Abstract
Background
Cyclocarya paliurus (Batal.) Iljinskaja is a common endemic tree species and used as a Chinese medicine. The main active components in the leaves of this plant are polysaccharides. However, the temporal patterns of gene expression underlying the synthesis of polysaccharides in C. paliurus at different leaf developmental stages and its relationship with the polysaccharide content and antioxidant activities has not been reported to date.
Methods
RNA-seq was used to investigate the biosynthesis pathway of polysaccharides at the four developmental stages of C. paliurus leaves. The content and the antioxidant activities of polysaccharides were measured with typical biochemical methods and the identified correlations were statistically evaluated.
Results
Sixty-nine differentially expressed genes were found in the leaves during different developmental stages of C. paliurus. These are associated with glycosyltransferases and belong to 18 families. During different developmental stages of C. paliurus, the polysaccharide content first increased and then decreased, and the UDP-glucose 4-epimerase gene was found to be significantly positively correlated with the polysaccharide content. The clearance rates of DPPH radicals, superoxide anion radicals, hydroxyl radicals, and the reducing power of polysaccharides in the leaves of C. paliurus at different developmental stages showed a dose-dependent relationship with the concentration of polysaccharides.
Conclusions
The smallest fully expanded leaves are suitable for high-quality tea, and leaves with sizes below the largest fully expanded leaves are suitable for industrial production of polysaccharides.
Keywords: Cyclocarya paliurus, Polysaccharide biosynthesis, Antioxidant activity, Industrial production
Introduction
Cyclocarya paliurus, the only species of the genus Cyclocarya of the Juglandaceae family, is commonly known as the “sweet tea tree” in China and is traditionally used as a Chinese medicine. It grows on the cloudy highlands of southern China, including the Provinces of Jiangxi, Hunan, and Zhejiang (Xie et al., 2013; Yang et al., 2018). In China, C. paliurus has been traditionally used as a health food for more than 1000 years in China (Yang et al., 2018). In 2013, the leaves of C. paliurus have been approved as a food resource by the National Health and Family Planning Commission of China (Wang et al., 2015). The leaves of C. paliurus contain an abundance of natural bioactive components including steroids, saponins, polysaccharides, flavonoids, triterpenoids, and phenolic compounds (Xie et al., 2016). The main chemical components of C. paliurus leaves and their biological activities receive increasingly scientific attention (Cao et al., 2019; Zhai et al., 2018).
Polysaccharides extracted from C. paliurus leaves were found to have notable bioactivities, e.g., antioxidant (Xie et al., 2010a), hypoglycemic (Xie et al., 2006), anticancer (Xie et al., 2013), antimicrobial (Xie et al., 2012), and immunomodulatory bioactivities (Xie et al., 2014). Previous work mainly focused on extraction methods of polysaccharides from C. paliurus leaves (Xie et al., 2014; Xie et al., 2010a; Xie et al., 2010b). It has been shown that the applied extraction techniques significantly affect the yield, physicochemical properties, and biological activities of polysaccharides (Song et al., 2019). Different types of polysaccharides were extracted from C. paliurus using various methods, and the resulting monosaccharide composition and molecular weight differed (Kakar et al., 2020). For example, Xie et al. (2010b) found that C. paliurus polysaccharide was composed of D-xylose, L-arabinose, D-glucose, D-galactose, L-rhamnose, and D-mannose at a molar ratio of 1.00:9.67:9.65:4.96:3.29:2.70, respectively. Yang et al. (2016) reported that a different polysaccharide was composed of rhamnose, arabinose, xylose, mannose, glucose, and galactose at a molar ratio of 1.00:2.23:0.64:0.49:0.63:4.16, respectively. C. paliurus polysaccharide showed notable bioactivities, such as antioxidant (Xie et al., 2010a), hypoglycemic (Xie et al., 2006), anticancer (Xie et al., 2013), antimicrobial (Xie et al., 2012), and immunomodulatory activities (Xie et al., 2014). In addition, the polysaccharide content and their antioxidant activity of C. paliurus leaves collected from different areas and populations were studied (Liu et al., 2018; Zhou et al., 2019). Fu et al. (2015) reported the existence of seasonal and genotypic variation of the water-soluble polysaccharide content of C. paliurus leaves. Yang et al. (2017) studied changes of water-soluble polysaccharides of C. paliurus under different light regimes. Many studies have reported changes of polysaccharide contents and antioxidant activities in C. paliurus leaves of different planting regions, populations, and seasons. However, how the differences in polysaccharide content and antioxidant activity develop over the different developmental stages in the leaves of C. paliurus still remains unclear. Wang et al. (2001) reported that the water-soluble polysaccharide content of sixth-class tea leaves (i.e., fully developed leaves) was twice as high as that of first-class tea leaves (i.e., tender leaves). Petropoulos et al. (2018) reported that the antioxidant activity of Cichorium spinosum leaves varied at the developmental stage. At present, Chinese enterprises always collect young leaves for their good taste and shape, while mature leaves are usually collected to extract polysaccharides. Therefore, a better understanding of the content and antioxidant activity of polysaccharides in C. paliurus leaves at different developmental stages can help the improvement of the production processes and thus, increase economic benefits.
Recently, temporal gene expression profiles that characterize the time-dynamics of expression of specific genes related to the secondary metabolites of plants have attracted increasing scientific attention (Li et al., 2016; Liu et al., 2015; Mueller, Chiou & Leng, 2008; Shivakumar, Johnson & Zimmer, 2019; Sweetman et al., 2012; Wong et al., 2017). For example, Li et al. (2016) reported that Camellia sinensis leaf transcriptomes differed among three growth stages, which is also the case for differentially expressed unigenes related to metabolic pathways, biosynthesis of secondary metabolites, phenylpropanoid biosynthesis, and carbon fixation during photosynthesis. Shivakumar, Johnson & Zimmer (2019) identified differentially expressed genes (DEGs) involved in oil production using transcriptome analysis of the leaves of Bergera koenigii at different developmental stages. However, the temporal patterns of the gene expression of polysaccharide synthesis in C. paliurus at different leaf developmental stages have not been reported so far. Moreover, no systematic study has investigated the variation in the gene expression underlying polysaccharide synthesis and the relationship of these genes with the polysaccharide content and antioxidant activities in C. paliurus leaves during development also remain unknown.
Based on transcriptomic sequencing data, this study investigated the temporal gene expression pattern of polysaccharides biosynthesis. The content and antioxidant activity of C. paliurus polysaccharides at different developmental stages were also studied. Moreover, the relationship between DEGs and the content of antioxidant activity of C. paliurus was analyzed. These results are helpful to further develop the polysaccharide product of C. paliurus.
Materials & methods
Plant materials
C. paliurus leaves were collected from Zhuzhang Village (E118°48′28″, N28°5′57″), Longquan City, and Lishui City, Zhejiang Province, China, on May 1, 2018. The developmental stages of C. paliurus leaves have been defined by Guo et al. (2017). The F1 stage refers to the smallest fully expanded leaf, while the F4 stage refers to leaves with full leaf enlargement and full leaf thickness. F2 and F3 refer to the developmental stages between the smallest and largest leaves. According to the methods described by Li et al. (2017), different developmental stages of C. paliurus leaves were separately sampled at the same time on the same tree. Three plants were randomly selected and used as three biological replicates. A portion of the samples was immediately frozen in liquid nitrogen and stored at −80 °C for metabolomic and transcriptomic analyses. Another portion of the sample was brought back to the laboratory on ice and was dried at 70 °C to a constant weight, and then ground to a powder for polysaccharide extraction and analysis.
Metabolite extraction, multiple reactions monitoring, and parameter setting
The freeze-dried leaves of C. paliurus were used for metabolite extraction and multiple reaction monitoring (MRM) by Metware Biotechnology Co., Ltd. (Wuhan, China) as previously described in Lin et al. (2020). Metabolites with variable importance in project (VIP) ≥1 and fold changes ≥2 or ≤0.5 were defined as significantly different in content. The MRM of each species was repeated three times.
The biosynthesis pathway of polysaccharides in C. paliurus leaves
The total RNA was extracted and four cDNA libraries at four different leaf developmental stages were constructed according to the manufacturer’s protocol Paired-end sequencing of the library was performed on HiSeq XTen sequencers (Illumina, San Diego, CA, USA) by Sangon Biotech Co., Ltd. (Shanghai, China).
The raw high-throughput sequencing data or raw reads, including gene ID and sequence, were stored in the FASTA file format. The raw data were further treated and annotated according to the protocol described by Lin et al. (2020).
Bowtie2 (version 2.3.2) was used to map the quality control sequences to the assembled transcripts and RSeQC (version 2.6.1) was used for the statistical analysis of the aligned result (Lin et al., 2020). Salmon (version 0.8.2) was used to calculate the read counts and expression values of unigenes (Lin et al., 2020). Gene expression between samples were compared afterh the influence of gene lengths and sequencing discrepancies were eliminated by transcripts per million (TPM) (Lin et al., 2020). Principal component analysis (PCA) and principal co-ordinates analysis (PCoA) were performed to assess the distance and difference between samples (Chen, You & Shan, 2019). DESeq2 (version 1.12.4) was used to determine DEGs between two samples and genes were considered as significantly differentially expressed at a q-value < 0.001 and |FoldChange| > 2 (Chen, You & Shan, 2019). When the normalized expression of a gene was zero between two samples, its expression value was adjusted to 0.01 (as 0 cannot be plotted on a log plot) (Lin et al., 2020). If the normalized expression of a specific gene in two libraries was lower than 1, further differential expression analysis would be conducted without this gene (Lin et al., 2020).
Glycosyltransferase (GT) genes were identified via dbCAN HMM and sequence similarity was identified based on the CAZy database (E-Value < 1e−15, http://bcb.unl.edu/dbCAN/blast.php) (Zhang et al., 2018).
Ten glycosyltransferase genes, involved in polysaccharide biosynthesis, were randomly selected for quantitative real-time PCR (RT-qPCR) verification. These genes have gene expression (i.e., TPM value) ≥5 in at least three of the four developmental stages. The reverse transcriptase reaction of DNase-treated RNA was conducted using HiScript II reverse transcriptase according to the manufacturer’s instructions (Vazyme, Nanjing, China). Specific primers for glycosyltransferase genes were designed using Primer Premier 5.0 (Table S1). The constitutively expressed gene (the β-Actin-1 gene) was used as internal control (Zhao et al., 2018). RT-qPCR was performed using CFX Connect (BioRAD, USA). The relative expression of genes was calculated by the 2−∆∆Ct method. All RT-qPCRs were performed in three biological and three technical replicates.
Determination of the polysaccharide content
Extraction of polysaccharides
The leaves were dried at 50 °C for 96 h until a constant weight was obtained. Then, the leaves were ground to a fine powder and stored at room temperature until further analysis. The polysaccharides of C. paliurus leaves were extracted according to a previously reported method with minor modification (Fu et al., 2015). Briefly, a total of 2 g leaf powder was extracted with 80 ml of 80% ethanol at 90 °C for 60 min to remove most of the pigments, small sugar molecules, and impurities. Insoluble residues were dried and then extracted twice with 80 ml distilled water at 90 °C for 60 min. The extracts were filtered, and the filtrate was obtained after centrifugation at 4500×g for 15 min.
Content determination
The water-soluble polysaccharide content was measured using the phenol-sulphuric acid colorimetric method (Dubois et al., 1956). The absorbance was measured at 490 nm. The concentration of the water-soluble polysaccharides was quantitatively determined via the calibration curve, using glucose as standard.
Measurement of the antioxidant activities of polysaccharides
Preparation of polysaccharides
The powder of the dried leaves was treated with refluxing petroleum ether twice for 2 h each to remove lipids and pigments, and the residue was air dried. Then, 20 g of samples were firstly refluxed twice with 200 mL of 80% (v/v) ethanol at 90 °C for 1 h to remove most of the small molecules. Subsequently, polysaccharides from the leaves were extracted twice with 400 mL of deionized water at 100 °C for 2 h. The extracted supernatant was collected by centrifugation at 4,000 rpm for 20 min, concentrated, and precipitated with four volumes of 95% ethanol (v/v) at 4 °C overnight, followed by centrifugation. The resultant precipitate was redissolved in distilled water, and the isolated proteins were removed approximately 10–12 times by the Sevag method (Staub, 1965), dialyzed with 14,000 Da molecular weight cutoff membrane against distilled water for 48 h, and lyophilized to obtain the partially purified C. paliurus polysaccharides.
Scavenging activity of DPPH radical
The DPPH radical scavenging capacity of polysaccharide samples was analyzed according to the method of Xie et al. Vc was used as a positive control. The ability to scavenge DPPH radical was calculated as follows: Scavenging activity (%) = 1 − (A2 − A1)/A0 × 100%. Here, a mixture of 0.5 mL of DPPH-ethanol solution plus 0.5 mL ethanol was used as negative control A0, a mixture of 0.5 mL ethanol plus 0.5 mL sample solution was used as blank A1, and a mixture of 0.5 mL of DPPH-ethanol solution plus 0.5 mL sample solution was used as A2.
Scavenging activity of superoxide radical
The scavenging capacities for the superoxide radical were assessed using the method previously described by Xie et al. (2016). Vc was used as positive control. The superoxide radical scavenging activity was calculated as follows: Scavenging activity (%) = 1 − A1/A0 × 100%, where A0 represents the change speed of absorbance of the control group in the superoxide radical generation system and A1 represents the change speed of absorbance of the sample.
Scavenging activity of hydroxyl radicals
The scavenging activity of hydroxyl radicals was assessed using the method described by Xie et al. (2016) Vc was used as positive control. The hydroxyl radicals scavenging effect was calculated using the following formula: Scavenging activity (%) = (A2 −A1)/(A0 − A1) × 100%, where A1 represents ultrapure water instead of sample, A2 represents the polysaccharide sample, and A0 represents ultrapure water instead of polysaccharide sample and H2O2.
The ferric reducing-antioxidant power
The FRAP assay was conducted according to the procedure described in the literature (He et al., 2017), with minor modification. Briefly, the FRAP reagent was prepared from 20 mmol/L iron (III) chloride solution, 10 mmol/L TPTZ solution in 40 mmol/L HCl, and 300 mmol/L sodium acetate buffer (pH 3.6) at a volume ratio of 1:1:10, respectively. The FRAP reagent was warmed in a water bath at 37 °C before use. Then, 0.1 mL of the sample and 0.4 ml ultrapure water were added to 0.5 mL of the FRAP solution and the absorbance was determined at 593 nm after 10 min. The results were expressed as VC μg/mL of polysaccharide samples.
Statistical analysis
The data are shown as means ± standard deviation (SD) of three independent replicates. One-way analysis of variance (ANOVA) was calculated by SPSS 17 software (SPSS Inc., Chicago, IL, USA) and was used to evaluate the significance at the 0.05 level. The correlation coefficients between genes and metabolites, as well as between genes and polysaccharide content, were calculated with the corrplot package in R-3.6.1.
Results
Sequencing and assembly
A total of 703,862,064 reads of sequencing data, including 541,694,618 raw reads and 499,710,194 clean reads were obtained. The average Q20 and Q30 values were 98.69% and 95.23%, respectively. The average GC content was 53.08% (Table 1). A total of 296,593 unigenes were assembled, and the longest and the shortest transcript had the same length. With regard to the unigenes, 61,444 were ≥500 bp (20.72%) and 19,068 were ≥1,000 bp (6.43%). The total length of the unigenes was 127,566,956 bp, and their average length was 430.11 bp (Table 2).
Table 1. Summary of sequencing quality.
| Sample | Raw reads | Clean reads | Clean bases | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| F1-1 | 60275244 | 57418316 | 8284605522 | 99.25% | 97.14% | 47.69% |
| F1-2 | 53598170 | 51222052 | 7420825268 | 99.28% | 97.24% | 48.39% |
| F1-3 | 43486608 | 40780112 | 5849968138 | 99.19% | 96.92% | 51.54% |
| F2-1 | 44202270 | 41685078 | 5992292304 | 99.23% | 97.03% | 52.97% |
| F2-2 | 61413864 | 58423068 | 8418014194 | 99.24% | 97.10% | 48.44% |
| F2-3 | 41315312 | 38853284 | 5606991844 | 99.23% | 96.99% | 54.26% |
| F3-1 | 42154028 | 38258314 | 5453521105 | 99.05% | 96.42% | 56.38% |
| F3-2 | 40111316 | 35177472 | 4690982062 | 97.65% | 91.86% | 60.67% |
| F3-3 | 34417588 | 30284380 | 4035084235 | 97.65% | 91.82% | 58.47% |
| F4-1 | 50535242 | 45373768 | 6098531357 | 97.93% | 92.51% | 49.69% |
| F4-2 | 51370720 | 46292002 | 6260829757 | 97.94% | 92.52% | 50.75% |
| F4-3 | 18814256 | 15942348 | 2274594827 | 98.68% | 95.18% | 57.74% |
| Summary | 541694618 | 499710194 | 70386240613 |
Note:
Q20: percentage of bases with a Phred value > 20; Q30: percentage of bases with a Phred value > 30.
Table 2. Length distribution of assembled transcripts and unigenes.
| Nucleotide length | Transcripts | Unigenes |
|---|---|---|
| Total | 574,747 | 296,593 |
| >=500 bp | 170,327 | 61,444 |
| >=1,000 bp | 56,093 | 19,068 |
| N50 | 624 | 478 |
| N90 | 240 | 225 |
| Max length | 76,464 | 76,464 |
| Min length | 201 | 201 |
| Total length | 289,576,116 | 127,566,956 |
| Average length | 503.83 | 430.11 |
Functional annotation and classification
All 296,593 assembled unigenes were searched and annotated in NR, NT, PFAM, KOG, CDD, TrEMBL, and Swiss-Prot databases using the BLAST algorithm (E-value ≤ 1E−5). Of these unigenes, 173,809 were annotated to the NR database, accounting for 58.60% of the total. A total of 118,930 (40.10%) unigenes were annotated into the Swissprot database. The numbers of unigenes annotated to NT, PFAM, GO, KOG, CDD, and TrEMBL databases were 104,770 (35.32%), 57,649 (19.44%), 145,508 (49.06%), 64,021 (21.59%), 89,954 (30.33%), and 118,930 (40.10%), respectively. A total of 191,829 (64.68%) sequences were annotated in at least one database (Table 3).
Table 3. Summary of unigene annotation against publicly available databases.
| Database | Number of genes | Percentage (%) |
|---|---|---|
| Annotated in CDD | 89,954 | 30.33 |
| Annotated in KOG | 64,021 | 21.59 |
| Annotated in NR | 173,809 | 58.6 |
| Annotated in NT | 104,770 | 35.32 |
| Annotated in PFAM | 57,649 | 19.44 |
| Annotated in Swissprot | 118,930 | 40.1 |
| Annotated in TrEMBL | 168,101 | 56.68 |
| Annotated in GO | 145,508 | 49.06 |
| Annotated in at least one database | 191,829 | 64.68 |
| Annotated in all database | 4,897 | 1.65 |
| Total genes | 296,593 | 100 |
Biosynthesis pathway of polysaccharides
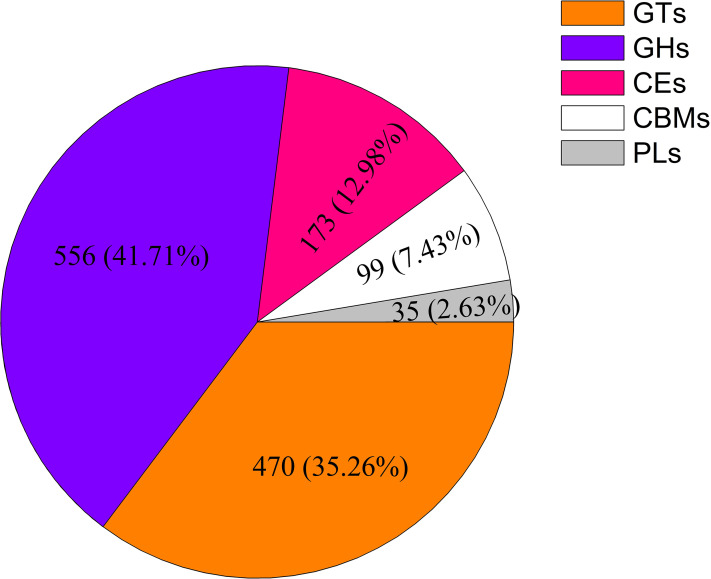
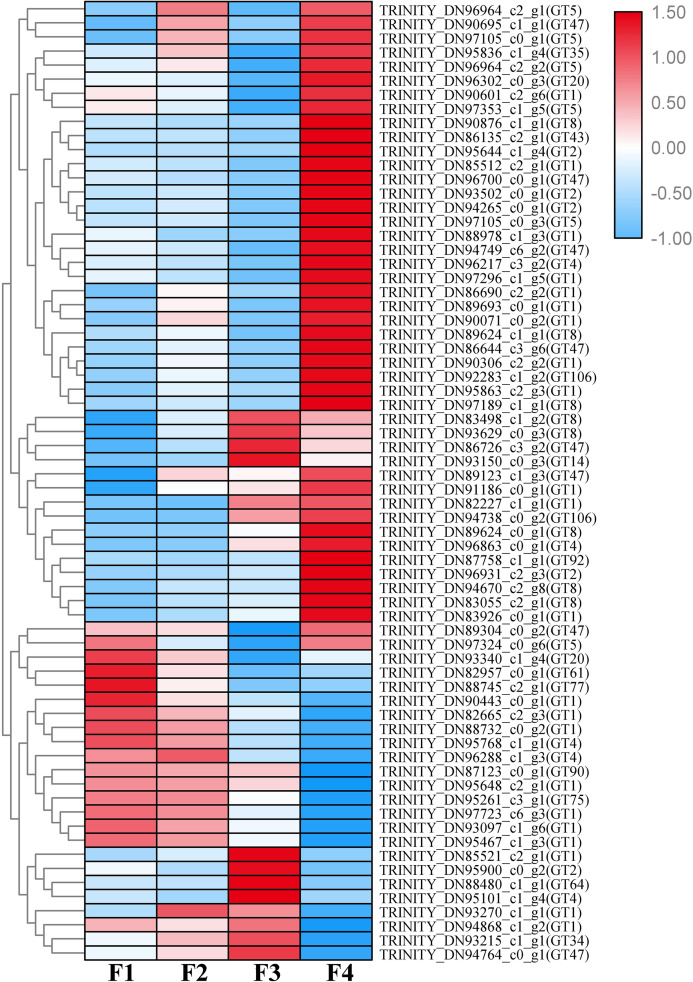
A total of 1,333 unigenes related to the carbohydrate metabolism were identified, including 470 GT genes (which belong to 57 GT families, Table S2), 556 glycoside hydrolases, 173 carbohydrate esterases, 99 carbohydrate-binding modules, and 35 polysaccharide lyases (Fig. 1). Sixty-nine DEGs associated with GT belonging to 18 GT families were identified (Fig. 2, Table S3).
Figure 1. Classification and number of carbohydrate-active enzyme families in Cyclocarya paliurus unigenes.
GT, glycosyltransferase; GH, glycoside hydrolase; CE, carbohydrate esterase; CBM, carbohydrate-binding module; PL, polysaccharide lyase.
Figure 2. Heat map of differentially expressed glycosyltransferase genes at four different developmental stages (i.e., F1, F2, F3, and F4) of Cyclocarya paliurus leaves.
The relative expression levels of genes are highlighted with different colors from blue (low) to red (high) across the F1, F2, F3, and F4 developmental stages.
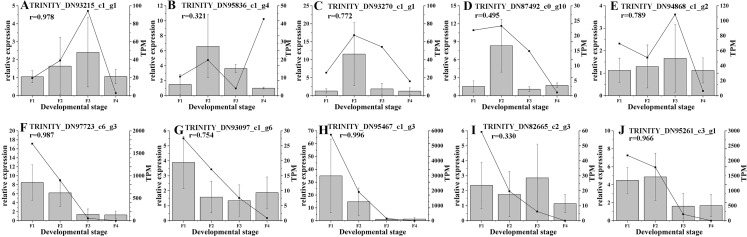
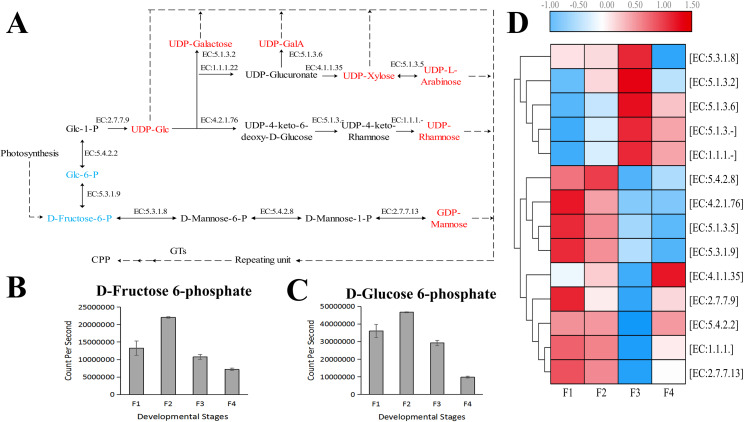
Based on the difference in expression levels of genes at four different developmental stages, 10 genes were screened for RT-qPCR (Fig. 3). All 10 selected genes had expression patterns similar to the RNA-seq data. Therefore, the data obtained in this study can be used as a tool to study the biosynthesis and metabolism-related genes of C. paliurus. Based on the results of transcriptome analysis and amino sugar and nucleotide sugar metabolism KEGG pathways, a pathway map for the biosynthesis of C. paliurus polysaccharide was proposed (Fig. 4A). In this pathway, the synthesis of C. paliurus polysaccharide can mainly be divided into three steps: first, formation of D-Fructose 6-phosphate; second, synthesis of multiple NDP-sugars; third, formation of polysaccharide under the action of different glycosyltransferases.
Figure 3. Real-time PCR validation of candidate unigenes involved in Cyclocarya paliurus polysaccharide biosynthesis by RNA-seq.
(A–J) Respectively indicate real-time PCR validation of different genes. The histograms show the relative gene expression levels obtained via real-time PCR. The transcripts per million (TPM) of each million mapped fragment of the transcriptome are represented by line graphs. The right y-axis indicates gene expression levels calculated as TPM. The left y-axis indicates relative gene expression levels obtained by real time PCR.
Figure 4. Putative biosynthesis pathway map and unigene expression pattern of Cyclocarya paliurus polysaccharide.
(A) The pathway of C. paliurus polysaccharide biosynthesis was constructed based on KEGG analysis. The full names of enzymes (with EC number) are provided in Table S1. The red font represents activated monosaccharides. The text formatted in blue font represents detected metabolites. (B)–(C) The bar graph shows the contents of these two metabolites across the F1, F2, F3, and F4 developmental stages. (D) The average expression level of the enzyme-encoding unigenes at different leaf stages is indicated with a heat map. The relative expression levels of genes are highlighted with different colors from blue (low) to red (high) across the F1, F2, F3, and F4 developmental stages.
Two metabolites that are part of the polysaccharide synthesis pathway were detected in the metabolome, including D-Fructose 6-phosphate and D-Glucose 6-phosphate (Figs. 4B, 4C). The contents of D-Fructose 6-phosphate and D-Glucose 6-phosphate increased first and then decreased over the four developmental stages.
Each enzyme in the pathway was associated with several unigenes (Table S3), indicating that each enzyme in the pathway was associated with multiple coding genes. Most single genes (12 genes) were annotated as genes encoding UDP-glucuronate decarboxylase (EC: 4.1.1.35), and mannose-1-phosphate guanylyltransferase (EC: 2.7.7.13), while the second largest number of unigenes (eight genes) were annotated as UDP-glucose 4-epimerase (EC: 5.1.3.2), and phosphomannomutase (EC: 5.4.2.8) encoding genes. The third largest number of unigenes (five genes) were annotated as genes encoding UDPglucose 6-dehydrogenase (EC: 1.1.1.22), UDP-glucose 4,6-dehydratase (EC: 4.2.1.76), and UDP-glucuronate 4-epimerase (EC: 5.1.3.6). Furthermore, four unigenes were annotated as genes encoding 3,5-epimerase/4-reductase (EC:5.1.3.-), 4-reductase (EC:1.1.1.-), and mannose-6-phosphate isomerase (EC:5.3.1.8). Two unigenes were annotated as genes encoding xylan 1,4-beta-xylosidase (EC:5.1.3.5). The expressions of eight genes peaked at F1 and F2 stages while the expressions of four genes peaked at the F3 stage (Fig. 4D).
Variation of polysaccharide contents of C. paliurus and its correlation with putative pathways of polysaccharides
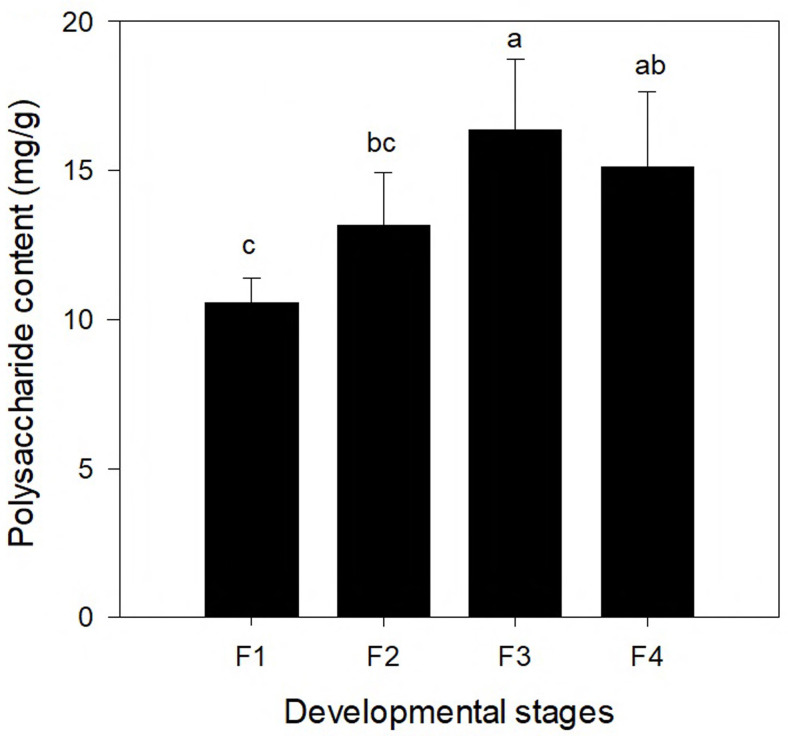
To compare the changes of polysaccharide content at different leaf development stages, the polysaccharide contents of leaves at four different developmental stages (F1, F2, F3, and F4) are shown in Fig. 5. The polysaccharide content differed significantly among the four different leaf stages. With continuing development of leaves, the polysaccharide content increased first and the highest content was found at the F3 stage (16.373 mg/g), which was followed by a decrease. The polysaccharide content in the leaves at the F4 stage was slightly lower than that at the F3 stage (but the difference was not significant).
Figure 5. Polysaccharide content in leaves of Cyclocarya paliurus at four developmental stages (F1, F2, F3, and F4).
Correlation analysis was conducted between the gene expression of genes of the polysaccharide synthesis pathway and the polysaccharide content (Table S4). One gene (TRINITY_DN83861_c1_g2, which encodes UDP-glucose 4-epimerase) was significantly positively correlated with the polysaccharide content (r = 0.037; p = 0.962), which encodes UDP-glucose 4-epimerase. Six genes (TRINITY_DN87479_c0_g1, TRINITY_DN92023_c2_g3, TRINITY_DN92244_c2_g3, TRINITY_DN93742_c2_g1, TRINITY_DN95705_c0_g3, and TRINITY_DN96635_c0_g1) were significantly negatively correlated with the polysaccharide content.
Variation of the antioxidant activities of C. paliurus polysaccharides
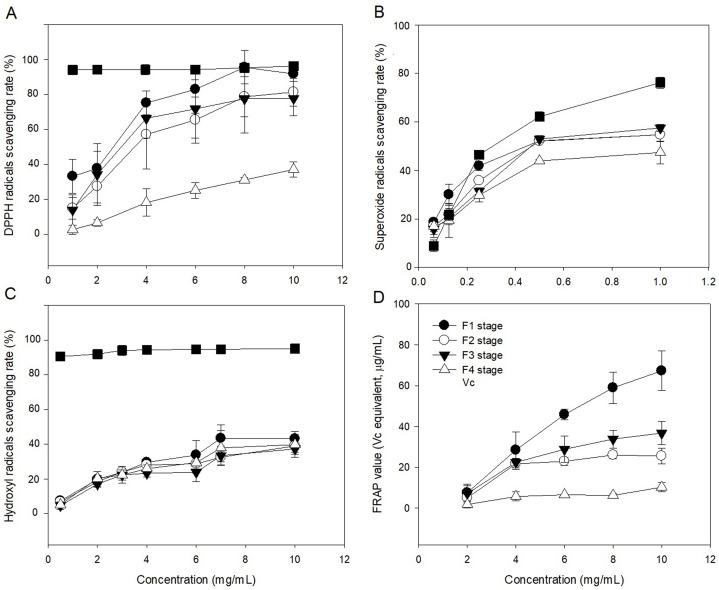
The radical (DPPH, OH, and O2−) scavenging activities of polysaccharide in leaves at different developmental stages were evaluated in comparison with Vc (Figs. 6A–6C). The ability of polysaccharide and Vc to scavenge DPPH radicals was measured and the results are shown in Fig. 6A. The polysaccharide from leaves of C. paliurus at different developmental stages has a concentration-dependent DPPH free radicals scavenging activity (1–10 mg/mL). The F4-stage polysaccharide exhibited DPPH free radical scavenging activities below 50% within 1–10 mg/mL. With increased polysaccharide concentration, the polysaccharide extracts at the F1 stage showed similar DPPH scavenging activity with Vc between 8 and 10 mg/mL. At a concentration of 1–10 mg/mL, the scavenging rates of polysaccharide at F2 and F3 stages were lower than the rate in Vc, but higher than at the F4 stage. Among the extracted polysaccharides, the F1 stage achieved the best DPPH scavenging ability.
Figure 6. Antioxidant activities of Cyclocarya paliurus polysaccharides in vitro.
(A) Scavenging activity of DPPH radicals; (B) scavenging activity of superoxide radicals; (C) scavenging activity of hydroxyl radicals; (D) reducing power.
The abilities of polysaccharide and Vc to scavenge superoxide radicals were measured and the results are shown in Fig. 6B. The polysaccharide from leaves of C. paliurus at different developmental stages showed concentration-dependent superoxide free radicals scavenging activity (0.0625–1 mg/mL). The F4-stage polysaccharide exhibited superoxide free radical scavenging activities below 50% within 0.0625-1 mg/mL. With increasing polysaccharide concentration, the superoxide free radical scavenging rates of polysaccharides in the leaves at F1, F2 and F3 stages were similar, but lower than that of Vc. The superoxide anion scavenging rate of polysaccharides was lowest at the F4 stage.
The abilities of polysaccharide and Vc to scavenge hydroxyl radicals were measured and the results are shown in Fig. 6C. The polysaccharide from leaves of C. paliurus at different developmental stages showed concentration-dependent hydroxyl radicals scavenging activity (0.5–10 mg/mL). The hydroxyl free radical scavenging rate of polysaccharides at the four developmental stages was lower than that of Vc. At high concentrations (10 mg/ml), the scavenging activity of polysaccharides in the leaves at the developmental stages of F1, F2, F3, and F4 stages were 43.154%, 39.045%, 37.258%, and 39.757%, respectively. The scavenging rate of hydroxyl radicals by polysaccharides was highest at the F1 stage.
The polysaccharides from leaves of C. paliurus at different developmental stages showed concentration-dependent ferric reducing-antioxidant power (2–10 mg/mL) (Fig. 6D). With increasing polysaccharide concentration, the ferric reducing-antioxidant power of polysaccharides in the leaves had different growth rates at four developmental stages. Among which, the growth rate was highest at the F1 stage and lowest at the F4 stage. At polysaccharide concentrations of 10 mg/mL, the reducing power of polysaccharides in the leaves at the four developmental stages of F1, F2, F3, and F4 were 67.262%, 25.498%, 36.749%, and 10.251%, respectively.
Discussion
Transcriptome analysis associated with polysaccharide synthesis
In this study, the polysaccharide contents of C. paliurus leaves were found to increase first and peaked at the F3 stage. Contents at the F4 stage were lower but the difference was not statistically significance. Similar trends were reported in the other plants. For example, Robakidze & Bobkova (2003) reported that the content of water-soluble polysaccharide was higher in older needles of Picea obovata. Wang et al. (2001) reported that the water-soluble polysaccharide content in sixth-class tea leaves (fully developed leaves) was twice as high as that of first-class tea leaves (young leaves). In addition, the peaks of two monosaccharide precursors (D-Fructose 6-phosphate and D-Glucose 6-phosphate) appeared earlier than the polysaccharide peaks (F2 stage vs. F3 stage). This indicates that in C. paliurus, the synthesis of polysaccharide accompanying the consumption of photosynthate increased with the development of leaves. Compared to stage F3, the slightly lower polysaccharide content in the leaves of C. paliurus at the F4 stage may be the result of flower bud differentiation at that stage, which may have led to the consumption of leaf nutrients (Dickmann & Gordon, 1975).
Active nucleotide sugars (NDP-sugars) are key precursors for plant polysaccharide biosynthesis. Previous studies showed that C. paliurus polysaccharides are composed of various NDP sugars, such as UDP-galactose (UDP-Gal), UDP-galacturonic acid (UDP-GalA), UDP-arabinose (UDP-Ara), UDP-rhamnose (UDP-Rha), GDP-mannose (GDP-Man), UDP-xylose (UDP-Xyl), and UDP-glucose (UDP-Glc) (Wang et al., 2019; Wang et al., 2015). Among them, GDP-Man and UDP-Glc are mainly derived from fructose-6-phosphate and glucose-6-phosphate, while other NDP-sugars are mainly converted from UDP-Glc under the actions of isomerase, dehydrogenase, and decarboxylase (Yin et al., 2011). Glucose-6-phosphate has two important metabolic branches, a fructose-6-P branch and a glucose-1-P branch; therefore, enzymes that catalyze these two branch reactions are most important in the biosynthesis of polysaccharides (Zhang et al., 2019). According to the results of the present study, the gene expression of these enzymes showed different changing trends. Although the contents of glucose-6-phosphate and fructose-6-phosphate followed similar changing trends in the pathway of polysaccharide synthesis, the difference in their content still affected the resulting content of NDP sugars. The genes encoding isomerase, dehydrogenase, and decarboxylase were found to have different expression levels at different developmental stages of C. paliurus leaves. These differing expression levels are indicative of a tendency for specific NDP-sugar synthesis, resulting in differences in the type and proportion of NDP-sugars at different developmental stages; moreover, the structure and monosaccharide composition ratio of polysaccharides may also differ.
Transfer of sugar moieties from activated donors to specific acceptors under the formation of glycosidic bonds is a key downstream step of polysaccharide biosynthesis (Shen et al., 2017). NDP-sugars are transferred to the residues of glycoconjugates and polysaccharides via action of various GTs, where they form plant polysaccharide (Pauly et al., 2013; Wang et al., 2012). GTs (EC 2.4.x.y) are enzymes that catalyze the transfer of sugar from active donors to specific receptor molecules. GTs are involved in a variety of biological processes in plants, including plant development, signal transduction, and plant defense (Yuan et al., 2018). GTs are dominant in unigenes associated with the carbohydrate metabolism. A total of 463 and 430 GT genes identified in A. thaliana and D. officinale were assigned to 42 and 35 families, respectively (Zhang et al., 2016). The present study identified 470 GTs in C. paliurus that were divided into 57 GT families (Table S2). The number of GT genes and families in C. paliurus exceeds that in A. thaliana and D. officinale (Zhang et al., 2016). In this study, 69 DEGs associated with GT were found to belong to 18 GT families (Fig. 3), which have different functions (Zhang et al., 2016). The families with the largest number of unigenes in the GT family were GT1 (68, 14.47%), GT2 (53, 11.28%), and GT47 (41, 8.72%). Among them, the families GT106, GT7, and GT3 are specific to C. paliurus. These specificities may reflect the unique polysaccharide synthesis of C. paliurus.
Different expression of these genes at four developmental stages indicates that they may exert different roles in the process of polysaccharide biosynthesis. Up-regulated GTs are probably involved in the synthesis of soluble polysaccharides in mature leaves, while down-regulated GTs are likely mainly used to build plant cell walls and other morphological structures at the juvenile stage. Genes with similar expression trends encode multiple glycosyltransferases, suggesting that a variety of glycosyltransferases co-mediate the development of the polysaccharide biosynthesis and morphological structure. The transcripts obtained in this study provide a basis for the development of highly efficient and sustainable natural production of C. paliurus polysaccharides.
Correlation analysis between the accumulation of polysaccharides and biosynthesis-related gene expression identified a significant correlation between 10 unigenes and the polysaccharide content. This provides evidence for the change of polysaccharide content during the development of C. paliurus leaves. Yang et al. (2016) showed that the monosaccharide composition of C. paliurus polysaccharide was rhamnose, arabinose, xylose, mannose, glucose, and galactose at a molar ratio of 1.00:2.23:0.64:0.49:0.63:4.16, respectively. Among these, the content of galactose was highest, identifying it as an important monosaccharide component of C. paliurus polysaccharide. The UDP-galactose synthase gene was significantly positively correlated with the polysaccharide content, which further identifies galactose as an important component of C. paliurus polysaccharide. However, other nucleotide sugars did not yield a significant positive correlation, which is likely because of their low monosaccharide content in C. paliurus polysaccharide or because synthetic nucleotide sugars were synthesized through other synthetic pathways. The results identified the relationship between polysaccharide content and polysaccharide synthesis pathway genes, which helps to increase polysaccharide content through molecular means and thus, to increase economic benefits.
Antioxidant activities of polysaccharide
As a stable free radical, DPPH has been widely employed to evaluate the free radical-scavenging ability of many natural compounds (Wang et al., 2014). The DPPH free radical scavenging ability of polysaccharides could be due to the presence of hydroxyls and carboxyls in the polysaccharide structure (mainly galacturonic acid). These act as hydrogen donators for scavenging the DPPH free radical, thus reducing the effects of oxidative stress (Hadidi et al., 2020). The superoxide radical is a weak oxidant, which is continuously produced by specific enzymes in biological systems and can induce the production of hydroxyl radicals and lipid peroxidation, thus damaging DNA, enzymes, and other biological molecules (Li & Shah, 2014). The possible mechanism underlying the polysaccharide scavenging of superoxide anions has been suggested to be associated with the dissociation energy of the OH bond, resulting from the number of carboxyl groups and aldehyde groups attached (Yuan et al., 2015). The hydroxyl group may subsequently provide electrons to reduce the radicals to more stable forms and/or may directly react with the free radicals to terminate the radical chain reaction thus causing the antioxidation of polysaccharides (Jing et al., 2009). The superoxide anion scavenging rate of polysaccharides was lowest at the F4 stage was lowest, which might be due to polysaccharides with lower content of carboxylic groups.
The hydroxyl radical possesses the highest activity among reactive oxygen species, enabling it to unspecifically attack adjacent biomolecules and induce oxidative damage in cells (Xu et al., 2018). The hydroxyl scavenging ability is related to the number of hydroxyl or amino groups in the polysaccharide (Guo et al., 2005). The hydroxyl radicals scavenging rate of polysaccharide were highest at the F1 stage was highest, which might be because polysaccharides had higher amino acid content at this stage. The reducing power of a compound is a useful indicator of its potential antioxidant activity (You et al., 2014). The reducing power was generally associated with the presence of reductones; therefore, polysaccharides at the F1 stage might contain higher levels of reductone, which could react with free radicals to stabilize and block radical chain reactions (Hou et al., 2012).
In the radical scavenging activity tests, the polysaccharides in leaves at different developmental stages showed different antioxidant activities. Many factors can affect the antioxidant capacity of polysaccharides, e.g., their uronic acid content, molecular weight, and monosaccharide composition (Wang et al., 2018). Several studies have postulated that the protein or peptide moiety in polysaccharides is partly responsible for their radical scavenging effect (Wang et al., 2016). Additionally, polysaccharides with lower molecular weights have more exposed reducing ends with which to accept and eliminate free radicals than polysaccharides with higher molecular weights; thus, polysaccharides with lower molecular weights might possess better antioxidant activity (Wu et al., 2020). The expression of genes in the polysaccharide biosynthesis pathway differs at different developmental stages, which may lead to different molecular weights of polysaccharides at different developmental stages, thus, in turn, leading to different antioxidant activities.
According to the results of the four antioxidant activity indexes, the polysaccharides at the F1 stage had the best antioxidant activity, followed by those at the F3 stage, while those at the F4 stage had the lowest activity. However, the polysaccharide content of leaves at the F1 stage was lowest and the biomass of leaves was also the lowest because of their small leaf size and leaf number. In summary, the leaves at the F1 stage are suitable for high-quality tea, but not suitable as raw material for polysaccharide products. Instead, the leaves at the F3 stage were more suitable for polysaccharide products.
Conclusions
In this study, within the transcriptome database of C. paliurus, 1,333 carbohydrate-active enzymes were identified (including 470 glycosyltransferases) were identified. Sixty-nine DEGs associated with glycosyltransferases belong to 18 families. The UDP-glucose 4-epimerase gene was significantly positively correlated with the polysaccharide content. A pathway map for the biosynthesis of C. paliurus polysaccharide was proposed. The results provide a solid basis for the study of the synthesis of polysaccharides of C. paliurus. The polysaccharide contents and their antioxidant activities in the leaves of C. paliurus were assessed at four different developmental stages. The leaves at the F1 stage are suitable for high-quality tea because of the good taste and the high antioxidant ability of the contained polysaccharide. At the F3 stage, the leaves are suitable for industrial production of polysaccharide for their high polysaccharide content and relatively higher antioxidant ability of polysaccharide. These results provide clear guidance for the application of C. paliurus leaves for food, functional food, or medicinal applications.
Supplemental Information
*Indicates significant correlation (P-value < 0.05, r > 0.9).
Funding Statement
This work was financially supported by Zhejiang Provincial Key Research and Development Program (2018C02021) and the Ten Thousand Talent Program of Zhejiang Province (2019R52043). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Junmin Li, Email: lijm@tzc.edu.cn.
Hongfei Lu, Email: luhongfei@zstu.edu.cn.
Additional Information and Declarations
Competing Interests
Jianmei Wang is employed by Zhejiang Yuanyang Agriculture development Co. Ltd. The authors declare that they have no competing interests.
Author Contributions
Weida Lin performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Huanwei Chen conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Jianmei Wang conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Yongli Zheng conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Qiuwei Lu analyzed the data, prepared figures and/or tables, and approved the final draft.
Ziping Zhu analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Na Li analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Zexin Jin conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Junmin Li conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Hongfei Lu conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Samples collection were permitted by Forest Research Institute of Longquan City, Zhejiang Province.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
All raw data are available at NCBI: PRJNA548403.
Data Availability
References
- Cao et al. (2019).Cao YN, Fang SZ, Fu XX, Shang XL, Yang WX. Seasonal variation in phenolic compounds and antioxidant activity in leaves of Cyclocarya paliurus (Batal.) Iljinskaja. Forests. 2019;10(8):624. doi: 10.3390/f10080624. [DOI] [Google Scholar]
- Chen, You & Shan (2019).Chen B, You WJ, Shan TZ. Myomaker, and myomixer-myomerger-minion modulate the efficiency of skeletal muscle development with melatonin supplementation through Wnt/β-catenin pathway. Experimental Cell Research. 2019;385(2):111705. doi: 10.1016/j.yexcr.2019.111705. [DOI] [PubMed] [Google Scholar]
- Dickmann & Gordon (1975).Dickmann DI, Gordon JC. Incorporation of C-photosynthate into protein during leaf development in young populus plants. Plant Physiology. 1975;56(1):23–27. doi: 10.1104/pp.56.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois et al. (1956).Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Fu et al. (2015).Fu XX, Zhou XD, Deng B, Shang XL, Fang SZ. Seasonal and genotypic variation of water-soluble polysaccharide content in leaves of Cyclocarya paliurus. Southern Forests: A Journal of Forest Science. 2015;77(3):231–236. doi: 10.2989/20702620.2015.1010698. [DOI] [Google Scholar]
- Guo et al. (2017).Guo F, Guo YF, Wang P, Wang Y, Ni DJ. Transcriptional profiling of catechins biosynthesis genes during tea plant leaf development. Planta. 2017;246(6):1139–1152. doi: 10.1007/s00425-017-2760-2. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2005).Guo ZY, Xing RE, Liu S, Yu HH, Wang PB, Li CP, Li PC. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorganic & Medicinal Chemistry Letters. 2005;15:4600–4603. doi: 10.1016/j.bmcl.2005.06.095. [DOI] [PubMed] [Google Scholar]
- Hadidi et al. (2020).Hadidi M, Amoli PI, Jelyani AZ, Hasiri Z, Rouhafza A, Ibarz A, Khaksar FB, Tabrizi ST. Polysaccharides from pineapple core as a canning by-product: extraction optimization, chemical structure, antioxidant and functional properties. International Journal of Biological Macromolecules. 2020;163(1):2357–2364. doi: 10.1016/j.ijbiomac.2020.09.092. [DOI] [PubMed] [Google Scholar]
- He et al. (2017).He MJ, Zeng JY, Zhai L, Liu YG, Wu HC, Zhang RF, Li ZT, Xia EG. Effect of in vitro simulated gastrointestinal digestion on polyphenol and polysaccharide content and their biological activities among 22 fruit juices. Food Research International. 2017;102(2):156–162. doi: 10.1016/j.foodres.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Hou et al. (2012).Hou Y, Wang J, Jin WH, Zhang H, Zhang QB. Degradation of Laminaria japonica fucoidan by hydrogen peroxide and antioxidant activities of the degradation products of different molecular weights. Carbohydrate Polymers. 2012;87(1):153–159. doi: 10.1016/j.carbpol.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Jing et al. (2009).Jing KY, Lin L, Zhao MW, Po HL, Wen QW, Jian YW. Acidic degradation and enhanced antioxidant activities of exopolysaccharides from Cordyceps sinensis mycelial culture. Food Chemistry. 2009;117(4):641–646. doi: 10.1016/j.foodchem.2009.04.068. [DOI] [Google Scholar]
- Kakar et al. (2020).Kakar MU, Naveed M, Saeed M, Zhao SC, Rasheed M, Firdoos S, Manzoor R, Deng Y, Dai R. A review on structure, extraction, and biological activities of polysaccharides isolated from Cyclocarya paliurus (Batalin) Iljinskaja. International Journal of Biological Macromolecules. 2020;156:420–429. doi: 10.1016/j.ijbiomac.2020.04.022. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017).Li H, Huang W, Wang GL, Wang WL, Cui X, Zhuang J. Trnascriptomic analysis of the biosynthesis, recycling, and distribution of ascorbic acid during leaf development in tea plant (Camellia sinensis (L.) O Kuntze) Scientific Reports. 2017;7(1):46212. doi: 10.1038/srep46212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Shah (2014).Li SQ, Shah NP. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chemistry. 2014;165(4):262–270. doi: 10.1016/j.foodchem.2014.05.110. [DOI] [PubMed] [Google Scholar]
- Li et al. (2016).Li CF, Xu YX, Ma JQ, Jin JQ, Huang DJ, Yao MZ, Ma CL, Chen L. Biochemical and transcriptomic analyses reveal different metabolite biosynthesis profiles among three color and developmental stages in ‘Anji Baicha’ (Camellia sinensis) BMC Plant Biology. 2016;16(1):195. doi: 10.1186/s12870-016-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2020).Lin WD, Li YL, Lu QW, Lu HF, Li JM. Combined analysis of the metabolome and transcriptome identified candidate genes involved in phenolic acid biosynthesis in the leaves of Cyclocarya paliurus. International Journal of Molecular Sciences. 2020;21(4):1337. doi: 10.3390/ijms21041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu Y, Fang SZ, Zhou MM, Shang XL, Yang WX, Fu XX. Geographic variation in water-soluble polysaccharide content and antioxidant activities of Cyclocarya paliurus leaves. Industrial Crops and Products. 2018;121:180–186. doi: 10.1016/j.indcrop.2018.05.017. [DOI] [Google Scholar]
- Liu et al. (2015).Liu J, Yang D, Liu ST, Li SG, Xu GR, Zheng GL, Xie LP, Zhang RQ. Microarray: a global analysis of biomineralization-related gene expression profiles during larval development in the pearl oyster, Pinctada fucata. BMC Genomics. 2015;16(1):325. doi: 10.1186/s12864-015-1524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, Chiou & Leng (2008).Mueller HG, Chiou JM, Leng XY. Inferring gene expression dynamics via functional regression analysis. BMC Bioinformatics. 2008;9(1):60. doi: 10.1186/1471-2105-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly et al. (2013).Pauly M, Gille S, Liu LF, Mansoori N, De Souza A, Schultink A, Xiong GY. Hemicellulose biosynthesis. Planta. 2013;238(4):627–642. doi: 10.1007/s00425-013-1921-1. [DOI] [PubMed] [Google Scholar]
- Petropoulos et al. (2018).Petropoulos SA, Fernandes A, Vasileios A, Ntatsi G, Barros L, Ferreira ICFR. Chemical composition and antioxidant activity of Cichorium spinosum L. leaves in relation to developmental stage. Food Chemistry. 2018;239:946–952. doi: 10.1016/j.foodchem.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Robakidze & Bobkova (2003).Robakidze EA, Bobkova KS. Carbohydrate accumulation in siberian spruce needles of various ages. Russian Journal of Plant Physiology. 2003;50(4):509–515. doi: 10.1023/A:1024724907949. [DOI] [Google Scholar]
- Shen et al. (2017).Shen CJ, Guo H, Chen HL, Shi YJ, Meng YJ, Lu JJ, Feng SG, Wang HZ. Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using RNA-Seq. Scientific Reports. 2017;7:187. doi: 10.1038/s41598-017-00292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar, Johnson & Zimmer (2019).Shivakumar VS, Johnson G, Zimmer EA. Transcriptome analysis of the curry tree (Bergera koenigii L., Rutaceae) during leaf development. Scientific Reports. 2019;9(1):4230. doi: 10.1038/s41598-019-40227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2019).Song YR, Han AR, Lim TG, Kang JH, Hong HD. Discrimination of structural and immunological features of polysaccharides from persimmon leaves at different maturity stages. Molecules. 2019:24. doi: 10.3390/molecules24020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub (1965).Staub AM. Removal of proteins: Sevag method. Methods in Carbohydrate Chemistry. 1965;5:5–6. [Google Scholar]
- Sweetman et al. (2012).Sweetman C, Wong DCJ, Ford CM, Drew DP. Transcriptome analysis at four developmental stages of grape berry (Vitis vinifera cv. Shiraz) provides insights into regulated and coordinated gene expression. BMC Genomics. 2012;13(1):691. doi: 10.1186/1471-2164-13-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang Y, Alonso AP, Wilkerson CG, Keegstra K. Deep EST profiling of developing fenugreek endosperm to investigate galactomannan biosynthesis and its regulation. Plant Molecular Biology. 2012;79(3):243–258. doi: 10.1007/s11103-012-9909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2016).Wang JQ, Hu SZ, Nie SP, Yu Q, Xie MY. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Medicine and Cellular Longevity. 2016;2016(64):5692852-13. doi: 10.1155/2016/5692852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang YF, Jia JX, Ren XJ, Li BH, Zhang Q. Extraction, preliminary characterization and in vitro antioxidant activity of polysaccharides from Oudemansiella radicata mushroom. International Journal of Biological Macromolecules. 2018;120(21):1760–1769. doi: 10.1016/j.ijbiomac.2018.09.209. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang ZB, Pei JJ, Ma HL, Cai PF, Yan JK. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohydrate Polymers. 2014;109:49–55. doi: 10.1016/j.carbpol.2014.03.057. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2001).Wang DF, Wang CH, Li J, Zhao GW. Components and activity of polysaccharides from coarse tea. Journal of Agricultural and Food Chemistry. 2001;49(1):507–510. doi: 10.1021/jf000029m. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2015).Wang ZJ, Xie JH, Kan LJ, Wang JQ, Shen MY, Li WJ, Nie SP, Xie MY. Sulfated polysaccharides from Cyclocarya paliurus reduce H2O2-induced oxidative stress in RAW264.7 cells. International Journal of Biological Macromolecules. 2015;80(Part 3):410–417. doi: 10.1016/j.ijbiomac.2015.06.031. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2019).Wang XJ, Zhao XL, Lv YJ, Hu MH, Fan LD, Li QY, Cai C, Li GY, Yu GL. Extraction, isolation and structural characterization of a novel polysaccharide from Cyclocarya paliurus. International Journal of Biological Macromolecules. 2019;132:864–870. doi: 10.1016/j.ijbiomac.2019.03.148. [DOI] [PubMed] [Google Scholar]
- Wong et al. (2017).Wong YC, Teh HF, Mebus K, Ooi TEK, Kwong QB, Koo KL, Ong CK, Mayes S, Chew FT, Appleton DR, Kulaveerasingam H. Differential gene expression at different stages of mesocarp development in high- and low-yielding oil palm. BMC Genomics. 2017;18(1):470. doi: 10.1186/s12864-017-3855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. (2020).Wu HY, Li MM, Yang XR, Wei Q, Sun LZY, Zhao JC, Shang HM. Extraction optimization, physicochemical properties and antioxidant and hypoglycemic activities of polysaccharides from roxburgh rose ( Rosa roxburghii Tratt.) leaves. International Journal of Biological Macromolecules. 2020;165:517–529. doi: 10.1016/j.ijbiomac.2020.09.198. [DOI] [PubMed] [Google Scholar]
- Xie et al. (2006).Xie MY, Li L, Nie SP, Wang XR, Lee FSC. Determination of speciation of elements related to blood sugar in bioactive extracts from Cyclocarya paliurus leaves by FIA-ICP-MS. European Food Research and Technology. 2006;223(2):202–209. doi: 10.1007/s00217-005-0173-0. [DOI] [Google Scholar]
- Xie et al. (2013).Xie JH, Liu X, Shen MY, Nie SP, Zhang H, Li C, Gong DM, Xie MY. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chemistry. 2013;136(3–4):1453–1460. doi: 10.1016/j.foodchem.2012.09.078. [DOI] [PubMed] [Google Scholar]
- Xie et al. (2014).Xie JH, Shen MY, Nie SP, Zhao Q, Li C, Xie MY. Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydrate Polymers. 2014;101(3):479–483. doi: 10.1016/j.carbpol.2013.09.075. [DOI] [PubMed] [Google Scholar]
- Xie et al. (2012).Xie JH, Shen MY, Xie MY, Nie SP, Chen Y, Li C, Huang DF, Wang YX. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohydrate Polymers. 2012;89(1):177–184. doi: 10.1016/j.carbpol.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Xie et al. (2016).Xie JH, Wang ZJ, Shen MY, Nie SP, Gong B, Li HS, Zhao Q, Li WJ, Xie MY. Sulfated modification, characterization and antioxidant activities of polysaccharide from Cyclocarya paliurus. Food Hydrocolloids. 2016;53:7–15. doi: 10.1016/j.foodhyd.2015.02.018. [DOI] [Google Scholar]
- Xie et al. (2010a).Xie JH, Xie MY, Shen MY, Nie SP, Li C, Wang YX. Optimisation of microwave-assisted extraction of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja using response surface methodology. Journal of the Science of Food and Agriculture. 2010a;90(8):1353–1360. doi: 10.1002/jsfa.3935. [DOI] [PubMed] [Google Scholar]
- Xie et al. (2010b).Xie JH, Xie M, Nie S, Shen M, Wang Y, Li C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chemistry. 2010b;119(4):1626–1632. doi: 10.1016/j.foodchem.2009.09.055. [DOI] [Google Scholar]
- Xu et al. (2018).Xu YQ, Niu XJ, Liu NY, Gao YK, Wang LB, Xu G, Li XG, Yang Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chemistry. 2018;243(4):26–35. doi: 10.1016/j.foodchem.2017.09.107. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang WX, Liu Y, Fang SZ, Ding HF, Zhou MM, Shang XL. Variation in growth, photosynthesis and water-soluble polysaccharide of Cyclocarya paliu rus under different light regimes. iForest—Biogeosciences and Forestry. 2017;10(2):468–474. doi: 10.3832/ifor2185-010. [DOI] [Google Scholar]
- Yang et al. (2016).Yang ZW, Ouyang KH, Zhao J, Chen H, Xiong L, Wang WJ. Structural characterization and hypolipidemic effect of Cyclocarya paliurus polysaccharide in rat. International Journal of Biological Macromolecules. 2016;91:1073–1080. doi: 10.1016/j.ijbiomac.2016.06.063. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2018).Yang ZW, Wang J, Li JG, Xiong L, Chen H, Liu X, Wang N, Ouyang K, Wang WJ. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocarya paliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydrate Polymers. 2018;183(3):11–20. doi: 10.1016/j.carbpol.2017.11.033. [DOI] [PubMed] [Google Scholar]
- Yin et al. (2011).Yin YB, Huang JL, Gu XG, Bar-Peled M, Xu Y. Evolution of plant nucleotide-sugar interconversion enzymes. PLOS ONE. 2011;6 doi: 10.1371/journal.pone.0027995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You et al. (2014).You QH, Yin XL, Zhang SN, Jiang ZH. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydrate Polymers. 2014;99:1–10. doi: 10.1016/j.carbpol.2013.07.088. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2015).Yuan QX, Xie YF, Wang W, Yan YH, Ye H, Jabbar S, Zeng XX. Extraction optimization, characterization and antioxidant activity in vitro of polysaccharides from mulberry (Morus alba L.) leaves. Carbohydrate Polymers. 2015;128(11):52–62. doi: 10.1016/j.carbpol.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Yuan et al. (2018).Yuan YD, Yu MY, Jia ZH, Song XE, Liang YQ, Zhang JC. Analysis of Dendrobium huoshanense transcriptome unveils putative genes associated with active ingredients synthesis. BMC Genomics. 2018;19(1):978. doi: 10.1186/s12864-018-5305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai et al. (2018).Zhai LX, Ning ZW, Huang T, Wen B, Liao CH, Lin CY, Zhao L, Xiao HT, Bian ZX. Cyclocarya paliurus leaves tea improves dyslipidemia in diabetic mice: a lipidomics-based network pharmacology study. Frontiers in Pharmacology. 2018;9:973. doi: 10.3389/fphar.2018.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang JX, He CM, Wu KL, Da Silva JAT, Zeng SJ, Zhang XH, Yu ZM, Xia HQ, Duan J. Transcriptome analysis of Dendrobium officinale and its application to the identification of genes associated with polysaccharide synthesis. Frontiers in Plant Science. 2016;7:5. doi: 10.3389/fpls.2016.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang N, Tang Z, Zhang J, Li X, Yang Z, Yang C, Zhang Z, Huang Z. Comparative transcriptome analysis reveals the genetic basis underlying the biosynthesis of polysaccharides in Hericium erinaceus. Botanical Studies. 2019;60 doi: 10.1186/s40529-019-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang H, Yohe T, Huang L, Entwistle S, Wu P, Yang ZL, Busk PK, Xu Y, Yin YB. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Research. 2018;46(W1):W95–W101. doi: 10.1093/nar/gky418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2018).Zhao S, Zhang XM, Su YP, Chen YL, Liu Y, Sun M, Qi GH. Transcriptome analysis reveals dynamic fat accumulation in the Walnut Kernel. International Journal of Genomics. 2018;2018(6):8931651-13. doi: 10.1155/2018/8931651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2019).Zhou MM, Lin Y, Fang SZ, Liu Y, Shang XL. Phytochemical content and antioxidant activity in aqueous extracts of Cyclocarya paliurus leaves collected from different populations. PeerJ. 2019;7:e6492. doi: 10.7717/peerj.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
*Indicates significant correlation (P-value < 0.05, r > 0.9).
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental Files: Table S5 (Figure 4D), S6 (Figure 5), S7 (Figure 6A), S8 (Figure 6B), S9 (Figure 6C), S10 (Figure 6D).