Supplemental Digital Content is Available in the Text.
A retrospective study followed up for a mean of 4.9 years showed that progression of myopic maculopathy was detected in 18.9% among highly myopic schoolchildren aged 4 to 17 years.
Key words: myopic maculopathy, high myopia, peripapillary diffuse choroidal atrophy, parapapillary gamma zone, children
Abstract
Purpose:
To investigate the progression of myopic maculopathy and associated factors in highly myopic Chinese children.
Methods:
In this retrospective observational case series, biometric fundus features were morphometrically measured on photographs. Myopic maculopathy was defined as recommended by the Meta-analysis of Pathologic Myopia Study Group.
Results:
The study included 274 children (mean age: 11.7 ± 2.5 years; mean refractive error: −7.66 ± 1.87 diopters [D]) with a mean follow-up of 4.9 ± 1.2 years. Myopic maculopathy progression was detected in 52 eyes (18.9%; 95% confidence interval [CI]: 14.3–23.7%). In multivariable analysis, myopic maculopathy progression was associated with a decrease in refractive error (odds ratio [OR]: 0.72; 95% CI: 0.56–0.92; P < 0.001) (i.e., higher myopization) and enlargement of parapapillary gamma zone (OR: 7.68; 95% CI: 1.63–36.2; P = 0.002). Incident peripapillary diffuse choroidal atrophy, noted in 47 of 236 eyes (20.0%; 95% CI: 14.8–25.2%), was correlated with a decrease in refractive error (OR: 0.70; 95% CI: 0.54–0.92; P = 0.009) (i.e., higher myopization) and greater gamma zone enlargement (OR: 8.28; 95% CI: 1.33–51.7; P = 0.02).
Conclusion:
Myopia in schoolchildren may have a considerable risk of progressing to myopic maculopathy. Enlargement of parapapillary gamma zone was a main independent risk factor.
In the past three decades, the prevalence of myopia increased markedly among the young generation, in particular in East Asia.1,2 It is predicted that high myopia, specifically pathologic myopia with myopic maculopathy, could become the most common cause for irreversible blindness worldwide.3,4 Because pathologic high myopia seen in adults (>50 years of age) and schoolchildren differ in their associations with the level of education, it is unclear whether the high myopia of adults and schoolchildren have the same risk of progressing to the pathologic stage of myopic maculopathy (MM).5,6 Myopic maculopathy is clinically significant and increases the risk of irreversible visual impairment. However, the progression of MM in young individuals has remained to be elusive yet.7–9
Besides myopia onset at a young age of <10 years, other risk factors for the eventual development of high myopia and pathologic myopia have not been identified in large studies yet.8 A previous case series study described the presence of peripapillary diffuse choroidal atrophy (PDCA) in children and adolescents as a potential biomarker for pathologic myopia development in adulthood.10 Although previous investigations on longitudinal changes of MM focused on adults, the timing of the development and progression of myopic fundus changes in children and adolescents and the factors associated with it are unclear.9,11–15 We, therefore, conducted this study to: 1) evaluate the prevalence of PDCA and the presence and degree of MM in highly myopic children; 2) explore PDCA and MM changes during a follow-up of ≥4 years; and 3) find factors associated with PDCA and MM prevalence and their changes over time.
Methods
Bilateral highly myopic children who were examined from August 2010 and followed up for at least four years at the Beijing Tongren Hospital until August 2018 were included into this retrospective study. The study procedures were approved by the Ethics Committee of the Tongren Hospital, Capital Medical University, and followed the Declaration of Helsinki. All participants provided written informed consents. We defined high myopia as a myopic refractive error (spherical equivalent) of less than −6.0 diopters (D).16 The inclusion criteria were a baseline age of less than 18 years and a refractive error of <−6.0 D. Children with ocular disorders (except for MM), history of an ocular surgery, and poor-quality photographs of the fundus were excluded.
All participants underwent the same ocular examinations at baseline and during the last visit. Ocular examinations conducted for all patients consisted of refractometry and measurement of best-corrected visual acuity using a retroilluminated visual chart with tumbling-E optotypes (MC-3; Topcon Corporation, Japan), slit-lamp–based examination of the anterior segment, and color digital fundus photography (CR-DGI or CR-2, Canon Inc, Tokyo, Japan). The refractive error was determined by cycloplegic refractometry using auto-refractometry (Autorefractor KR-8900; Topcon, Tokyo, Japan).
Biometric measurements of fundus structures (Figure 1) were conducted using the ImageJ software (version 1.43u; developed by Wayne Rasband, National Institute of Health, Bethesda, MD; available in the public domain at http://rsb.info.nih.gov/ij/index.html). The technique has been described in detail previously (see Text, Supplemental Digital Content 1, http://links.lww.com/IAE/B343, which demonstrates the definitions of biometric fundus features).17–20 We corrected the image magnification by the optic media of the eye by applying Bengtsson's21 method and used the refractive error as the parameter for the calculations.
Fig. 1.
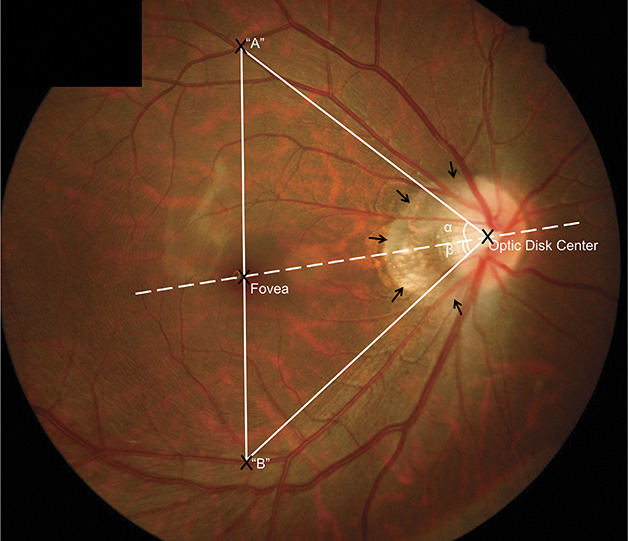
Illustration of measured fundus features. Fundus photograph showing the angle between the temporal arterial arcade and the disk center-fovea line with the optic disk as the vertex of the angle (angle α and angle β). The vertical distance between the temporal superior and temporal inferior arterial arcades was the distance between point A and point B, which were the crossing points of a vertical line passing through the fovea and crossing the temporal superior arterial arcade A and crossing the temporal inferior arterial arcade B. Black arrows: Parapapillary gamma zone.
The degree of fundus tessellation was graded in the macular region and in four peripapillary regions. It ranged from Grade “0” (no visibility of the large choroidal vessels outside the region of the parapapillary region) to Grade “3” (marked visibility).22 The contrast, brightness, background pigmentation, and photographic quality of the images were taken into account. The subjective evaluation of the photographs was repeatedly calibrated using standard photographs during the study. Myopic maculopathy was classified into four categories as recommended by the META-PM Study Group.4 We distinguished PDCA from macular diffuse choroidal atrophy (Figure 2). “Pathologic myopia” was defined by the presence of structural changes in the posterior segment of the eye (including posterior staphyloma, myopic maculopathy, and high myopia-associated optic neuropathy).16 Progression of MM was defined as an increase from Category 0/Category 1 to Category 2 or higher, and as progression of PDCA to macular diffuse choroidal atrophy. The development or enlargement of combined parapapillary gamma zone was also recorded. The assessment of the interobserver and intraobserver variability was assessed (see Text, Supplemental Digital Content 2, http://links.lww.com/IAE/B344, which shows the results of intraobserver and interobserver variability of measurements).
Fig. 2.
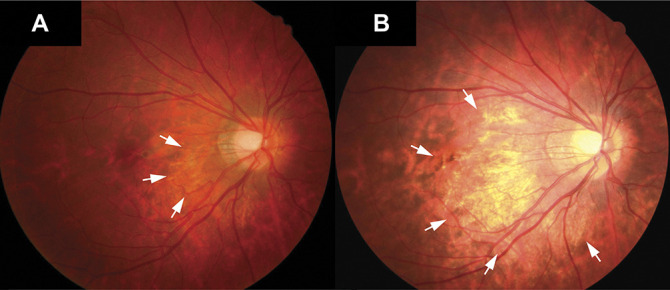
Progression of peripapillary diffuse atrophy. Fundus photograph showing the progression of a slight peripapillary diffuse atrophy to a diffuse choroidal atrophy during the follow-up. A. Image from the right eye obtained at the first visit with a refractive error of −6.50 D at an age of 10 years. Slight peripapillary diffuse atrophy was visible during the first examination (white arrows). B. Four years later, peripapillary diffuse atrophy had progressed toward the fovea (white arrows), with a refractive error of −9.38 D.
For the statistical analyses, we used a commercially available software package (SPSS for Windows, version 25.0, IBM-SPSS, Chicago, IL). Only the right eyes were included into the analysis due to the highly significant correlation of refractive errors between both eyes of the same individual (correlation coefficient, r = 0.85). We calculated the spherical equivalent of the refractive error as: spherical error + 0.5 × cylindrical error. A descriptive analysis was presented as mean values ± SDs. We performed univariate analyses using the Mann–Whitney U test to compare subgroups with or without incident PDCA and with or without progression of MM. We then performed a stepwise multivariable binary regression analysis with the incidence of PDCA or progression of MM as the dependent variable and all variables that were significantly associated with the outcome parameter in the univariate analysis as independent parameters. The chi-square test was performed to detect significant differences in the frequencies of parameters. Odds ratios (OR) and 95% confidence intervals (CI) were calculated, and the statistical significance threshold was set at a P-value of <0.05.
Results
The study population consisted of 274 patients (141 girls, 51.4%) with a mean age of 11.8 ± 2.5 years (range, 4–17 years) and a mean refractive error of −7.65 ± 1.86 D (range, −17.50 to −6.00 D) at baseline, and with a mean age of 16.7 ± 2.6 years (range, 10–23 years) and a mean refractive error of −9.78 ± 2.15 D (range: −18.38 to −6.25 D) at the last visit (see Figure, Supplemental Digital Content 3, http://links.lww.com/IAE/B345, which shows the flowchart of the study population). The mean follow-up was 4.9 ± 1.2 years (range, 4–8 years) (Table 1). As compared to the children included into the study, the children with a follow-up of less than 4 years were older (14.0 ± 2.51 vs. 11.9 ± 2.34 years, P < 0.000) and had a less myopic refractive error (−6.89 ± 1.39 vs. −7.11 ± 1.44 D, P = 0.04). They did not differ significantly in sex (P = 0.89).
Table 1.
Ocular Characteristics of the Study Population at Baseline and at the Last Study Visit
| Characteristics | First Visit | Last Visit | P |
| Best-corrected visual acuity (logMAR), mean (SD) | 0.05 (0.13) | 0.03 (0.10) | <0.001* |
| Refractive error, mean (SD), D | −7.66 (1.87) | −9.78 (2.17) | <0.001* |
| Horizontal optic disk diameter, mean (SD), mm | 1.10 (0.33) | 1.24 (0.31) | <0.001* |
| Vertical optic disk diameter, mean (SD), mm | 1.32 (0.37) | 1.59 (0.31) | <0.001* |
| Minimal optic disk diameter, mean (SD), mm | 1.08 (0.31) | 1.25 (0.69) | <0.001* |
| Maximal optic disk diameter, mean (SD), mm | 1.34 (0.38) | 1.63 (0.31) | <0.001* |
| Disk–fovea distance, mean (SD), mm | 3.79 (0.88) | 4.67 (0.60) | <0.001* |
| Optic disk ovality, mean (SD) | 1.26 (0.19) | 1.38 (0.30) | <0.001* |
| Distance between the optic center and the outer border of parapapillary gamma zone, mean (SD), mm | 0.97 (0.33) | 1.33 (0.36) | <0.001* |
| Distance between the optic center and the optic disk border on the disk–fovea line, mean (SD), mm | 0.57 (0.17) | 0.63 (0.17) | <0.001* |
| Parapapillary gamma zone maximal width, mean (SD), mm | 0.38 (0.21) | 0.62 (0.28) | <0.001* |
| Vertical distance between the temporal superior and temporal inferior arterial arcade, mean (SD), mm | 5.96 (1.39) | 7.10 (1.45) | <0.001* |
| Distance between the optic center and the superior temporal arcade, mean (SD), mm | 4.73 (0.99) | 5.81 (0.84) | <0.001* |
| Distance between the optic center and the inferior temporal arcade, mean (SD), mm | 4.92 (1.07) | 5.91 (0.79) | <0.001* |
| Angle between maximal disk diameter and the horizontal line, mean (SD), ° | 78.5 (29.9) | 81.8 (22.9) | 0.145* |
| Angle between horizontal line and superior arcade, mean (SD), ° | 41.9 (7.48) | 40.1 (7.13) | 0.009* |
| Angle between horizontal line and inferior arcade, mean (SD), ° | 35.1 (6.76) | 33.8 (6.24) | 0.016* |
| Presence of PDCA, number (%) | 39 (14.2) | 86 (31.4) | <0.001† |
| Proportion of macular fundus tessellation, n (%) | <0.001† | ||
| Grade 0 | 24 (8.8) | 2 (0.7) | |
| Grade 1 | 181 (66.1) | 116 (42.3) | |
| Grade 2 | 50 (18.2) | 108 (39.4) | |
| Grade 3 | 19 (6.9) | 48 (17.5) | |
| Degree of macular fundus tessellation, mean (SD) | 1.25 (0.71) | 1.75 (0.75) | <0.001* |
| Degree of fundus tessellation in superior parapapillary region, mean (SD) | 1.44 (0.73) | 1.84 (0.71) | <0.001* |
| Degree of fundus tessellation in inferior parapapillary region, mean (SD) | 1.70 (0.79) | 2.16 (0.70) | <0.001* |
| Degree of fundus tessellation in nasal parapapillary region, mean (SD) | 1.50 (0.72) | 1.88 (0.71) | <0.001* |
| Degree of fundus tessellation in temporal parapapillary region, mean (SD) | 1.44 (0.81) | 1.93 (0.79) | <0.001* |
| Category of myopic maculopathy, n (%) | <0.001† | ||
| Category 0 | 97 (35.4) | 58 (21.2) | |
| Category 1 | 139 (50.7) | 130 (47.4) | |
| Category 2 and 3 | 38 (13.9) | 86 (31.4) |
Mann–Whitney U test.
Chi-square test.
logMAR, logarithm of minimum angle of resolution.
Myopic Maculopathy Progression
The MM prevalence at baseline was 35.4% (97/274), 50.7% (139/274), and 13.9% (38/274) for Categories 0, 1, and 2, respectively (Table 2). We detected an MM progression in 52 of 274 eyes (18.9%; 95% CI: 14.3–23.7%). MM progressed from Category 0 to 2 in four eyes of 97 eyes (4.1%; 95% CI: 0.1–8.2%), from Category 1 to 2 in 44 of 139 eyes (31.9%; 95% CI: 24.0–39.8%), and from Category 2 to 3 with development of patchy atrophies in one of 38 eyes (2.6%; 95% CI: −2.6 to 7.8%) (Figure 3). In addition, three eyes (7.7%; 95% CI: −1.1 to 16.4%) showed a spatial progression of a peripapillary diffuse choroidal atrophy in direction to the foveal region. Individuals with diffuse choroidal atrophy at baseline had a younger age (P = 0.01) compared to individuals without the presence of diffuse choroidal atrophy. However, they did not differ significantly in the enlargement of the maximal width of gamma zone, change in the distance between the optic center and outer border of gamma zone, and the rate of MM progression (Table 3).
Table 2.
Distribution of the Presence of PDCA and of the Categories (C0 to C3) of Myopic Maculopathy at Baseline and at the Last Study Visit
| Age, Years | No. Study Participants | Follow-Up | Refractive Error, Mean (SD), D | Presence of PDCA | C0 | C1 | C2 | C3 |
| 4 | 3 | Baseline | −7.88 ± 1.81 | 1 | 0 | 2 | 1 | 0 |
| Last visit | −13.33 ± 1.94 | 2 | 0 | 1 | 2 | 0 | ||
| 5 | 3 | Baseline | −7.62 ± 0.63 | 0 | 1 | 2 | 0 | 0 |
| Last visit | −12.08 ± 2.57 | 2 | 0 | 1 | 2 | 0 | ||
| 6 | 5 | Baseline | −8.31 ± 0.66 | 2 | 0 | 3 | 2 | 0 |
| Last visit | −11.25 ± 2.33 | 3 | 0 | 2 | 3 | 0 | ||
| 7 | 8 | Baseline | −6.89 ± 0.69 | 3 | 1 | 4 | 3 | 0 |
| Last visit | −9.61 ± 2.26 | 4 | 1 | 3 | 4 | 0 | ||
| 8 | 10 | Baseline | −7.33 ± 2.08 | 4 | 4 | 2 | 4 | 0 |
| Last visit | −10.45 ± 2.19 | 6 | 2 | 2 | 6 | 0 | ||
| 9 | 12 | Baseline | −7.42 ± 2.42 | 3 | 5 | 4 | 3 | 0 |
| Last visit | −9.91 ± 1.88 | 3 | 2 | 7 | 3 | 0 | ||
| 10 | 28 | Baseline | −6.78 ± 1.13 | 3 | 11 | 14 | 3 | 0 |
| Last visit | −9.38 ± 1.84 | 10 | 4 | 14 | 10 | 0 | ||
| 11 | 36 | Baseline | −7.55 ± 1.44 | 5 | 15 | 16 | 5 | 0 |
| Last visit | −9.85 ± 1.85 | 10 | 11 | 15 | 10 | 0 | ||
| 12 | 60 | Baseline | −7.64 ± 2.34 | 6 | 24 | 30 | 6 | 0 |
| Last visit | −9.83 ± 2.56 | 18 | 10 | 32 | 18 | 0 | ||
| 13 | 49 | Baseline | −7.83 ± 1.88 | 7 | 18 | 24 | 7 | 0 |
| Last visit | −9.66 ± 2.09 | 13 | 13 | 23 | 13 | 0 | ||
| 14 | 26 | Baseline | −7.59 ± 1.29 | 1 | 8 | 17 | 1 | 0 |
| Last visit | −9.44 ± 1.76 | 7 | 6 | 13 | 6 | 1 | ||
| 15 | 23 | Baseline | −8.16 ± 1.83 | 1 | 8 | 14 | 1 | 0 |
| Last visit | −9.18 ± 1.91 | 4 | 8 | 11 | 4 | 0 | ||
| 16 | 9 | Baseline | −8.96 ± 2.05 | 2 | 2 | 5 | 2 | 0 |
| Last visit | −9.90 ± 2.58 | 3 | 1 | 5 | 3 | 0 | ||
| 17 | 2 | Baseline | −10.62 ± 0.18 | 0 | 0 | 2 | 0 | 0 |
| Last visit | −11.18 ± 1.33 | 1 | 0 | 1 | 1 | 0 |
Fig. 3.
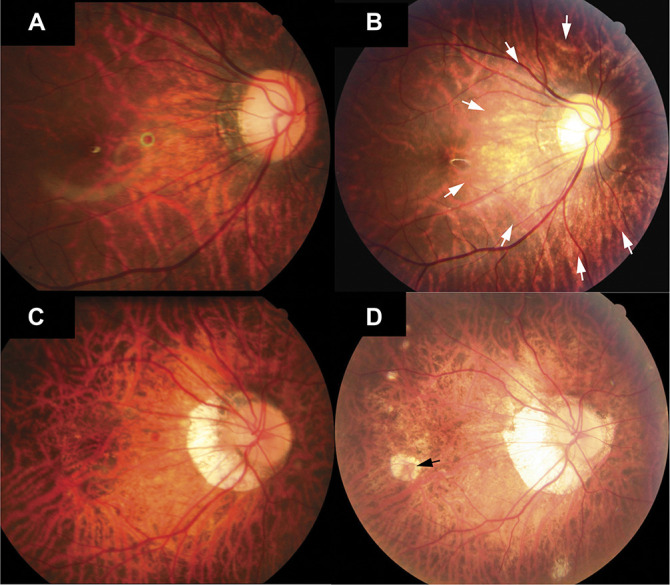
Progression of myopic maculopathy. Fundus photographs of two patients (images A and B and images C and D) showing the progression of myopic maculopathy during the follow-up. A. Image obtained at the first visit at an age of 9 years with a refractive error of −13.50 D and with the macula defined as Category 1. B. The fundus had progressed to Category 2 as peripapillary diffuse atrophy was visible (white arrows) 4 years later with a refractive error of −14.25 D. C. Image of the second patient at the first visit at an age of 14 years, with a refractive error of −11.00 D, and with the macula defined as Category 2. D. Eight years after the first visit, the macula had progressed to Category 3 with patchy atrophy (black arrow) and with a refractive error of −18.38 D.
Table 3.
Progression Patterns in Eyes With and Without Diffuse Choroidal Atrophy at Baseline
| Characteristic | Diffuse Choroidal Atrophy | P | |
| Yes (38 Eyes) | No (236 Eyes) | ||
| Age at baseline, mean (SD), years | 10.7 (2.86) | 11.9 (2.37) | 0.01 |
| Change in parapapillary gamma zone maximal width, mean (SD), mm | 0.26 (0.30) | 0.27 (0.18) | 0.79* |
| Change in distance between optic center and outer border of gamma zone, mean (SD), mm | 0.32 (0.46) | 0.36 (0.25) | 0.46* |
| Myopic maculopathy progression, number (%) | 4 (10.5) | 48 (20.3) | 0.11† |
| Time of follow-up, mean (SD), years | 5.1 (0.9) | 4.9 (1.1) | 0.30* |
Mann–Whitney U test.
Chi-square test.
Compared to patients with a stable myopic fundus, the patients with MM progression had a significantly larger progression of myopic refractive error (P < 0.001), a greater enlargement of the maximal width of gamma zone (P < 0.001), a larger increase in the disk–fovea distance (P < 0.001) and in the optic disk ovality (P < 0.001), a larger increase in the vertical optic disk diameter (P < 0.001) and in the maximal optic disk diameter (P < 0.001), a greater increase in the vertical distance between the temporal superior and temporal inferior arterial arcade (P = 0.008), a greater increase in the distance between the optic disk center and the temporal superior (P < 0.001) and temporal inferior arterial arcades (P < 0.001), and a greater progression of the macular and peripheral superior, temporal, and nasal fundus tessellation (P < 0.001). It was not significantly associated with any of the baseline parameters (Table 4).
Table 4.
Ocular Characteristics of the Whole Participants With and Without Myopic Maculopathy Progression Using Mann–Whitney U Test
| Characteristic | Progression | P | |
| Yes (52 Eyes) | No (222 Eyes) | ||
| Baseline | |||
| Age, mean (SD), years | 11.4 (2.8) | 11.8 (2.4) | 0.42 |
| Best-corrected visual acuity (logMAR), mean (SD) | 0.04 (0.09) | 0.06 (0.14) | 0.86 |
| Refractive error, mean (SD), D | −7.63 (1.90) | −7.65 (1.85) | 0.78 |
| Distance between optic center and outer border of gamma zone, mean (SD), mm | 0.97 (0.33) | 0.98 (0.33) | 0.87 |
| Parapapillary gamma zone maximal width, mean (SD), mm | 0.44 (0.23) | 0.44 (0.25) | 0.78 |
| Follow-up | |||
| Best-corrected visual acuity (logMAR), mean (SD) | 0.01 (0.04) | 0.03 (0.11) | 0.63 |
| Refractive error, mean (SD), D | −10.40 (2.39) | −9.63 (2.08) | 0.04 |
| Distance between optic center and outer border of gamma zone, mean (SD), mm | 1.45 (0.45) | 1.30 (0.33) | 0.02 |
| Parapapillary gamma zone maximal width, mean (SD), mm | 0.81 (0.40) | 0.68 (0.31) | 0.05 |
| Degree of macular fundus tessellation, mean (SD) | 2.25 (0.65) | 1.62 (0.72) | <0.001 |
| Degree of fundus tessellation in the superior parapapillary region, mean (SD) | 2.37 (0.56) | 1.72 (0.70) | <0.001 |
| Degree of fundus tessellation in the inferior parapapillary region, mean (SD) | 2.67 (0.47) | 2.04 (0.69) | <0.001 |
| Degree of fundus tessellation in the nasal parapapillary region, mean (SD) | 2.38 (0.53) | 1.76 (0.70) | <0.001 |
| Degree of fundus tessellation in the temporal parapapillary region, mean (SD) | 2.65 (0.48) | 1.76 (0.75) | <0.001 |
| Changes in variables | |||
| Change in refractive error, mean (SD), D | −2.77 (1.57) | −1.98 (1.22) | 0.001 |
| Change in parapapillary gamma zone maximal width, mean (SD), mm | 0.37 (0.26) | 0.25 (0.18) | 0.001 |
| Change in degree of macular fundus tessellation, mean (SD) | 0.79 (0.57) | 0.44 (0.52) | <0.001 |
| Change in degree of fundus tessellation in the superior parapapillary region, mean (SD) | 0.65 (0.52) | 0.35 (0.49) | <0.001 |
| Change in degree of fundus tessellation in the inferior parapapillary region, mean (SD) | 0.52 (0.58) | 0.45 (0.53) | 0.50 |
| Change in degree of fundus tessellation in the nasal parapapillary region, mean (SD) | 0.63 (0.49) | 0.32 (0.50) | <0.001 |
| Change in degree of fundus tessellation in the temporal parapapillary region, mean (SD) | 0.83 (0.62) | 0.41 (0.53) | <0.001 |
| Time of follow-up, mean (SD), years | 5.0 (1.1) | 4.9 (1.1) | 0.46 |
logMAR, logarithm of minimum angle of resolution.
In the multivariable analysis, we first dropped due to collinearity of the following parameters: change in the maximal optic disk diameter (variance inflation factor [VIF], 42.1), elongation of the disk–fovea distance (VIF, 28.2), increase in the distance between the optic disk center and the superior (VIF, 16.0) and inferior arterial arcades (VIF, 18.7), and change in the vertical optic disk diameter (VIF, 38.5). Due to a lack of statistical significance, we then dropped in a step-by-step manner the parameter of a change in optic disk ovality (P = 0.08). In the final model, MM progression was associated with a larger increase in myopic refractive error (i.e., a more negative refractive error) (OR, 0.72; 95% CI: 0.56–0.92; P < 0.001) and with a greater enlargement of the maximal width of gamma zone (OR, 7.68; 95% CI: 1.63–36.2; P = 0.002).
Incidence of Peripapillary Diffuse Choroidal Atrophy
At baseline, we detected a PDCA in 38 of 274 eyes (13.9%; 95% CI: 10.0–18.3%) (Table 2). The PDCA was located temporal to the optic nerve head in all of these eyes. We observed an incident PDCA in 47 of the remaining 236 eyes (20.0%; 95% CI: 14.8–25.2%). The incident PDCA was located temporal to the optic disk in 46 of 47 eyes (97.9%) and inferior to the optic nerve head in one eye.
In univariate analysis, patients with incident PDCA differed from individuals with no change in the status of PDCA in the change of refractive error, distance between the optic disk and the superior arterial arcade, maximal gamma zone width, optic disk–fovea distance, optic disk vertical diameter, and progression of the macular and peripheral fundus tessellation (P < 0.001). They did not differ significantly in any of the baseline parameters, except for the fundus tessellation parameters (Table 5).
Table 5.
Comparison Between Eyes With Incident Peripapillary Diffuse Choroidal Atrophy and Stable Eyes Using Mann–Whitney U Test
| Characteristic | Incident Group (47 Eyes) | Stable Group (189 Eyes) | P |
| Baseline | |||
| Age, mean (SD), years | 11.5 (2.8) | 12.0 (2.2) | 0.35 |
| Best-corrected visual acuity (logMAR), mean (SD) | 0.04 (0.09) | 0.04 (0.11) | 0.25 |
| Refractive error, mean (SD), D | −7.54 (1.81) | −7.37 (1.38) | 0.80 |
| Distance between optic center and outer border of gamma zone, mean (SD), mm | 0.93 (0.27) | 0.92 (0.26) | 0.66 |
| Parapapillary gamma zone maximal width, mean (SD), mm | 0.41 (0.19) | 0.39 (0.21) | 0.43 |
| Degree of macular fundus tessellation, mean (SD) | 1.38 (0.57) | 0.98 (0.49) | <0.001 |
| Degree of fundus tessellation in the superior parapapillary region, mean (SD) | 1.66 (0.64) | 1.16 (0.55) | <0.001 |
| Degree of fundus tessellation in the inferior parapapillary region, mean (SD) | 2.11 (0.67) | 1.39 (0.65) | <0.001 |
| Degree of fundus tessellation in the nasal parapapillary region, mean (SD) | 1.70 (0.59) | 1.24 (0.58) | <0.001 |
| Degree of fundus tessellation in the temporal parapapillary region, mean (SD) | 1.74 (0.60) | 1.11 (0.59) | <0.001 |
| Follow-up | |||
| Best-corrected visual acuity (logMAR), mean (SD) | 0.02 (0.04) | 0.01 (0.05) | 0.10 |
| Refractive error, mean (SD), D | −10.3 (2.32) | −9.40 (1.69) | 0.02 |
| Distance between optic center and outer border of gamma zone, mean (SD), mm | 1.39 (0.32) | 1.24 (0.29) | 0.005 |
| Parapapillary gamma zone maximal width, mean (SD), mm | 0.77 (0.29) | 0.64 (0.29) | 0.008 |
| Degree of macular fundus tessellation, mean (SD) | 2.19 (0.65) | 1.43 (0.58) | <0.001 |
| Degree of fundus tessellation in the superior parapapillary region, mean (SD) | 2.30 (0.55) | 1.55 (0.59) | <0.001 |
| Degree of fundus tessellation in the inferior parapapillary region, mean (SD) | 2.64 (0.48) | 1.89 (0.65) | <0.001 |
| Degree of fundus tessellation in the nasal parapapillary region, mean (SD) | 2.32 (0.52) | 1.60 (0.61) | <0.001 |
| Degree of fundus tessellation in the temporal parapapillary region, mean (SD) | 2.64 (0.48) | 1.56 (0.62) | <0.001 |
| Changes in variables | |||
| Change in refractive error, mean (SD), D | −2.82 (1.63) | −2.00 (1.18) | 0.002 |
| Change in degree of macular fundus tessellation, mean (SD) | 0.81 (0.57) | 0.45 (0.52) | <0.001 |
| Change in degree of fundus tessellation in the superior parapapillary region, mean (SD) | 0.64 (0.54) | 0.39 (0.50) | 0.003 |
| Change in degree of fundus tessellation in the inferior parapapillary region, mean (SD) | 0.53 (0.58) | 0.50 (0.54) | 0.85 |
| Change in degree of fundus tessellation in the nasal parapapillary region, mean (SD) | 0.62 (0.49) | 0.36 (0.52) | 0.001 |
| Change in degree of fundus tessellation in the temporal parapapillary region, mean (SD) | 0.89 (0.59) | 0.46 (0.54) | <0.001 |
| Change in parapapillary gamma zone maximal width, mean (SD), mm | 0.31 (0.19) | 0.22 (0.16) | 0.003 |
| Time of follow-up, mean (SD), years | 5.0 (1.1) | 4.9 (1.2) | 0.32 |
logMAR, logarithm of minimum angle of resolution.
In the multivariable binary regression analysis with incident PDCA as the dependent variable, we first dropped due to collinearity of the parameters of distance between the optic disk and the superior arterial arcade (VIF, 13.2) and the disk-fovea distance (VIF, 6.60). Due to a lack of statistical significance, we then dropped the parameters of change in the vertical optic disk diameter (P = 0.35). In the final model, incident PDCA was significantly associated with a higher change in myopic refractive error (i.e., a more negative refractive error) (OR, 0.70; 95% CI: 0.54–0.92; P = 0.009) and with a greater enlargement of the maximal width of gamma zone (OR, 8.28; 95% CI: 1.33–51.7; P = 0.02).
A higher prevalence of PDCA at baseline was correlated with the baseline parameters of younger age (OR, 0.74; 95% CI: 0.64–0.86; P < 0.001), more myopic refractive error (OR, 0.73; 95% CI: 0.59–0.89; P = 0.002), and larger width of gamma zone (OR, 73.1; 95% CI: 13.3–403; P < 0.001).
In linear multivariable analysis, the enlargement of the maximal width of the parapapillary gamma zone was associated with an increase in myopic refractive error (beta, 0.33; B, 0.05; 95% CI: 0.03–0.06; P < 0.001) and incidence of PDCA (beta, 0.15; B, 0.07; 95% CI: 0.01–0.12; P = 0.02). If the parameter of MM stage progression was added, the latter was significantly correlated (beta, 0.22; B, 0.10; 95% CI: 0.05–0.16; P = 0.001), whereas PDCA was not (P = 0.85).
Discussion
In our study population, the prevalence of MM was 35.4, 50.7, and 13.9% for Categories 0, 1, and 2, respectively. We detected a progression of MM in 52 eyes (18.9%). Risk factors were a more marked myopization and greater enlargement of gamma zone. The prevalence of PDCA was 13.9% at baseline, and its incidence was 20.0%, with the risk factors of a higher amount of myopization and greater enlargement of gamma zone.
The findings obtained in our study on the prevalence of various MM categories can hardly be compared with other studies, due to varying definitions of MM and differences in the study population. Kobayashi et al23 reviewed the medical records of 46 children with a mean age of 5.4 ± 2.1 years and a mean refractive error of −8.4 ± 3.8 D and reported that a mild chorioretinal atrophy around the optic disk was found in 13 eyes (16%). Koh et al examined 16 Chinese men (mean age, 21.8 ± 1.3 years; mean refractive error, −10.88 ± 1.25 D) and found the prevalence of fundus tessellation to be 86%, whereas none of the participants showed a Category 2 of MM.24 A study by Li et al,15 which included Chinese individuals (mean age, 21.6 ± 12.2 years; mean refractive error, −10.18 ± 3.38 D), found that MM progressed in 97 (14.8%) of 657 eyes during a 2-year follow-up period. Differences in the duration of follow-up and composition of study population may explain the differences in the rate of progression between studies. In previous studies, the patients with MM progression as compared to individuals with a stable macula were older, had a greater axial elongation, and greater change in myopic refractive error. Also, in our study, an increase in myopic refractive error was a risk factor for the progression of MM, whereas age was not significantly associated. Interestingly, studies on elderly highly myopic patients revealed that the risk of MM progression increased with older age.13,14,25–27 The relatively young age in our study population might have been the reason that age was not significantly associated with a higher risk of MM progression. Another reason could be that our study population was potentially composed of two subgroups, one with an early onset of myopia and a genetic basis of myopia in the family, and a second subgroup with an onset of myopia after the age of 10 to 13 years with a predominantly environmental etiology of myopia.8 The young age and the composition of the study population may also be the reasons why a potential difference between individuals with diffuse choroidal atrophy at baseline as compared to those without diffuse choroidal atrophy at baseline in the progression pattern did not reach a level of statistical significance.
In a previous study, the presence of PDCA, in association with a segmental thinning of the choroid, has been discussed to be a biomarker in children with high myopia for the eventual development of pathologic myopia in later life.10,28 In the present hospital-based study, the incidence of PDCA (47/236 eyes or 20.0%) was associated with a higher amount of myopization (OR, 0.70) and a greater enlargement of gamma zone (OR, 8.28). It was, however, not significantly associated with a higher risk of an MM progression, which was associated with the same risk factors. It may suggest that the incidence of PDCA and the progression of MM occurred concurrently, which is likely because the development of a diffuse choroidal atrophy is the hallmark of Category 2 of MM.4 Because the prevalence of Category 3 of MM was low in this study population and PDCA shares similar morphological features with category two of MM, this study may not be able to address the question whether PDCA signified an increased risk for progression of MM (to Category 3 or 4) or whether PDCA and MM were just sequels of the same development.
The enlargement of parapapillary gamma zone was a main risk factor for the progression of MM and for an increased incidence of PDCA. According to recent investigations, the enlargement of gamma zone in eyes with increasing myopia may occur in two steps. In the first step, the Bruch membrane opening shifts into the temporal direction toward the macula.29 It leads to and explains the overhanging of the Bruch membrane at the nasal optic disk border and the lack of Bruch membrane at the temporal disk border, i.e., the gamma zone. With an axial elongation beyond the length of 26.5 mm, the Bruch membrane opening enlarges into all directions, such that the gamma zone is present also at the nasal optic disk border and thus encircles the optic disk.29 The shift and subsequent enlargement of the Bruch membrane is strongly dependent on axial elongation and potentially other, yet unknown parameters.30 From a practical viewpoint, it may indicate that an increase in the gamma zone may be a biomarker for an increased risk for the progression of MM.
According to previous studies on adults, progression of MM correlated with a decrease in best-corrected visual acuity.11,13,14 It was in contrast to this study, in which best-corrected visual acuity did not differ significantly between eyes with stable versus progressing MM. A possible reason for this discrepancy might have been that the MM did not affect the foveal region in our study.
Our study has limitations. First, the study was performed in a third referral center in North China, so the results obtained may not be directly generalizable to the general population or to populations of non-Chinese ethnicity. Second, we did not measure the axial length, instead we sued the refractive error as measured under cycloplegia as a surrogate for axial length. Because the refractive error is dependent on other additional factors besides axial length, any change in the corneal or lens refractive power might have influenced a change in the refractive error. Third, diffuse choroidal atrophy was diagnosed on fundus photograph through its yellowish appearance. Changes in the background pigmentation during the study period might have influenced its detection. Fourth, parapapillary gamma zone was measured on fundus photographs. Availability of optical coherence tomography images of the optic disk and the peripapillary region would have facilitated the outlining of parapapillary gamma zone. Fifth, the differences between participants and nonparticipants may have produced a selection bias in the progression of MM and PDCA.
In conclusion, progression of MM during a mean follow-up of 4.9 years was detected in 18.9% of highly myopic schoolchildren aged 4 to 17 years. Enlarging gamma zone may be a biomarker for progression of MM and may be measured in schoolchildren with progressing myopia. Considering the relatively high progression rate, the findings suggest that myopia in schoolchildren may have a risk of converting into pathologic myopia in later life.
Footnotes
Supported by National Natural Science Foundation of China (grant nos. 81670884, nos. 81873684).
None of the authors has any financial/conflicting interests to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
Contributor Information
Yin Guo, Email: guoy.168@163.com.
Lijuan Liu, Email: lijuan_l@aliyun.com.
Ping Tang, Email: tp2200@vip.sina.com.
Yanyun Lv, Email: 1489043665@qq.com.
Min Wu, Email: wmi9501wmi@126.com.
Xu Liang, Email: hbliangxu1985@126.com.
Lin Zhang, Email: zhanglin_2015@126.com.
Jost. B. Jonas, Email: jost.jonas@medma.uni-heidelberg.de.
References
- 1.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123:1036–1042. [DOI] [PubMed] [Google Scholar]
- 2.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet 2012;379:1739–1748. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Wang Y, Wang S, et al. High myopia and glaucoma susceptibility. The Beijing Eye Study. Ophthalmology 2007;114:216–220. [DOI] [PubMed] [Google Scholar]
- 4.Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International classification and grading system for myopic maculopathy. Am J Ophthalmol 2015;159:877–883.e7. [DOI] [PubMed] [Google Scholar]
- 5.Jonas JB, Xu L, Wang YX, et al. Education-related parameters in high myopia: adults versus school children. PLoS One 2016;11:e0154554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res 2005;24:1–38. [DOI] [PubMed] [Google Scholar]
- 7.Lin H, Long E, Ding X, et al. Prediction of myopia development among Chinese school-aged children using refraction data from electronic medical records: a retrospective, multicentre machine learning study. PLoS Med 2018;15:e1002674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan IG, French AN, Ashby RS, et al. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res 2018;62:134–149. [DOI] [PubMed] [Google Scholar]
- 9.Wang SK, Guo Y, Liao C, et al. Incidence of and factors associated with myopia and high myopia in Chinese children, based on refraction without cycloplegia. JAMA Ophthalmol 2018;136:1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoi T, Jonas JB, Shimada N, et al. Peripapillary diffuse atrophy in children as sign of eventual pathologic myopia in adults. Ophthalmology 2016;123:1783–1787. [DOI] [PubMed] [Google Scholar]
- 11.Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology 2002;109:704–711. [DOI] [PubMed] [Google Scholar]
- 12.Liu HH, Xu L, Wang YX, et al. Prevalence and progression of myopic retinopathy in Chinese adults: the Beijing Eye Study. Ophthalmology 2010;117:1763–1768. [DOI] [PubMed] [Google Scholar]
- 13.Fang Y, Yokoi T, Nagaoka N, et al. Progression of myopic maculopathy during 18-year follow-up. Ophthalmology 2018;125:863–877. [DOI] [PubMed] [Google Scholar]
- 14.Yan YN, Wang YX, Yang Y, et al. Ten-year progression of myopic maculopathy: the Beijing Eye Study 2001–2011. Ophthalmology 2018;125:1253–1263. [DOI] [PubMed] [Google Scholar]
- 15.Li ZX, Liu R, Xiao O, et al. Progression of myopic maculopathy in highly myopic Chinese eyes. Invest Ophthalmol Vis Sci 2019;60:1096–1104. [DOI] [PubMed] [Google Scholar]
- 16.Flitcroft DI, He M, Jonas JB, et al. IMI—defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci 2019;60:M20–M30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas JB, Weber P, Nagaoka N, Ohno-Matsui K. Temporal vascular arcade width and angle in high axial myopia. Retina 2018;38:1839–1847. [DOI] [PubMed] [Google Scholar]
- 18.Guo Y, Liu LJ, Tang P, et al. Optic disc-fovea distance and myopia progression in school children: the Beijing Children Eye Study. Acta Ophthalmol 2018;96:e606–e613. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y, Liu LJ, Tang P, et al. Parapapillary gamma zone and progression of myopia in school children: the Beijing Children Eye Study. Invest Ophthalmol Vis Sci 2018;59:1609–1616. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Liu LJ, Xu L, et al. Parapapillary beta zone in primary school children in Beijing: associations with outdoor activity. Invest Ophthalmol Vis Sci 2014;55:918–925. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson B. The variation and covariation of cup and disc diameters. Acta Ophthalmol 1976;54:804–818. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Liu L, Zheng D, et al. Prevalence and associations of fundus tessellation among junior students from Greater Beijing. Invest Ophthalmol Vis Sci 2019;60:4033–4040. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Ohno-Matsui K, Kojima A, et al. Fundus characteristics of high myopia in children. Jpn J Ophthalmol 2005;49:306–311. [DOI] [PubMed] [Google Scholar]
- 24.Koh VT, Nah GK, Chang L, et al. Pathologic changes in highly myopic eyes of young males in Singapore. Ann Acad Med Singapore 2013;42:216–224. [PubMed] [Google Scholar]
- 25.Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 2010;117:1595–1611. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Wen F, Li H, et al. The types and severity of high myopic maculopathy in Chinese patients. Ophthalmic Physiol Opt 2012;32:60–67. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Pan CW, Ohno-Matsui K, et al. Myopia-related fundus changes in Singapore adults with high myopia. Am J Ophthalmol 2013;155:991–999. [DOI] [PubMed] [Google Scholar]
- 28.Yokoi T, Zhu D, Bi H, et al. Parapapillary diffuse choroidal atrophy in children is associated with extreme thinning of parapapillary choroid. Invest Ophthalmol Vis Sci 2017;58:901–906. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Xu L, Wei WB, et al. Size and shape of Bruch's membrane opening in relationship to axial length, gamma zone and macular Bruch's membrane defects. Invest Ophthalmol Vis Sci 2019;60:2591–2598. [DOI] [PubMed] [Google Scholar]
- 30.Jonas JB, Ohno-Matsui K, Jiang WJ, Panda-Jonas S. Bruch membrane and the mechanism of myopization: a new theory. Retina 2017;37:1428–1440. [DOI] [PubMed] [Google Scholar]


