Abstract
Cocaine- and amphetamine-regulated transcript encodes an eponymous peptide, CARTp, which exerts diverse pharmacologic actions in the central and peripheral nervous systems, as well as in several endocrine organs, including pancreas. Here we review those diverse actions, the physiological relevance of which had remained unestablished until recently. With the identification of a CARTp receptor, GPR160, the physiologic importance and therapeutic potential of CARTp or analogs are being revealed. Not only is the CARTp-GPR160 interaction essential for the circadian regulation of appetite and thirst but also for the transmission of nerve injury-induced pain. Molecular approaches now are uncovering additional physiologically relevant actions and the development of acute tissue-specific gene compromise approaches may reveal even more physiologically relevant actions of this pluripotent ligand/receptor pair.
Keywords: satiation, allodynia, addiction, vagus nerve, obesity
The initial excitement that follows the identification of a previously unrecognized, endogenous hormone continues to grow when genetic mutations are created in experimental animals and the consequences of loss of function mutations are reported in human populations. All too often, however, that initial enthusiasm for the hormone’s potential as a therapeutic agent wanes because the development of clinically relevant ligands is stalled by the failure of efforts to identify a cognate receptor. Until such a receptor is identified, the field progresses along with a host of suggestive evidences, descriptions of localization, potential sites of action, and observations of changes in gene transcription or hormone levels under a variety of experimental conditions or human disease states. Enthusiasm is suspended by many in the field, but never completely abandoned by some investigators, particularly those who value the promise of the development of novel ligands for the treatment of common diseases that have not been controlled by available pharmacologic tools. One such example of this barrier to progress recently may have been overcome, and the promise of treatment of a wide variety of human disease states has been renewed.
Discovery and Early Descriptions of Cocaine- and Amphetamine-Regulated Transcript Peptide Actions
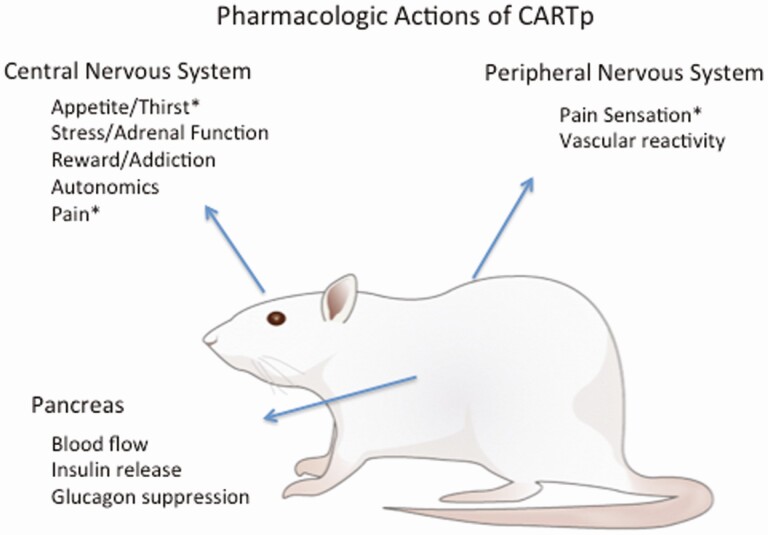
The transcript identified to be upregulated by psychostimulants in rodent brain, cocaine- and amphetamine-regulated transcript (CART) (1), was found to encode a large peptide, CARTp, which exerts multiple pharmacologic effects in rodents including in pancreas, as well as central and peripheral nervous systems (Fig. 1). But the physiological relevance of those actions remained uncertain until the recent identification of a CARTp receptor (2). Over 20 years ago, CARTp encoding messenger RNA was observed in rat islet tumors and normal islets of Langerhans (3), and in situ hybridization combined with immunostaining revealed the presence of CARTp in delta cells of the rat islet. CARTp-immunoreactivity was subsequently observed in pancreatic nerves (4,5). Although originally hypothesized to act within the islet to enhance proliferation during development, prior studies failed to identify proliferative effects in cultured INS-1 cells (4). Glucose homeostasis was altered in embryonic CART knockout (KO) mice (6); animals displayed impaired insulin secretion to glucose challenge at 40 weeks of age in vivo and impaired insulin secretion from harvested islets in vitro. CARTp was demonstrated to potentiate glucose-stimulated insulin secretion in islets treated with glucagon-like peptide-1 (GLP-1) or forskolin, but the peptide was, if anything, inhibitory to glucose-stimulated insulin secretion in the absence of agents that elevated protein kinase A activity in those tissues (7).
Figure 1.
Summary of diverse pharmacologic actions of CART peptide, described in experimental animals, discussed in the text. Asterisks indicate actions demonstrated to be physiologically relevant.
Emerging Evidence of Potential Physiological Relevance
More recently, Wierup and colleagues (8) provided evidence suggesting a physiologically relevant role for endogenous CARTp in beta cell function. Using a small interfering RNA approach they demonstrated CART silencing to alter the expression of transcription factors involved in insulin production and secretion. Thus, not only was insulin secretion compromised in CART KO animals, a potential role for endogenous CARTp in islet cell survival had been established. More work from the same group (9) revealed the expression of CART in alpha as well as beta cells and the upregulation of CART expression in islets from diabetic humans and mice. Importantly, that work also identified an action of CARTp to inhibit glucagon secretion from human and mouse islets leading these authors to suggest that CART or analogs of the peptide might be developed as antidiabetic agents. But one major problem remained that seemed to block the development of therapeutic agents: no unique CARTp receptor had yet to be identified.
An additional role for CARTp in endocrine function initially was proposed when an extensive distribution of CARTp-positive cells was identified in hypothalamus—in particular, in arcuate, paraventricular, supraoptic, and anterior periventricular nuclei (10). Koylu et al also identified CARTp-positive cells in the anterior pituitary gland, although the staining there was not as intense as that observed in nerve fibers in the adjacent neural lobe (10). Those observations, paired with the detection of CARTp-positive cells in the adrenal medulla, led the authors to hypothesize a role for CARTp in the hypothalamo-pituitary-adrenal (HPA) axis (10). An even more extensive characterization of CARTp staining subsequently characterized the distribution in both rat and human brains (11). That study revealed colocalization of CARTp with several other neuropeptides known to be involved in energy homeostasis and thus support for a role of CARTp-producing neurons in metabolism was introduced. A literature has developed indicating CARTp to exert significant effects on autonomic function (12-16), thermoregulation (17), energy homeostasis (18), and appetite (see following discussion). In terms of the potential actions of CARTp within the HPA axis, 2 groups independently identified actions of CARTp on neurons producing corticotropin-releasing hormone (CRH). Vrang and colleagues observed increased c-Fos staining in CRH and oxytocin neurons following central administration of CARTp and a concomitant increase in plasma corticosterone and oxytocin levels (19). Plasma glucose levels also increased following CARTp administration into the lateral cerebroventricle, which may have reflected activation of oxytocinergic projections to sympathetic centers in brainstem (19). Stanley and coworkers similarly administered CARTp centrally and observed, at a dose of the peptide that inhibited food intake, a significant elevation in plasma adrenocorticotropin and corticosterone levels (20). Plasma levels of prolactin increased transiently as did plasma growth hormone concentrations at a single time point. When a lower dose of CARTp was administered directly into the paraventricular nucleus, a transient but significant increase in plasma adrenocorticotropin, but not prolactin or growth hormone, levels was observed. These results were matched by hypothalamic explant studies where CARTp stimulated the release of CRH, neuropeptide Y, thyrotropin-releasing hormone, and alpha-melanocyte–stimulating hormone. In summary, anatomic and pharmacologic studies strongly suggested that CARTp was an important contributor to the control of the HPA axis, but once again, in the absence of potent selective antagonists, the physiological relevance could not be firmly established. A receptor would have to be identified.
Identification of a Potential CARTp Receptor
Our group had developed a strategy with which we identified potential receptors for neuronostatin (21) and phoenixin (22), 2 novel peptides we discovered using a bioinformatics approach (23,24). We also had been able with our deductive reasoning strategy to identify potential receptors for proinsulin-connecting peptide (25) and adropin (26). We were impressed by the broad pharmacologic portfolio of actions of CARTp and curious why no cognate receptor had been identified. There existed in the literature strong evidence that CARTp signaled via a G protein-coupled receptor (GPCR) (27-29), and thus we utilized our receptor identification approach to determine if one of the list of orphan GPCRs cataloged by International Union of Basic and Clinical Pharmacology (30) might be a receptor for CARTp. We employed both in vivo and in vitro approaches in our search. The existing literature suggested a role for CARTp in the transmission of neuropathic pain (31-34), including peptide expression in dorsal root ganglion of the lumbar region and pain responses elicited by intrathecal administration of the CARTp. In an established model [chronic constriction of the sciatic nerve (CCI)] of pain, we searched for transcripts that might be elevated in dorsal horn laminae 1 and 2 of the spinal cord on the ipsilateral side of the injury, compared to the contralateral side, and identified several possible candidates. From that list of candidates, we then examined databases for nonorphan GPCRs, known to be elevated in pain states. Those receptor sequences were then used to search for homologous orphan GPCRs that were known to be expressed in tissues where CARTp had been demonstrated to exert pharmacologic effects. We quickly focused on the orphan GPCR, GPR160, as our top candidate. Indeed we characterized the increased expression of GPR160 on the ipsilateral side of the nerve injury by immunostaining, reverse transcription polymerase chain reaction, and RNA-Seq analysis (2).
Just demonstrating expression changes in the pain model wasn’t enough to convince us that GPR160 was necessary for the transmission of neuropathic pain. Instead, we compromised GPR160 expression using intrathecal injections of a small interfering RNA construct designed to selectively target the Gpr160 transcript and found that this approach not only prevented the onset of both spinal injury–induced CCI and spared-nerve injury–induced (35) mechano-allodynia but also reversed established pain in the CCI model (2). Passive immunoneutralization of endogenous GPR160 also resulted in a reversal of CCI-induced mechano-allodynia. The effects were observed in male and female animals. Our task then became the final link, that CARTp bound to GPR160. In human KATO III cells, knockdown of Gpr160 prevented the ability of CARTp to increase cfos expression and CARTp co-immunoprecipitated with GPR160 from KATO III cells membrane extracts. In addition, Gpr160 knockdown abrogated CARTp-induced extracellular signal-regulated kinase phosphorylation in cultured PC-12 cells. Physical association of CARTp with GPR160 was demonstrated using a proximity ligation assay. We believe we had identified at least 1 CARTp receptor (2).
Is GPR160 Necessary for Other Actions of CARTp?
Using the passive immunoneutralization approach, we presented evidence that CARTp requires the presence of GPR160 to exert its pharmacologic and physiologic anorexigenic actions (36). CARTp had long been known to exert anorexigenic action when administered into the ventricular system or rodents (17,37-41), and both hypothalamic and brainstem sites of action had been hypothesized. In fact, as early as 1998, Kristensen and colleagues had demonstrated that passive immunoneutralization of endogenous CARTp levels resulted in exaggerated normal, light-entrained food intake in rats (42), and more recently, Lee and colleagues demonstrated a similar effect of anti-CARTp antibody when injected into the nucleus solitarius of the rodent brainstem (43), a site of abundant Gpr160 expression (36). Those authors also established that the anorexigenic action of CART in brainstem was due to delivery of the peptide via vagal afferents to appetite-related areas adjacent to the area postrema (43) and that the anorexigenic effect of cholecystokinin required release of CARTp into brainstem feeding centers. Cholecystokinin isn’t the only gut hormone known to control the activity of vagal afferents; in fact, GLP-1 receptors are present in nodose ganglion and peripheral administration of the GLP-1 inhibits food intake via an effect on those afferents and possibly GLP-1 receptors in brainstem (44). CARTp interacting with GPR160 appears also to be important in the control of thirst (36,45), an effect that at least temporally differs from that on feeding (36,46).
Translational Potential for the Development of CARTp Analogs or Mimetics
Embryonic KOs of the CART gene in mice resulted in varying phenotypes. Wierup and colleagues (6) reported that CART KO mice, provided standard lab chow, displayed impaired insulin responses to glucose even before any significant changes in body weight were observed, compared to wild-type (WT) controls. In addition, pancreatic islets isolated from KO mice released less insulin in response to glucose than islets from WT mice. Another group (35) compared CART homozygous KO mice to heterozygous CART KO and WT mice on a high-fat diet. Homozygous male KO mice consumed more food and gained more bodyweight and fat mass than heterozygous male KO mice and WT mice. In females, both homozygous and heterozygous mice ate more food and gained more body weight and fat mass than WT females (35). These were global gene compromise models, and therefore the reason for the observed phenotypes can only be hypothesized. Was it due to the loss of the protropic effect on the beta cell or perhaps the loss of the inhibitory effect on glucagon secretion? Could it have been due to the loss of CARTp’s actions in the central nervous system? Even site-specific gene compromise of CARTp production might not address the issue of site of deficit since CARTp circulates in blood and those levels, as well as tissue concentrations, are affected by multiple factors including leptin (15) and adrenal steroids (47). However, these KO animals still have important value. It would be interesting to know if reintroduction of CART expression in these animals or simply daily or continuous infusion of exogenous peptide would reverse some of the phenotypes observed. If so, then there might be promise for treatment of individuals described next.
Current Challenges
As previously described, mutations in the gene encoding CARTp resulted in obese phenotypes. Could the same result from mutations in the gene encoding GPR160? Single nucleotide polymorphisms have been cataloged in human tumors (https://www.ncbi.nlm.nih.gov/snp/?term=GPR160); however, patient populations expressing mutations in Gpr160 have yet to our knowledge been described, at least in terms of a characteristic phenotype. We would hypothesize that if they did exist, the clinical presentation of those individuals would mirror that of the families described next. It is, however, possible that more than 1 receptor for CARTp exists, although compromise of GPR160 levels in rodents did abrogate both pharmacologic and pathophysiologic actions of CARTp (2). Just the same, at least 1 group has suggested that in addition to signaling via a Gi/o mechanism, CARTp may signal via a Gs mechanism (48), and therefore either GPR160 must signal via alternative G proteins dependent upon cell type and physiologic condition or more than 1 receptor may exist. This will require further validation.
Prospects for the Future
Information from human mutation studies revealed that alteration in CART prehormone production and processing resulted in profound metabolic and behavioral phenotypes, suggesting that therapeutic administration of CARTp or mimetics might provide some benefit. The first report of mutations in the CART gene detailed the result of a Leu34Phe in an adolescent with early onset obesity (49). A cosegregating history of obesity and diabetes was noted on his mother’s side of the family, as was a history of anxiety and depression (50). When this mutation was introduced into AtT20 cells, evidence suggesting altered processing of the CART prohormone was observed (51). Altered posttranslational processing and intracellular trafficking of CARTp subsequently was demonstrated by Yanik and colleagues (52) in AtT20 cells transfected with the mutant gene (Leu34Phe), and importantly, these authors demonstrated that circulating mature (ie, bioactive) CARTp levels were reduced in an obese kindred bearing the mutation. Indeed, work by some of the same investigators (53) revealed several single-nucleotide polymorphisms in the CART gene that correlated with extreme obesity. Another group reported in a separate population of children that the A1475G mutation was significantly associated with overweight and obesity (54). Thus, mutations in the CART gene have been identified, which cosegregate with obesity, and the underlying reason for the development of that phenotype might well be the altered processing and trafficking within the cell of the CART prohormone.
Could the metabolic consequences of low levels of mature bioactive CARTp be overcome by replacement of the missing peptide in these individuals? This alone highlights the importance of identifying a cognate CARTp receptor. Unless the gene deficit itself can be corrected, perhaps the best approach would be to see whether the administration of exogenous WT CARTp or a stable analog might ameliorate some of the devastating consequences of improper processing and trafficking of the CART gene product. The identification of a CARTp receptor now enables the development of selective and/or biased receptor ligands with which to either drive the CARTp/GPR160 tandem to inhibit appetite and address metabolic issues or, in the case of pain sensation, interrupt that signaling. Challenges to be overcome in those efforts will include issues of delivery and site-selective targeting, in addition to the pharmacokinetic properties of peptide analogs. Just the same, the possibility that safe, effective, and tissue-specific ligands could be developed will certainly contribute to our ability to gain additional insight into the cellular basis of several pathologies and, with any luck, enable the development of novel therapeutic agents.
Acknowledgments
Due to space limitations the authors regret not being able to reference the entire existing literature on CARTp. We have relied on public database searches to select the findings described here and recommend that approach to readers seeking a broader exposure to the literature.
Financial Support: Work related to this review was provided by NIH grants DK118340 (GLCY), NS113257 (DS and GLCY), and HL121456 (WS).
Additional Information
Disclosures: WKS, DS, and GLCY are holders of patent no. 10,85,378 (Methods for Treating Pain Using Anti-GPR160 Antibodies).
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Douglass J, Daoud S. Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene. 1996;169(2):241-245. [DOI] [PubMed] [Google Scholar]
- 2. Yosten GL, Harada CM, Haddock C, et al. GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. J Clin Invest. 2020;130(5):2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jensen PB, Kristensen P, Clausen JT, et al. The hypothalamic satiety peptide CART is expressed in anorectic and non-anorectic pancreatic islet tumors and in the normal islet of Langerhans. FEBS Lett. 1999;447(2-3):139-143. [DOI] [PubMed] [Google Scholar]
- 4. Wierup N, Kuhar M, Nilsson BO, Mulder H, Ekblad E, Sundler F. Cocaine- and amphetamine-regulated transcript (CART) is expressed in several islet cell types during rat development. J Histochem Cytochem. 2004;52(2):169-177. [DOI] [PubMed] [Google Scholar]
- 5. Kasacka I, Janiuk I, Lewandowska A, Bekisz A, Lebkowski W. Distribution pattern of CART-containing neurons and cells in the human pancreas. Acta Histochem. 2012;114(7):695-699. [DOI] [PubMed] [Google Scholar]
- 6. Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahrén B, Sundler F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept. 2005;129(1-3):203-211. [DOI] [PubMed] [Google Scholar]
- 7. Wierup N, Björkqvist M, Kuhar MJ, Mulder H, Sundler F. CART regulates islet hormone secretion and is expressed in the beta-cells of type 2 diabetic rats. Diabetes. 2006;55(2):305-311. [DOI] [PubMed] [Google Scholar]
- 8. Shcherbina L, Edlund A, Esguerra JL, et al. Endogenous beta-cell CART regulates insulin secretion and transcription of beta-cell genes. Mol Cell Endocrinol. 2017;447:52-60. [DOI] [PubMed] [Google Scholar]
- 9. Abels M, Riva M, Bennet H, et al. CART is overexpressed in human type 2 diabetic islets and inhibits glucagon secretion and increases insulin secretion. Diabetologia. 2016;59(9):1928-1937. [DOI] [PubMed] [Google Scholar]
- 10. Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9(11):823-833. [DOI] [PubMed] [Google Scholar]
- 11. Elias CF, Lee CE, Kelly JF, et al. Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol. 2001;432(1):1-19. [DOI] [PubMed] [Google Scholar]
- 12. Matsumura K, Tsuchihashi T, Abe I. Central human cocaine- and amphetamine-regulated transcript peptide 55-102 increases arterial pressure in conscious rabbits. Hypertension. 2001;38(5):1096-1100. [DOI] [PubMed] [Google Scholar]
- 13. Dun NJ, Dun SL, Kwok EH, Yang J, Chang J. Cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat sympatho-adrenal axis. Neurosci Lett. 2000;283(2):97-100. [DOI] [PubMed] [Google Scholar]
- 14. Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391(1):115-132. [PubMed] [Google Scholar]
- 15. Elias CF, Lee C, Kelly J, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21(6):1375-1385. [DOI] [PubMed] [Google Scholar]
- 16. Iliff JJ, Alkayed NJ, Golshani KJ, Weinstein J, Traystman RJ, West GA. In vivo cerebrovascular effects of cocaine- and amphetamine-regulated transcript (CART) peptide. J Cardiovasc Pharmacol. 2008;52(1):82-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skibicka KP, Alhadeff AL, Grill HJ. Hindbrain cocaine- and amphetamine-regulated transcript induces hypothermia mediated by GLP-1 receptors. J Neurosci. 2009;29(21):6973-6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lau J, Farzi A, Qi Y, et al. CART neurons in the arcuate nucleus and lateral hypothalamic area exert differential controls on energy homeostasis. Mol Metab. 2018;7:102-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141(2):794-801. [DOI] [PubMed] [Google Scholar]
- 20. Stanley SA, Small CJ, Murphy KG, et al. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001;893(1-2):186-194. [DOI] [PubMed] [Google Scholar]
- 21. Yosten GL, Redlinger LJ, Samson WK. Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol. 2012;303(9):R941-R949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stein LM, Tullock CW, Mathews SK, et al. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am J Physiol Regul Integr Comp Physiol. 2016;311(3):R489-R496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Samson WK, Zhang JV, Avsian-Kretchmer O, et al. Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular, and metabolic functions. J Biol Chem. 2008;283(46):31949-31959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yosten GL, Lyu RM, Hsueh AJ, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol. 2013;25(2):206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yosten GL, Kolar GR, Redlinger LJ, Samson WK. Evidence for an interaction between proinsulin C-peptide and GPR146. J Endocrinol. 2013;218(2):B1-B8. [DOI] [PubMed] [Google Scholar]
- 26. Stein LM, Yosten GL, Samson WK. Adropin acts in brain to inhibit water drinking: potential interaction with the orphan G protein-coupled receptor, GPR19. Am J Physiol Regul Integr Comp Physiol. 2016;310(6):R476-R480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci. 2001;21(19):7474-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384(1-2):198-202. [DOI] [PubMed] [Google Scholar]
- 29. Somalwar AR, Choudhary AG, Sharma PR, et al. Cocaine- and amphetamine-regulated transcript peptide (CART) induced reward behavior is mediated via Gi/o dependent phosphorylation of PKA/ERK/CREB pathway. Behav Brain Res. 2018;348:9-21. [DOI] [PubMed] [Google Scholar]
- 30. Alexander SPH, Christopoulos A, Davenport AP, et al. ; CGTP Collaborators . The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br J Pharmacol. 2019;176(Suppl 1):S21-S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Damaj MI, Zheng J, Martin BR, Kuhar MJ. Intrathecal CART (55-102) attenuates hyperlagesia and allodynia in a mouse model of neuropathic but not inflammatory pain. Peptides. 2006;27(8):2019-2023. [DOI] [PubMed] [Google Scholar]
- 32. Kozsurek M, Lukácsi E, Fekete C, Wittmann G, Réthelyi M, Puskár Z. Cocaine- and amphetamine-regulated transcript peptide (CART) is present in peptidergic C primary afferents and axons of excitatory interneurons with a possible role in nociception in the superficial laminae of the rat spinal cord. Eur J Neurosci. 2007;26(6):1624-1631. [DOI] [PubMed] [Google Scholar]
- 33. Ohsawa M, Dun SL, Tseng LF, Chang J, Dun NJ. Decrease of hindpaw withdrawal latency by cocaine- and amphetamine-regulated transcript peptide to the mouse spinal cord. Eur J Pharmacol. 2000;399(2-3):165-169. [DOI] [PubMed] [Google Scholar]
- 34. Zacharko-Siembida A, Kulik P, Szalak R, Lalak R, Arciszewski MB. Co-expression patterns of cocaine- and amphetamine-regulated transcript (CART) with neuropeptides in dorsal root ganglia of the pig. Acta Histochem. 2014;116(2):390-398. [DOI] [PubMed] [Google Scholar]
- 35. Asnicar MA, Smith DP, Yang DD, et al. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142(10):4394-4400. [DOI] [PubMed] [Google Scholar]
- 36. Haddock CJ, Almeida-Pereira G, Stein LM, et al. Signaling in rat brainstem via Gpr160 is required for the anorexigenic and antidipsogenic actions of cocaine- and amphetamine-regulated transcript peptide. Am J Physiol Regul Integr Comp Physiol. 2021;320(3):R236-R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393(6680):72-76. [DOI] [PubMed] [Google Scholar]
- 38. Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281(6):R1862-R1867. [DOI] [PubMed] [Google Scholar]
- 39. Zheng H, Patterson C, Berthoud HR. Fourth ventricular injection of CART peptide inhibits short-term sucrose intake in rats. Brain Res. 2001;896(1-2):153-156. [DOI] [PubMed] [Google Scholar]
- 40. Aja S, Ewing C, Lin J, Hyun J, Moran TH. Blockade of central GLP-1 receptors prevents CART-induced hypophagia and brain c-Fos expression. Peptides. 2006;27(1):157-164. [DOI] [PubMed] [Google Scholar]
- 41. Smedh U, Moran TH. Peptides that regulate food intake: separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1418-R1426. [DOI] [PubMed] [Google Scholar]
- 42. Thim L, Kristensen P, Larsen PJ, Wulff BS. CART, a new anorectic peptide. Int J Biochem Cell Biol. 1998;30(12):1281-1284. [DOI] [PubMed] [Google Scholar]
- 43. Lee SJ, Krieger JP, Vergara M, et al. Blunted vagal cocaine- and amphetamine-regulated transcript promotes hyperphagia and weight gain. Cell Rep. 2020;30(6):2028-2039.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krieger JP. Intestinal glucagon-like peptide-1 effects on food intake: Physiological relevance and emerging mechanisms. Peptides. 2020;131:170342. [DOI] [PubMed] [Google Scholar]
- 45. Ruginsk SG, Uchoa ET, Elias LL, Antunes-Rodrigues J, Llewellyn-Smith IJ. Hypothalamic cocaine- and amphetamine-regulated transcript and corticotrophin releasing factor neurons are stimulated by extracellular volume and osmotic changes. Neuroscience. 2011;186:57-64. [DOI] [PubMed] [Google Scholar]
- 46. Aja S, Robinson BM, Mills KJ, Ladenheim EE, Moran TH. Fourth ventricular CART reduces food and water intake and produces a conditioned taste aversion in rats. Behav Neurosci. 2002;116(5):918-921. [PubMed] [Google Scholar]
- 47. Vicentic A, Dominguez G, Hunter RG, Philpot K, Wilson M, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide levels in blood exhibit a diurnal rhythm: regulation by glucocorticoids. Endocrinology. 2004;145(9):4119-4124. [DOI] [PubMed] [Google Scholar]
- 48. Sathanoori R, Olde B, Erlinge D, Göransson O, Wierup N. Cocaine- and amphetamine-regulated transcript (CART) protects beta cells against glucotoxicity and increases cell proliferation. J Biol Chem. 2013;288(5):3208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. del Giudice EM, Santoro N, Cirillo G, D’Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes. 2001;50(9):2157-2160. [DOI] [PubMed] [Google Scholar]
- 50. Miraglia del Giudice E, Santoro N, Fiumani P, Dominguez G, Kuhar MJ, Perrone L. Adolescents carrying a missense mutation in the CART gene exhibit increased anxiety and depression. Depress Anxiety. 2006;23(2):90-92. [DOI] [PubMed] [Google Scholar]
- 51. Dominguez G, del Giudice EM, Kuhar MJ. CART peptide levels are altered by a mutation associated with obesity at codon 34. Mol Psychiatry. 2004;9(12):1065-1066. [DOI] [PubMed] [Google Scholar]
- 52. Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology. 2006;147(1):39-43. [DOI] [PubMed] [Google Scholar]
- 53. Guérardel A, Barat-Houari M, Vasseur F, et al. Analysis of sequence variability in the CART gene in relation to obesity in a Caucasian population. BMC Genet. 2005;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rigoli L, Munafò C, Di Bella C, Salpietro A, Procopio V, Salpietro C. Molecular analysis of the CART gene in overweight and obese Italian children using family-based association methods. Acta Paediatr. 2010;99(5):722-726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.