Abstract
Peripheral artery disease (PAD) is associated with impaired lower extremity function. We hypothesized that contrast-enhanced magnetic resonance imaging (CE-MRI) based arterial signal enhancement (SE) measures are associated with markers of PAD. A total of 66 participants were enrolled, 10 were excluded due to incomplete data, resulting in 56 participants for the final analyses (36 PAD, 20 matched controls). MR imaging was performed post reactive hyperemia using bilateral thigh blood-pressure cuffs. First pass-perfusion images were acquired at the mid-calf region with a high-resolution saturation recovery gradient echo pulse sequence, and arterial SE was measured for the lower extremity arteries. As expected, peak walking time (PWT) was reduced in PAD patients compared to controls (282 (248–317) sec, vs. 353 (346–360) sec; p=0.002), and post-exercise ankle brachial index (ABI) decreased in PAD patients but not in controls (PAD: 0.75 ± 0.2, 0.60 (0.5–0.7); p<0.001; vs. Controls: 1.17 ± 0.1, 1.19 (1.1–1.2); p=0.50). Intraclass correlation coefficients were excellent for inter- and intra-observer variability of arterial tracings (n=10: 0.95 (95%-confidence interval [CI]: 0.94–0.96), n=9: 1.0 (CI: 1.0–1.0). Minimum arterial SE was reduced in PAD patients compared with matched controls (128 (110–147) A.U. vs. 192 (149–234) A.U., p=0.003). Among PAD patients but not in controls the maximum arterial SE was associated with the estimated glomerular filtration rate (eGFR), a marker of renal function (n=36, ß=1.37, R2=0.12, p=0.025). In conclusion, CE-MRI first-pass arterial perfusion is impaired in PAD patients compared to matched controls and associated with markers of lower extremity ischemia.
Keywords: peripheral artery disease, magnetic resonance imaging, arterial signal enhancement, cross-sectional leg muscle area, peak walking time
Peripheral artery disease (PAD) is associated with impaired blood flow in the lower extremities.1,2 It is estimated that more 200 million people have PAD worldwide3 including about 12% of the adult U.S. population and PAD is significantly associated with adverse cardiovascular outcomes.4–6 Previous studies demonstrated that contrast-enhanced MRI (CE-MRI) is suitable to visualize functional or pathologic changes in the lower extremity arteries.7,8 CE-MRI can visualize arterial anatomy with high sensitivity and specificity8,9 and quantitatively assess first-pass perfusion of the major lower extremity arteries.10,11,12 Previously, researchers have demonstrated that peak-exercise measurement of lower limb perfusion with first-pass MRI distinguishes PAD from controls independent of exercise workload13 and that tissue perfusion correlates with walking distance.14,15,16 For this study, we have hypothesized that CE-MRI-based arterial signal enhancement (SE) is associated with markers of PAD. We used a validated peak detection algorithm to identify physiological and fixed time points in the arterial input function taken from the more symptomatic leg during first-pass CE-MRI for the posterior tibialis (PT), anterior tibialis (AT) and peroneal (PE) arteries.17 We have determined associations between CE-MRI arterial signal enhancement measures and markers of PAD in both PAD patients and matched controls.
Methods
Men and women with a resting ankle brachial index (ABI) < 0.9 and life-style limiting intermittent claudication (IC) were recruited18 between July 2013 and July 2016 from the Houston Methodist Hospital and the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC), Houston, TX. All study participants received standard of care during this observational imaging study. Patients with contraindications to MRI or with an estimated glomerular filtration rate (eGFR) ≤ 40 mL/min/1.73.m2 were excluded from this study. Matched controls without PAD were also recruited at the same sites. In all 66 participants were enrolled. The target leg was defined as the one with the lower ABI or as the more symptomatic leg. This study obtained approval from the local institutional review board (IRB) and all participants provided informed consent.
CE-MR imaging was performed with a 3.0T system (Siemens Magnetom Trio or Verio, Erlangen, Germany) with a 36-element bilateral lower extremity coil. Participants were positioned on the MRI table feet first in the supine position and imaging was performed at the mid-calf level. Initial localizers were performed with a field of view (FOV) of 19.9 × 39.9 cm. A previously described reactive hyperemia protocol was implemented. Briefly, bilateral MRI compatible blood-pressure cuffs were placed above the knee and inflated to supra systolic levels (170 mmHg) for 3.5 minutes.17 Subsequently imaging was commenced and a gadolinium-based contrast agent (GBCA) was administered and blood pressure cuffs were deflated rapidly, as described previously.17 Briefly, CE-MRI was performed with high resolution saturation recovery gradient echo (GRE) pulse sequence (repetition time [TR] = 2.7 ms; echo time [TE] = 1.23 ms; slice thickness [ST] = 10 mm; bandwidth = 1021 Hz/px; flip angle [FA] = 30°; FOV= 17.5 × 35.0 cm; matrix = 144 × 288, number of averages = 1, temporal resolution = 409 ms). A GBCA was administered intravenously (gadopentetate dimeglumine [Magnevist, Bayer Inc.] at 0.2 mmol/kg, or gadobutrol [Gadavist, Bayer Inc.] at 0.1 mmol/kg) with flow rates of 2–4 ml/s which was followed by a 20 ml saline flush.
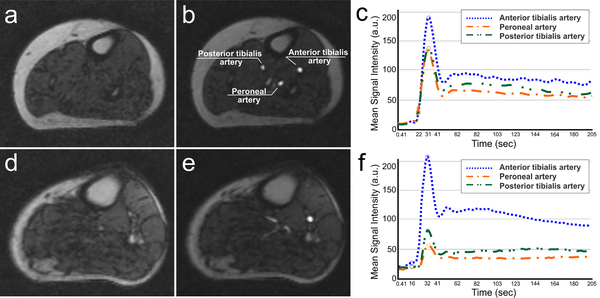
MRI scans were saved in the DICOM format and subsequently transferred to a workstation for further processing. The arterial lumen of the AT, PT and PE arteries were segmented, as available, with Sante DICOM Editor Version 3.0 (Santesoft LTD, Greece). The lumen was segmented in a single frame and the contours were propagated to all remaining frames. Due to the imaging protocol with a bilateral lower extremity coil, leg motion was limited effectively. Care was taken to account for frames with significant leg motion. In order to determine reproducibility and quality of the lumen segmentations, detailed inter- and intra-observer reproducibility analyses were conducted. The frame numbers (time points) were extracted from the arterial signal enhancement curves (Figure 1). The local pre-contrast arrival frame number (timing) was confirmed visually as the frame before any arterial signal enhancement was apparent. For each of the 3 main arteries (AT, PT and PE), the peak arterial signal in arbitrary units (A. U.) was extracted as the maximum value across all segmented frames. The minimum post peak enhancement was extracted as the minimum value between the peak and the recirculation peak of the gadolinium bolus. The level of arterial signal enhancement was measured as the difference between the local pre-contrast arrival signal intensity [SI] and the peak arterial SI. SE was a difference measure and not an absolute quantity. The minimum SE and the maximum SE variables were defined as either the minimum or maximum SE values across the 3 arteries (AT, PT, and PE). Frame numbers were converted to time by multiplying with the temporal resolution (409 ms) of the GRE pulse sequence. Arterial SE slope, or wash-in slope, was calculated as the slope of the line connecting the pre-arrival arterial SI with the peak (maximum) arterial SI.19 Cross-sectional leg muscle area (CSLMA) was measured, as describe previously.17
Figure 1 –
(a, d): Contrast-enhanced magnetic resonance images of a control and PAD patient at pre-contrast arrival; (b, e): Contrast-enhanced magnetic resonance images of a control and PAD patient peak arterial signal enhancement; (c, f): arterial signal enhancement from a control and a PAD patient (panels: curves represent the time points prior to contrast arrival, peak arterial signal enhancement, the minimum prior to the re-circulation peak).
All variables were expressed as mean (standard deviation), median (interquartile range [IQR]) for non-normal variables, percentages, or frequencies. Variable normality was determined with the Shapiro-Wilk test. Group differences for categorical variables were analyzed with the Chi-square or Fisher exact test. Continuous variables were analyzed with an independent samples Student’s T-test and the Mann-Whitney-Wilcoxon test was used for non-normal variables. Associations between MRI signal enhancement measures and markers of PAD were determined with linear regression analyses. Correlation analyses were performed and the strength of the correlation was described as weak (r < 0.3), medium (0.3 ≤ r < 0.5), or strong (r ≥ 0.5).20 Inter-observer and intra-observer variability was measured with the intraclass correlation coefficient (ICC) using a 2-way random-effects model.21 All tests were 2-sided and a p-value < 0.05 was considered statistically significant. The statistical analyses were performed with Stata Statistical Software: Release 13 (College Station, Texas, StataCorp LP).
Results
A total of 66 participants were enrolled and 5 PAD patients among them did not return for the baseline MRI visit, 4 more were excluded due to incomplete MRI exams, protocol deviations, or poor image quality, and 1 more was excluded due to not performing the exercise ABI, resulting in 56 participants included in the final analysis (36 PAD, 20 controls). PAD patients and controls without PAD were matched appropriately and there were no differences in age, gender, race, or body mass index (BMI, Table 1). Compared with controls, PAD patients were more likely diabetic (47% vs. 15%, p=0.021), with a history of smoking (89% vs. 40%, p<0.001), hypertensive (92% vs. 60%, p=0.011), hyperlipidemic (94% vs. 60%, p=0.002), be on lipid lowering therapy (89% vs. 45%, p=0.001), and have a history of lower extremity revascularization (61% vs. 0%, p=0.001).
Table 1.
Baseline patient characteristics.
| Variable | PAD patients (N= 36) |
Controls (N= 20) |
P-value |
|---|---|---|---|
| Age (years) | 69 ± 9.0 | 65 ± 6.7 | 0.10 |
| Men | 27 (75%) | 12 (60%) | 0.36 |
| Black | 12 (33%) | 4 (20%) | 0.36 |
| Body mass index (kg/m2) | 27 ± 4.9 | 29 ± 5.3 | 0.19 |
| Resting ABI (A.U.) | 0.75 ± 0.2 | 1.17 ± 0.1 | <0.001 |
| Post-treadmill ABI (A.U.) | 0.60 (0.5–0.7) | 1.19 (1.1–1.2) | <0.001 |
| Smoker | 32 (89%) | 8 (40%) | <0.001 |
| Diabetes mellitus | 17 (47%) | 3 (15%) | 0.021 |
| Hypertension (history) | 33 (92%) | 12 (60%) | 0.011 |
| Hyperlipidemia | 34 (94%) | 12 (60%) | 0.002 |
| Heart rate (bpm) | 78 ± 19 | 71 ± 10 | 0.13 |
| Hematocrit (%) | 41 (39–42) | 412 (40–44) | 0.46 |
| eGFR (ml/min/1.73m2) | 78 ± 22 | 78 ± 18 | 0.95 |
| Anticoagulation | 9 (25%) | 3 (15%) | 0.51 |
| ACE inhibitor | 17 (47%) | 5 (25%) | 0.15 |
| Beta blocker | 18 (50%) | 7 (35%) | 0.40 |
| Claudication onset time (sec) | 222 (180–264) | 353 (346–360) | <0.001 |
| Peak walking time (sec) | 282 (248–317) | 353 (346–360) | 0.002 |
| Complete 6 min treadmill | 19 (53%) | 19 (95%) | 0.002 |
| Cholesterol-lowering drug use | 32 (89%) | 9 (45%) | 0.001 |
| Coronary artery disease | 15 (42%) | 5 (25%) | 0.26 |
| Low extremities revascularization | 22 (61%) | 0 (0%) | 0.001 |
| Family history of coronary heart disease | 14 (39%) | 8 (40%) | 0.07 |
Values are reported as mean (standard deviation), medians and interquartile range (IQR), as number (percentage). PAD: peripheral artery disease, BMI: body mass index, eGFR: estimated glomerular filtration rate, ABI: ankle brachial index. Hematocrit controls: n=15; Post-treadmill ABI PAD patients: n=35; Claudication onset time PAD patients: n=35; Peak walking time PAD patients: n=35.
Resting ABIs were significantly lower (0.75 ± 0.2 vs. 1.17 ± 0.1, p<0.001) and peak walking time (PWT) was shorter (282 (248–317) sec. vs. 353 (346–360) sec., p=0.002) in PAD patients compared with matched controls (Table 1). The changes between rest and exercise ABI showed that post-treadmill walking ABIs were significantly decreased in PAD patients (0.76 ± 0.2, 0.60 (0.5–0.7); p<0.001) but remained unchanged in controls (1.17 ± 0.1, 1.19 (1.1–1.2); p=0.50), as expected.
In a sub-group analysis, PAD patients were divided into 2 groups, 1) those who completed the 6-minute treadmill walking test (treadmill completers, n=19) and 2) those who did not (treadmill non-completers, n=17). Non-completers compared with treadmill completers had significantly lower resting ABIs (0.65 ± 0.2 vs. 0.84 ± 0.2, p=0.011), shorter PWT (190 (148–232) sec. vs. 360 (360–360) sec., p<0.001), and claudication onset time (COT, 122 (80–164) sec. vs. 232 (163–301) sec., p=0.005).
Inter-observer variability was assessed for 2 readers using 10 scans and intra-observer reproducibility was assessed for 9 scans. Intra-observer and inter-observer variability of the arterial tracings, as measured with ICC coefficients, was excellent for both. Similarly, inter- and intra-observer reproducibility was assessed for CSLMA for 2 readers, using 20 scans each. ICCs of CSLMA were excellent for both inter- and intra-observer variability (Table 2).
Table 2.
Inter-reader and intra-reader reproducibility.
| Variability of arterial |
CSLMA tracings |
|||||
|---|---|---|---|---|---|---|
| N (patients) | ICC | Confidence Interval (95%) | N (patients) | ICC | Confidence Interval (95%) | |
| Inter-reader correlation | 10 | 0.95 | 0.94 – 0.96 | 20 | 0.91 | 0.62 – 0.97 |
| Intra-reader correlation | 9 | 1.0 | 1.0 – 1.0 | 20 | 0.99 | 0.97 – 1.0 |
ICC and confidence interval were calculated using a two-way model. CSLMA: Cross-sectional leg muscle area. ICC: intra-class correlation.
Minimum SE and maximum SE were significantly reduced in PAD patients compared with matched controls (128 (110–147) A.U. vs. 192 (149–234) A.U., p=0.003; and 265 ± 81 A.U. vs. 314 ± 89 A.U., p=0.040). When considering individual arteries, the SE of the PE and PT arteries were significantly reduced in PAD patients compared with controls (168 ± 154 A.U. vs. 218 ± 87 A.U., p=0.022; 163 ± 73 A.U. vs. 234 ± 110 A.U., p=0.007) but not for the AT artery (235 ± 201 A.U. vs. 289 ± 93 A.U., p=0.05; Table 3).
Table 3.
Magnetic resonance imaging measurements.
| Variable | PAD Patients (N=36) | Controls (N=20) | P-value |
|---|---|---|---|
| AT arterial SE (A.U.) | 235 ± 201 | 289 ± 93 | 0.05 |
| PE arterial SE (A.U.) | 168 ± 154 | 218 ± 87 | 0.022 |
| PT arterial SE (A.U.) | 163 ± 73 | 234 ± 110 | 0.007 |
| Minimum arterial SE (A.U.) | 128 (110–147) | 192 (149–234) | 0.003 |
| Maximum arterial SE (A.U.) | 265 ± 82 | 314 ± 89 | 0.040 |
| AT: SI at pre-contrast arrival | 23 ± 7.7 | 22 ± 4.2 | 0.37 |
| PE: SI at pre-contrast arrival | 21 (19–23) | 19 (17–21) | 0.09 |
| PT: SI at pre-contrast arrival | 22 (20–25) | 20 (18–23) | 0.29 |
| AT: SE slope | 15 (12–18) | 17 (11–22) | 0.46 |
| PE: SE slope | 9.9 (7.5–12) | 12 (8.2–15) | 0.41 |
| PT: SE slope | 6.2 (4.9–7.5) | 8.0 (5.6–10) | 0.14 |
| Cross sectional leg area (cm2) | 92 ± 24 | 103 ± 21 | 0.09 |
| Cross sectional leg muscle area (cm2) | 53 ± 15 | 62 ± 16 | 0.05 |
| Cross sectional AM area (cm2) | 9.2 (8.2–10) | 11 (9.9–13) | 0.013 |
| Cross sectional LM area (cm2) | 4.6 (4.1–5.1) | 4.7 (3.8–5.6) | 0.82 |
| Cross sectional DM area (cm2) | 3.3 (2.7–3.8) | 3.2 (2.5–3.9) | 0.91 |
| Cross sectional SM area (cm2) | 21 (19–23) | 24 (21–28) | 0.12 |
| Cross sectional GM area (cm2) | 15 (13–17) | 18 (16–21) | 0.036 |
| % muscle of cross-sectional leg area (%) | 58 ± 8.6 | 59 ± 6.6 | 0.45 |
Values are reported as mean (standard deviation), medians and interquartile range (IQR). Leg measurements were done on the more symptomatic side. PAD: peripheral artery disease, SE: signal enhancement, A.U.: arbitrary units. PE: peroneal artery, PT: posterior tibialis artery, AT: anterior tibialis artery. Minimum and maximum arterial SE refer to the artery with the lowest and highest SE among PE, PT, AT, respectively. Leg muscle groups: AM: anterior muscle, LM: lateral muscle, DM: deep posterior muscle, SM: soleus muscle, GM: gastrocnemius muscle.
AT arterial SE controls: n=19; PT arterial SE controls: n=18; SI at pre-contrast arrival of AT controls: n=19; SI at pre-contrast arrival of PT controls: n=18; SE slope of AT controls: n=19; SE slope of PT controls: n=18; PE artery SE PAD patients: n=35; SI at pre-contrast arrival of PE PAD patients: n=35; SE slope of PE PAD patients: n=35; SE slope of PT PAD patients: n=35.
A categorical analysis by artery showed no significant differences for a preferential artery with minimum and maximum SE between PAD patients and controls (Supplementary Table 1a). However, among PAD patients and among matched controls the AT had the highest frequency of having the maximum SE across the PT, PE, and AT. Conversely, for minimum SE there was no difference in the frequency among the PT, PE, and AT. (Supplementary Table 1b).
In PAD patients compared to matched controls there were significant differences of the cross-sectional area of the anterior muscle and the gastrocnemius muscle, but not for the lateral muscle (LM), soleus muscle (SM), deep posterior muscle (DM), and CSLMA (Table 3). There were no significant differences in CSLMA between PAD treadmill completers and non-completers (data not shown).
The maximum arterial SE and the AT arterial SE were significantly associated with the eGFR in a pooled analysis (Table 4). The CSLMA was significantly associated with the BMI in the pooled analysis (Table 5).
Table 4.
Pooled linear regression analyses for signal enhancement parameters with clinical measures.
| Independent Variable | N | β | Standard Error | R2 | Adjusted r2 | P-value | |
|---|---|---|---|---|---|---|---|
| Minimum arterial SE (A.U.) | Resting ABI | 56 | 109 | 34 | 0.16 | 0.14 | 0.002 |
| Δ of ABI | 55 | 79 | 53 | 0.04 | 0.02 | 0.15 | |
| Claudication onset time (sec) | 24 | −0.21 | 0.12 | 0.12 | 0.08 | 0.09 | |
| Peak walking time (sec) | 55 | 0.12 | 0.12 | 0.02 | <0.01 | 0.32 | |
| Body mass index (kg/m2) | 56 | −1.93 | 2.01 | 0.02 | <0.01 | 0.34 | |
| eGFR (ml/min/1.73m2) | 56 | −0.01 | 0.50 | <0.01 | −0.02 | 0.99 | |
| Cross-sectional leg muscle area (cm2) | 56 | 1.12 | 0.62 | 0.06 | 0.04 | 0.08 | |
| Maximum arterial SE (A.U.) | Resting ABI | 56 | 91 | 41 | 0.08 | 0.01 | 0.032 |
| Δ of ABI | 55 | 92 | 61 | 0.04 | 0.02 | 0.13 | |
| Claudication onset time (sec) | 24 | −0.15 | 0.20 | 0.02 | −0.02 | 0.48 | |
| Peak walking time (sec) | 55 | 0.04 | 0.14 | <0.01 | −0.02 | 0.79 | |
| Body mass index (kg/m2) | 56 | −0.84 | 2.33 | <0.00 | −0.02 | 0.72 | |
| eGFR (ml/min/1.73m2) | 56 | 1.48 | 0.54 | 0.12 | 0.11 | 0.008 | |
| Cross-sectional leg muscle area (cm2) | 56 | 1.86 | 0.69 | 0.12 | 0.10 | 0.010 | |
| AT arterial SE (A.U.) | Resting ABI | 55 | 100 | 48 | 0.08 | 0.06 | 0.041 |
| Δ of ABI | 54 | 45 | 73 | 0.01 | −0.01 | 0.54 | |
| Claudication onset time (sec) | 24 | −0.13 | 0.24 | 0.01 | −0.03 | 0.60 | |
| Peak walking time (sec) | 54 | −0.06 | 0.16 | <0.01 | −0.02 | 0.70 | |
| Body mass index (kg/m2) | 55 | 0.10 | 2.69 | <0.01 | −0.02 | 0.97 | |
| eGFR (ml/min/1.73m2) | 55 | 1.74 | 0.62 | 0.13 | 0.11 | 0.007 | |
| Cross-sectional leg muscle area (cm2) | 55 | 2.23 | 0.81 | 0.12 | 0.11 | 0.008 | |
| PE arterial SE (A.U.) | Resting ABI | 55 | 126 | 35 | 0.20 | 0.18 | 0.001 |
| Δ of ABI | 55 | 41 | 56 | 0.01 | −0.01 | 0.47 | |
| Claudication onset time (sec) | 24 | −0.23 | 0.12 | 0.14 | 0.10 | 0.07 | |
| Peak walking time (sec) | 55 | 0.15 | 0.12 | 0.03 | 0.01 | 0.21 | |
| Body mass index (kg/m2) | 55 | −2.52 | 2.07 | 0.03 | 0.01 | 0.23 | |
| eGFR (ml/min/1.73m2) | 55 | −0.09 | 0.52 | <0.01 | −0.02 | 0.87 | |
| Cross-sectional leg muscle area (cm2) | 55 | 0.68 | 0.66 | 0.02 | <0.01 | 0.31 | |
| PT arterial SE (A.U.) | Resting ABI | 54 | 89 | 44 | 0.07 | 0.05 | 0.05 |
| Δ of ABI | 53 | 126 | 66 | 0.07 | 0.05 | 0.06 | |
| Claudication onset time (sec) | 24 | −0.18 | 0.18 | 0.05 | <0.01 | 0.31 | |
| Peak walking time (sec) | 53 | 0.21 | 0.14 | 0.04 | 0.02 | 0.16 | |
| Body mass index (kg/m2) | 54 | −1.84 | 2.51 | 0.01 | −0.01 | 0.47 | |
| eGFR (ml/min/1.73m2) | 54 | 0.12 | 0.64 | <0.01 | −0.02 | 0.85 | |
| Cross-sectional leg muscle area (cm2) | 54 | 1.57 | 0.81 | 0.07 | 0.05 | 0.06 | |
ABI: ankle brachial index, Δ of ABI: difference between resting ABI and post-treadmill ABI, eGFR: estimated glomerular filtration rate, SE: signal enhancement, A.U.: arbitrary units. AT: anterior tibialis artery, PE: peroneal artery, PT: posterior tibialis artery.
Table 5.
Linear regression analyses for magnetic resonance imaging muscle area parameters with clinical measures.
| Independent Variable | N | β | Standard Error | R2 | Adjusted r2 | P-value | |
|---|---|---|---|---|---|---|---|
|
Pooled analyses | |||||||
| Cross-sectional leg muscle area (cm2) | Resting ABI Δ of ABI Claudication onset time (sec) Peak walking time (sec) |
56 | 11 | 7.80 | 0.04 | 0.02 | 0.17 |
| 55 | 17 | 11 | 0.04 | 0.02 | 0.13 | ||
| 24 | 0.04 | 0.04 | 0.05 | 0.01 | 0.28 | ||
| 55 | 0.03 | 0.03 | 0.02 | <0.01 | 0.28 | ||
| Body mass index (kg/m2) | 56 | 1.69 | 0.37 | 0.28 | 0.27 | <0.001 | |
| eGFR (ml/min/1.73m2) | 56 | 0.15 | 0.10 | 0.04 | 0.02 | 0.15 | |
|
Control group | |||||||
| Cross-sectional leg muscle area (cm2) | Resting ABI Δ of ABI Peak walking time (sec) |
20 | −44 | 39.5 | 0.06 | 0.01 | 0.28 |
| 20 | 58 | 26.5 | 0.21 | 0.16 | 0.043 | ||
| 20 | 0.29 | 0.18 | 0.13 | 0.09 | 0.11 | ||
| Body mass index (kg/m2) | 20 | 0.63 | 0.71 | 0.04 | −0.01 | 0.38 | |
| eGFR (ml/min/1.73m2) | 20 | 0.27 | 0.21 | 0.09 | 0.04 | 0.21 | |
|
PAD group | |||||||
| Cross-sectional leg muscle area (cm2) | Resting ABI Δ of ABI Claudication onset time (sec) Peak walking time (sec) |
36 | 3.13 | 12 | <0.01 | −0.03 | 0.79 |
| 35 | −2.67 | 14 | <0.01 | −0.03 | 0.85 | ||
| 24 | 0.04 | 0.04 | 0.05 | 0.01 | 0.28 | ||
| 35 | <0.00 | 0.03 | <0.01 | −0.03 | 0.90 | ||
| Body mass index (kg/m2) | 36 | 2.20 | 0.39 | 0.49 | 0.47 | <0.001 | |
| eGFR (ml/min/1.73m2) | 36 | 0.12 | 0.12 | 0.03 | <0.01 | 0.32 | |
|
Treadmill completers PAD | |||||||
| Cross-sectional leg muscle area (cm2) | Resting ABI Δ of ABI Claudication onset time (sec) |
18 | 1.72 | 1.23 | 0.11 | 0.05 | 0.18 |
| 18 | 1.72 | 1.23 | 0.11 | 0.05 | 0.18 | ||
| 8 | 0.06 | 0.08 | 0.09 | −0.06 | 0.47 | ||
| Body mass index (kg/m2) | 19 | 2.75 | 0.63 | 0.53 | 0.50 | <0.001 | |
| eGFR (ml/min/1.73m2) | 19 | 0.28 | 0.17 | 0.13 | 0.08 | 0.13 | |
|
Treadmill non-completers PAD | |||||||
| Cross-sectional leg muscle area (cm2) | Resting ABI Δ of ABI Claudication onset time (sec) Peak walking time (sec) |
17 | −3.03 | 24 | <0.01 | −0.07 | 0.90 |
| 16 | −8.61 | 27 | 0.01 | −0.06 | 0.75 | ||
| 16 | 0.02 | 0.05 | 0.01 | −0.06 | 0.74 | ||
| 16 | −0.06 | 0.05 | 0.09 | 0.02 | 0.27 | ||
| Body mass index (kg/m2) | 17 | 1.94 | 0.48 | 0.52 | 0.49 | 0.001 | |
| eGFR (ml/min/1.73m2) | 17 | −0.01 | 0.16 | <0.01 | −0.07 | 0.95 | |
PAD: peripheral artery disease, ABI: ankle brachial index, Δ of ABI: difference between resting ABI and post-treadmill ABI, eGFR: estimated glomerular filtration rate.
In separate analyses only among PAD patients but not in controls the maximum arterial SE and the AT arterial SE were significantly associated with the eGFR (Supplementary Table 2a). Also in PAD patients but not in controls the slope of the AT arterial SE was significantly associated with eGFR (Supplementary Table 2b). Conversely, the maximum arterial SE and PE arterial SE were inversely associated with the BMI in controls but not in PAD patients (Supplementary Table 2a).
Among treadmill non-completers the maximum arterial SE and the AT arterial SE were significantly associated with the eGFR (Supplementary Table 3). CSLMA was significantly associated with the BMI among treadmill completers, and treadmill non-completers (Table 5).
In a pooled analysis, maximum arterial SE was significantly associated with the CSLMA (Table 4).
The slope of the AT arterial SE was significantly associated with the CSLMA in PAD patients but not in controls (Supplementary Table 2b).
Among treadmill non-completers, the maximum arterial SE was positively associated with the CSLMA (Supplementary Table 3). The slope of the AT arterial SE was significantly associated with the CSLMA among treadmill non-completers (Supplementary Table 2b) but not in treadmill completers. There were no significant associations between the slopes of the PE and PT arterial SE and CSLMA (Supplementary Table 2b).
The pre-contrast arrival SI of the AT and PT of the more symptomatic leg were significantly correlated with the contralateral side for PAD patients but not for matched controls (r=0.49, p=0.003 vs. r=0.51, p=0.002; and r=0.40, p=0.09 vs. r=0.36, p=0.12). Arterial SE between the target and contralateral leg was significantly correlated with the AT artery (r=0.73, p<0.001 vs. r=0.43, p=0.009) and the PE artery (r=0.68, p=0.001 vs. r=0.56, p<0.001) in both controls and PAD patients, but PT arterial SE was only significant in controls and not in PAD patients (r=0.62, p=0.008 vs. r=0.15, p=0.42; Supplementary Table 4).
A pooled analysis of SE variables with clinical measures showed significant associations for the minimum arterial SE (n=56, ß=109, R2=0.16, p=0.002), maximum arterial SE (n=56, ß=91, R2=0.08, p=0.032), the AT arterial SE (n=55, ß=100, R2=0.08, p=0.041), and the PE arterial SE (n=55, ß=126, R2=0.18, p=0.001) with the resting ABI (Table 4).
In PAD patients, the PE arterial SE was significantly associated with the resting ABI (Supplementary Table 5). The SE slope of the AT was inversely associated with the changes between resting and exercise ABI (Supplementary Table 6).
Among treadmill non-completers, the maximum arterial SE was inversely associated with PWT and positively associated with CSLMA (Table 6, and Supplementary Table 3 ).
Table 6.
Linear regression analyses for signal enhancement parameters with clinical measures of PAD in PAD treadmill completers and non-completers.
| Independent Variable | N | β | Standard Error | R2 | Adjusted r2 | P-value | |
|---|---|---|---|---|---|---|---|
|
Treadmill completers PAD | |||||||
| Minimum arterial SE (A.U.) | Resting ABI | 19 | 93 | 48 | 0.18 | 0.13 | 0.07 |
| Δ of ABI | 19 | −67 | 57 | 0.07 | 0.02 | 0.26 | |
| Claudication onset time (sec) | 8 | −0.28 | 0.23 | 0.19 | 0.05 | 0.28 | |
| Maximum arterial SE (A.U.) | Resting ABI | 19 | 79 | 74 | 0.06 | 0.01 | 0.30 |
| Δ of ABI | 19 | −19 | 86 | <0.01 | −0.06 | 0.81 | |
| Claudication onset time (sec) | 8 | −0.62 | 0.43 | 0.25 | 0.13 | 0.20 | |
| AT arterial SE (A.U.) | Resting ABI | 19 | 112 | 98 | 0.07 | 0.02 | 0.27 |
| Δ of ABI | 19 | −63 | 114 | 0.02 | −0.04 | 0.58 | |
| Claudication onset time (sec) | 8 | −0.47 | 0.58 | 0.10 | −0.05 | 0.45 | |
| PE arterial SE (A.U.) | Resting ABI | 19 | 179 | 53 | 0.40 | 0.37 | 0.003 |
| Δ of ABI | 19 | −57 | 76 | 0.03 | −0.02 | 0.46 | |
| Claudication onset time (sec) | 8 | −0.30 | 0.15 | 0.39 | 0.29 | 0.10 | |
| PT arterial SE (A.U.) | Resting ABI | 19 | −19 | 78 | <0.01 | −0.06 | 0.81 |
| Δ of ABI | 19 | −54 | 87 | 0.02 | −0.04 | 0.55 | |
| Claudication onset time (sec) | 8 | −0.56 | 0.43 | 0.22 | 0.09 | 0.24 | |
|
Treadmill non-completers PAD | |||||||
| Minimum arterial SE (A.U.) | Resting ABI | 17 | 74 | 86 | 0.05 | −0.02 | 0.40 |
| Δ of ABI | 16 | 30 | 97 | 0.01 | −0.06 | 0.76 | |
| Claudication onset time (sec) | 16 | −0.21 | 0.19 | 0.08 | 0.01 | 0.29 | |
| Peak walking time (sec) | 16 | −0.29 | 0.18 | 0.16 | 0.10 | 0.13 | |
| Maximum arterial SE (A.U.) | Resting ABI | 17 | 24 | 137 | <0.01 | −0.06 | 0.87 |
| Δ of ABI | 16 | 147 | 145 | 0.07 | <0.01 | 0.33 | |
| Claudication onset time (sec) | 16 | −0.08 | 0.30 | <0.01 | −0.07 | 0.80 | |
| Peak walking time (sec) | 16 | −0.61 | 0.26 | 0.28 | 0.23 | 0.033 | |
| AT arterial SE (A.U.) | Resting ABI | 17 | 59 | 158 | 0.01 | −0.06 | 0.71 |
| Δ of ABI | 16 | 36 | 171 | <0.01 | −0.07 | 0.84 | |
| Claudication onset time (sec) | 16 | <0.00 | 0.34 | <0.01 | −0.07 | 1.00 | |
| Peak walking time (sec) | 16 | −0.74 | 0.29 | 0.33 | 0.28 | 0.021 | |
| PE arterial SE (A.U.) | Resting ABI | 16 | 76 | 102 | 0.04 | −0.03 | 0.47 |
| Δ of ABI | 16 | 47 | 111 | 0.01 | −0.06 | 0.68 | |
| Claudication onset time (sec) | 16 | −0.22 | 0.22 | 0.07 | <0.01 | 0.33 | |
| Peak walking time (sec) | 16 | −0.24 | 0.22 | 0.08 | 0.01 | 0.30 | |
| PT arterial SE (A.U.) | Resting ABI | 17 | 15 | 107 | <0.01 | −0.07 | 0.89 |
| Δ of ABI | 16 | 121 | 114 | 0.07 | 0.01 | 0.31 | |
| Claudication onset time (sec) | 16 | −0.34 | 0.22 | 0.15 | 0.09 | 0.14 | |
| Peak walking time (sec) | 16 | −0.22 | 0.23 | 0.06 | −0.01 | 0.37 | |
PAD: peripheral artery disease, ABI: ankle brachial index, Δ of ABI: difference between resting ABI and post-treadmill ABI, SE: signal enhancement, A.U.: arbitrary units. AT: anterior tibialis artery, PE: peroneal artery, PT: posterior tibialis artery.
Among PAD treadmill completers, the PE arterial SE was associated with the resting ABI (Table 6). The SE slope of the AT was inversely associated with the changes between the resting and exercise ABI in PAD treadmill completers (Supplementary Table 6).
Discussion
The primary findings of this study are that CE-MRI-based first-pass arterial perfusion is impaired in PAD patients compared with matched controls and is associated with measures of lower extremity ischemia. The minimum and maximum arterial SE were significantly reduced in PAD patients compared with matched controls. Among PAD patients but not in matched controls, maximum arterial SE was associated with eGFR, an established marker of renal function. In a pooled analysis of PAD patients and controls, CE-MRI-based minimum and maximum arterial SE were significantly associated with the resting ABI. The inter- and intra-observer agreement of the imaging based measures were excellent.
It has been shown that in healthy volunteers MRI-based phase-contrast quantitative flow measurements of the lower extremity arteries depend on age, gender, and calf muscle volume.22 In our study, maximum arterial SE was positively associated with cross-sectional leg muscle area in PAD treadmill non-completers but not in matched controls or treadmill completers.
Previous research showed that PAD patients who underwent exercise conditioning had a marked improvement in functional capacity, plethysmograph-based calf blood flow, and peak walking time, however, the change in blood flow did not correlate with the increase in PWT.23 In this study, maximum arterial SE was significantly reduced in PAD patients compared with matched controls, and we observed an association with CSLMA only in claudicants who did not complete the 6 minute graded treadmill test.
Atkins and Gardner demonstrated that the ABI was not correlated with lower extremity functional strength.24 In this study linear regression analyses indicate that the ABI is significantly associated with the minimum and maximum arterial SE for the pooled analysis. We also have observed that PAD treadmill non-completers compared with completers had a significantly lower resting ABI and an earlier onset of claudication pain, indicating a relationship between hemodynamics and leg function.
Szuba et al. reported that maximal calf blood flow does not predict treadmill walking distance.25 Our results indicate that treadmill non-completers versus completers had a significant shorter COT, as expected, and that among all PAD patients, maximum arterial SE was not associated with PWT.
Maximum arterial SE was associated with eGFR, a known marker of PAD, in PAD patients but not in matched controls, suggesting a potential link with kidney function which is a known marker of lower extremity ischemia.26 The eGFR has also been previously associated with an impaired mirocvascular circulation in PAD patients, indicating an important link between kidney function and macrovascular and microvascular disease in PAD patients.17
Leg muscle area has been previously studied in PAD patients.27,2 The WALCS II study found that a decrease in calf muscle area, measured by computed tomography over a period of 2 years, was associated with an increased loss of mobility in PAD patients when compared to non-PAD subjects.2 In this study, CSLMA in PAD patients was associated with the BMI, and the maximum arterial SE was associated with the CSLMA only in treadmill non-completers but not in completers, or among matched controls.
This study has limitations. The study population was limited to PAD patients with life-style limiting claudication who had no clinical indication for revascularization during the recruitment phase. Participants also had no rest pain or critical limb ischemia, an important group for whom the proposed MR measures have to be assessed in future studies. The proposed technique is subject to all limitations of MR imaging including contraindications to MRI and GBCA. Future work will need to determine the clinical feasibility of incorporating the proposed technique with clinically performed MRI scans with contrast. However, non-invasive CE-MRI may be useful to quantitatively assess PAD.
In conclusion, first-pass arterial perfusion, as measured with CE-MRI, is impaired in PAD patients compared with matched controls and is associated with clinical measures of lower extremity ischemia. Arterial perfusion measures are highly reproducible and could be of interest as surrogate markers to assess response to clinical management and novel PAD therapies.
Supplementary Material
Supplementary Table 1 – Categorical arterial group analysis by preferential minimum or maximum arterial signal enhancement: a) – between Control and PAD groups; b) – by Control and PAD group.
Supplementary Table 2a – Linear regression analyses for signal enhancement parameters and markers of PAD in matched controls and PAD patients.
Supplementary Table 2b – Linear regression analyses for the slopes of arterial signal enhancement variables and markers of PAD in matched controls and PAD patients.
Supplementary Table 3 – Linear regression analyses for signal enhancement parameters and markers of PAD in PAD treadmill completers and non-completers.
Supplementary Table 5 – Linear regression analyses for signal enhancement parameters with measures of treadmill walking and exercise ankle brachial index.
Supplementary Table 4 – Correlation analyses of arterial magnetic resonance imaging measures between the more symptomatic and contralateral leg.
Supplementary Table 6 – Linear regression analyses for the slopes of arterial signal enhancement variables with measures of treadmill walking and exercise ankle brachial index.
Acknowledgements:
We thank all study participants for their cooperation. This work was supported with funding from the National Institutes of Health (R01HL137763, K25HL121149 both to GB), American Heart Association (13BGIA16720014 to GB).
Footnotes
Disclosures: There are no conflicts of interest to disclose.
References
- 1.Lumsden AB, Rice TW, Chen C, Zhou W, Lin PH, Bray P, Morrisett J, Nambi V, Ballantyne C. Peripheral arterial occlusive disease: magnetic resonance imaging and the role of aggressive medical management. World J Surg 2007;31:695–704. [DOI] [PubMed] [Google Scholar]
- 2.McDermott M, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, Liao Y, Criqui M. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation 2009:1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu J, Santulli G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018;275:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509–1526. [DOI] [PubMed] [Google Scholar]
- 5.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2011;58:2020–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC-II). J Vasc Surg 2007;45 Suppl S:5. [DOI] [PubMed] [Google Scholar]
- 7.Koelemay MJ, Lijmer JG, Stoker J, Legemate DA, Bossuyt PM. Magnetic resonance angiography for the evaluation of lower extremity arterial disease: a meta-analysis. JAMA : the journal of the American Medical Association 2001;285:1338–1345. [DOI] [PubMed] [Google Scholar]
- 8.Nelemans PJ, Leiner T, de Vet HC, van Engelshoven JM. Peripheral arterial disease: meta-analysis of the diagnostic performance of MR angiography. Radiology 2000;217:105–114. [DOI] [PubMed] [Google Scholar]
- 9.Visser K, Hunink MG. Peripheral arterial disease: gadolinium-enhanced MR angiography versus color-guided duplex US--a meta-analysis. Radiology 2000;216:67–77. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Tyan Yu.-Ch., Lai J-J, Chang Ch.-Ch. Automated Determination of Arterial Input Function for Dynamic Susceptibility Contrast MRI from Regions around Arteries Using Independent Component Analysis. Radiology Research and Practice 2016:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, Butany J, Wasserman BA, Johnstone DM, Silver FL, Mikulis DJ. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology 2009;72:627–634. [DOI] [PubMed] [Google Scholar]
- 12.Oostendorp M, Post MJ, Backes WH. Vessel growth and function: depiction with contrast-enhanced MR imaging. Radiology 2009;251:317–335. [DOI] [PubMed] [Google Scholar]
- 13.Isbell DC, Epstein FH, Zhong X, DiMaria JM, Berr SS, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging 2007;25:1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins JL, Jones WS, Duscha BD, Allen JD, Kraus WE, Regensteiner JG, Hiatt WR, Annex BH. Relationship between leg muscle capillary density and peak hyperemic blood flow with endurance capacity in peripheral artery disease. J Appl Physiol 2011;111:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimnich OA, Singh J, Bismuth J, Shah DJ, Brunner G. Magnetic resonance imaging based modeling of microvascular perfusion in patients with peripheral artery disease. J Biomech 2019;93:147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stylianopoulos T, Jain RK. Combining two strategies to improve perfusion and drug delivery in solid tumors. Proc Natl Acad Sci U S A 2013;110:18632–18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner G, Bismuth J, Nambi V, Ballantyne CM, Taylor A, Lumsden AB, Morrisett JD, Shah DJ. Calf Muscle Perfusion As Measured With Magnetic Resonance Imaging To Assess Peripheral Arterial Disease. Med Biol Eng Comput 2016;54(11):1667–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicolai SP, Kruidenier LM, Rouwet EV, Bartelink ML, Prins MH, Teijink JA. Ankle brachial index measurement in primary care: are we doing it right? Br J Gen Pract 2009;59:422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalifa F, Soliman A, El-Baz A, Abou El-Ghar M, El-Diasty T, Gimel’farb G, Ouseph R, Dwyer AC. Models and methods for analyzing DCE-MRI: a review. Medical physics 2014;41:124301. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 21.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological bulletin 1979;86:420–428. [DOI] [PubMed] [Google Scholar]
- 22.Klein WM, Bartels LW, Bax L, van der Graaf Y, Mali WP. Magnetic resonance imaging measurement of blood volume flow in peripheral arteries in healthy subjects. J Vasc Surg 2003;38:1060–1066. [DOI] [PubMed] [Google Scholar]
- 23.Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation 1990;81:602–609. [DOI] [PubMed] [Google Scholar]
- 24.Atkins LM, Gardner AW. The relationship between lower extremity functional strength and severity of peripheral arterial disease. Angiology 2004;55:347–355. [DOI] [PubMed] [Google Scholar]
- 25.Szuba A, Oka RK, Harada R, Cooke JP. Limb hemodynamics are not predictive of functional capacity in patients with PAD. Vascular medicine (London, England) 2006;11:155–163. [DOI] [PubMed] [Google Scholar]
- 26.Baber U, Mann D, Shimbo D, Woodward M, Olin JW, Muntner P. Combined role of reduced estimated glomerular filtration rate and microalbuminuria on the prevalence of peripheral arterial disease. The American journal of cardiology 2009;104:1446–1451. [DOI] [PubMed] [Google Scholar]
- 27.Brunner G, Nambi V, Yang E, Kumar A, Virani SS, Kougias P, Shah D, Lumsden A, Ballantyne CM, Morrisett JD. Automatic Quantification of Muscle Volumes in Magnetic Resonance Imaging Scans of the Lower Extremities. Magnetic Resonance Imaging 2011;29:1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 – Categorical arterial group analysis by preferential minimum or maximum arterial signal enhancement: a) – between Control and PAD groups; b) – by Control and PAD group.
Supplementary Table 2a – Linear regression analyses for signal enhancement parameters and markers of PAD in matched controls and PAD patients.
Supplementary Table 2b – Linear regression analyses for the slopes of arterial signal enhancement variables and markers of PAD in matched controls and PAD patients.
Supplementary Table 3 – Linear regression analyses for signal enhancement parameters and markers of PAD in PAD treadmill completers and non-completers.
Supplementary Table 5 – Linear regression analyses for signal enhancement parameters with measures of treadmill walking and exercise ankle brachial index.
Supplementary Table 4 – Correlation analyses of arterial magnetic resonance imaging measures between the more symptomatic and contralateral leg.
Supplementary Table 6 – Linear regression analyses for the slopes of arterial signal enhancement variables with measures of treadmill walking and exercise ankle brachial index.