Abstract
Renal cell carcinoma (RCC) is a common urological malignancy. Despite early detection and surgical treatment, some lesions recur late at distant sites. The most common dissemination sites are lung, bone, and liver. Skin metastases are not common, and the incidence and clinical manifestations are poorly established in the literature. We report here the case of a male patient with an isolated scalp cutaneous metastase of RCC, 7 years after radical nephrectomy. An excisional biopsy was performed and confirmed metastatic RCC.
Keywords: Cutaneous metastasis, recurrence, renal cell carcinoma, skin metastasis
INTRODUCTION
Renal cell carcinoma (RCC) accounts for approximately 2%–3% of adult malignancies.[1]
Despite early detection and effective surgical treatment, some lesions recur at distant sites, most of them within 5 years.[2] However, “late metastases” also occur. In medical literature, “late” is usually defined as >10 years from the removal of the primary tumor and without evidence of local recurrence.[3]
Until recently, only few case reports have been published, ascertaining the rarity of this presentation and the need for a high suspicion index in making this diagnosis.[4]
CASE REPORT
A 75-year-old male presented in January 2018 with a 3-month history of an enlarging scalp nodule. On observation, the lesion was a 5 cm red-violet nodule, with a firm consistency, well-defined borders and not adherent to deep planes [Figure 1].
Figure 1.
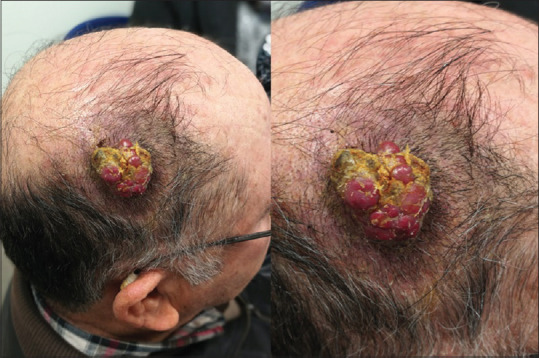
Scalp lesion
The patient was submitted to a cranial computed tomography (CT)-scan [Figure 2] that revealed the right parietal lesion with 48 mm × 24 mm diameter localized in the subcutaneous tissue, exophytic, with ovoid morphology and no temporal muscle invasion. There were no other cranial lesions.
Figure 2.
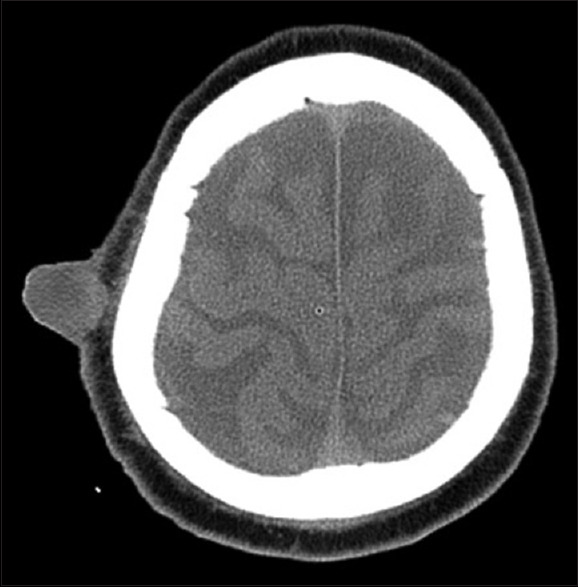
Cranial computed tomography-scan revealing the right parietal subcutaneous lesion
The patient had a 5 cm intraparenchymal left kidney tumor history in 2011. He was submitted to a left radical nephrectomy and diagnosed with a clear-cell RCC, Furhman grade 4, and negative surgical margins (pT1b). The Thoraco-Abdomino-Pelvic CT-scan (TAP-CT) showed no metastases. There was no evidence of metastases during the follow-up.
An excisional biopsy was performed, and the scalp was reconstructed with a full-thickness supraclavicular skin graft [Figure 3]. Histopathologic examination revealed an expanded dermis and subcutis by clear-cell proliferation, with gland formation, compatible with metastatic RCC [Figure 4]. The surgical margins were negative.
Figure 3.
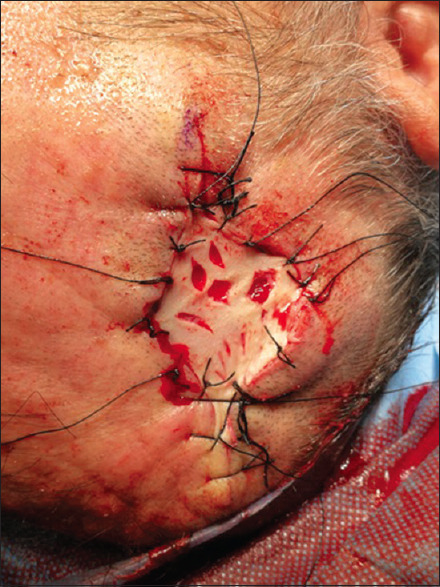
Scalp reconstruction with a full-thickness supraclavicular skin graft after excisional biopsy
Figure 4.
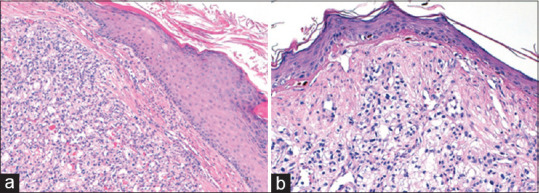
(a and b) Solid proliferation of clear cells infiltrating the dermis, admixed with a network of small, thin-walled vessels (H and E, ×4 and ×20, respectively)
A TAP-CT was performed, and no other distance metastases were identified.
DISCUSSION
Solid tumors × cutaneous metastases are uncommon: they occur in 0.7%–9%. Tumor invasion can take place through different routes: Hematogenous, lymphatic, by contiguity, or iatrogenic implantation.[2]
Evaluating the largest retrospective studies, in >81,000 reported cases, the most common primary tumors metastasizing to the skin depend on the gender of the affected individual and include the breast (69%), colon (9%), lung (4%) and ovary (4%) in women, and lung (24%), colon (19%), and head-and-neck cancers (12%) in men.[5]
The incidence of cutaneous metastasis in RCC ranges from 2.4% to 6.4%.[4]
The most common site for RCC cutaneous metastasis is the scalp and face. Highly angioinvasive tumors, such as lung and renal cancers, tend to originate unlimited, early metastases, with an unpredictable pattern of location, usually far away from the primary tumor.[6] It has been proposed that the normal pathway of dissemination to the head-and-neck area is through the lung. The alternate pathway of dissemination of tumor cells through the vertebral venous plexus explains the absence of lung metastasis in patients who have isolated metastasis in the head and neck.[7]
The cutaneous metastasis usually appears within 6 months to 5 years from the initial diagnosis and indicate progression or recurrence of malignancy following treatment. Sometimes, it may even be the primary presentation of the disease.[1] It is believed that metastatic cells can remain dormant for decades after the primary tumor has been removed. The mechanisms capable of inducing dormancy may be reversible, leading to late recurrences.
The cutaneous metastases are usually single lesions that grow rapidly and are bluish-red in color and sometimes pulsating.[8] Macroscopic differential diagnosis includes commonly angioma, cutaneous horn, and basal cell carcinoma.
The presented case showed the predominance of clear cells in the hematoxylin and eosin staining section, so differential diagnosis includes primary tumors of skin/appendages and metastatic tumors with clear cell morphology. Clear-cell tumors remain a challenge for the pathologist, who is frequently asked to determine the primary anatomic site of the malignancy based on the cytomorphology of the aspirated material. Due to the cytomorphological overlap of various clear-cell tumors, clinicopathological correlation, and immunohistochemistry (IHC) aid in diagnosis making. IHC panel includes pancytokeratin (indicating epithelial nature), vimentin, calponin, S100, HMB45, and specific markers for metastatic tumors such as CD10 or PAX8 for RCC.[1]
Local treatment of single metastases from RCC is possible. If the metastatic lesion aims to be resectable, the surgical approach is possible. Sometimes, it is possible to do various radiotherapy modalities in unresectable lesions as part of a multimodal approach, including systemic therapy with angiogenesis/multikinase inhibitor agents. It is always important to restage with a contrast-enhanced CT-scan or magnetic resonance of the abdomen and chest. Bone scan and brain CT-scan may be used in the presence of clinical or laboratory signs and symptoms.
These patients have, in general, a poor prognosis. Systemic metastases portend a poor prognosis for RCC, conventionally with 1-year survival of <50%, 5-year survival of 5%–30%, and 10-year survival of 0%–5%. Patients presenting with synchronous metastases fare worse, with many patients dying from disease progression within 1–2 years. For patients with asynchronous metastases, the metastasis-free interval has proved to be a useful prognostic factor because it reflects the time of disease progression.[9] The survival after the diagnosis of skin metastasis ranged from 3 to 15 months (mean 7 months).[4]
Controversy exists on the optimal RCC follow-up. Some argue that follow-up with imaging is not cost-effective after 5 years; however, late metastases are more likely to be solitary and justify more aggressive therapy with curative intent. Several authors designed scoring systems and nomograms to quantify the likelihood of patients developing tumor recurrences, metastases, and subsequent death.[10,11] In authors ‘opinion prolonged and thorough follow-up is mandatory in all patients, even in those with low clinical and pathological stages, with a CT-scan of the abdomen and chest and all body examination each year during the first 3 years after treatment, and every 2 years after the 3rd-year follow-up. It is essential a complete and periodical dermatologic examination, with a biopsy of any recently appearing cutaneous lesion, since this can help in early detection of recurrences. In patients presenting with cutaneous lesions at unusual sites, RCC should always be an important differential diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient (s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh A, Mohan G, Chaturvedi S, Khan SA. Cytodiagnosis of a cutaneous clear cell malignancy: Metastatic renal cell carcinoma on chin. J Clin Diagn Res. 2016;10:ED12–4. doi: 10.7860/JCDR/2016/15869.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Rueda P, Ruiz-López P, Ramírez-Negrín MA, Fuentes-Suárez A, Toussaint-Caire S, Vega-Memije ME. Cutaneous metastasis of renal cell carcinoma: A case report and review of the literature. Gac Med Mex. 2015;151:533–7. [PubMed] [Google Scholar]
- 3.Friberg S, Nyström A. Cancer metastases: Early dissemination and late recurrences. Cancer Growth Metastasis. 2015;8:43–9. doi: 10.4137/CGM.S31244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorairajan LN, Hemal AK, Aron M, Rajeev TP, Nair M, Seth A, et al. Cutaneous metastases in renal cell carcinoma. Urol Int. 1999;63:164–7. doi: 10.1159/000030440. [DOI] [PubMed] [Google Scholar]
- 5.Mueller TJ, Wu H, Greenberg RE, Hudes G, Topham N, Lessin SR, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021–6. doi: 10.1016/j.urology.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 6.de Paula TA, da Silva PS, Berriel LG. Renal cell carcinoma with cutaneous metastasis: Case report. J Bras Nefrol. 2010;32:213–5. [PubMed] [Google Scholar]
- 7.Jindal T, Sinha RK, Mukherjee S, Karmakar D. Calvarial and cutaneous metastasis as the primary presentation of a renal cell carcinoma. BMJ Case Rep. 2014;2014:1–3. doi: 10.1136/bcr-2013-202830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbasi F, Alizadeh M, Noroozinia F, Moradi A. Cutaneous metastasis of bilateral renal cell carcinoma. J Pak Med Assoc. 2013;63:111–3. [PubMed] [Google Scholar]
- 9.Campbell SC, Lane BR. Malignant renal tumors. In: Wein AJ, editor. Campbell-walsh Urology. 11th ed. Philadelphia: Elsevier; 2016. pp. 1314–64. [Google Scholar]
- 10.Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 11.Karakiewicz PI, Suardi N, Capitanio U, Jeldres C, Ficarra V, Cindolo L, et al. A preoperative prognostic model for patients treated with nephrectomy for renal cell carcinoma. Eur Urol. 2009;55:287. doi: 10.1016/j.eururo.2008.07.037. [DOI] [PubMed] [Google Scholar]


