Abstract
Objective:
Despite high rates of prenatal insomnia, efficacious treatment options for this population are quite limited. Early evidence from randomized controlled trials (RCTs) support the efficacy of face-to-face cognitive-behavioral therapy for insomnia (CBTI) for prenatal insomnia. Yet, as many patients are unable to access this specialist-driven care, a critical need exists to increase its accessibility. This RCT examined the efficacy internet-based digital CBTI in pregnant women with insomnia.
Methods:
Single-site RCT. A total of 91 pregnant women (29.03 ± 4.16 years) nearing/entering the third trimester who screened positive for clinical insomnia on the Insomnia Severity Index (ISI) were randomized to digital CBTI or digital sleep education control. The ISI, Pittsburgh Sleep Quality Index (PSQI), Edinburgh Postnatal Depression Scale (EPDS), and Pre-Sleep Arousal Scale’s Cognitive factor (PSAS-C) served as study outcomes, which were collected before treatment and after treatment during pregnancy, then six weeks after childbirth.
Results:
From pre to posttreatment, CBTI patients reported reductions in ISI (−4.91 points, p < 0.001) and PSQI (−2.98 points, p < 0.001) and increases in nightly sleep duration by 32 min (p = 0.008). Sleep symptoms did not change during pregnancy in the control group. After childbirth, CBTI patients, relative to controls, slept longer by 40 min per night (p = 0.01) and reported better sleep maintenance. No pre or postnatal treatment effects on depression or cognitive arousal were observed.
Conclusions:
Digital CBTI improves sleep quality and sleep duration during pregnancy and after childbirth. To better optimize outcomes, CBTI should be tailored to meet the changing needs of women as the progress through pregnancy and early parenting.
Name:
Insomnia and Rumination in Late Pregnancy and the Risk for Postpartum Depression.
URL:
clinicaltrials.gov. Registration: NCT03596879.
Keywords: Pregnancy, Perinatal, Postpartum, Cognitive arousal, Depression, RCT
1. Introduction
Sleep disturbance and insufficient sleep are endemic to pregnant women [1–6]. Over half of pregnant women meet the diagnostic criteria for insomnia disorder [7] or endorse clinically significant insomnia symptoms [1,5,8]. Compelling evidence including prospective data indicate that these sleep disturbances increase across pregnancy and are most severe in the third trimester [2,4,8–10], although some evidence suggests that the prevalence of insomnia is unchangingly high across pregnancy [1]. Insomnia and insufficient sleep during pregnancy are associated with negative maternal outcomes such as depression and suicidal ideation [7,11–15], cognitive-emotional dysregulation [12,16,17], and reduced quality of life [17] among myriad other consequences [10]. Despite alarmingly high rates of prenatal insomnia and known associated complications, empirically supported treatment options for insomnia have been very limited for pregnant women [18].
In the broader US adult population, individuals with insomnia often seek treatment via prescription and/or over-the-counter (OTC) sleep aids [19,20]. However, the Food and Drug Administration (FDA) categorizes most prescription sleep-promoting medications as pregnancy Category C or D and thus are not considered safe for use during pregnancy [21]. The FDA designates common OTC sedating antihistamine medications, namely doxylamine and diphenhydramine, as Category A or B, respectively. These medications are typically recommended for use during pregnancy for allergy symptoms and nausea. However, no large randomized controlled trials (RCTs) have been conducted to thoroughly examine the efficacy and safety profiles of these OTC antihistamines for mother and child based on medication dosage and duration needed to alleviate perinatal insomnia [21,22]. Therefore, no sleep aids, prescription or OTC, are presently considered safe and efficacious for insomnia during pregnancy. Even so, over 90% of pregnant women self-treat insomnia symptoms with OTC antihistamines [21].
Cognitive-behavioral therapy for insomnia (CBTI), the guideline recommended treatment for insomnia [23], is highly effective and confers benefits over pharmacotherapy including superior long-term insomnia outcomes [24,25]. Recent evidence has begun to support the efficacy of CBTI during pregnancy. An open-label trial showed medium to large effect sizes in reducing sleep disturbances including latency to sleep and wake after sleep onset for pregnant women who completed a five-week CBTI group therapy program [26]. Randomized controlled trials (RCTs) also support CBTI efficacy in this population. CBTI delivered in individual and group formats produce superior treatment effects on insomnia symptoms during pregnancy compared to control [27,28]. Not only is CBTI efficacious in pregnancy, but pregnant women perceive CBTI as a more credible treatment option than pharmacotherapy and acupuncture [22], thus supporting its potential for patient engagement and real-world uptake.
Despite emerging efficacy and positive appraisals for CBTI for prenatal insomnia, access to this treatment is severely limited. Few US adults with insomnia have access to behavioral sleep medicine specialists who provide CBTI treatment [29,30]. This limited access is due to a shortage of CBTI practitioners and, further complicating matters, uneven geographic distribution of certified practitioners clustered in select urban areas [29]. Efforts to increase CBTI access have leveraged web and mobile health technology and clinical trials is the general US adult population show efficacy for CBTI delivered automated mobile health apps [31–39].
Despite the critical need for safe alternatives to pharmacological treatment in this population and the shortage of healthcare providers capable of delivering CBTI, the efficacy of web-based CBT-I in this population has not been established. Not only do pregnant women face the same access barriers as insomnia patients in the broader population [29,30], but pregnant women also have additional unique logistical barriers including managing other recurring prenatal health appointments, reserving medical leave for other prenatal and postnatal appointments, and preserving personal time off for maternity leave. Thus, it is critical to increase access to insomnia treatment for pregnant women that maximizes flexibility while preserving efficacy. As digital CBTI has proven highly efficacious at improving sleep in broader adult populations [40], leveraging this technology to increase reach and accessibility of insomnia care for pregnant women has immense potential to improve sleep and mental wellbeing in this vulnerable population.
The present study was a single-site RCT comparing digital CBTI and digital sleep education control for the treatment of clinically significant insomnia symptoms in pregnant women nearing/entering the third trimester. We targeted pregnant women in mid-to-late pregnancy based on evidence that prenatal sleep quality is worst in the third trimester [2,4,8–10]. We examined acute treatment effects in the prenatal period as well as longer term effects in early postpartum, which is a transition period of sleep instability for mother and child. We hypothesized that pregnant patients receiving digital CBTI would report greater improvements in sleep (decreased insomnia symptoms, decreased sleep disturbance, increased sleep duration), depressive symptoms, and nocturnal cognitive arousal relative to control patients. In addition, we hypothesized that these treatment group differences would be maintained six weeks after childbirth.
2. Methods
2.1. Study design
In a 6-hospital healthcare system, we randomized patients nearing or entering the third trimester of pregnancy in a randomized controlled trial comparing the efficacy of digital CBTI versus digital sleep education control. The study was approved by the Internal Review Board at the site where the study was located. All patients provided written consent to participate. The trial was registered at the US National Institutes of Health (ClinicalTrials.gov) #NCT03596879.
2.2. Study populations
Pregnant women receiving prenatal care in the health system were invited to be assessed for eligibility. Inclusion criteria were clinically significant insomnia symptoms on a validated self-report instrument (ISI score ≥ 10 [41], see Measures below) and gestational age between 25 and 30 weeks at time of eligibility screening. Exclusion criteria included high risk pregnancy per self-report (any reason), being in the care of the maternal-fetal medicine team for high risk pregnancy (any reason) per electronic medical records, multiple pregnancy, prescription or over-the-counter sleep aid use at the time of screening, alcohol or recreational drug use at time of screening, current rotating and/or night shift work, epilepsy or seizures, bipolar disorder, diagnosis of a sleep disorder that is untreated (other than insomnia), and severe depression (Edinburgh Postnatal Depression Scale [EPDS] score ≥ 19 [42], see Measures below).
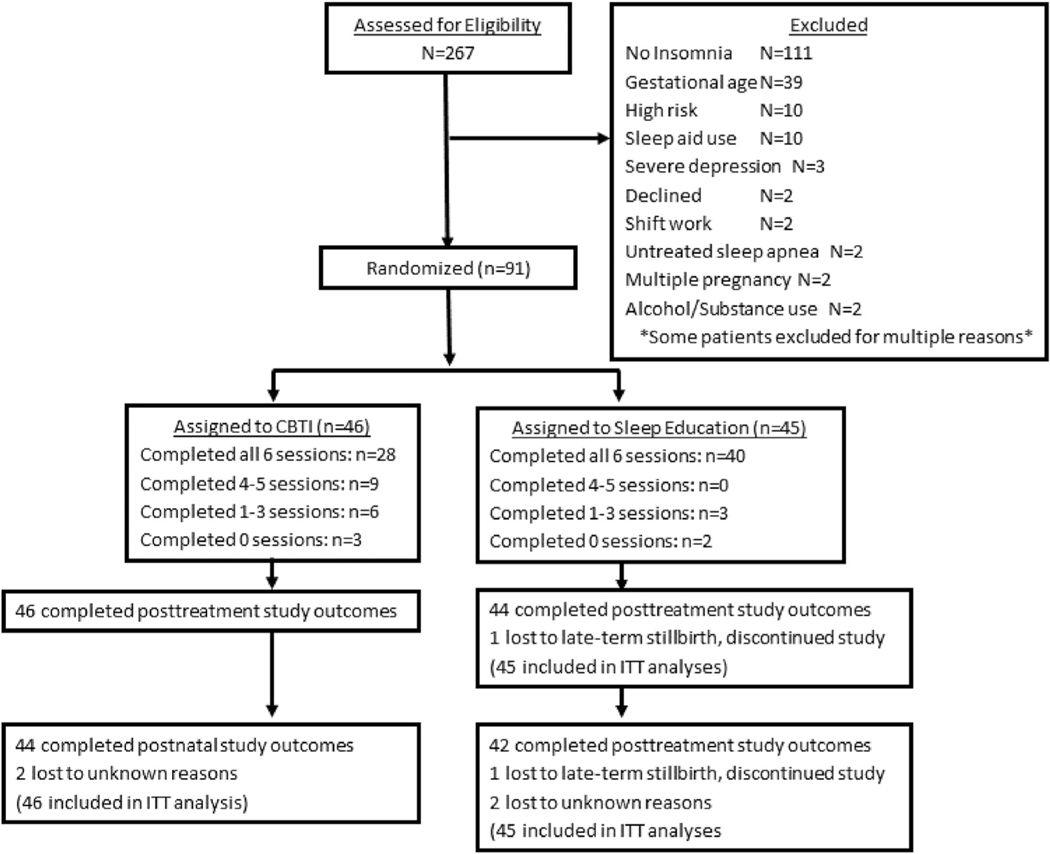
Invitations advertising a study on perinatal sleep (without mentioning either that we were focused on poor sleep or that we were evaluating sleep treatments) were sent via email and phone calls to 3585 patients. A total of 535 women contacted us with interest in our study. Of these patients, 272 women consented to participating in the study, 267 of whom provided sufficient data for full eligibility determination, which were collected between September 12, 2018 and March 9, 2019. Of these 267 screeners, 156 women met insomnia symptom inclusion criteria for the RCT. However, 65 of these women were not enrolled into the trial for the following reasons: completed eligibility screening after gestational age limit for RCT inclusion (n = 39), high risk pregnancy (most cases were considered high risk due to age; n = 10), sleep medications (only doxylamine and diphenhydramine reported; n = 10), severe depression (n = 3), did not want to participate (n = 2), shift work (n = 2), patient-reported untreated sleep apnea (n = 2), multiple pregnancy (n = 2), and current alcohol or recreational substance use (n = 2). See Fig. 1 for study flowchart.
Fig. 1.
Flow chart of study enrollment and participation.
2.3. Study interventions
Enrolled patients were randomly assigned to receive either digital CBTI or digital sleep education control. For randomization, we used blocked randomization with a block size of 10 and an allocation ratio of 1:1. Research assistants uninvolved in hypothesis generation or data analysis generated randomization allocation, enrolled patients, and assigned patients to interventions. Due to the nature of the interventions, patients were not blinded to intervention, but they were not informed as to whether their treatment was considered active or control.
2.3.1. Digital cognitive behavioral therapy for insomnia
Patients randomized to CBTI completed the Sleepio program via the internet (www.sleepio.com, Big Health Inc.). Sleepio is among several currently available digital CBTI programs and was selected for this study because it is evidence-based, standardized, fully automated, and has been tested in multiple RCTs comprising several thousand patients including several of our own trials [31–39]. In the present study, patients were granted access to Sleepio until either (A) they completed six sessions of digital CBTI or (B) they gave birth. The intervention covered behavioral components (sleep restriction, stimulus control), cognitive components (eg, cognitive restructuring, paradoxical intention), progressive muscle relaxation, and sleep hygiene. Only one modification was made to accommodate our pregnant patients: in sleep restriction, time in bed could not be prescribed as <6 h, which is consistent with other perinatal CBTI trials [27,43]. Sessions were directed by an animated ‘virtual therapist’ who reviews and guides progress with the patient based on submitted sleep data. Participants were granted access to new sessions weekly (ie, new session became available a week after completing a previous session).
2.3.2. Digital sleep education control
Patients randomized to the online sleep education condition received six weekly emails based on the National Institutes of Health guide to healthy sleep [44]. Information was provided on the basics of sleep regulation; relationships between sleep and health problems such as obesity, diabetes, and cardiovascular disease; effects of sleep-disruptive substances such as caffeine, nicotine, and alcohol; and tips on creating a sleep-conductive bedroom environment. Psychoeducation and sleep hygiene were selected because they are common in clinical practice, especially in primary care [45,46], and also because they are commonly used as an attention control in clinical trials of insomnia. Importantly, sleep education is not considered an effective standalone treatment for insomnia [47].
2.4. Study outcomes
All study outcomes were assessed via online surveys hosted by Qualtrics. Assessment schedule included assessments at (1) pretreatment: 1–2 weeks before treatment, (2) posttreatment: a week after completing treatment or upon discontinuing treatment (indicated by informing study personnel of their discontinuation or after two weeks of not engaging in treatment), and finally (3) postnatal follow-up: six weeks after childbirth. All pretreatment measures retained their original assessment windows, whereas posttreatment and follow-up measures were modified to assess symptoms over ‘the prior week’ to ensure that symptom levels after treatment were estimated for a uniform time period across measures and to minimize likelihood of administering posttreatment assessments after childbirth (note: all posttreatment assessments in the present study were completed before childbirth).
Insomnia Severity Index (ISI) measured global insomnia symptom severity [41,48], which was a primary outcome in this RCT. Scores range from 0 to 28 with higher scores indicating greater severity. ISI ≥10 indicates clinically significant insomnia symptoms. Per standard practice, treatment response was operationalized as a reduction of ≥6 points on the scale and remission was defined as ISI ≤7.
Global sleep disturbance was measured using the Pittsburgh Sleep Quality Index [49] (PSQI), which was a primary outcome in this RCT. The PSQI measures a wide range of sleep parameters over the past month including sleep duration, sleep latency, sleep aid use, and sleep difficulties related to insomnia, breathing difficulties, environmental stimuli, and other factors. A global cutoff score of PSQI >5 is the original cutoff for differentiating good vs poor sleepers, which has been widely supported. We used item-level data to classify patients as having sleep onset insomnia symptoms (item 5a: endorsing inability to fall asleep within 30 m on ≥3 nights/week) and sleep maintenance insomnia symptoms (item 5b: endorsing difficulties with nighttime awakening and/or early morning awakenings on ≥3 nights/week). This method of classifying sleep onset insomnia symptoms and sleep maintenance insomnia symptoms with the PSQI has been supported by prior research [5,50–52]. In addition to measuring sleep disturbances, we used the PSQI to assess habitual sleep duration (item #4a) as a secondary outcome due to CBTI’s documented inconsistent effects on sleep duration. We operationalized women sleeping <6 h as short sleepers, which is consistent with current practices for objective and self-reported sleep duration [53,54].
Edinburgh Postnatal Depression Scale (EPDS) measured depression [42], which served as a secondary outcome. Scores range from 0 to 30 with higher scores indicating greater severity. EPDS scores ≥10 suggest clinically significant minor or major depression [55]. EPDS scores ≥19 represent severe depressive symptoms [56]. To assess suicidal ideation, we examined item #10 and operationalized any level of endorsement as a positive case.
Presleep Arousal Scale – Cognitive factor (PSAS-C) [57] measures trait tendency for cognitive arousal while trying to fall asleep at night, which served as a secondary outcome. Example items from the PSAS-C are “review or ponder events of the day” and “can’t shut off thoughts.” Scores range from 8 to 40 with higher scores indicating greater nocturnal cognitive arousal. PSAS-C scores >19 may indicate clinically significant insomnia and affective symptoms [58].
2.5. Sociodemographics, health information, and potential covariates
At screening, patients provided standard sociodemographic and health information including age, race, and parity. In addition, patients reported whether they snore (Yes/No) and estimated the timing of their insomnia onset, which we dichotomized into pre-pregnancy vs gestational onset for analysis. We collected data in both conditions on number of sessions completed to assess adherence. In postnatal assessments, patients were asked ‘Over the past week, has your baby been difficult to soothe or has your baby been colicky?’ to which patients responded on a 4-point Likert-type scale (1 = No, my baby has not been fussy much at all and is very easy to soothe to 4 = Yes, my baby has been very colicky and/or difficult to soothe). Body mass index (BMI) as an indicator of obesity (operationalized as BMI ≥35) was derived from electronic medical records from visit notes closest to the screening date.
2.6. Analysis plan
Power analyses indicated that with an anticipated sample size of n = 90 and anticipating medium-large posttreatment group differences in insomnia outcomes (Cohen’s d = 0.65), we would have 0.86 power to detect effects at a significance level of α = 0.05. Study outcomes were downloaded directly from Qualtrics and all analyses were performed in SPSS version 25 (IBM Corp) with a significance value of 0.05. Our primary analyses were conducted using an intent-to-treat approach using last observation carried forward, which is a conservative approach and includes all randomized patients. We first examined descriptive data for sample characteristics and compared treatment conditions on sociodemographics, pregnancy-related information, snoring, insomnia onset timing, pretreatment values of study outcomes, and treatment adherence. Independent samples t-tests were used to compare groups on continuous variables, whereas chi-square analysis was used to compare groups on dichotomous variables. To examine treatment effects, we ran two series of repeated measures analysis of covariance (ANCOVA) models and univariate ANCOVA models. First, we used repeated measures ANCOVA to analyze acute main effects of Time and interaction effects of Treatment X Time on study outcomes from pre to posttreatment. When a significant Treatment X Time effect was observed, we conducted posthoc paired samples t-tests to examine within group changes in study outcomes. For all study outcomes, we then conducted univariate ANCOVAs to test for group differences in posttreatment values while controlling for relevant covariates. This process was then repeated to examine changes in study outcomes from pretreatment to postnatal follow-up. In all ANCOVA models, we entered timing of insomnia onset (pre-pregnancy vs gestational) as a covariate to test whether treatment outcomes were associated with insomnia symptom onset. In addition, we entered pretreatment group differences as covariates into all ANCOVA models to control for potential confounds. In ANCOVA models testing postnatal outcomes, we controlled for reported infantile colic due to potential effects on maternal sleep and mood. When significant treatment effects were observed, we conducted posthoc analyses to compare rates of treatment response and disease remission when applicable.
3. Results
3.1. Sample characteristics and differences by treatment condition
A total of 91 women (aged 29.03 ± 4.16y, gestational week: 27.76 ± 0.87) participated in this study. We sought a total RCT sample size of 90. However, the number of eligible patients with insomnia exceeded this target by one in our last wave of recruitment. Instead of excluding this patient from the trial, she was randomly assigned to treatment (1:1 ratio). Trial recruitment ended when we reached our target sample size. The sample mostly identified as non-Hispanic white (51.6%) or non-Hispanic black (31.9%). Prior to treatment, mean ISI scores were in the clinical range (14.49 ± 3.47), mean PSQI scores indicated elevated sleep disturbances in the sample (9.36 ± 2.84), and women estimated sleeping an average of 6.29 ± 1.23 h per night (24.6% of the sample reported sleeping < 6 h/night before treatment). Pretreatment mean EPDS scores were slightly below the clinical cutoff for possible depression (8.45 ± 4.47) and mean PSAS-C scores were elevated (22.34 ± 6.54) indicating high nocturnal cognitive arousal. Please see Table 1 for additional sociodemographic, sleep, and pregnancy-related information.
Table 1.
Sample demographics and characteristics (n = 91)
| All subjects | Sleep Education | CBTI | ||
|---|---|---|---|---|
| Sample size | 91 | 45 | 46 | |
| Age (M±SD) | 29.03 ± 4.16y | 29.16 ± 4.11 | 28.91 ± 28.91 | t(89) = −0.28, p = 0.78 |
| Poverty (n; %) | 16/90; 17.8% | 9/44; 20.5% | 7/46; 15.2% | χ2 = 0.42, p = 0.52 |
| Obesity (BMI ≥ 35) (n; %) | 12/86; 14.0% | 3/42; 7.1% | 9/44; 20.5% | χ2 = 3.17, p = 0.08 |
| Multiparous (n; %) | 59/91; 64.8% | 15/45; 33.3% | 17/46; 37.0% | χ2 = 0.13, p = 0.72 |
| Snore (n; %) | 28/91; 30.8% | 9/45; 20.0% | 19/46; 41.3% | χ2 = 4.85, p = 0.03 |
| Gestational onset of insomnia (n; %) | 20/91; 22.0% | 11/45; 24.4% | 9/46; 19.6% | χ2 = 0.32, p = 0.57 |
| Race (n; %) | χ2 = 0.38, p = 1.00 | |||
| White | 47; 51.6% | 23; 51.1% | 24; 52.2% | |
| Black | 29; 31.9% | 14; 31.1% | 15; 32.6% | |
| Asian | 6; 6.6% | 3; 6.7% | 3; 6.5% | |
| Middle Eastern or Arabic | 4; 4.4% | 2; 4.4% | 2; 4.3% | |
| Hispanic or Latino | 2; 2.2% | 1; 2.2% | 1; 2.2% | |
| Multiracial | 3; 3.3% | 2; 4.4% | 2.2% | |
| Number of sessions completed (M±SD) | 5.14 ± 1.75 | 5.47 ± 1.58 | 4.83 ± 1.88 | t(89) = −1.76, p = 0.08 |
Note: Age = age (in years) at study screening. BMI = body mass index derived from electronic medical records. Obesity was operationalized as BMI ≥35 as this cutoff is a robust and sensitive predictor of sleep-disordered breathing in pregnant women. M±SD = mean and standard deviation. n; % = number of participants and percentage within that group. t = t-statistic from independent samples t-test comparing means between sleep education and CBTI conditions. χ2 = chi-square value derived from comparing treatment conditions on binary variables. p = significant value. Age, income, parity, insomnia onset, and race were all reported by patients. Poverty was operationalized as <$20,000 annual household income
Next, we compared treatment groups on sociodemographics, pretreatment symptoms, and treatment compliance indices. Sociodemographic characteristics did not differ between treatment conditions. However, CBTI patients, relative to controls, reported higher rates of snoring (Table 1). Despite CBTI patients reporting greater pretreatment depressive symptoms (Table 2), the two groups did not differ in frequency of pretreatment suicidal ideation (CBTI vs control: 4.3% vs 6.7%, χ2 = 0.24, p = 0.63). Additionally, an association suggesting that CBTI patients completed fewer sessions that controls approached significance (Table 1). Attrition was low overall, and rates did not differ significantly by group: 1.1% of posttreatment outcome data were missing (1 control, 0 CBTI patients) and 5.5% of six-week postnatal follow-up data were missing (3 controls, 2 CBTI patients).
Table 2.
Comparing digital CBTI and sleep education control on sleep and cognitive-emotional outcomes during pregnancy and after childbirth.
| Pretreatment M±SD |
Posttreatment M±SD |
Postnatal M±SD |
Δ Pre- to posttreatment | Δ Pre- to 6-week Postnatal | |
|---|---|---|---|---|---|
| ISI | t(89) = 1.17, p = 0.25 | F(1,85) = 4.15, p < 0.05 | F(1,84) = 2.56, p = 0.11 | F(1,85) = 8.22, p = 0.005 | F(1,84) = 2.56, 0.11 |
| Control | 14.07 ± 3.37 | 12.89 ± 4.84 | 9.91 ± 5.64 | t(44) = −1.45, p = 0.16 | - |
| CBTI | 14.91 ± 3.55 | 10.00 ± 5.66 | 8.96 ± 5.46 | t(45) = −5.61, p < 0.001, d = 0.86 | - |
| PSQI | t(89) = 0.76, p = 0.45 | F(1,85) = 9.97, p = 0.002 | F(1,84) = 0.27, p = 0.61 | F(1,85) = 16.72, p < 0.001 | F(1,84) = 1.16, p = 0.13 |
| Control | 9.13 ± 3.06 | 9.29 ± 4.12 | 7.53 ± 3.47 | t(44) = 0.27, p = 0.79 | - |
| CBTI | 9.59 ± 2.61 | 6.61 ± 2.99 | 7.00 ± 3.22 | t(45) = −6.31, p < 0.001, d = 0.93 | - |
| Sleep Duration | t(89) = 0.19, p = 0.85 | F(1,85) = 6.78, p = 0.01 | F(1,84) = 6.50, p = 0.01 | F(1,85) = 4.94, p = 0.03 | F(1,84) = 4.67, p = 0.03 |
| Control | 6.27 ± 1.21 | 5.94 ± 1.32 | 5.44 ± 1.16 | t(44) = −1.51, p = 0.14 | t(44) = −3.97, p < 0.001, d = 0.60 |
| CBTI | 6.32 ± 1.26 | 6.85 ± 1.17 | 6.10 ± 1.53 | t(45) = 2.79, p = 0.008, d = 0.41 | t(45) = −0.88, p = 0.39 |
| EPDS | t(89) = −2.19, p = 0.03 | F(1,85) = 0.62, p = 0.43 | F(1,84) = 0.00,p = 0.95 | F(1,86) = 4.02, p < 0.05 | F(1,84) = 2.52, p = 0.12 |
| Control | 9.47 ± 4.62 | 5.93 ± 5.29 | 5.18 ± 5.40 | - | - |
| CBTI | 7.46 ± 4.14 | 5.87 ± 4.28 | 4.76 ± 4.07 | - | - |
| PSAS-C | t(89) = −0.47, p = 0.64 | F(1,85) = 0.22, p = 0.64 | F(1,84) = 0.04, p = 0.84 | F(1,85) = 0.58, p = 0.45 | F(1,84) = 0.08, p = 0.78 |
| Control | 22.67 ± 7.02 | 16.51 ± 6.35 | 14.11 ± 5.53 | - | - |
| CBTI | 22.02 ± 6.09 | 15.70 ± 6.16 | 14.35 ± 5.96 | - | - |
Note: In the Pretreatment column, t-statistics were derived from independent samples t-tests comparing mean levels of study outcomes between treatment conditions. In the Δ Pre-to posttreatment and Δ Pre-to 6-week Postnatal columns, F-ratios were derived from repeated measures analysis of covariance (ANCOVA) models for Treatment X Time effects, and t-statistics were derived from paired samples t-tests which were only conducted if the corresponding Treatment X Time interaction was significant. In the Posttreatment and 6-week Postnatal columns, F-ratios were derived from univariate ANCOVA models. All ANOVA models presented in this table included the following covariates: insomnia onset timing, snoring, pretreatment depressive symptoms (except in repeated measures ANCOVA models), number of sessions completed, and maternal reports of infantile colic (postnatal models only). M = mean. SD = standard deviation. ISI = insomnia severity index. PSQI = Pittsburgh sleep quality index. Sleep duration = average total sleep time per night, reported in hours in half-hour increments, derived from PSQI item 4a. EPDS = Edinburgh postnatal depression scale. PSAS-C = presleep arousal scale, cognitive factor.
3.2. Treatment effects on sleep and cognitive-emotional symptoms
All repeated measures and univariate ANCOVA models examining Treatment X Time interactions and group differences controlled for insomnia onset timing and group differences in snoring, depressive symptoms, and number of sessions completed as potential confounds. In addition, models predicting postnatal study outcomes controlled for maternal reports of infantile colic.
3.2.1. Insomnia
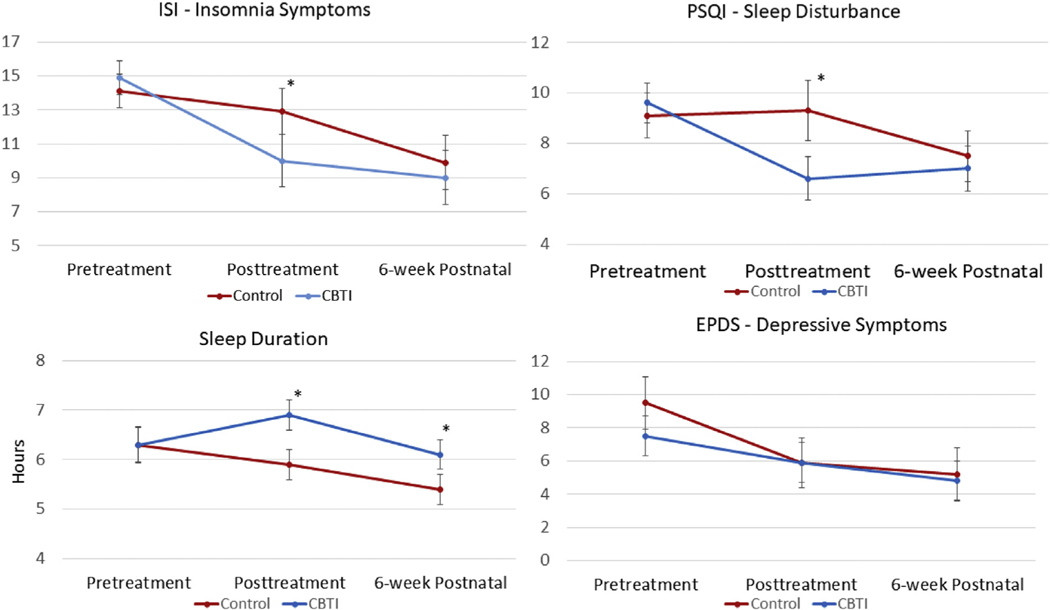
We first conducted a repeated measures ANCOVA examining changes in ISI from pre to posttreatment as estimated by Treatment as a between-subjects factor (see Table 2 for full results and Fig. 2 for visual representation of data). In the full sample, insomnia symptoms did not change from pre to posttreatment [F(1,85) = 1.58, p = 0.21]. However, we observed a significant Treatment X Time interaction [F(1,85) = 8.22, p = 0.005]. Paired samples t-tests showed that ISI scores significantly decreased in the CBTI group by 4.91 points [t(45) = −5.61, p < 0.001, Cohen’s d = 0.86], whereas no significant change in ISI was observed in the control group. We observed no significant Covariate X Time effects. A univariate ANCOVA comparing posttreatment ISI scores between groups showed that CBTI patients reported lower insomnia symptoms on the ISI by 2.89 points after treatment than controls [F(1,85) = 4.15, p < 0.05, see Table 2 for means]. Consistent with these posttreatment differences, treatment response rates were higher in the CBTI group than control (37.0% vs 13.3%, χ2 = 6.72, p = 0.01) and a similar group difference in remission rates approached significance (34.8% vs 17.8%, χ2 = 3.39, p = 0.07).
Fig. 2.
Means and 95% confidence intervals of sample mean standard errors presented for insomnia symptoms, sleep disturbance, sleep duration, and depressive symptoms by condition (CBTI vs Control) at pretreatment, posttreatment, and 6-week postnatal follow-up (* represents significant group differences in mean outcome scores per univariate ANCOVA).
To explore which aspects of nocturnal sleep improved with treatment, posthoc chi-square tests compared rates of sleep onset insomnia symptoms and sleep maintenance insomnia symptoms before and after treatment between conditions. Before treatment, CBTI and control conditions did not differ in rates of sleep onset insomnia (41.3% vs 44.4%, χ2 = 0.09, p = 0.76) or sleep maintenance insomnia (84.8% vs 77.8%, χ2 = 0.74, p = 0.39). Yet, after treatment, CBTI patients, relative to controls, reported lower rates of both sleep onset insomnia (15.2% vs 35.7%, χ2 = 4.92, p = 0.03) and sleep maintenance insomnia (67.4% vs 85.7%, χ2 = 4.06, p = 0.04).
Next, we ran a repeated measures ANCOVA examining changes in ISI from pretreatment to six-week postnatal follow-up. We observed a significant main effect of time [F(1,84) = 11.32, p = 0.001] revealing that ISI scores for the full sample were 5.06 points lower after childbirth relative to pretreatment levels. The Treatment X Time interaction, however, was non-significant and a univariate ANCOVA revealed that postnatal ISI scores did not differ between treatment conditions (Table 2).
Although Treatment X Time effects were not observed after childbirth, we conducted exploratory posthoc comparisons of sleep onset and maintenance symptom rates. CBTI and control patients did not differ on rates of postnatal sleep onset insomnia (6.8% vs 7.3%, χ2 = 0.01, p = 0.93). However, CBTI patients, relative to controls, reported lower rates of sleep maintenance insomnia after childbirth (65.9% vs 85.4%, χ2 = 4.32, p = 0.04).
3.2.2. Sleep disturbance
To examine changes in global sleep disturbance, we conducted a repeated measures ANCOVA examining changes in PSQI from pre to posttreatment as estimated by Treatment as a between-subjects factor (see Table 2 for full results and Fig. 2 for visual representation of data). No main effect of time was observed [F(1,85) = 0.75, p = 0.39], but we observed a significant Treatment X Time interaction [F(1,85) = 16.72, p < 0.001]. Paired samples t-tests showed that PSQI scores significantly decreased in the CBTI group by 2.98 points [t(45) = −6.31, p < 0.001, Cohen’s d = 0.93], whereas no significant change in PSQI was observed in the control group. A univariate ANCOVA comparing posttreatment PSQI scores showed that CBTI patients reported lower levels of sleep disturbance after treatment relative to control patients [F(1,85) = 9.97, p = 0.002]. Among poor sleepers before treatment (PSQI > 5), 44.2% (19/45) of CBTI patients were re-classified as good sleepers after treatment, which was nearly 3 times greater than the frequency of 15.0% (6/40) in the control group (χ2 = 7.20, p = 0.007). We observed no significant Covariate X Time effects.
Next, we ran a repeated measures ANCOVA examining changes in PSQI from pretreatment to postnatal follow-up. A main effect of time was observed such that PSQI scores in the full sample decreased by 2.10 points between pretreatment and postnatal follow-up [F(1,84) = 5.13, p = 0.03]. However, the Treatment X Time interaction was non-significant [F(1,84) = 1.16, p = 0.13, Table 2] as were Covariate X Time effects. A univariate ANCOVA revealed that postnatal PSQI scores did not differ between treatment conditions [F(1,84) = 0.27, p = 0.61, Table 2].
3.2.3. Sleep duration
To examine changes in sleep duration, we conducted a repeated measures ANCOVA examining changes in sleep duration from pre to posttreatment as estimated by Treatment as a between-subjects factor (see Table 2 for full results and Fig. 2 for visual representation of data). We observed a significant main effect of time such that mean sleep duration in the full sample increased by 7 min per night [F(1,84) = 3.97, p < 0.05]. Importantly, we observed a significant Treatment X Time interaction [F(1,85) = 4.94, p = 0.03]. Paired samples t-tests showed that sleep duration significantly increased in the CBTI group by 32 min per night [t(45) = 2.79, p = 0.008, Cohen’s d = 0.41], whereas no significant change in sleep duration was observed in the control group. A univariate ANCOVA comparing posttreatment sleep duration showed that CBTI patients reported longer sleep than controls by 55 min [6 h 51 min vs 5 h 56 min; F(1,85) = 6.78, p = 0.01, Table 2]. We observed no significant Covariate X Time effects.
Next, we ran a repeated measures ANCOVA examining changes in sleep duration from pretreatment to postnatal follow-up as estimated by Treatment (Table 2). The main effect of time on sleep duration between pretreatment and postnatal follow-up was non-significant (p = 0.27). However, the Treatment X Time interaction was again significant [F(1,84) = 4.67, p = 0.03]. Relative to pretreatment sleep duration, paired samples t-tests showed that sleep duration decreased by 30 min per night after childbirth in the control group [t(44) = −3.97, p < 0.001, Cohen’s d = 0.60], but that postnatal sleep duration in the CBTI group did not differ from pretreatment sleep duration. A univariate ANCOVA revealed that CBTI patients slept significantly longer than controls after childbirth by 40 min per night [6 h 6 min vs 5 h 26 min; F(1,84) = 6.50, p = 0.01, Table 2].
3.2.4. Depression
See Fig. 2 for visual representation of data. A repeated measures ANCOVA examining changes in EPDS from pre to posttreatment showed no significant main effect of time (p = 0.94), but revealed a significant Treatment X Time interaction [F(1,86) = 4.02, p < 0.05, Table 2]. However, a univariate ANCOVA showed that posttreatment EPDS scores did not differ between CBTI and control patients [F(1,85) = 0.62, p = 0.43], indicating that the significant Treatment X Time interaction was due to pretreatment group differences in depressive symptoms (Table 2). No Covariates X Time interactions were significant.
A repeated measures ANCOVA examining EPDS changes from pretreatment to postnatal follow-up revealed a significant main effect of time such that EPDS scores decreased by 3.48 points from pretreatment to postnatal follow-up [F(1,85) = 7.50, p = 0.008]. However, no Treatment X Time interaction was observed [F(1,85) = 2.52, p = 0.12] and a univariate ANCOVA showed that postnatal EPDS scores did not differ by treatment condition [F(1,85) = 0.00, p = 0.95].
Analyses comparing frequency rates of suicidal ideation between conditions revealed a statistical trend to suggest that CBTI may reduce suicidal ideation as posttreatment rates were lower in the CBTI than in the control group (0.0% vs 6.7%, χ2 = 3.17, p = 0.08). Specifically, all three patients in the control condition who were suicidal before treatment remained suicidal after treatment. In comparison, both of the 2 CBTI patients who were suicidal before treatment reported no suicidal ideation at posttreatment. Later, at six-week postnatal follow-up, 1 CBTI patient endorsed suicidal ideation (2.2%) compared to two controls (4.4%), but the difference was non-significant (p = 0.54).
3.2.5. Nocturnal cognitive arousal
We then ran these analyses predicting changes and treatment group differences on the PSAS-C. From pre to posttreatment, no main effect of time (p = 0.27) or Treatment X Time interaction (p = 0.45) was observed. Between pretreatment and postnatal follow-up, a main effect of time was observed as PSAS-C decreased in the full sample by 7.99 points [F(1,84) = 4.09, p = 0.046], whereas no Treatment X Time effect was observed (Table 2).
4. Discussion
In a sample of 91 perinatal women, we evaluated the efficacy of digital CBTI in comparison to digital sleep education control for prenatal insomnia. Evidence supported the efficacy of digital CBTI for this population with improvement in sleep onset and maintenance symptoms and sleep duration. Importantly, this RCT was the first to show that CBTI during pregnancy protects against sleep loss after childbirth. Despite these improvements in sleep, we observed no treatment effects of digital CBTI on depressive symptoms or cognitive arousal. Taken together, these data suggest that digital CBTI may be a viable treatment option for pregnant women with insomnia, but that it likely requires modifications to maximize outcomes in pregnant and postpartum women.
4.1. Digital CBTI improves sleep during pregnancy
The primary sleep complaint prior to treatment was the inability to maintain sleep, thus confirming sleep maintenance difficulties as the most common feature of prenatal insomnia [1,2,5]. Regarding CBTI efficacy, women in the CBTI arm of the present trial, relative to those receiving sleep education, were more likely to respond to treatment, slept longer at night, and were less likely to have difficulty falling and staying asleep during pregnancy. Indeed, the ability to fall asleep normalized in the CBTI group during pregnancy. Nevertheless, rates of difficulty staying asleep remained above normal limits after treatment in both groups despite significant improvement with CBTI. This refractory sleep maintenance insomnia suggests that standard CBTI is presently ill-designed to adequately address these difficulties in pregnant women. Important to emphasize here is that some aspects of pregnancy that disrupt sleep may not be fully amenable to CBTI. For instance, awakenings related to frequent urination or difficulty finding a comfortable position that are common in late pregnancy [5] may not be solved by CBTI strategies. Even so, successful implementation of CBTI may minimize the duration of these awakenings.
These RCT results are highly consistent with a recently published RCT of digital CBTI in pregnant women by Felder and colleagues that showed that CBTI outperformed waitlist control for improving prenatal insomnia and sleep efficiency [59]. Despite promising results for digital CBTI in these two RCTs, digital CBTI produced remission rates <50% in both trials, which may suggest that standard CBTI may not be ideally equipped to treat prenatal insomnia.
Although Felder and colleagues’ RCT [59] and our own treated pregnant women with the same digital CBTI program, some notable differences in study outcomes were produced. First, we observed significant effects on sleep duration before and after childbirth, whereas Felder’s team did not. We cannot precisely identify why trial findings diverged on sleep duration during pregnancy, but it is notable that women in our trial reported sleeping <6.5 h per night before treatment, whereas average pretreatment sleep duration in Felder’s trial neared 7 h. This may have created a ceiling effect in their study. Regarding postpartum outcomes, Felder’s team did not report postpartum outcomes, thus it is presently unknown whether CBTI during pregnancy had longer lasting effects after childbirth in their trial. Regarding non-sleep outcomes, Felder’s trial observed antidepressant effects for CBTI, whereas our study did not. CBTI effects on depression are often modest especially for patients with mild depressive symptoms before treatment [60,61], and it is possible that our smaller sample size may have limited our ability to detect such effects.
4.2. Digital vs face-to-face CBTI for perinatal insomnia
Findings from Felder’s RCT [59] and our own are mostly consistent with results from an RCT supporting the efficacy of CBTI of clinician-administered face-to-face CBTI to substantially improve sleep quality and modestly alleviate depressive symptoms during pregnancy in women with insomnia disorder [27]. In all three studies, pretreatment ISI scores were ~15, whereas posttreatment scores were 8 in the face-to-face CBTI RCT and 9 in this trial for digital CBTI (posttreatment ISI mean not reported in Felder’s RCT [59]). Notably, posttreatment means in this study and Manber’s face-to-face RCT were not in full remission range, thus indicating the need for refinements to this treatment modality or additional treatment options to achieve remission in many pregnant women with insomnia.
Even so, early evidence suggests that face-to-face CBTI may be more efficacious for perinatal insomnia than presently available digital CBTI programs that are not tailored to pregnancy or postpartum. Despite similar changes in mean ISI scores in the active treatment arms of this RCT and Manber’s face-to-face CBTI trial, we observed lower remission rates after digital CBTI than previously observed for face-to-face CBTI (35% in digital CBTI vs 64% in face-to-face CBTI [27], based on the same operationalization of remission). Indeed, Felder’s RCT produced [59] remission rates of 44% with digital CBTI, which was lower than Manber’s face-to-face CBTI.
Important to emphasize, however, is that differential rates of remission between CBTI and control appear similar for digital and face-to-face CBTI: 17e22% difference in remission rates between digital CBTI and control, compared with 12% difference in remission rates in face-to-face RCT [27]. It is unclear why control remission rates were so high in the previous face-to-face study (52%) compared with 18e22% in the digital CBTI trials. However, high remission rates in the control condition of Manber’s RCT may be attributable in part to including psychoeducation on maternal and infant sleep (potentially normalizing dramatic changes in maternal sleep), differences in method of contact (digital control vs face-to-face control), sampling differences (eg, patients who engage in face-to-face treatment may be more highly motivated than those who engage in digital treatment), or some combination. Even so, these early data suggest that, for pregnant women with insomnia, face-to-face CBTI (in-person or real-time video telemedicine) may be more efficacious than digital CBTI for which currently available programs are not tailored for pregnancy or postpartum.
4.3. Digital CBTI improves sleep after childbirth
In early postpartum, patients who received digital CBTI were less likely to have sleep maintenance insomnia symptoms and slept an average of 40 min longer per night. To emphasize the magnitude of difference between groups, patients in the control condition reported sleeping an average of <6 h per night, which is linked to serious morbidity and early mortality [54,62], whereas treated patients averaged > 6 h per night. Our study is the first trial to provide evidence to suggest that improving sleep during pregnancy via CBTI also benefits postpartum sleep and possibly offers protection for new mothers from against postnatal disruptions to maternal sleep. In other words, although many new moms experience frequent nighttime awakenings due to infant feeding or poor infant sleep, moms who receive cognitive and behavioral direction in promoting good sleep may be better equipped to re-initiate sleep after middle-of-the-night awakenings, whereas those without this guidance may be susceptible to prolonged and excessive nighttime wake bouts. This is consistent with prospective data showing that CBTI (face-to-face and digital) produces long-term improvements in sleep and prevents the development of mental illness over months and years in the broader patient population [32,63].
Although digital CBTI produces improvement in postnatal sleep duration, we also observed that postnatal insomnia symptoms decreased significantly in both the CBTI and sleep education control conditions and that few patients reported difficulty falling asleep in early postpartum. This pattern may reflect two non-mutually exclusive possibilities. For women who developed insomnia gestationally, childbirth may represent a removal of the insomnia trigger and thus allow some sleep normalization in postpartum. In addition, newborn infants have highly dysregulated sleep and also need to feed during the night, thus high levels of sleep restriction in the newborn period may have washed out some of the postnatal treatment effects on insomnia symptoms by sleep depriving moms. Thus, it remains unclear to what extent insomnia symptoms re-emerge after infant sleep patterns stabilize and whether mothers who received CBTI may be protected against worsening insomnia later in the postpartum period.
4.4. The need to enhance the antidepressant effects of CBTI
Digital CBTI in the general adult population improves depressive symptoms [31,32,35,64], and the prior face-to-face and digital CBTI RCTs for prenatal insomnia revealed small but significant effects on depression in patients with mild symptoms before treatment [27,59]. In the present study, however, patients who received digital CBTI did not report lower depressive symptoms during pregnancy following treatment or at postnatal follow-up. Given that RCTs of CBTI show modest to no therapeutic benefit for perinatal depressive symptoms, it appears that standard CBTI—irrespective of delivery method—may be ill-designed to reduce perinatal depressive symptoms in a substantial and clinically meaningful way.
Even so, we observed a statistical trend to suggest that CBTI may reduce suicidal ideation after treatment. In other trials, improving sleep quality with digital CBTI effectively reduces two-year risk of developing clinical depression [32]. It is possible that reducing insomnia symptoms (particularly difficulty falling asleep, which is closely linked to perinatal depression and suicidality [12]) and extending sleep duration may reduce suicidal thoughts and behaviors and protect women against perinatal increases in depression. Larger RCTs with follow-up assessments across the postpartum year are needed to better characterize potential protective qualities of insomnia treatments for depression and suicidal ideation.
4.5. Standard CBTI does not reduce cognitive arousal: a critical limitation
Digital CBTI had no effect on nocturnal cognitive arousal. This is consistent with evidence from a prior face-to-face CBTI RCT also showing minimal or no effect of standard CBTI on cognitive arousal [61]. Given that individuals with insomnia have cognitive-emotional profiles similar to patients with depression and anxiety [65–69], it is unsurprising that 1–2 sessions of cognitive therapy in a standard 6-session CBTI protocol has minimal impact on the manner in which poorly sleeping pregnant women process stress and other negative information. Augmentation strategies to enhance CBTI effects on cognitive arousal (eg, rumination, worry) may improve acute treatment response by reducing faulty cognitive processing known to fuel insomnia [70,71] and may even enhance long-term outcomes for treatment responders by reducing a robust risk factor for insomnia relapse [72]. As pregnant women, especially those with insomnia, endorse high levels of cognitive arousal and stress-related sleep reactivity, which are linked to depression and suicidal ideation during pregnancy [11,12], directly targeting concomitant cognitive arousal and depressive symptoms should be primary goals of insomnia therapy in perinatal populations.
4.6. Limitations
The present study should be interpreted in light of certain limitations. Our primary limitation concerns a lack of follow-up assessments beyond the newborn period. Longer-term prospective data are needed to improve our understanding of the durability of these effects in new mothers and the potential for prevention of depression and other consequences of insomnia including quality of life, and economic outcomes. Indeed, our postnatal follow-up was during the newborn period, which is typically marked by chronic sleep deprivation due to nighttime infant feeding and unstable infant sleep patterns. Postnatal sleep studies should assess sleep at least throughout the first postpartum year and account for key environmental factors that may impact maternal sleep and wellbeing such as maternity leave and partner support. It is possible that this transition period of sleep instability may have masked therapeutic effects of CBTI on insomnia symptoms that could potentially re-emerge after infant sleep stabilizes. Future studies should characterize patterns of insomnia re-emergence and relapse across treatment conditions as well as trajectories of postpartum depressive symptoms over the longer-term. In addition, we did not collect sleep diary data in the present study, which would have enriched our study outcomes. Special consideration may be given to sleep pattern stability, especially after childbirth. If CBTI patients continued to implement behavioral sleep strategies, it is possible that they may have more stable sleep patterns, even in the newborn period. Future studies should collect sleep diary data in pregnant and postpartum women to determine whether CBTI helps moms stabilize their sleep schedules.
Another key limitation regards a lack of objective sleep assessments and physiological markers of cognitive arousal. CBTI produces minimal to no effect on objective sleep disturbance in non-pregnant patient populations [73]. And although patient-reported outcomes best represent the clinical experience of insomnia, there has been a resurgence of interest in objective sleep disturbance within insomnia [74], given its association with morbidity, mortality, and treatment-resistance [53,75–78]. Notably, cognitive arousal while trying to fall asleep is associated with objective sleep disturbances [79–84], thus enhancing CBTI effects on cognitive arousal (both objective and self-report measures) has potential to improve objective sleep. Lastly, our study was underpowered to test treatment effects on suicidal ideation, which has a relatively low base rate, especially considering that severely depressed women were excluded. Notably, just 3 controls and 2 CBTI patients endorsed suicidal ideation before treatment. Although both CBTI patients denied suicidal ideation after treatment, whereas all controls continued to endorse this symptom, the analysis was non-significant due to insufficient statistical power.
4.7. Tailoring CBTI for perinatal women with insomnia
Data from this RCT and prior CBTI trials suggest that standard CBTI is not currently designed to maximally treat prenatal insomnia. Further, digital interventions may be at an added disadvantage due to the lack of personalization to the variable perinatal experience. Given the unique factors that trigger and/or perpetuate perinatal sleep problems (eg, pregnancy/maternal-specific worries and concerns, physical discomfort, urinary frequency, fetal activity, difficult infant temperament and sleep [5,7,12,85–87]), combined with potential obstacles to insomnia therapy adherence for new and expectant mothers (eg, difficulty adhering to sleep restriction), perinatal insomnia patients will likely best benefit from mobile health interventions designed to account for the experiences of pregnancy and new motherhood.
Indeed, CBTI–whether delivered by sleep or mental health clinicians or digitally–should be better tailored for new and expectant mothers. In the present study, the only modification was to ensure that time-in-bed was never restricted below 6 h. In the prior face-to-face CBTI RCT, the authors also included this modification in addition to adding an extra 30 min to time-in-bed (added to self-reported habitual sleep duration) and providing perinatal psychoeducation. It is possible that these additional modifications enhanced their treatment outcomes in this patient population. Potential modifications to consider may be scheduling limited naps to ensure opportunity to obtain adequate sleep [43], augmenting CBTI with mindfulness meditation to reduce cognitive arousal [88–90] and to improve healthy coping with perinatal stress and sleep problems [91–95], and implementing postpartum follow-up visits to monitor maternal sleep and to teach mothers behavioral interventions to improve infant sleep [96–98].
5. Conclusions
Despite over half of pregnant women experiencing clinically significant insomnia, few empirically supported insomnia treatment options are available for this population. Early evidence indicates that CBTI is efficacious for improving sleep during pregnancy, which also has longer-term benefits for the postnatal period but perhaps to a lesser extent. Although evidence supports CBTI efficacy when delivered through a fully-automated digital intervention, treatment outcomes may be superior for pregnant women receiving the intervention from a specialist with expertise in maternal and infant sleep, who may be better able to navigate patient factors unique to the perinatal period. Even so, digital CBTI represents a more widely available and more cost-effective alternative for pregnant women with insomnia who have difficulty accessing CBTI specialty care. Future studies are needed to better tailor CBTI—face-to-face and digital—for treating insomnia in the perinatal period.
Acknowledgements
This study was funded by the American Academy of Sleep Medicine (198-FP-18, PI: Kalmbach). Dr. Cheng’s effort was supported by the National Heart, Lung, and Blood Institute (K23 HL13866, PI: Cheng). Dr. Swanson’s effort was funded by the National Heart, Lung, and Blood Institute (K23 HL122461, PI: Swanson). We would like to thank Colin Espie, Christopher Miller, and everyone at Sleepio for their support of our research.
Disclosure statement
Dr. Kalmbach reports receiving nonfinancial support from Big Health Inc (provision of Sleepio for use in this clinical trial). Dr. Cheng has received research support from Harmony Biosciences. Dr. Henry reports being an employee of Big Health Inc, which owns Sleepio. Dr. Roth has received research support from Aventis, Cephalon, Glaxo Smith Kline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth and Xenoport and has acted as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, Astra Zeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, Glaxo Smith Kline, Hypnion, Impax, Intec, Intra-Cellular, Jazz, Johnson & Johnson, King, Lundbeck, McNeil, Medici Nova, Merck & Co., Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Procter-Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. No other financial or non-financial interests exist. Dr. Drake has received financial research support from Eisai Co., Procter & Gamble, Jazz, Suven; and has served on speakers bureau for Harmony Biosciences.
Footnotes
Conflict of interest
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2020.03.016.
References
- [1].Mindell JA, Cook RA, Nikolovski J. Sleep patterns and sleep disturbances across pregnancy. Sleep Med 2015;16(4):483–8. [DOI] [PubMed] [Google Scholar]
- [2].Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs 2000;29(6):590–7. [DOI] [PubMed] [Google Scholar]
- [3].Hutchison BL, Stone PR, McCowan LM, et al. A postal survey of maternal sleep in late pregnancy. BMC Pregnancy Childbirth 2012;12(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Facco FL, Kramer J, Ho KH, et al. Sleep disturbances in pregnancy. Obstet Gynecol 2010;115(1):77–83. [DOI] [PubMed] [Google Scholar]
- [5].Kalmbach DA, Cheng P, Sangha R, et al. Insomnia, short sleep, and snoring in mid-to-late pregnancy: disparities related to poverty, race, and obesity. Nat Sci Sleep 2019;11:301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fernández-Alonso AM, Trabalón-Pastor M, Chedraui P, et al. Factors related to insomnia and sleepiness in the late third trimester of pregnancy. Arch Gynecol Obstet 2012;286(1):55–61. [DOI] [PubMed] [Google Scholar]
- [7].Drheim SK, Bjorvatn B, Eberhard-Gran M. Insomnia and depressive symptoms in late pregnancy: a population-based study. Behav Sleep Med 2012;10(3):152–66. [DOI] [PubMed] [Google Scholar]
- [8].Kızılırmak A, Timur S, Kartal B. Insomnia in pregnancy and factors related to insomnia. Sci World J 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep 2004;27(7): 1405–17. [DOI] [PubMed] [Google Scholar]
- [10].Reichner CA. Insomnia and sleep deficiency in pregnancy. Obstet Med 2015;8(4):168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Palagini L, Cipollone G, Masci I, et al. Stress-related sleep reactivity is associated with insomnia, psychopathology and suicidality in pregnant women: preliminary results. Sleep Med 2019;56:145–50. [DOI] [PubMed] [Google Scholar]
- [12].Kalmbach DA, Cheng P, Ong JC, et al. Depression and suicidal ideation in pregnancy: exploring relationships with insomnia, short sleep, and nocturnal rumination. Sleep Med 2019;65:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Volkovich E, Tikotzky L, Manber R. Objective and subjective sleep during pregnancy: links with depressive and anxiety symptoms. Arch Wom Ment Health 2016;19(1):173–81. [DOI] [PubMed] [Google Scholar]
- [14].Okun ML, Mancuso RA, Hobel CJ, et al. Poor sleep quality increases symptoms of depression and anxiety in postpartum women. J Behav Med 2018;41(5): 703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Manber R, Steidtmann D, Chambers AS, et al. Factors associated with clinically significant insomnia among pregnant low-income Latinas. J Wom Health 2013;22(8):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Swanson LM, Pickett SM, Flynn H, et al. Relationships among depression, anxiety, and insomnia symptoms in perinatal women seeking mental health treatment. J Wom Health 2011;20(4):553–8. [DOI] [PubMed] [Google Scholar]
- [17].Mourady D, Richa S, Karam R, et al. Associations between quality of life, physical activity, worry, depression and insomnia: a cross-sectional designed study in healthy pregnant women. PloS One 2017;12(5):e0178181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bacaro V, Benz F, Pappaccogli A, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev 2019:101234. [DOI] [PubMed] [Google Scholar]
- [19].Roehrs T, Roth T. Insomnia pharmacotherapy. Neurotherapeutics 2012;9(4): 728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002;6(2):97–111. [DOI] [PubMed] [Google Scholar]
- [21].Okun ML, Ebert R, Saini B. A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol 2015;212(4):428–41. [DOI] [PubMed] [Google Scholar]
- [22].Sedov ID, Goodman SH, Tomfohr-Madsen LM. Insomnia treatment preferences during pregnancy. J Obstet Gynecol Neonatal Nurs 2017;46(3): e95–104. [DOI] [PubMed] [Google Scholar]
- [23].Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;165(2):125–33. [DOI] [PubMed] [Google Scholar]
- [24].Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev 2009;13(3):205–14. [DOI] [PubMed] [Google Scholar]
- [25].Mitchell MD, Gehrman P, Perlis M, et al. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract 2012;13(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tomfohr-Madsen LM, Clayborne ZM, Rouleau CR, et al. Sleeping for two: an open-pilot study of cognitive behavioral therapy for insomnia in pregnancy. Behav Sleep Med 2017;15(5):377–93. [DOI] [PubMed] [Google Scholar]
- [27].Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol 2019;133(5):911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cain MA, Brumley J, Louis-Jacques A, et al. A pilot study of a sleep intervention delivered through group prenatal care to overweight and obese women. Behav Sleep Med 2019:1–11. [DOI] [PubMed] [Google Scholar]
- [29].Thomas A, Grandner M, Nowakowski S, et al. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med 2016;14(6):687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med 2018;33(6):955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cheng P, Luik AI, Fellman-Couture C, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cheng P, Kalmbach DA, Joseph C, et al. Depression prevention via digital CBT for insomnia: a randomized controlled trial. Sleep 2019;42(10):zsz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pillai V, Anderson JR, Cheng P, et al. The anxiolytic effects of cognitive behavior therapy for insomnia: preliminary results from a web-delivered protocol. J Sleep Med Disord 2015;2(2):a–7. [PMC free article] [PubMed] [Google Scholar]
- [34].Freeman D, Sheaves B, Goodwin GM, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry 2017;4(10):749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Espie CA, Emsley R, Kyle SD, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry 2019;76(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep 2012;35(6): 769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bostock S, Luik AI, Espie CA. Sleep and productivity benefits of digital cognitive behavioral therapy for insomnia: a randomized controlled trial conducted in the workplace environment. J Occup Environ Med 2016;58(7): 683–9. [DOI] [PubMed] [Google Scholar]
- [38].Barnes CM, Miller JA, Bostock S. Helping employees sleep well: effects of cognitive behavioral therapy for insomnia on work outcomes. J Appl Psychol 2017;102(1):104. [DOI] [PubMed] [Google Scholar]
- [39].McGrath ER, Espie CA, Power A, et al. Sleep to lower elevated blood pressure: a randomized controlled trial (SLEPT). Am J Hypertens 2017;30(3):319–27. [DOI] [PubMed] [Google Scholar]
- [40].Zachariae R, Lyby MS, Ritterband LM, et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomniaea systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2016;30:1–10. [DOI] [PubMed] [Google Scholar]
- [41].Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987;150(6):782–6. [DOI] [PubMed] [Google Scholar]
- [43].Swanson LM, Flynn H, Adams-Mundy JD, et al. An open pilot of cognitive-behavioral therapy for insomnia in women with postpartum depression. Behav Sleep Med 2013;11(4):297–307. [DOI] [PubMed] [Google Scholar]
- [44].Health NIo. Your guide to healthy sleep. Southern Medical Association; 2011. [Google Scholar]
- [45].Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev 2015;22:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Grandner MA, Chakravorty S. Insomnia in primary care: misreported, mishandled, and just plain missed. J Clin Sleep Med 2017;13(8):937–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep 2006;29(11):1415–9. [PubMed] [Google Scholar]
- [48].Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- [49].Buysse DJ, Reynolds III CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- [50].Fichtenberg NL, Putnam SH, Mann NR, et al. Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh Sleep Quality Index. Am J Phys Med Rehabil 2001;80(5):339–45. [DOI] [PubMed] [Google Scholar]
- [51].Fichtenberg NL, Zafonte RD, Putnam S, et al. Insomnia in a post-acute brain injury sample. Brain Inj 2002;16(3):197–206. [DOI] [PubMed] [Google Scholar]
- [52].Kalmbach DA, Conroy DA, Falk H, et al. Poor sleep is linked to impeded recovery from traumatic brain injury. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vgontzas AN, Fernandez-Mendoza J, Liao D, et al. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 2013;17(4):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kalmbach DA, Pillai V, Arnedt JT, et al. DSM-5 insomnia and short sleep: comorbidity landscape and racial disparities. Sleep 2016;39(12):2101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Matthey S, Henshaw C, Elliott S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scaleeimplications for clinical and research practice. Arch Wom Ment Health 2006;9(6):309–15. [DOI] [PubMed] [Google Scholar]
- [56].McCabe-Beane JE, Segre LS, Perkhounkova Y, et al. The identification of severity ranges for the Edinburgh postnatal depression scale. J Reprod Infant Psychol 2016;34(3):293–303. [Google Scholar]
- [57].Nicassio PM, Mendlowitz DR, Fussell JJ, et al. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther 1985;23(3):263–71. [DOI] [PubMed] [Google Scholar]
- [58].Puzino K, Frye SS, LaGrotte CA, et al. Am I (hyper) aroused or anxious? Clinical significance of pre-sleep somatic arousal in young adults. J Sleep Res 2019: e12829. [DOI] [PubMed] [Google Scholar]
- [59].Felder JN, Epel ES, Neuhaus J, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psychiatry 2020. 10.1001/jamapsychiatry.2019.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Manber R, Bernert RA, Suh S, et al. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. Focus 2014;12(1):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kalmbach DA, Cheng P, Arnedt JT, et al. Treating insomnia improves depression, maladaptive thinking, and hyperarousal in postmenopausal women: comparing cognitive-behavioral therapy for insomnia (CBTI), sleep restriction therapy, and sleep hygiene education. Sleep Med 2019;55:124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Sivertsen B, Pallesen S, Glozier N, et al. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Publ Health 2014;14(1):720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Castronovo V, Galbiati A, Sforza M, et al. Long-term clinical effect of group cognitive behavioral therapy for insomnia: a case series study. Sleep Med 2018;47:54–9. [DOI] [PubMed] [Google Scholar]
- [64].Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry 2016;3(4): 333–41. [DOI] [PubMed] [Google Scholar]
- [65].Baglioni C, Spiegelhalder K, Lombardo C, et al. Sleep and emotions: a focus on insomnia. Sleep Med Rev 2010;14(4):227–38. [DOI] [PubMed] [Google Scholar]
- [66].Fernandez-Mendoza J, Calhoun SL, Bixler EO, et al. Sleep misperception and chronic insomnia in the general population: the role of objective sleep duration and psychological profiles. Psychosom Med 2011;73(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fernandez-Mendoza J, Vela-Bueno A, Vgontzas AN, et al. Cognitive-emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosom Med 2010;72(4):397–403. [DOI] [PubMed] [Google Scholar]
- [68].Pillai V, Drake CL. Sleep and repetitive thought: the role of rumination and worry in sleep disturbance. In: Babson KA, Feldner MT, editors. Sleep and affect. London: Elsevier; 2015. p. 201–25. [Google Scholar]
- [69].Fernandez-Mendoza J, Shaffer ML, Olavarrieta-Bernardino S, et al. Cognitive-emotional hyperarousal in the offspring of parents vulnerable to insomnia: a nuclear family study. J Sleep Res 2014;23(5):489–98. [DOI] [PubMed] [Google Scholar]
- [70].Harvey AG. Unwanted intrusive thoughts in insomnia. 2005. [Google Scholar]
- [71].Harvey AG. A cognitive theory and therapy for chronic insomnia. J Cognit Psychother 2005;19(1):41. [Google Scholar]
- [72].Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore J Sci Heal 2009;5(1):30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mitchell L, Bisdounis L, Ballesio A, et al. The impact of cognitive behavioural therapy for insomnia on objective sleep parameters: a meta-analysis and systematic review. Sleep Med Rev 2019;47:90–102. [DOI] [PubMed] [Google Scholar]
- [74].Dietch JR, Taylor DJ. The enigma of objective and subjective measurement of response to Cognitive Behavioral Therapy for Insomnia: call to action. Sleep Med Rev 2019;47:119–21. [DOI] [PubMed] [Google Scholar]
- [75].Bathgate CJ, Edinger JD, Krystal AD . Insomnia patients with objective short sleep duration have a blunted response to cognitive behavioral therapy for insomnia. Sleep 2017;40(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Miller CB, Espie CA, Bartlett DJ, et al. Acceptability, tolerability, and potential efficacy of cognitive behavioural therapy for Insomnia Disorder subtypes defined by polysomnography: a retrospective cohort study. Sci Rep 2018;8(1): 6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fernandez-Mendoza J. The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention. Curr Opin Psychiatr 2017;30(1):56–63. [DOI] [PubMed] [Google Scholar]
- [78].Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep Med Rev 2003;7(3):203–14. [DOI] [PubMed] [Google Scholar]
- [79].Hall M, Buysse D, Reynolds C, et al. Stress-related intrusive thoughts disrupt sleep onset and continuity. Sleep Res 1996;25:163. [Google Scholar]
- [80].Wuyts J, De Valck E, Vandekerckhove M, et al. The influence of pre-sleep cognitive arousal on sleep onset processes. Int J Psychophysiol 2012;83(1): 8–15. [DOI] [PubMed] [Google Scholar]
- [81].Galbiati A, Giora E, Sarasso S, et al. Repetitive thought is associated with both subjectively and objectively recorded polysomnographic indices of disrupted sleep in insomnia disorder. Sleep Med 2018;45:55–61. [DOI] [PubMed] [Google Scholar]
- [82].Gross RT, Borkovec T . Effects of a cognitive intrusion manipulation on the sleep-onset latency of good sleepers. Behav Ther 1982;13(1):112–6. [Google Scholar]
- [83].Zoccola PM, Dickerson SS, Lam S. Rumination predicts longer sleep onset latency after an acute psychosocial stressor. Psychosom Med 2009;71(7): 771–5. [DOI] [PubMed] [Google Scholar]
- [84].Kalmbach DA, Buysse DJ, Cheng P, et al. Nocturnal cognitive arousal is associated with objective sleep disturbance and indicators of physiologic hyperarousal in good sleepers and individuals with insomnia disorder. Sleep Med 2020;71:151e60. 10.1016/j.sleep.2019.11.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Krystal AD. Insomnia in women. Clin Cornerstone 2003;5(3):41–50. [DOI] [PubMed] [Google Scholar]
- [86].Martini J, Asselmann E, Einsle F, et al. A prospective-longitudinal study on the association of anxiety disorders prior to pregnancy and pregnancy-and child-related fears. J Anxiety Disord 2016;40:58–66. [DOI] [PubMed] [Google Scholar]
- [87].Bayer JK, Hiscock H, Hampton A, et al. Sleep problems in young infants and maternal mental and physical health. J Paediatr Child Health 2007;43(1–2): 66–73. [DOI] [PubMed] [Google Scholar]
- [88].Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behav Ther 2008;39(2):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ong JC, Manber R, Segal Z, et al. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep 2014;37(9):1553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Deyo M, Wilson KA, Ong J, et al. Mindfulness and rumination: does mindfulness training lead to reductions in the ruminative thinking associated with depression? EXPLORE J Sci Heal 2009;5(5):265–71. [DOI] [PubMed] [Google Scholar]
- [91].Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Arch Wom Ment Health 2008;11(1):67–74. [DOI] [PubMed] [Google Scholar]
- [92].Goodman JH, Guarino A, Chenausky K, et al. CALM Pregnancy: results of a pilot study of mindfulness-based cognitive therapy for perinatal anxiety. Arch Wom Ment Health 2014;17(5):373–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Beddoe AE, Yang C-PP, Kennedy HP, et al. The effects of mindfulness-based yoga during pregnancy on maternal psychological and physical distress. J Obstet Gynecol Neonatal Nurs 2009;38(3):310–9. [DOI] [PubMed] [Google Scholar]
- [94].Wong MY, Ree MJ, Lee CW. Enhancing CBT for chronic insomnia: a randomised clinical trial of additive components of mindfulness or cognitive therapy. Clin Psychol Psychother 2016;23(5):377–85. [DOI] [PubMed] [Google Scholar]
- [95].Felder JN, Laraia B, Coleman-Phox K, et al. Poor sleep quality, psychological distress, and the buffering effect of mindfulness training during pregnancy. Behav Sleep Med 2018;16(6):611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Gradisar M, Jackson K, Spurrier NJ, et al. Behavioral interventions for infant sleep problems: a randomized controlled trial. Pediatrics 2016;137(6): e20151486. [DOI] [PubMed] [Google Scholar]
- [97].Hiscock H, Bayer J, Gold L, et al. Improving infant sleep and maternal mental health: a cluster randomised trial. Arch Dis Child 2007;92(11):952–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wolfson A, Lacks P, Futterman A. Effects of parent training on infant sleeping patterns, parents’ stress, and perceived parental competence. J Consult Clin Psychol 1992;60(1):41. [DOI] [PubMed] [Google Scholar]