Abstract
Monoclonal antibodies (mAbs) are important therapeutic glycoproteins, but their large size and structural complexity make them difficult to rapidly characterize. Top-down mass spectrometry (MS) has potential to overcome challenges of other common approaches by minimizing sample preparation and preserving endogenous modifications. However, comprehensive mAb characterization requires generation of many, well-resolved fragments and remains challenging. While ETD retains modifications and cleaves disulfide bonds – making it attractive for mAb characterization – it can be less effective for precursors having high m/z values. Activated ion electron transfer dissociation (AI-ETD) uses concurrent infrared photoactivation to promote product ion generation and has proven effective in increasing sequence coverage of intact proteins. Here we present the first application of AI-ETD to mAb sequencing. For the standard NIST mAb we observe a high degree of complementarity between fragments generated using standard ETD with a short reaction time and AI-ETD with a long reaction time. Most importantly, AI-ETD reveals disulfide-bound regions that have been intractable, thus far, for sequencing with top-down MS. We conclude AI-ETD has the potential to rapidly and comprehensively analyze intact mAbs.
Keywords: Fragmentation, Mass spectrometry, Peptides and proteins, Immunology
Graphical Abstract

Introduction
Monoclonal antibodies (mAbs) are effective therapies that target specific antigens to diagnose or treat a host of diseases from cancer to viral infections.1–8 Their large size (~150 kDa), post-translational modifications (PTMs) and numerous disulfide bond linkages between and within the light and heavy chains necessitate extensive sequence and structure analysis.1, 9, 10 While tandem mass spectrometry (MS/MS) techniques such as shotgun proteomics provide comprehensive analysis for clinical mAb screening, improvements in mass spectrometry could reduce cost and time required to get the drugs to the patient.11–21
Top-down mass spectrometry (TD MS) can provide robust and rapid characterization of intact biologics without digestion steps.22,23, 24 However, comprehensive sequence coverage remains challenging owing to difficulties in effectively dissociating these large molecules across the entire sequence. Beam-type collisional activation, i.e., higher-energy collisional dissociation (HCD),25 or ion trap collisional activation dissociation (CAD),26 preferentially cleaves the weakest bonds to generate spectra dominated by fragments produced only through a low number of the lowest energy dissociation pathways, providing incomplete information about a mAb.27, 28 Consequently, electron-based dissociation methods have enabled the most extensive sequencing of intact mAbs to date.29–33 Electron-driven dissociation via ion-electron (i.e., electron-capture dissociation, ECD)34 and ion-ion reactions (i.e., electron-transfer dissociation, ETD)35 can generate extensive backbone cleavage while retaining modifications and simultaneously cleaving disulfide bonds, important factors for characterizing mAbs.29, 36–45
While ETD presents several advantages, caveats exist. For example, Fornelli and co-authors observed that even averaging thousands of spectra and including multiple charge states and reaction times, they could only achieve mAb sequence coverage of 35% with ETD alone.18, 30, 31 Non-covalent interactions that occur across the gas-phase precursor ion are the main barrier that limits ETD. That is, following electron transfer backbone bonds may be cleaved; however these noncovalent interactions prevent the separation of the ETD-generated (c/z•-type) fragments, i.e., electron transfer without dissociation (ETnoD).46 ETnoD is especially pervasive for precursors with low charge density (e.g., m/z > 1,000), which permits a high degree of secondary structure, or a high number of disulfide bonds.44, 46, 47 Delivery of supplemental vibrational activation of the precursor ion population either during or after the electron transfer event can reduce ETnoD and boost ETD efficiency.48–51,33 One way to accomplish this is to collisionally activate all ETD products using HCD after the ion-ion reaction (EThcD).52–54 Unfortunately for mAb analysis, ETD alone provided more coverage than EThcD, although a combination of the two fragmentation methods enhanced the sequence coverage to approximately 31%.46 This underwhelming performance by EThcD was largely attributed to its inability to effectively disrupt the secondary structure of the immunoglobulin-like domains.31
Activated ion ETD (AI-ETD)51, 55, 56 bombards the precursor ion population during ETD with infrared photons. These photons are tuned so that they provide optimal energy to vibrationally excite the precursor and disrupt the non-covalent interactions. We have shown AI-ETD to provide excellent performance for both large proteins (up to ~66 kDa)56 and proteins rich in disulfide bonds.47 Here we examined the utility of AI-ETD for sequencing of the intact NIST monoclonal antibody on a modified Fusion Lumos Orbitrap platform. With a significant quantity (>100 μg) of highly pure mAb in hand, direct infusion was used to rapidly screen different AI-ETD laser powers and ETD reaction times.30 For AI-ETD, laser power and reaction time were varied generating distinct populations of fragment ions. For example, an increase of reaction times and laser powers revealed more fragments from disulfide-bound regions, suggesting that higher energy IR photons disrupted structures that stemmed from disulfide connectivity. Further, our results indicated that AI-ETD can provide substantially more information about the sequence of an intact mAb than ETD alone and that ETD and AI-ETD were complementary – especially when using different ion-ion reaction times. With this technique we achieved over 60% sequence coverage of the intact mAbs using AI-ETD for TD-MS.
Experimental Methods
All experiments were performed on an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific, San Jose, CA) that has been previously modified with a Firestar T-100 Synrad 60-W CO2 continuous wave laser (Mukiltwo, WA) for AI-ETD57 (see Supporting Information for more details).
Results and Discussion
Owing to its well-characterized features,28 we selected the NIST intact mAb standard to assess AI-ETD performance. Aiming to develop a comprehensive and fast approach we performed direct infusion of the NIST mAb standard. Shown in Figure S1, the major glycoforms of the NIST mAb were confirmed through intact mass analysis. These measurements provided a global overview of the antibody’s features, but therapeutic mAbs required unambiguous characterization of their PTMs and sequence. To achieve this, we selected the most abundant precursor population and tested AI-ETD performance by varying the reaction times until an apparent maximum sequence coverage was achieved for each laser power (Figure S2). AI-ETD robustly generated mAb fragments at moderate laser powers; at 12 and 18 W more than 300 products were assigned over broad reaction time ranges of 40 to 400 ms and 15 to 220 ms, respectively. At higher powers of 24 W and 30 W, the laser’s influence on the ion fragmentation was more prominent and the precursor ions likely fragmented multiple times generating unconventional product ions.
As previously reported31, 46, 58, 59, the ETD reaction duration generated distinct spectra and AI-ETD recapitulated this trend(Figure S3). For instance, the spectrum resulting from a 5 ms ETD reaction provided large product ions with average charge states of 8+, across the whole spectrum (Figure 1A). With both longer ion-ion reaction times and irradiation at 18 W, charge-reduced products and products resulting from multiple electron transfer events distributed across the entire m/z range with average charge states of 4+ (Figure 1B). To better illustrate these differences, we magnified and annotated the region from 1,500 to 1,550 m/z from each of these tandem mass spectra. In the experiment using long reaction duration and AI-ETD at 18 W the resolving power was sufficient to delineate fragments of similar m/z, such as the light chain c30 and heavy chain z•28 species, which were observed as well-resolved isotopic distributions (Figure S4). The main challenge of the prolonged reaction times was the presence of low intensity, internal fragments, a well-known caveat of overexposure during ETD fragmentation.60, 61 In contrast, short ETD reaction times preserve large, highly charged fragments that were difficult to deconvolute due to overlapping signals in greatly congested regions of the spectrum. Nevertheless, combining short ETD reaction times with longer AI-ETD reaction times resulted in almost 400 unique bonds broken and over 60% mAb sequence coverage. Future experiments using targeted approaches, e.g., MS3 or parallel ion parking with proton transfer reactions,58, 62–66 in combination with AI-ETD may make these internal regions more accessible while also improving deconvolution of highly charged fragments from shorter reaction times.
Figure 1.
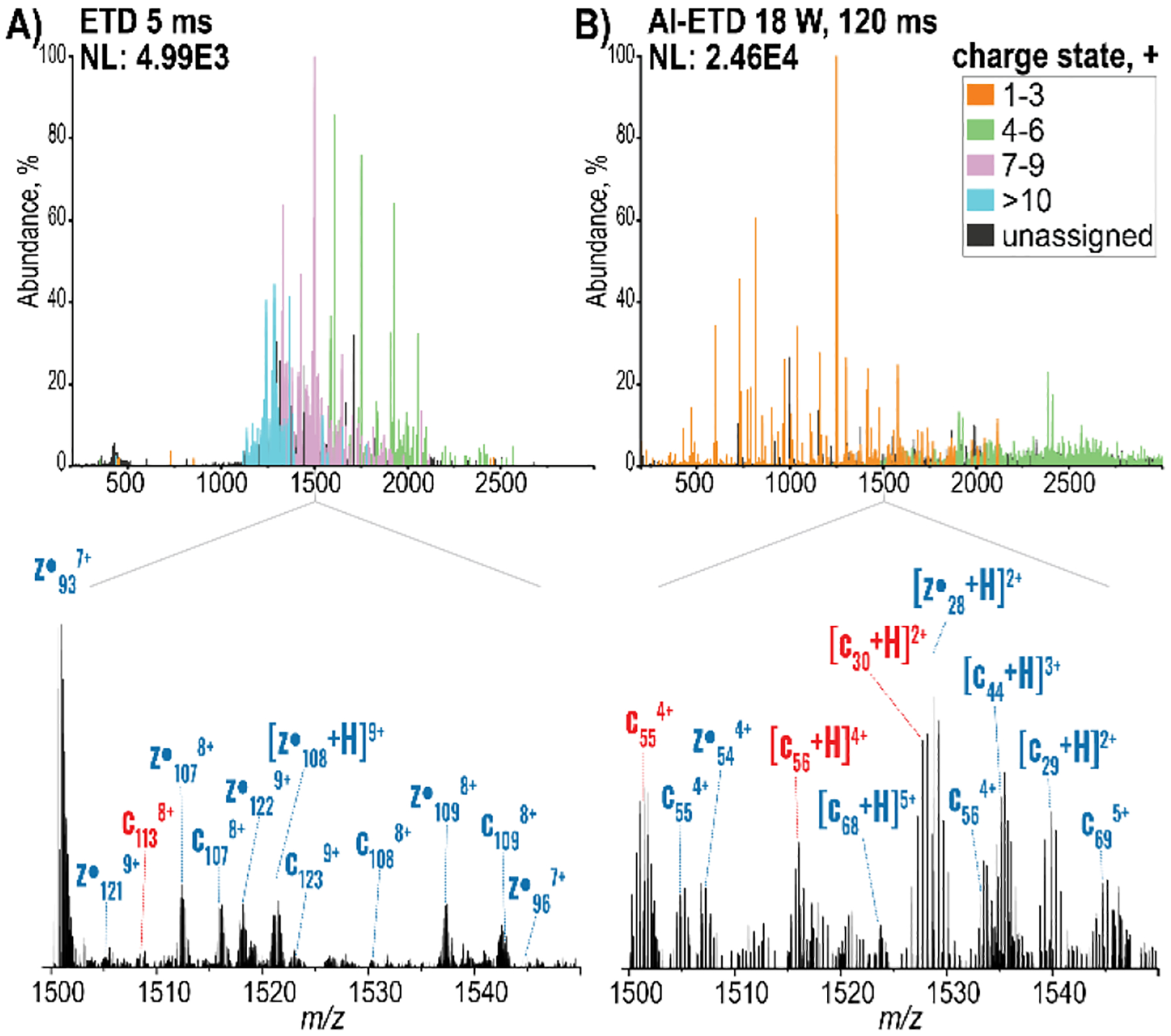
Distinct patterns were evident in spectra generated using short and long reaction times. The spectra obtained from the accumulation of 200 scans at a resolving power of 240,000 at 200 m/z using A) ETD for 5 ms B) AI-ETD 18 W laser power for 120 ms. The peaks in the top spectra are colored according to their assigned charge states. Below, the annotated region from 1,500 to 1,550 m/z denotes the best matching ions for the heavy chain in blue and the light chain in red. NL = normalized level
The modifications generated from disulfide cleavage pathways revealed how the disulfide connectivity influenced the observed product ion populations. Since disulfides contributed to maintaining protein structure in solution, they promoted gas-phase structures that were conducive to ETnoD. AI-ETD disrupted the residual gas-phase structures after ETD cleaved the backbone and disulfide bond. For instance, the fragmentation patterns for the reaction time and the disulfide bond network were decipherable by tracking abundance of specific ion populations and cleavage sites at 18 W laser power (Figure S5). With the extended reaction times, b- and c-type ions dominated within the disulfide bound regions near the N-terminal regions of the heavy and light chains, while small y- and z•-type fragments dominated at the C-terminal region. Thus, the extended time provided the opportunity for specific reactions at the disulfide and backbone bond(s) and generated shorter fragments (<15 kDa). Products whose formation required more than two intra-disulfide cleavages, were rarely observed, and only captured in short reactions, as they were presumably lost as internal fragments with prolonged ETD exposure. The diversity of fragments from short and long reaction times prompted us to employ a pairwise comparison between different activation conditions. The results suggested that a greater number of complementary fragments were generated when AI-ETD was performed using different reaction times or laser powers, or when ETD alone was performed, relative to when other fragmentation techniques (Figure S6). Two conditions that afforded the highest number of cleavage sites for the light and heavy chains included one short and one long reaction time for each chain (Figure 2). More importantly, the number of unique bonds broken under each condition was greater than the number of overlapping bond cleavages between the two (Figure 2). Only 55 of 248 bonds (~22%) were broken under both conditions for the heavy chain, and 52 of 169 total bonds (~31%) were redundant between the two analyses of the light chain. The optimal conditions for the light chain involved higher vibrational energy at longer reaction times (AI-ETD at 18 W for 80 ms), while 5 ms ETD reaction was preferred for the heavy chain. Effective fragmentation of the light chain necessitates breakage of the interchain disulfide bonds and therefore, AI-ETD with longer reaction times and higher laser power was the most efficient at sequencing this region.
Figure 2.

The average signal (% TIC) of matched fragments obtained under the two conditions listed as plotted according to an amino acid residue position in the heavy (top) and (bottom) light chains. The intrachain disulfide bonds are shaded in light blue and the interchain disulfide bond is shown in pink. The Venn diagrams illustrate the number of distinct and overlapping bonds broken under each condition.
ETD is a charge-state dependent reaction,35, 60, 67 so additional fragment populations were expected from precursors with higher charge states. However, a pairwise comparison for different precursors showed that ETD provided considerably fewer unique cleavage sites, especially in comparison to AI-ETD (Figure S7). This supported that an invariable set of fragment ion populations consistently contributed to ETnoD products due to the disulfide bond network and stable gas-phase structures (Figure S8). The fragmentation patterns of ETD were highly dependent on the disulfide bonds, so they could validate disulfide connectivity and provide important structural information in future studies.
Beyond the amino acid sequence, glycan and glycosylation site information were also relevant for the antibody function and specificity.68–72 Briefly, we successfully reconstructed several fragments with intact glycans using ETD and AI-ETD (Figure S9). Unfortunately, these low abundant fragments were insufficient to confidently localize the precise site of modification. The expected glycan-containing fragments had relatively large masses, between 20 to 53 kDa, so another experimental set-up may have been more conducive to glycan analysis. For instance, using a higher mass range and lowering the IRM pressure would have both transmitted and detected these ions more readily.
The complementarity determining region (CDR) was also an important region for sequencing the mAb because it engendered specificity by targeting distinct antigens.11, 73 In a single AI-ETD experiment (18 W, 100 ms), 78% of amino acids in this region were confirmed by observed product ions (Figure 3) suggesting that this technique alone can provide considerable insight into the sequence of this region.
Figure 3.

The fragment ion map for the NIST variable region for AI-ETD at 18 W, 100 ms.
Finally, we assessed the relative value of other fragmentation techniques for mAb sequencing. The highly abundant 48+ precursor was selected in all experiments, and when compared to ETD, EThcD, and HCD, AI-ETD at 18 W for 120 ms, yielded the highest total sequence coverage of 51% (Figure S10). The results with AI-ETD demonstrated that improvements in fragmentation techniques can provide considerable success for top-down sequencing of mAbs.
Conclusions
Rapid, comprehensive analysis of mAbs is a pressing need in biopharmaceutical research. TD-MS of intact mAbs expedites sample processing and data acquisition, and ETD-based methods have proven valuable for generating moderate sequence coverage of the intact mAb.29, 31, 46 Here we demonstrate that AI-ETD also provides myriad benefits for analysis of the intact mAb. Namely, AI-ETD improves fragmentation of disulfide-enclosed regions, yields a greater number of total sequencing ions, and as a supplemental activation method, considerably outperforms EThcD. Combination of multiple reaction times for a given precursor has been used to boost coverage in previous studies,24, 46 and it proves valuable here as well. However, the improved sequencing power of AI-ETD does not approach the nearly complete coverage from shotgun proteomics.16, 28 Major challenges remain for TD MS/MS involving the generation and analysis of complex product ion spectra. For the application of AI-ETD some of these issues are circumvented by restricting the m/z range to include well-resolved peaks and by limiting the fragment library to include well-known modifications. To that end, many product ions remain unexplained. Other important information is also missing including the localization of glycosylation site(s). While experimental MS/MS conditions could be optimized to detect large modified fragments, additional technological advances such as improved resolving power for heavy product ions74, 75 are required to confidently detect these ions. Other techniques to reduce spectral complexity including parallel ion parking with proton transfer reactions can increase the number of assigned peaks and the confidence in their identity and contribute to disulfide mapping. An additional factor that requires attention is the complexity and throughput required for mAb characterization. The direct infusion method used in this study is ideal for comparing MS/MS results with a highly pure mAb but is low throughput. The optimal MS/MS methods established here are adaptable to include online separation which would also increase the sensitivity and throughput of AI-ETD for characterizing mAbs. In addition to technological advancements, informatic tools are also required to confidently identify all product ions and completely profile mAb heterogeneity. With continued efforts, TD MS/MS using AI-ETD has the potential to rapidly and efficiently provide exhaustive structural information on therapeutic mAbs, including their sequence, disulfide linkages, and post-translational modifications.
Supplementary Material
Figure 4.

Number of disulfide cleavages for the observed fragments by EThcD at NCE 20% and AI-ETD at 18 W for 5 and 120 ms are plotted according to the amino acid residue position in the heavy and light chains. The intrachain disulfide bonds are shaded in light blue, and the interchain disulfide bond is shown in pink.
Acknowledgements
We gratefully acknowledge support from NIH Grant R35GM118110.
References
- 1.Beck A; Wurch T; Bailly C; Corvaia N, Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol 2010, 10, 345. [DOI] [PubMed] [Google Scholar]
- 2.Marston HD; Paules CI; Fauci AS, Monoclonal Antibodies for Emerging Infectious Diseases — Borrowing from History. N. Engl. J. Med 2018, 378 (16), 1469–1472. [DOI] [PubMed] [Google Scholar]
- 3.Kisalu NK; Idris AH; Weidle C; Flores-Garcia Y; Flynn BJ; Sack BK; Murphy S; Schön A; Freire E; Francica JR; Miller AB; Gregory J; March S; Liao H-X; Haynes BF; Wiehe K; Trama AM; Saunders KO; Gladden MA; Monroe A; Bonsignori M; Kanekiyo M; Wheatley AK; McDermott AB; Farney SK; Chuang G-Y; Zhang B; Kc N; Chakravarty S; Kwong PD; Sinnis P; Bhatia SN; Kappe SHI; Sim BKL; Hoffman SL; Zavala F; Pancera M; Seder RA, A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat. Med 2018, 24 (4), 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AminJafari A; Ghasemi S, The possible of immunotherapy for COVID-19: A systematic review. Int. Immunopharmacol 2020, 83, 106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y; Liu Q; Men T; Liang Y; Niu H; Wang J, A microfluidic system based on the monoclonal antibody BCMab1 specifically captures circulating tumor cells from bladder cancer patients. J. Biomater. Sci. Polym. Ed 2020, 1–12. [DOI] [PubMed] [Google Scholar]
- 6.Kumar R; Shrivastava T; Samal S; Ahmed S; Parray HA, Antibody-based therapeutic interventions: possible strategy to counter chikungunya viral infection. Appl. Microbiol. Biotechnol 2020, 104 (8), 3209–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.London M; Gallo E, Epidermal growth factor receptor (EGFR) involvement in epithelial-derived cancers and its current antibody-based immunotherapies. Cell Biol Int 2020. [DOI] [PubMed] [Google Scholar]
- 8.Kaplon H; Muralidharan M; Schneider Z; Reichert JM, Antibodies to watch in 2020. MAbs 2020, 12 (1), 1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H; May K, Disulfide bond structures of IgG molecules. mAbs 2012, 4 (1), 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parr MK; Montacir O; Montacir H, Physicochemical characterization of biopharmaceuticals. J. Pharm. Biomed. Anal 2016, 130, 366–389. [DOI] [PubMed] [Google Scholar]
- 11.Beck A; Sanglier-Cianférani S; Van Dorsselaer A, Biosimilar, Biobetter, and Next Generation Antibody Characterization by Mass Spectrometry. Anal. Chem 2012, 84 (11), 4637–4646. [DOI] [PubMed] [Google Scholar]
- 12.Beck A; Wagner-Rousset E; Ayoub D; Van Dorsselaer A; Sanglier-Cianférani S, Characterization of Therapeutic Antibodies and Related Products. Anal. Chem 2013, 85 (2), 715–736. [DOI] [PubMed] [Google Scholar]
- 13.Rogstad S; Faustino A; Ruth A; Keire D; Boyne M; Park J, A Retrospective Evaluation of the Use of Mass Spectrometry in FDA Biologics License Applications. J. Am. Soc. Mass Spectrom 2017, 28 (5), 786–794. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z; Pan H; Chen X, Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spectrom. Rev 2009, 28 (1), 147–176. [DOI] [PubMed] [Google Scholar]
- 15.Lundell N; Schreitmüller T, Sample Preparation for Peptide Mapping— A Pharmaceutical Quality-Control Perspective. Anal. Biochem 1999, 266 (1), 31–47. [DOI] [PubMed] [Google Scholar]
- 16.Ayoub D; Jabs W; Resemann A; Evers W; Evans C; Main L; Baessmann C; Wagner-Rousset E; Suckau D; Beck A, Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. mAbs 2013, 5 (5), 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M; Gucinski AC; Boyne MT, Performance metrics for evaluating system suitability in liquid chromatography—Mass spectrometry peptide mass mapping of protein therapeutics and monoclonal antibodies. mAbs 2015, 7 (6), 1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornelli L; Srzentić K; Huguet R; Mullen C; Sharma S; Zabrouskov V; Fellers RT; Durbin KR; Compton PD; Kelleher NL, Accurate Sequence Analysis of a Monoclonal Antibody by Top-Down and Middle-Down Orbitrap Mass Spectrometry Applying Multiple Ion Activation Techniques. Anal. Chem 2018, 90 (14), 8421–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotham VC; Brodbelt JS, Characterization of Therapeutic Monoclonal Antibodies at the Subunit-Level using Middle-Down 193 nm Ultraviolet Photodissociation. Anal. Chem 2016, 88 (7), 4004–4013. [DOI] [PubMed] [Google Scholar]
- 20.Fodor S; Zhang Z, Rearrangement of terminal amino acid residues in peptides by protease-catalyzed intramolecular transpeptidation. Anal. Biochem 2006, 356 (2), 282–290. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y; Li X; Liu Y-H; Richardson D; Li H; Shameem M; Yang X, Simultaneous monitoring of oxidation, deamidation, isomerization, and glycosylation of monoclonal antibodies by liquid chromatography-mass spectrometry method with ultrafast tryptic digestion. mAbs 2016, 8 (8), 1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandra K; Vandenheede I; Sandra P, Modern chromatographic and mass spectrometric techniques for protein biopharmaceutical characterization. J. Chromatogr. A 2014, 1335, 81–103. [DOI] [PubMed] [Google Scholar]
- 23.Thompson NJ; Rosati S; Rose RJ; Heck AJR, The impact of mass spectrometry on the study of intact antibodies: from post-translational modifications to structural analysis. Chem. Commun 2013, 49 (6), 538–548. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H; Cui W; Gross ML, Mass spectrometry for the biophysical characterization of therapeutic monoclonal antibodies. FEBS Letters 2014, 588 (2), 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen JV; Macek B; Lange O; Makarov A; Horning S; Mann M, Higher-energy C-trap dissociation for peptide modification analysis. Nat. Methods 2007, 4, 709. [DOI] [PubMed] [Google Scholar]
- 26.McLafferty FW; Bente PF; Kornfeld R; Tsai S-C; Howe I, Metastable ion characteristics. XXII. Collisional activation spectra of organic ions. J. Am. Chem. Soc 1973, 95 (7), 2120–2129. [Google Scholar]
- 27.Bondarenko PV; Second TP; Zabrouskov V; Makarov AA; Zhang Z, Mass measurement and top-down HPLC/MS analysis of intact monoclonal antibodies on a hybrid linear quadrupole ion trap-Orbitrap mass spectrometer. J. Am. Soc. Mass Spectrom 2009, 20 (8), 1415–24. [DOI] [PubMed] [Google Scholar]
- 28.Formolo T; Ly M; Levy M; Kilpatrick L; Lute S; Phinney K; Marzilli L; Brorson K; Boyne M; Davis D; Schiel J, Determination of the NISTmAb Primary Structure. In State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study, American Chemical Society: 2015; Vol. 1201, pp 1–62. [Google Scholar]
- 29.Tsybin YO; Fornelli L; Stoermer C; Luebeck M; Parra J; Nallet S; Wurm FM; Hartmer R, Structural Analysis of Intact Monoclonal Antibodies by Electron Transfer Dissociation Mass Spectrometry. Anal. Chem 2011, 83 (23), 8919–8927. [DOI] [PubMed] [Google Scholar]
- 30.Mao Y; Valeja SG; Rouse JC; Hendrickson CL; Marshall AG, Top-Down Structural Analysis of an Intact Monoclonal Antibody by Electron Capture Dissociation-Fourier Transform Ion Cyclotron Resonance-Mass Spectrometry. Anal. Chem 2013, 85 (9), 4239–4246. [DOI] [PubMed] [Google Scholar]
- 31.Fornelli L; Damoc E; Thomas PM; Kelleher NL; Aizikov K; Denisov E; Makarov A; Tsybin YO, Analysis of Intact Monoclonal Antibody IgG1 by Electron Transfer Dissociation Orbitrap FTMS. Mol. Cell Proteomics 2012, 11 (12), 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange O; Damoc E; Wieghaus A; Makarov A, Enhanced Fourier transform for Orbitrap mass spectrometry. Int. J. Mass Spectrom 2014, 369, 16–22. [Google Scholar]
- 33.Riley NM; Coon JJ, The Role of Electron Transfer Dissociation in Modern Proteomics. Anal. Chem 2018, 90 (1), 40–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zubarev RA; Kelleher NL; McLafferty FW, Electron Capture Dissociation of Multiply Charged Protein Cations. A Nonergodic Process. J. Am. Chem. Soc 1998, 120 (13), 3265–3266. [Google Scholar]
- 35.Syka JEP; Coon JJ; Schroeder MJ; Shabanowitz J; Hunt DF, Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Pro. Natl. Acad. Sci. U.S.A 2004, 101 (26), 9528–9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zubarev RA; Kruger NA; Fridriksson EK; Lewis MA; Horn DM; Carpenter BK; McLafferty FW, Electron Capture Dissociation of Gaseous Multiply-Charged Proteins Is Favored at Disulfide Bonds and Other Sites of High Hydrogen Atom Affinity. J. Am. Chem. Soc 1999, 121 (12), 2857–2862. [Google Scholar]
- 37.Tan L; Durand KL; Ma X; Xia Y, Radical cascades in electron transfer dissociation (ETD) – implications for characterizing peptide disulfide regio-isomers. Analyst 2013, 138 (22), 6759–6765. [DOI] [PubMed] [Google Scholar]
- 38.Cole SR; Ma X; Zhang X; Xia Y, Electron Transfer Dissociation (ETD) of Peptides Containing Intrachain Disulfide Bonds. J. Am. Soc. Mass Spectrom 2012, 23 (2), 310–320. [DOI] [PubMed] [Google Scholar]
- 39.Liu F; van Breukelen B; Heck AJR, Facilitating protein disulfide mapping by a combination of pepsin digestion, electron transfer higher energy dissociation (EThcD), and a dedicated search algorithm SlinkS. Mol. Cell Proteomics 2014, 13 (10), 2776–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark DF; Go EP; Desaire H, Simple Approach to Assign Disulfide Connectivity Using Extracted Ion Chromatograms of Electron Transfer Dissociation Spectra. Anal. Chem 2013, 85 (2), 1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S-L; Jiang H; Lu Q; Dai S; Hancock WS; Karger BL, Mass Spectrometric Determination of Disulfide Linkages in Recombinant Therapeutic Proteins Using Online LC−MS with Electron-Transfer Dissociation. Anal. Chem 2009, 81 (1), 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni W; Lin M; Salinas P; Savickas P; Wu S-L; Karger BL, Complete Mapping of a Cystine Knot and Nested Disulfides of Recombinant Human Arylsulfatase A by Multi-Enzyme Digestion and LC-MS Analysis Using CID and ETD. J. Am. Soc. Mass Spectrom 2013, 24 (1), 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y; Lu Q; Wu S-L; Karger BL; Hancock WS, Characterization and comparison of disulfide linkages and scrambling patterns in therapeutic monoclonal antibodies: using LC-MS with electron transfer dissociation. Anal. Chem 2011, 83 (8), 3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lermyte F; Sobott F, Electron transfer dissociation provides higher-order structural information of native and partially unfolded protein complexes. Proteomics 2015, 15 (16), 2813–2822. [DOI] [PubMed] [Google Scholar]
- 45.Fornelli L; Ayoub D; Aizikov K; Beck A; Tsybin YO, Middle-Down Analysis of Monoclonal Antibodies with Electron Transfer Dissociation Orbitrap Fourier Transform Mass Spectrometry. Anal. Chem 2014, 86 (6), 3005–3012. [DOI] [PubMed] [Google Scholar]
- 46.Fornelli L; Ayoub D; Aizikov K; Liu X; Damoc E; Pevzner PA; Makarov A; Beck A; Tsybin YO, Top-down analysis of immunoglobulin G isotypes 1 and 2 with electron transfer dissociation on a high-field Orbitrap mass spectrometer. J. Proteomics 2017, 159, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rush MJP; Riley NM; Westphall MS; Coon JJ, Top-Down Characterization of Proteins with Intact Disulfide Bonds Using Activated-Ion Electron Transfer Dissociation. Anal. Chem 2018, 90 (15), 8946–8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swaney DL; McAlister GC; Wirtala M; Schwartz JC; Syka JEP; Coon JJ, Supplemental Activation Method for High-Efficiency Electron-Transfer Dissociation of Doubly Protonated Peptide Precursors. Anal. Chem 2007, 79 (2), 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia Y; Han H; McLuckey SA, Activation of Intact Electron-Transfer Products of Polypeptides and Proteins in Cation Transmission Mode Ion/Ion Reactions. Anal. Chem 2008, 80 (4), 1111–1117. [DOI] [PubMed] [Google Scholar]
- 50.Pitteri SJ; Chrisman PA; McLuckey SA, Electron-Transfer Ion/Ion Reactions of Doubly Protonated Peptides: Effect of Elevated Bath Gas Temperature. Anal. Chem 2005, 77 (17), 5662–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ledvina AR; Beauchene NA; McAlister GC; Syka JEP; Schwartz JC; Griep-Raming J; Westphall MS; Coon JJ, Activated-Ion ETD (AI-ETD) Improves the Ability of ETD to Identify Peptides in a Complex Mixture. Anal. Chem 2010, 82 (24), 10068–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frese CK; Altelaar AF; van den Toorn H; Nolting D; Griep-Raming J; Heck AJ; Mohammed S, Toward full peptide sequence coverage by dual fragmentation combining electron-transfer and higher-energy collision dissociation tandem mass spectrometry. Anal. Chem 2012, 84 (22), 9668–73. [DOI] [PubMed] [Google Scholar]
- 53.Frese CK; Zhou H; Taus T; Altelaar AF; Mechtler K; Heck AJ; Mohammed S, Unambiguous phosphosite localization using electron-transfer/higher-energy collision dissociation (EThcD). J. Proteome Res 2013, 12 (3), 1520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mommen GP; Frese CK; Meiring HD; van Gaans-van den Brink J; de Jong AP; van Els CA; Heck AJ, Expanding the detectable HLA peptide repertoire using electron-transfer/higher-energy collision dissociation (EThcD). Proc. Natl. Acad. Sci. U.S.A 2014, 111 (12), 4507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riley NM; Westphall MS; Coon JJ, Activated Ion Electron Transfer Dissociation for Improved Fragmentation of Intact Proteins. Anal. Chem 2015, 87 (14), 7109–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riley NM; Westphall MS; Coon JJ, Sequencing Larger Intact Proteins (30–70 kDa) with Activated Ion Electron Transfer Dissociation. J. Am. Soc. Mass Spectrom 2018, 29 (1), 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley NM; Westphall MS; Hebert AS; Coon JJ, Implementation of Activated Ion Electron Transfer Dissociation on a Quadrupole-Orbitrap-Linear Ion Trap Hybrid Mass Spectrometer. Anal. Chem 2017, 89 (12), 6358–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coon JJ; Ueberheide B; Syka JEP; Dryhurst DD; Ausio J; Shabanowitz J; Hunt DF, Protein identification using sequential ion/ion reactions and tandem mass spectrometry. Proc. Natl. Acad. Sci. U.S.A 2005, 102, 9463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coon JJ; Shabanowitz J; Hunt DF; Syka JEP, Electron transfer dissociation of peptide anions. J. Am. Soc. Mass Spectrom 2005, 16 (6), 880–882. [DOI] [PubMed] [Google Scholar]
- 60.Rose CM; Rush MJP; Riley NM; Merrill AE; Kwiecien NW; Holden DD; Mullen C; Westphall MS; Coon JJ, A Calibration Routine for Efficient ETD in Large-Scale Proteomics. J. Am. Soc. Mass Spectrom 2015, 26 (11), 1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Compton PD; Strukl JV; Bai DL; Shabanowitz J; Hunt DF, Optimization of electron transfer dissociation via informed selection of reagents and operating parameters. Anal. Chem 2012, 84 (3), 1781–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang L; English AM; Bai DL; Ugrin SA; Shabanowitz J; Ross MM; Hunt DF; Wang W-H, Analysis of Monoclonal Antibody Sequence and Post-translational Modifications by Time-controlled Proteolysis and Tandem Mass Spectrometry. Mol. Cell Proteomics 2016, 15 (4), 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chrisman PA; Pitteri SJ; McLuckey SA, Parallel Ion Parking of Protein Mixtures. Anal. Chem 2006, 78 (1), 310–316. [DOI] [PubMed] [Google Scholar]
- 64.Anderson LC; Karch KR; Ugrin SA; Coradin M; English AM; Sidoli S; Shabanowitz J; Garcia BA; Hunt DF, Analyses of Histone Proteoforms Using Front-end Electron Transfer Dissociation-enabled Orbitrap Instruments. Mol. Cell Proteomics 2016, 15 (3), 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ugrin SA; English AM; Syka JEP; Bai DL; Anderson LC; Shabanowitz J; Hunt DF, Ion-Ion Proton Transfer and Parallel Ion Parking for the Analysis of Mixtures of Intact Proteins on a Modified Orbitrap Mass Analyzer. J. Am. Soc. Mass Spectrom 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huguet R; Mullen C; Srzentić K; Greer JB; Fellers RT; Zabrouskov V; Syka JEP; Kelleher NL; Fornelli L, Proton Transfer Charge Reduction Enables High-Throughput Top-Down Analysis of Large Proteoforms. Anal. Chem 2019, 91 (24), 15732–15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Good DM; Wirtala M; McAlister GC; Coon JJ, Performance characteristics of electron transfer dissociation mass spectrometry. Mol. Cell Proteomics 2007, 6, 1942–51. [DOI] [PubMed] [Google Scholar]
- 68.Wright A; Morrison SL, Effect of glycosylation on antibody function: implications for genetic engineering. Trends Biotechnol. 1997, 15 (1), 26–32. [DOI] [PubMed] [Google Scholar]
- 69.Mimura Y; Church S; Ghirlando R; Ashton PR; Dong S; Goodall M; Lund J; Jefferis R, The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol. Immunol 2000, 37 (12), 697–706. [DOI] [PubMed] [Google Scholar]
- 70.Higel F; Seidl A; Sörgel F; Friess W, N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm 2016, 100, 94–100. [DOI] [PubMed] [Google Scholar]
- 71.Arnold JN; Wormald MR; Sim RB; Rudd PM; Dwek RA, The Impact of Glycosylation on the Biological Function and Structure of Human Immunoglobulins. Annu. Rev. Immunol 2007, 25 (1), 21–50. [DOI] [PubMed] [Google Scholar]
- 72.Prien JM; Stöckmann H; Albrecht S; Martin SM; Varatta M; Furtado M; Hosselet S; Wang M; Formolo T; Rudd PM; Schiel JE, Orthogonal Technologies for NISTmAb N-Glycan Structure Elucidation and Quantitation. In State-of-the-Art and Emerging Technologies for Therapeutic Monoclonal Antibody Characterization Volume 2. Biopharmaceutical Characterization: The NISTmAb Case Study, American Chemical Society: 2015; Vol. 1201, pp 185–235. [Google Scholar]
- 73.Mian IS; Bradwell AR; Olson AJ, Structure, function and properties of antibody binding sites. J. Mol. Biol 1991, 217 (1), 133–151. [DOI] [PubMed] [Google Scholar]
- 74.Li H; Wolff JJ; Van Orden SL; Loo JA, Native Top-Down Electrospray Ionization-Mass Spectrometry of 158 kDa Protein Complex by High-Resolution Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem 2014, 86 (1), 317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valeja SG; Kaiser NK; Xian F; Hendrickson CL; Rouse JC; Marshall AG, Unit Mass Baseline Resolution for an Intact 148 kDa Therapeutic Monoclonal Antibody by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem 2011, 83 (22), 8391–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marty MT; Baldwin AJ; Marklund EG; Hochberg GKA; Benesch JLP; Robinson CV, Bayesian Deconvolution of Mass and Ion Mobility Spectra: From Binary Interactions to Polydisperse Ensembles. Anal. Chem 2015, 87 (8), 4370–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senko MW; Beu SC; McLafferty FW, Determination of monoisotopic masses and ion populations for large biomolecules from resolved isotopic distributions. J. Am. Soc. Mass Spectrom 1995, 6 (4), 229–233. [DOI] [PubMed] [Google Scholar]
- 78.Hunter JD, Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng 2007, 9 (3), 90–95. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


