was considered low. Recommendations included sonographic surveillance later in gestation to monitor for fetal well-being and head size prior to delivery. Delivery at a community hospital near the family’s home was felt to be appropriate, given the absence of neonatal complications in most infants with CMS. Evaluation by an experienced pediatric neurodevelopmental specialist, non-sedated brainstem auditory evoked response (BAER) testing, and a postnatal non-sedated brain MR were also recommended. Finally, genetic testing for the mutation segregating in the family was offered.
Delivery History
The remainder of the pregnancy was uneventful. A subsequent ultrasound performed at 37 + 5 weeks showed a normal amniotic fluid index and further progression of lateral ventriculomegaly, measuring 28 mm bilaterally. A non-stress test was normal at that time.
At 40 + 6 weeks, the patient presented to her community hospital with increasing frequency of contractions, and labor progressed as expected. Vaginal delivery produced a live female baby weighing 3.3 kg. The postnatal inpatient course was uneventful and the mother and infant were discharged home. Profound sensorineural hearing loss and auditory neuropathy were confirmed by BAER, although low-frequency otoacoustic emissions were present in the right ear indicating some cochlear function. The infant’s weight, length, and head circumference remained proportionate at the 75th percentile during the first year after birth. Ventricular shunting was not required. At 14 months, the child underwent placement of bilateral cochlear implants following a normal temporal bone CT study.
Postnatal Brain Imaging
Postnatal brain MRI confirmed the expected findings of CMS, including partial agenesis of the corpus callosum without a midline cyst, stable colpocephaly (Fig. 2a), small frontal horns, bifrontal subcortical heterotopia and polymicrogyria (Fig. 2b and c), and inferior cerebellar dysplasia. The findings were similar to those of the infant’s two older siblings (Fig. 3), although both of the older siblings had a midline cyst, requiring fenestration, and ventriculoperitoneal shunting in the brother only.
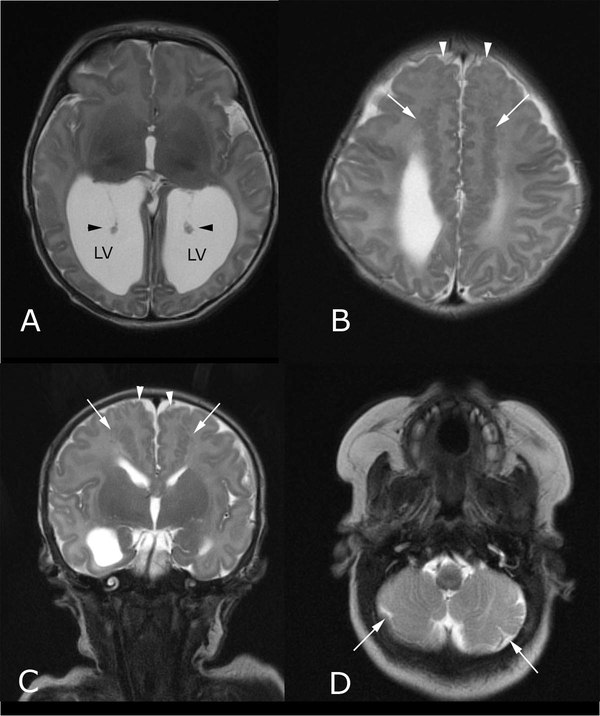
FIG. 2.
Postnatal brain MR imaging performed at 6 days of life shows findings consistent with Chudley–McCullough syndrome. (A) Axial T2-weighted image through the lower cerebral hemispheres shows disproportionate enlargement of the lateral ventricle (LV) atria (black arrowheads indicate choroid); (B) Axial and (C) coronal T2-weighted images through the bifrontal lobes show abnormal, serpiginous signal (white arrows in B,C) in the subcortical white matter, consistent with gray-matter heterotopia, and abnormal gyriform pattern medially (white arrowheads), consistent with polymicrogyria; (D) Axial T2-weighted image through the inferior cerebellar hemispheres shows an abnormally lobulated morphology of the cerebellum bilaterally (white arrows in D), with a disorganized folia pattern, indicating dysplasia. Inferior vermian dysplasia was also observed (not shown).
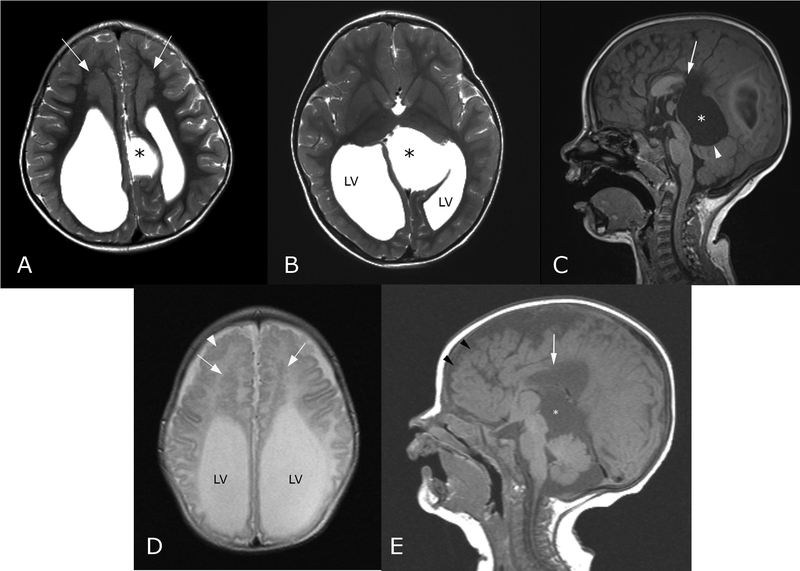
FIG. 3.
Brain MRI findings of Chudley–McCullough in two siblings. (A and B) Axial T2-weighted images through the lateral ventricles (LV) of the older sister at age 3 years shows ventriculomegaly and an abnormal left parasagittal cyst (*). Bifrontal subcortical heterotopia is thicker than seen in her newborn sister; (C) Sagittal T1-weighted image of the same older sister also shows the absence of the posterior corpus callosum (white arrow) and the mass effect upon the cerebellar vermis (arrowhead) by the near-midline cyst (*); (D) Axial T2-weighted image through the frontal lobes of the older brother, imaged at age 1 month, shows the same colpocephalic configuration of the lateral ventricles (LV) and slit-like frontal horns. Bifrontal heterotopia (arrows in D) and polymicrogyria (arrowhead in D) are readily apparent; (E) Sagittal T1-weighted image through the midline shows the same features as in the older sister, with frontal polymicrogyria (black arrowheads), partial agenesis of the corpus callosum (white arrow), and quadrigeminal plate cyst (*).
Although the developmental outcome of this child is not yet fully known due to her young age, the patient’s older sister was treated with a left cochlear implant for profound bilateral sensorineural hearing loss, and at age 8 years, requires specific support in written expression and in mathematics at school. Her neurological exam was notable for dysmetric vertical saccades and some mild motor delays, which improved over time. The brother was treated with VP shunting, as mentioned above, and his hearing loss was treated with hearing aids. At 7 years of age, his gross and fine motor function was normal. Similar to his sister, he exhibited hypometric saccades and mild end-gaze nystagmus bilaterally.
DISCUSSION
Ventriculomegaly is the most common prenatal brain imaging abnormality, and has a broad differential diagnosis that includes obstruction, injury, and genetic disorders [Plunk and Chapman, 2014; Weisstanner et al., 2015]. Upon recognition of abnormal fetal ventricular enlargement, it is important to identify potential causes of obstruction, such as cerebral aqueductal stenosis, midbrain/hindbrain malformation, obstructive cyst, or rarely, tumor. Isolated mild ventriculomegaly (atrial measurement 10–12 mm) is associated with a small increase in risk for abnormal neurodevelopmental outcome. Factors thought to be associated with increased risk of abnormal neurodevelopmental outcome include larger ventricle size, other brain abnormalities, other non-brain abnormalities, and/or genetic conditions known to be associated with neurodevelopmental disability.
Chudley–McCullough syndrome is characterized by profound sensorineural hearing loss and a distinctive brain malformation including ventriculomegaly with small frontal horns, varying degrees of corpus callosum agenesis, bilateral medial frontal polymicrogyria, bilateral frontal subcortical heterotopia, cerebellar dysplasia, and sometimes arachnoid cysts [Chudley et al., 1997; Lemire and Stoeber, 2000; Welch et al., 2003; Østergaard et al., 2004; Matteucci et al., 2006; Nadkarni et al., 2008; Alrashdi et al., 2011; Diaz-Horta et al., 2012; Doherty et al., 2012; Kau et al., 2012; Almomani et al., 2013; Krishnan et al., 2014]. Surprisingly, despite their impressive brain malformations, individuals with CMS do not tend to have seizures or prominent neurodevelopmental issues beyond those expected with profound hearing loss.
Although fetal “hydrocephalus” diagnosed by ultrasound has been reported [Østergaard et al., 2004; Doherty et al., 2012; Kau et al., 2012], to the best of our knowledge, diagnosis of CMS by fetal MRI has not been reported. To provide appropriate prenatal counseling, it is essential to distinguish CMS from other causes of ventriculomegaly or ACC in combination with additional brain malformations, which typically confer a very high-risk of severe neurodevelopmental disability. In addition, the 25% recurrence risk for CMS is markedly higher than the empiric recurrence risk for idiopathic ventriculomegaly and ACC. For the pregnancy described above, we had the advantage of family history; however, the presence of slit-like frontal horns should trigger close evaluation of the frontal sulcation pattern and subcortical white matter signal for indicators of neuronal migrational anomalies. In this case, the bifrontal subcortical heterotopia were evident by abnormal hypointense T2-weighted signal, matching that of cortex (Fig. 1c and d). Careful evaluation of the cerebellar hemisphere folia pattern may also reveal disorganized arrangement, particularly inferiorly, and could offer further support for CMS. The presence of frontal cortical dysplasia, medial frontal heterotopia, or inferior cerebellar dysplasia should raise suspicion for CMS and prompt consideration of confirmatory genetic testing.
Recognition of this constellation of fetal brain findings may allow for improved prenatal counseling and genetic testing. Identification of GPSM2 mutations in fetuses and infants with agenesis of the corpus callosum and heterotopia would greatly alter prognostic counseling and allow for early detection, monitoring and treatment for increased intracranial pressure due to hydrocephalus and hearing loss after birth.
Footnotes
Conflict of interest: none.
REFERENCES
- Almomani R, Sun Y, Aten E, Hilhorst-Hofstee Y, Peeters-Scholte CM, van Haeringen A, Hendriks YM, den Dunnen JT, Breuning MH, Kriek M, Santen GW. 2013. GPSM2 and Chudley-McCullough syndrome: A Dutch founder variant brought to North America. Am J Med Genet Part A 161A:973–976. [DOI] [PubMed] [Google Scholar]
- Alrashdi I, Barker R, Patton MA. 2011. Chudley-McCullough syndrome: Another report and a brief review of the literature. Clin Dysmorphol 20:107–110. [DOI] [PubMed] [Google Scholar]
- Chudley AE, McCullough C, McCullough DW. 1997. Bilateral sensorineural deafness and hydrocephalus due to foramen of Monro obstruction in sibs: A newly described autosomal recessive disorder. Am J Med Genet 68:350–356. [DOI] [PubMed] [Google Scholar]
- Diaz-Horta O, Sirmaci A, Doherty D, Nance W, Arnos K, Pandya A, Tekin M. 2012. GPSM2 mutations in Chudley-McCullough syndrome. Am J Med Genet Part A 158A:2972–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D, Chudley AE, Coghlan G, Ishak GE, Innes AM, Lemire EG, Rogers RC, Mhanni AA, Phelps IG, Jones SJ, Zhan SH, Fejes AP, Shahin H, Kanaan M, Akay H, Tekin M, FORGE Canada Consortium, Triggs-Raine B, Zelinski T. 2012. GPSM2 mutations cause the brain malformations and hearing loss in Chudley-McCullough syndrome. Am J Hum Genet 90:1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau T, Veraguth D, Schiegl H, Scheer I, Boltshauser E. 2012. Chudley-McCullough syndrome: Case report and review of the neuroimaging spectrum. Neuropediatrics 43:44–47. [DOI] [PubMed] [Google Scholar]
- Krishnan P, Chattopadhyay A, Saha M. 2014. Periventricular nodular heterotopia, frontonasal encephalocele, corpus callosal dysgenesis and arachnoid cyst: A constellation of abnormalities in a child with epilepsy. J Pediatr Neurosci 9:273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire EG, Stoeber GP. 2000. Chudley-McCullough syndrome: Bilateral sensorineural deafness, hydrocephalus, and other structural brain abnormalities. Am J Med Genet 90:127–130. [DOI] [PubMed] [Google Scholar]
- Matteucci F, Tarantino E, Bianchi MC, Cingolani C, Fattori B, Nacci A, Ursino F. 2006. Sensorineural deafness, hydrocephalus and structural brain abnormalities in two sisters: The Chudley-McCullough syndrome. Am J Med Genet Part A 140:1183–1188. [DOI] [PubMed] [Google Scholar]
- Nadkarni TD, Menon RK, Shah AH, Goel A. 2008. Chudley McCullough syndrome. Childs Nerv Syst 24:541–544. [DOI] [PubMed] [Google Scholar]
- Østergaard E, Pedersen VF, Skriver EB, Brøndum-Nielsen K. 2004. Brothers with Chudley-McCullough syndrome: Sensorineural deafness, agenesis of the corpus callosum, and other structural brain abnormalities. Am J Med Genet Part A 124A:74–78. [DOI] [PubMed] [Google Scholar]
- Plunk MR, Chapman T. 2014. The fundamentals of fetal magnetic resonance imaging: Part 2. Curr Probl Diagn Radiol 43:347–355. [DOI] [PubMed] [Google Scholar]
- Weisstanner C, Kasprian G, Gruber GM, Brugger PC, Prayer D. 2015. MRI of the FBrain. Clin Neuroradiol Suppl 2:189–196. [DOI] [PubMed] [Google Scholar]
- Welch KO, Tekin M, Nance WE, Blanton SH, Arnos KS, Pandya A. 2003. Chudley-McCullough syndrome:Expanded phenotype andreview of the literature. Am J Med Genet Part A 119A:71–76. [DOI] [PubMed] [Google Scholar]