Abstract
Primary malignant melanoma of esophagus is very rare, and its clinicopathologic and genetic features have not been extensively investigated. In this study, 20 tumors from 14 male and 6 female patients (40–79 years old) were evaluated. Dysphagia, chest pain, and weight loss were frequent symptoms. Thirteen melanomas, including two with multiple lesions, involved the distal third of esophagus. The median tumor diameter was 6 cm. Epithelioid morphology, moderate atypia, and pigmentation were typical findings. None of the patients had melanoma elsewhere, and all tumors exhibited a junctional peri-epithelial component consistent with a primary lesion. The median mitotic activity was 11 per 10 high-power fields (range, 0–31). Nine patients died of tumor within 4–22 months, however, two showed long-term (96 and 104 months) survival. In 15 cases, tissue for further immunohistochemical and molecular studies were available. BRAF, KIT, and NRAS mutation status was assessed by Sanger sequencing in all 15 tumors. The next-generation sequencing of 50 or 409 genes was performed in five and three cases, respectively. IGF1R expression indicating activation of the IGF axis was seen in 82%(9/11) of tumors. However, no BRAF mutations were identified. In 33% (5/15) of tumors, NRAS mutations were detected. KIT expression was seen in 50% (7/14) of melanomas including single KIT mutant. Two of three tumors evaluated with 409 genes panel revealed multiple driver mutations indicating sub-clonal expansion, whereas a single mutation (TSC1 p.H371Q) was the sole change in the third case. SF3B1 p.K666T and p.R625C mutations were detected in two cases. However, no co-occurrence of SF3B1 and GNAQ or GNA11 mutations, seen in uveal melanoma, was detected. FBXW7 p.R465C and p. R479G mutations, linked to cancer progression, were found in two of eight tumors. In summary, esophageal melanoma mutation profile indicates complexity of molecular mechanisms underlying its pathogenesis.
Introduction
Primary malignant melanoma of esophagus (from here on called “esophageal melanoma”) is an extremely rare neoplasm with the incidence estimated to be 0.03 per million in the USA [1]. Because of its rarity, only a few small series of esophageal melanoma are available [2–6] and most clinicopathologic data are derived from > 300 single case studies published since the first histologic description [7–9].
Esophageal melanoma is believed to develop from melanocytes anchored in the esophageal mucosa. Aberrant migration of melanoblasts to the esophagus can occur during their early migration from the neural crest to the epidermis and other sites [10–12]. Primary esophageal melanoma should not be confused with metastatic melanoma, which could present in any portion of the gastrointestinal tract. Although involvement of esophagus is uncommon, the differential diagnosis between metastatic and primary melanoma can be challenging [5, 13, 14]. Metastatic melanoma cells can infiltrate mucosa mimicking primary junctional changes [15]. Thus, a primary mucosal melanoma should be defined by identification of melanocytes at the epithelial–stromal junction and/or an adjacent melanoma in situ, and lack of primary cutaneous melanoma [16].
The mutation profile of esophageal melanoma remains incompletely characterized. Sanger sequencing data available of 30 cases are limited to BRAF, NRAS, and KIT mutation status [17–20]. More recent studies have employed next-generation sequencing for multiple targets, or whole-genome sequencing, on a few primary and metastatic tumors [21–24]. However, those studies did not clearly state the diagnostic criteria for primary esophageal melanoma so that metastatic melanomas might have been included.
The aim of this study was to evaluate the clinicopathologic and molecular genetic profile of 20 strictly defined primary esophageal melanomas containing junctional melanocytes.
Materials and methods
This study evaluated a series of 20 primary malignant melanomas of esophagus. Sixteen tumors were from the Armed Forces Institute of Pathology, Washington, D.C. Additional four tumors were contributed by co-authors. Demographic, clinical, and follow-up data were obtained according to the Institutional Review Board approvals.
Immunohistochemical studies
Expression of several antigens including melanocytic differentiation markers (human melanoma black [HMB]–45, KBA.62-melanoma associated antigen, Melan-A protein, microphthalmia-associated transcription factor [MITF], PNL-2-melanoma associated antigen, S100 protein, tyrosinase [TYR]) and CD34, CD117 (KIT), cytokeratin 8 (CK8), Cytokeratin cocktail (AE1/AE3), DOG1 (discovered on GIST1; also known as anoctamin 1, or ANO1), insulin–like growth factor 1 receptor (IGF1R), and Vimentin was evaluated immunohistochemically. Leica Bond-Max automated immunostainer (Leica, Bannockburn, IL) was used in this study. Detailed description of antibodies and immunohistochemical protocols is provided in supplemental data.
Molecular genetic studies
In 15 cases, formalin-fixed paraffin-embedded tissue blocks or unstained tumor sections were available. DNA was extracted from 5 to 10 5 μ sections using Maxwell® RSC DNA FFPE kit and Maxwell® RSC instrument (Promega, Madison, WI) following manufacturer’s protocol provided at www.promega.com. All 15 tumors were screened for BRAF, KIT, and NRAS mutations by PCR amplification and Sanger sequencing following previously published protocols [25, 26]. Subsequently, eight tumors with better-preserved DNA (cases 1, 6, 7, 8, 9, 12, 14, and 20) were evaluated by targeted next-generation sequencing. Ion Torrent™ (Life Technologies/Thermo Fisher Scientific, Waltham, MA) next-generation sequencing platform was used following manufacturer’s recommendations. Depending on the DNA quality either Ion AmpliSeq™ Cancer Hotspot Panel v2 Kit (50 gene targets) or Ion AmpliSeq™ Comprehensive Cancer Panel (409 gene targets) was employed to evaluate five and three tumors, respectively. Fifty genes targeted by the Cancer Hotspot Panel were included in the Comprehensive Cancer Panel. A list of all genes analyzed in this study is provided in supplemental data.
The data were processed by Torrent Server Suite 4.2 and sequences aligned to human genome reference sequence HG-19 (The Genome Reference Consortium). Variant calling was performed using Variant Caller v4.2, which is compatible with the Integrative Genomics Viewer (Broad Institute, Cambridge, MA), a high-performance visualization tool for interactive exploration of large, integrated data sets. Mutation nomenclature is based on Human Genome Mutation Society (www.hgvs.org) recommendations. The FATHMM (Functional Analysis Through Hidden Markov Models) scores predicting functional consequences of coding variants were obtained from the COSMIC (Catalog of Somatic Mutations in Cancer) at https://cancer.sanger.ac.uk or assessed using VarSome (The Human Genomic Variant Search Engine) at https://varsome.com.
Results
Demographic and clinicopathologic data
There were 14 males and 6 females (ratio 2.3:1). The median age at the diagnosis was 60 years for men and 63.5 years for women. Caucasian ethnicity was known in 16 cases. Demographic and clinicopathologic data are summarized in Table 1. Clinicopathologic characteristics of cases 5 and 8 were previously published [27, 28]. The latter was metachronous melanoma diagnosed 67 months after successful treatment of primary gastric melanoma located in the cardia [28]. Symptoms preceding the diagnosis most commonly included progressive dysphagia (87% of the cases), abdominal or chest pain (40%), and substantial weight loss (20%). Distal third of the esophagus was the most common location (13 cases), with five of these tumors seated at the esophagogastric junction. The tumor extended to or was limited to the mid-esophagus in four cases. The location was not specified in the remaining three cases. Two patients had multiple lesions. Most esophageal melanomas formed polypoid and lobulated endophytic masses. Tumor size, available in 19 cases, varied from 0.7 to 12 cm (median 6 cm). Ulceration was seen in 17 of 19 cases with suitable data. Metastases in local lymph nodes were detected at the time of surgery in 50% (9/18) of patients. Yet, in all cases, there was no evidence of co-existing or previous cutaneous melanoma.
Table 1.
Demographic and clinicopathologic data of 20 primary esophageal melanomas evaluated in this study
| Case | Sex | Age | Symptoms |
Tumor location | Tumor size (cm) | Local metastases at the surgery | Follow-up (in months) | ||
|---|---|---|---|---|---|---|---|---|---|
| Dysphagia | Pain | Weight loss | |||||||
| 1 | M | 40 | Unknown | Unknown | Unknown | Distal | 7 × 3 × 3 | No | Alive (96) |
| 2 | M | 45 | Yes | No | No | Distal | 5.5 | Yes | DOD (19) |
| 3 | M | 47 | Yes | No | No | Mid | Unknown | Unknown | DUNK (18) |
| 4 | M | 51 | Yes | Yes | No | Distal | 6 × 3 × 5 | Yes | DOD (10) |
| 5* | M | 57 | Yes | Yes | Yes | Mid | 7 | No | DOPC |
| 6 | M | 58 | Unknown | Unknown | Unknown | Unknown | 3 × 2.5 | Yes | DOD (20) |
| 7 | M | 59 | Yes | No | No | Mid to distal | 12.5 × 4 | Yes | DOD (11) |
| 8* | M | 61 | Yes | No | No | Mid to distal | 0.7 | No | DOD (16) |
| 9 | M | 62 | No | yes | No | Distal at E_G junction | 5 × 4 | Unknown | Unknown |
| 10 | M | 65 | No | Yes | No | Unknown | 6 × 6 × 3 | No | DOD (4) |
| 11 | M | 68 | Yes | Yes | Yes | Distal | 6 × 3.5 | Yes | DOD (22) |
| 12 | M | 72 | Unknown | Unknown | Unknown | Distal at E_G junction | 2.5 × 2.3 × 1 + two more lesions | No | DUNK (7) |
| 13 | M | 73 | Yes | No | No | Distal at E_G junction | 8 | Yes | Unknown |
| 14 | M | 79 | Yes | Yes | Yes | Distal | 7.5 × 4.5 | Yes | DOPC |
| 15 | F | 53 | Yes | No | No | Distal | 8 × 1.5 | Yes | DOD (16) |
| 16 | F | 55 | Yes | No | No | Distal | 4 × 1 | Yes | Unknown |
| 17 | F | 58 | Yes | No | No | Distal at E_G junction | 12 × 12 × 7.5 | Yes | DOD (4) |
| 18 | F | 69 | Unknown | Unknown | Unknown | Distal | Two lesions: 3.5 × 2.5 × 1.5; 3 × 2 × 1 | No | DUNK (104) |
| 19 | F | 71 | Unknown | Unknown | Unknown | Unknown | 2.1 × 1.3 × 0.7 | No | DUNK (8) |
| 20 | F | 78 | Yes | No | No | Distal at E_G junction | 7 × 4.5 × 4.5 | No | Unknown |
Histological features
A junctional peri-epithelial tumor component was present in all cases and at least focal melanin pigmentation in 85% (17/20) of cases. Pagetoid involvement of the overlying squamous epithelium was seen in 32% (6/19) of melanomas. Majority of tumors were composed of epithelioid cells. One tumor showed predominantly spindle cell morphology, and focal spindle or round cell component was seen in four cases and nuclear pleomorphism in five cases. Mitotic activity per 10 high-power fields (HPFs; 2 mm2) varied from 0 to 31 (median 12). Tumor necrosis was seen in 21% (4/19) and ulceration in 90% (17/19) of cases, respectively. Histopathologic data are summarized in Table 2. Representative histological images are shown in Figs. 1a, 2a. Additional figure illustrating junctional changes (atypical melanocytes disposed as single cells and as irregular nests along the basal layer of the esophageal epithelium) in case 19 is available in supplemental data.
Table 2.
Histopathologic features of 20 primary esophageal melanomas
| Case | Cell type | Pigment | Atypia | Mitoses/10 HPF | Tumor necrosis | Junctional activity | Pagetoid spread | Ulceration |
|---|---|---|---|---|---|---|---|---|
| 1 | Epithelioid to spindle | No | Moderate | 11 | Yes | Yes | No | Yes |
| 2 | Epithelioid | No | Moderate | 10 | No | Yes | No | Yes |
| 3 | Epithelioid | Yes | Moderate | 0 | No | Yes | No | Yes |
| 4 | Epithelioid | Yes | Moderate (focally severe) | 15 | No | Yes | No | Yes |
| 5* | Epithelioid | Yes | Moderate | 6 | No | Yes | No | Yes |
| 6 | Epithelioid | Yes | Moderate | 6 | No | Yes | Yes (f) | Yes |
| 7 | Epithelioid | Yes | Moderate (focally severe) | 14 | Yes | Yes | No | Yes |
| 8* | Epithelioid | Yes | Moderate | 4 | No | Yes | No | No |
| 9 | Epithelioid | Yes | Moderate | 4 | No | Yes | No | Yes |
| 10 | Epithelioid | Yes | Moderate | 23 | No | Yes | Yes | Yes |
| 11§ | Epithelioid | Yes | Moderate | n/a | n/a | Yes | n/a | n/a |
| 12 | Epithelioid to round cell | Yes | Moderate | 31 | No | Yes | Yes | Yes |
| 13 | Epithelioid to spindle | Yes | Moderate | 19 | Yes | Yes | No | Yes |
| 14 | Epithelioid | Yes | Moderate (focally severe) | 9 | No | Yes | No | Yes |
| 15 | Epithelioid | Yes | Moderate | 2 | No | Yes | No | Yes |
| 16 | Epithelioid | Yes | Moderate | 11 | Yes | Yes | Yes | Yes |
| 17 | Spindle to epithelioid | Yes | Severe | 14 | No | Yes | No | Yes |
| 18 | Epithelioid | No | Moderate (focally severe) | 7 | No | Yes | Yes (f) | Yes |
| 19 | Epithelioid | Yes | Moderate | 12 | No | Yes | Yes (f) | No |
| 20 | Epithelioid | Yes | Moderate | 29 | No | Yes | No | Yes |
Fig. 1.
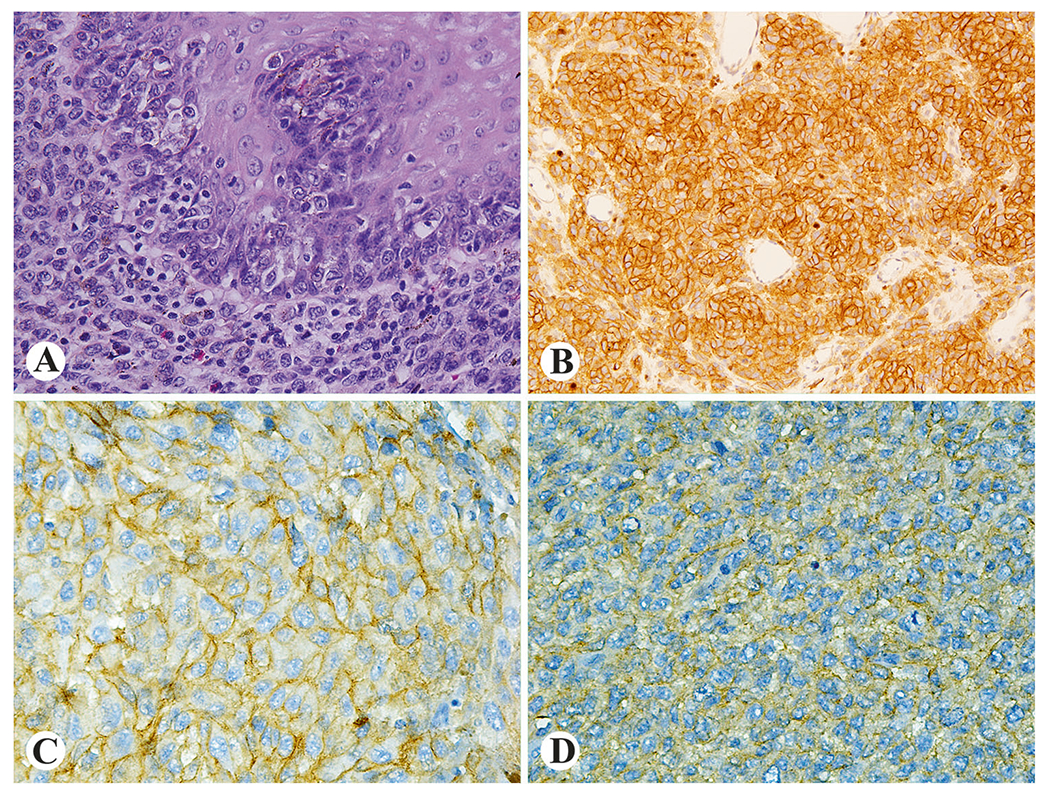
Examples of histologic and immunohistochemical findings in esophageal melanoma. Junctional changes (a) and prominent IGF1R expression (b) in case 6. Strong KIT expression in KIT-wild type case 7 (c) and weak KIT expression in KIT mutant case 20 (d)
Fig. 2.
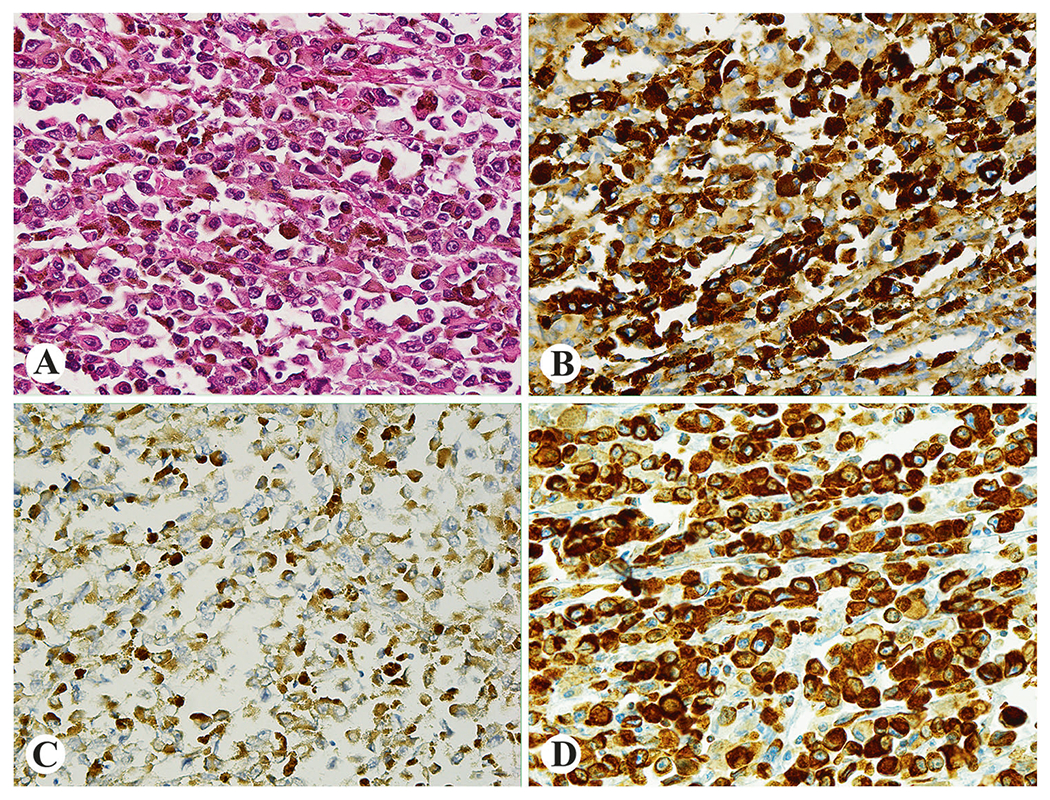
Histologic and immunohistochemical findings in case 10. Epithelioid melanoma with moderate atypia and prominent pigmentation (a), strong HMB45 expression (b), showing cytokeratin (c) and vimentin immunoreactivity (d)
Immunohistochemical features
All analyzed tumors were positive for at least for one marker of melanocytic differentiation (Fig. 2b). IGF1R expression was seen in 83% (9/11) evaluated tumors. Although IGF1R expression pattern was diffuse in all cases (Fig. 1b), intensity of immunohistochemical reactions varied from weak (n = 2) to moderate (n = 4) and strong (n = 3). Fifty percent (7/14) of esophageal melanomas showed variable KIT expression (Figs. 1c, d) with diffuse, strong immunoreactivity seen in two cases. In general, KIT expression was more prominent in junctional areas. No CD34 or DOG1 expression was detected. One tumor, case 20, revealed cytokeratin immunoreactivity (focal with CK8 antibody and more prominent with AE1/AE3 cytokeratin cocktail antibody). Vimentin was expressed in 89% (8/9) of melanomas. Representative images are shown in Figs. 2c, d. Immunohistochemical results are detailed in Table 3.
Table 3.
Immunohistochemical features of 20 primary esophageal melanomas
| Melanocytic differentiation markers |
Other markers |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | HMB45 | KBA.62 | Melan-A | MITF | PNL-2 | S100 | TYR | CD34 | CD117 (KIT) | DOG1 | IGFR1 | Keratin (CK8) | Keratins (pan-CK) | VIM |
| 1 | (+) | (+) | (+) | ND | (+) | (+) | (+) | (−) | (+) | ND | (+) | ND | (−) | ND |
| 2 | (+) | ND | ND | ND | ND | (+) | ND | ND | ND | ND | ND | ND | ND | ND |
| 3 | (+) | ND | ND | ND | ND | (+) | ND | ND | (+) | ND | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND | ND | (+) | ND | ND | ND | ND | ND | ND | ND | ND |
| 5 | (+) | (−) | (−) | (−) | (+) | (−) | (+) | (−) | (−) | (−) | (+) | (−) | (−) | (+) |
| 6 | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (−) | (−) | (+) | (−) | (−) | (+) |
| 7 | (+) | (−) | (+) | (−) | (+) | (+) | (+) | (−) | (+) | (−) | (−) | (−) | (−) | (+) |
| 8 | (+) | (−) | (+) | (+) | (+) | (+) | (+) | (−) | (−) | (−) | (+) | (−) | (−) | (+) |
| 9 | (−) | (+) | (+) | (+) | (−) | (+) | (−) | (−) | (−) | (−) | (+) | (−) | (−) | (+) |
| 10 | (+) | (+) | (+) | (−) | (+) | (+) | (+) | (−) | (−) | (−) | (−) | (+) | (+) | (+) |
| 11 | (+) | ND | ND | ND | ND | (+) | ND | ND | (+) | ND | ND | ND | ND | ND |
| 12 | (+) | (+) | (+) | (+) | (+) | (+) | (+) | (−) | (+) | (−) | (+) | (−) | (−) | (+) |
| 13 | ND | ND | ND | ND | ND | (+) | ND | ND | ND | ND | ND | ND | ND | ND |
| 14 | (+) | (−) | (−) | (−) | (+) | (+) | (+) | (−) | (−) | (−) | (+) | (−) | (−) | (−) |
| 15 | (+) | ND | ND | ND | ND | (+) | ND | ND | ND | ND | ND | ND | ND | ND |
| 16 | (+) | ND | ND | ND | ND | ND | ND | ND | (+) | ND | ND | ND | ND | ND |
| 17 | (+) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 18 | ND | ND | ND | ND | ND | (+) | ND | ND | ND | ND | ND | ND | ND | ND |
| 19 | (+) | (+) | (+) | ND | (+) | (+) | ND | ND | (−) | ND | (+) | (−) | (−) | ND |
| 20 | (+) | (−) | (+) | (−) | (+) | (+) | (+) | (−) | (+) | (−) | (+) | (−) | (−) | (+) |
ND not done
Molecular genetic features
Thirty-three percent (5/15) of tumors harbored NRAS mutations. A Q to K (n = 3) and Q to H (n = 1) substitutions at NRAS codon 61 were the most common change. In one case, p.A146T mutations were identified. One tumor contained a KRAS codon 13 mutation at relatively (20%) low frequency. Also, in one case, activating KIT mutation (p.L576P) was identified.
Two of three cases studied using Ion AmpliSeq™ Comprehensive Cancer Panel next-generation sequencing contained SF3B1 (splicing factor 3B subunit 1) mutations (p.R625C and p.K666T). A p.R625C co-occurred with nonsense mutation truncating mammalian target of rapamycin (mTOR) at p.Q1684, whereas p.K666T was detected in melanoma carrying NRAS p.Q61K and KIT p.L576P driver mutations in addition to cyclin-dependent kinase inhibitor 2B (CDKN2B) p.110N and ataxia telangiectasia and Rad3 related (ATR) p.V372G substitutions. In one case, tuberous sclerosis 1 (TSC1) p.H371Q was the only mutation identified. Two F-box and WD repeat domain containing 7 (FBXW7) mutants, p.R465C and p.R479G were found among eight analyzed cases. Tumor characterized by FBXW7 p.R465C also harbored KRAS p.G13C mutations at a low frequency. FATHMM scores describing pathogenicity of the missense variants identified in this study ranged from 0.80 to 0.99 supporting pathogenic potential (complete list provided in supplemental data).
In case 20, NRAS p.Q61K and KIT WT genotype, and NRAS WT and KIT p.L576P genotype were identified by Sanger sequencing and next-generation sequencing, respectively. This variation could be related to two different DNA samples evaluated by Sanger and next-generation sequencing.
No mutations were identified in the genes often indicated in melanoma [29, 30] such as BRAF (15 cases analyzed), CDKN2A, GAN11 and GNAQ, PIK3CA and TP53 (eight cases analyzed) BAP1 and NF1 (three cases analyzed). Sanger and next-generation sequencing results are detailed in Table 4 and supplemental data.
Table 4.
Results of esophageal melanoma genotyping including Sanger sequencing of BRAF, KIT, and NRAS (15 cases) and all mutations identified by the Ion Torrent™ next-generation sequencing with Ion AmpliSeq™ Cancer Hotspot Panel v2 Kit (five cases) and Ion AmpliSeq™ Comprehensive Cancer Panel (three cases)
| Gene |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Methods | ATR | BRAF | CDKN2B | FBXW7 | KIT | KRAS | NRAS | MTOR | SF3B1 | TSC1 |
| 1 | Ion AmpliSeq™ CCP Sanger sequencing | WT | WT | WT | WT | WT | WT | WT | WT | WT | p.H371Q |
| 3 | Sanger sequencing | WT | WT | WT | |||||||
| 5 | Sanger sequencing | WT | WT | WT | |||||||
| 6 | Ion AmpliSeq™ CHP Sanger sequencing | NIP | WT | WT | WT | WT | p.Q61K | NIP | NIP | NIP | |
| 7 | Ion AmpliSeq™ CHP Sanger sequencing | NIP | WT | NIP | p.R479G | WT | WT | WT | NIP | NIP | NIP |
| 8 | Ion AmpliSeq™ CHP Sanger sequencing | NIP | WT | NIP | WT | WT | WT | p.A146T | NIP | NIP | NIP |
| 9 | Ion AmpliSeq™ CHP Sanger sequencing | NIP | WT | NIP | WT | WT | WT | p.Q61K | NIP | NIP | NIP |
| 10 | Sanger sequencing | WT | WT | WT | |||||||
| 11 | Sanger sequencing | WT | WT | WT | |||||||
| 12 | Ion AmpliSeq™ CCP Sanger sequencing | WT | WT | WT | WT | WT | WT | WT | p.Q1684* | p.R625C | WT |
| 14 | Ion AmpliSeq™ CHP Sanger sequencing | NIP | WT | NIP | p.R465C | WT | p.G13C | WT | NIP | NIP | NIP |
| 16 | Sanger sequencing | WT | WT | p.Q61H | |||||||
| 17 | Sanger sequencing | WT | WT | WT | |||||||
| 19 | Sanger sequencing | WT | WT | WT | |||||||
| 20 | Ion AmpliSeq™ CCP Sanger sequencing | p.V372G | WT | p.D110N | WT | xp.L576P | WT | xxp.Q61K | WT | p.K666T | WT |
Complete next-generation sequencing data are provided in the Supplemental Table 2 and Supplemental Table 3
CCP Comprehensive Cancer Panel (409 gene targets), CHP Cancer Hotspot Panel v2 Kit (50 gene targets), NIP not included in this panel
next-generation sequencing result
Sanger sequencing result
Follow-up data
Metastatic disease at the surgery was diagnosed in 9 of 18 (50%) cases. Follow-up data were available on 16 patients. Two patients including one with local metastases died of postoperative complications. Nine patients died of disease within 4–22 months (mean survival 15 months), while 4 patients died of unknown causes within 8–104 months (mean survival 43 months). One patient was alive without disease 96 months after surgery; two patients, who survived 96 and 104 months, respectively, had no nodal metastases at surgery.
Discussion
This study analyzed 20 well-documented primary esophageal melanomas. Seventy percent of patients were male. Similar age distribution and predominance of male gender were reported in two recently published largest cohorts of 13 and 17 primary esophageal melanoma patients of Asian ethnicity [31, 32]. Male predominance among primary esophageal melanoma patients is reportedly not associated with alcohol and tobacco consumption, as seen in esophageal squamous cell carcinoma patients [32]. In this series, most of melanomas arose in the distal third of the esophagus, a location frequently indicated by previous studies [9]. Progressive dysphagia accompanied by upper abdominal pain and weight loss mirror reported main clinical symptoms [33].
Tumor ulceration was seen in almost all (17/19) analyzed esophageal melanomas. Presence of ulceration is a prognostic factor indicating shorter overall-survival for both Stage I and Stage II cutaneous melanoma patients [34]. In this study, one of two cases with long overall survival lacked ulceration. Also, mitotic rate has been considered to be a prognostic factor for cutaneous melanoma [35]. In this study, mitotic rates were slightly higher in esophageal melanomas with overall survival shorter than 12 months.
In general, the prognosis for esophageal melanoma is poor [8, 33]. A great majority of patients included in this series died of disease within several months. However, two long survivals of 104 and 96 months were documented. In both cases, no local lymph node metastases were diagnosed at the curative resection. Patients with esophageal melanoma at T1a stage revealed excellent prognosis compared with more advanced tumors with local lymph nodes metastases [36]. However, long-term survivals (up to 12 years) have been reported in few cases with submucosal invasion and local lymph node metastases treated by subtotal esophagectomy and adjuvant chemotherapy [37, 38].
In this series of esophageal melanomas, one tumor expressed keratins. This phenomenon has been previously described [39]. The tumor invasiveness and metastatic potential may correlate with keratin and vimentin coexpression [40]. Reported in this study, tumor coexpressing keratin and vimentin showed rapid progression and only 4-month overall survival.
Alterations of proteins forming MAPK (mitogen-activated protein kinase) pathway that communicates signals from cell surface to the nucleus, have been reported in different type of cancers including malignant melanoma [41]. NRAS (neuroblastoma RAS viral [v-ras] oncogene homolog) is a member of human RAS proto-oncogene family that encodes cell membrane-associated proteins involved in transduction of extracellular growth and differentiation signals [42]. Typically, oncogenic NRAS mutations cluster in exon 1 (G12/13) and exon 2 (Q61) and represent the second most common driver after BRAF mutations in melanoma [43]. About 5–20% of mucosal melanoma, depending on tumor location, harbor NRAS mutations [44]. In this study, five NRAS mutants were identified among 15 esophageal melanomas. Four substitutions were found in codon 61, a “hot-spot” for NRAS mutations in melanoma [45], whereas one tumor harbored NRAS p.A146T substitution. This mutation has not been reported in melanoma by COSMIC. However, it was identified in melanoma cell line A375 clones with acquired resistance to the dabrafenib GSK2118436, a BRAF inhibitor [46]. NRAS p.A146T mutation was detected in blastic plasmacytoid dendritic cell neoplasm, and in B- and T-cell acute lymphoblastic leukemia [47–49].
KIT, a transmembrane receptor tyrosine kinase, plays a crucial role in growth regulation, differentiation, migration, and proliferation of melanocytes. Somatic KIT mutations cause oncogenic signaling affecting both the mitogenactivated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathway [50]. KIT-mutants were mainly identified among mucosal, acral, and chronically sun-damaged skin tumors [51, 52]. In melanoma, 70% of KIT mutations were found in juxtamembrane domain (exon 11) with p.L576P substitution being a most common [53]. This mutation, initially reported in gastrointestinal stromal tumors, leads to pathologic activation of KIT tyrosine kinase activity [54]. In this study, p.L576P mutation was found in 7% (1/15) of esophageal melanomas. Previously, a few (n = 4) KIT mutations including p.L576P, p.H580_G592dup in the juxtamembrane domain, and p.F504L and p. A502_Y503insFA in extracellular domain, were reported in a combined cohort of 17 esophageal melanomas [18, 20, 21]. However, variable KIT expression was detected immunohistochemically in a higher number of cases. In this study, 50% of analyzed tumors revealed KIT positivity. As reported, KIT was stronger expressed in the in situ and junctional component than in the invasive part of the lesion [55]. Although response to tyrosine kinase inhibitors imatinib mesylate and sorafenib in KIT-mutated rectal melanoma have been reported in isolated cases [56, 57], larger studies have failed to confirm convincing therapeutic efficacy [58].
Two of three esophageal melanomas analyzed with Ion AmpliSeq™ Comprehensive Cancer Panel revealed mutations in the gene encoding splicing factor 3B subunit 1 (SF3B1), a component of the spliceosome. Identical SF3B1 p.R625C and p.K666T mutations were previously reported in uveal melanomas, colorectal and other mucosal melanomas, and have been associated with diverse alternative splicing events [59, 60]. Approximately 20% of each uvealharbored SF3B1 somatic mutations. In uveal melanoma, the presence of SF3B1 mutations is associated with mutational activation of GNAQ or GNA11 oncogenes [59]. In esophageal melanoma, no co-occurrence of SF3B1 and GNAQ or GNA11 mutations was identified. However, one SF3B1-mutant harbored KIT p.L576P and NRAS p.Q61K mutations. A recent study documented SF3B1 mutations in anorectal melanomas harboring RAS mutations [22]. Second of SF3B1-mutant esophageal melanomas harbored mTOR FAT-domain p.Gln1684* mutation. Previously, missense mTOR mutations were identified in mucosal melanoma and linked to a worse prognosis [61]. Although biological significance of mTOR nonsense mutation is unknown, mTOR inactivation may lead to deregulation of mTOR complex 1 and its tumor suppression function [62].
Two esophageal melanomas harbored mutations affecting the FBXW7 gene. FBXW7 (F-Box and WD repeat domain containing 7) encodes a member of the F-box protein family. The F-box proteins constitute one of the four subunits of ubiquitin protein ligase complex called SCFs (SKP1-cullin-F-box), which functions in phosphorylation-dependent ubiquitination. A recent melanoma study showed no association between the presence of FBXW7 and BRAF or RAS mutations and designated FBXW7 as a tumor-suppressor gene, a novel driver for a subset of melanomas [63]. In line with this observation, one of the FBXW7-mutant esophageal melanomas reported in this study was BRAF and RAS wild type. However, another tumor harbored a p.G13C KRAS-mutant subclone. In general, KRAS-mutants are very rare (< 1%) in melanoma and have not been reported in esophageal melanoma. However, concomitant of FBXW7 and KRAS mutations have been found in advanced colorectal carcinomas [64]. Two FBXW7 mutations identified in esophageal melanoma (p. R465C and p.R479G), which are considered to inactivate FBXW7 were previously reported in ovarian and head and neck squamous cell carcinomas [64].
In one esophageal melanoma, a p.H371Q mutation in tuberin-binding domain of TSC1 was identified as a sole alteration. Recent study reported TCS1 mutations in a spectrum of mucosal melanomas. In a few TSC1 mutants, including one esophageal melanoma, alteration of TSC1 was the only change and did not co-occur with NRAS, KIT, or BRAF mutations [65]. In contrast with those cases, tumors with multiple driver mutations were seen in this series and previously reported indicating high frequency of somatic mutation in melanoma [66]. Dynamic clonal changes might be responsible for the differences between Sanger and NGS-sequencing results in case 20, especially if different DNA samples are being evaluated.
In this study, no BRAF mutations were identified in esophageal melanomas. Previous investigations have reported a small number of BRAF-mutant esophageal melanomas [18, 20–24, 66, 67]. However, clinical and histological findings specific for primary esophageal melanoma were not clearly documented, so that the possibility of inclusion of metastatic cutaneous melanoma in those series cannot be excluded. In this study, both atypical junctional changes in the squamous epithelium and/or an adjacent melanoma in situ with no evidence for co-existing or previous cutaneous melanoma was documented in all cases.
In summary, activation of RAS_RAF_MEK pathway through the NRAS mutations seems to be essential for development of a subset of esophageal melanoma, whereas BRAF mutations are rare if they occur. Also, mutations of FBX7, KIT, SF3B1, and TSC1 being previously found in other mucosal melanomas may play significant role in this tumor with a complex pathogenesis. Further studies, such as RNA sequencing for fusion gene transcripts, may identify other molecular events underlying initiation and progression of this rare neoplasm.
Supplementary Material
Acknowledgements
We thank Dr. Leslie Sobin, MD, for support and reviewing this manuscript.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41379-018-0163-y) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Coté TR, Sobin LH. Primary melanomas of the esophagus and anorectum: epidemiologic comparison with melanoma of the skin. Melanoma Res. 2009;19:58–60. [DOI] [PubMed] [Google Scholar]
- 2.DiCostanzo DP, Urmacher C. Primary malignant melanoma of the esophagus. Am J Surg Pathol. 1987;11:46–52. [DOI] [PubMed] [Google Scholar]
- 3.Lohmann CM, Hwu WJ, Iversen K, et al. Primary malignant melanoma of the oesophagus: a clinical and pathological study with emphasis on the immunophenotype of the tumours for melanocyte differentiation markers and cancer/testis antigens. Melanoma Res. 2003;13:595–601. [DOI] [PubMed] [Google Scholar]
- 4.Li B, Lei W, Shao K, et al. Characteristics and prognosis of primary malignant melanoma of the esophagus. Melanoma Res. 2007;17:239–42. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez AA, Wu TT, Prieto VG, et al. Comparison of primary and metastatic malignant melanoma of the esophagus: clinicopathologic review of 10 cases. Arch Pathol Lab Med. 2008;132:1623–9. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Huang XY, Li Y, et al. Primary malignant melanoma of the esophagus: a study of clinical features, pathology, management and prognosis. Dis Esophagus. 2011;24:109–13. [DOI] [PubMed] [Google Scholar]
- 7.Garfinkle JM, Cahan WG. Primary melanocarcinoma of the esophagus; first histologically proved case. Cancer . 1952;5:921–6. [DOI] [PubMed] [Google Scholar]
- 8.Volpin E, Sauvanet A, Couvelard A, et al. Primary malignant melanoma of the esophagus: a case report and review of the literature. Dis Esophagus. 2002;15:244–9. [DOI] [PubMed] [Google Scholar]
- 9.Bisceglia M, Perri F, Tucci A, et al. Primary malignant melanoma of the esophagus: a clinicopathologic study of a case with comprehensive literature review. Adv Anat Pathol. 2011;18:235–52. [DOI] [PubMed] [Google Scholar]
- 10.De La Pava S, Nigogosyan G, Pickren JW, et al. Melanosis of the esophagus. Cancer . 1963;16:48–50. [DOI] [PubMed] [Google Scholar]
- 11.Tateishi R, Taniguchi H, Wada A, et al. Argyrophil cells and melanocytes in esophageal mucosa. Arch Pathol. 1974;98:87–9. [PubMed] [Google Scholar]
- 12.Ohashi K, Kato Y, Kanno J, et al. Melanocytes and melanosis of the oesophagus in Japanese subjects--analysis of factors effecting their increase. Virchows Arch A Pathol Anat Histopathol. 1990;417:137–43. [DOI] [PubMed] [Google Scholar]
- 13.Eng J, Pradhan GN, Sabanathan S, et al. Malignant melanoma metastatic to the esophagus. Ann Thorac Surg. 1989;48:287–88. [DOI] [PubMed] [Google Scholar]
- 14.Schneider A, Martini N, Burt ME. Malignant melanoma metastatic to the esophagus. Ann Thorac Surg. 1993;55:516–7. [DOI] [PubMed] [Google Scholar]
- 15.Littman CD. Metastatic melanoma mimicking primary bronchial melanoma. Histopathology. 1991;18:561–3. [DOI] [PubMed] [Google Scholar]
- 16.Allen AC, Spitz S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer . 1953;6:1–45. [DOI] [PubMed] [Google Scholar]
- 17.Wong CW, Fan YS, Chan TL, et al. Cancer Genome Project. BRAF and NRAS mutations are uncommon in melanomas arising in diverse internal organs. J Clin Pathol. 2005;58:640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekine S, Nakanishi Y, Ogawa R, et al. Esophageal melanomas harbor frequent NRAS mutations unlike melanomas of other mucosal sites. Virchows Arch. 2009;454:513–7. [DOI] [PubMed] [Google Scholar]
- 19.Terada T Amelanotic malignant melanoma of the esophagus: report of two cases with immunohistochemical and molecular genetic study of KIT and PDGFRA. World J Gastroenterol. 2009;15:2679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langer R, Becker K, Feith M, et al. Genetic aberrations in primary esophageal melanomas: molecular analysis of c-KIT, PDGFR, KRAS, NRAS and BRAF in a series of 10 cases. Mod Pathol. 2011;24:495–501. [DOI] [PubMed] [Google Scholar]
- 21.Furney SJ, Turajlic S, Stamp G, et al. Genome sequencing of mucosal melanomas reveals that they are driven by distinct mechanisms from cutaneous melanoma. J Pathol. 2013;230:261–9. [DOI] [PubMed] [Google Scholar]
- 22.Cosgarea I, Ugurel S, Sucker A, et al. Targeted next generation sequencing of mucosal melanomas identifies frequent NF1 and RAS mutations. Oncotarget. 2017;8:40683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Yan S, Liu Z, et al. Multiregional sequencing reveals genomic alterations and clonal dynamics in primary malignant melanoma of the esophagus. Cancer Res. 2018;78:338–47. [DOI] [PubMed] [Google Scholar]
- 25.Lasota J, Kowalik A, Wasag B, et al. Detection of the BRAF V600E mutation in colon carcinoma: critical evaluation of the imunohistochemical approach. Am J Surg Pathol. 2014;38:1235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasota J, Jasinski M, Sarlomo-Rikala M, et al. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niezychowska K, Zawadzki J, Wejman J. Primary malignant melanoma of the esophagus. A case report. Pol J Pathol. 1997;48:205–7. [PubMed] [Google Scholar]
- 28.Dabrowski A, Zinkiewicz K, Szumilo J, et al. Unusual clinical course of metachronous melanomas of the upper digestive system. World J Gastroenterol. 2005;11:2197–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–80. [DOI] [PubMed] [Google Scholar]
- 30.Hintzsche JD, Gorden NT, Amato CM, et al. Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res. 2017;27:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Tachimori Y, Hokamura N, et al. Diagnosis and surgical outcomes for primary malignant melanoma of the esophagus: a single-center experience. Ann Thorac Surg. 2013;96:1002–6. [DOI] [PubMed] [Google Scholar]
- 32.Shugeng Gao, Jiagen Li, Xiaoli Feng, et al. Characteristics and surgical outcomes for primary malignant melanoma of the esophagus. Sci Rep. 2016;6:23804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabanathan S, Eng J, Pradhan GN. Primary malignant melanoma of the esophagus. Am J Gastroenterol. 1989;84:1475–81. [PubMed] [Google Scholar]
- 34.Balch CM, Wilkerson JA, Murad TM, et al. The prognostic significance of ulceration of cutaneous melanoma. Cancer . 1980;45:3012–7. [DOI] [PubMed] [Google Scholar]
- 35.Attis MG, Vollmer RT. Mitotic rate in melanoma: a reexamination. Am J Clin Pathol. 2007;127:380–4. [DOI] [PubMed] [Google Scholar]
- 36.Kuwabara S, Ebihara Y, Nakanishi Y, et al. Primary malignant melanoma of the esophagus treated with subtotal esophagectomy: a case report. BMC Surg. 2017;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamdy FC, Smith JH, Kennedy A, et al. Long survival after excision of a primary malignant melanoma of the oesophagus. Thorax. 1991;46:397–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta V, Kochhar R, Sinha SK, et al. Primary malignant melanoma of the esophagus: long-term survival after radical resection. J Thorac Oncol. 2009;4:1180–2. [DOI] [PubMed] [Google Scholar]
- 39.Miettinen M, Franssila K. Immunohistochemical spectrum of malignant melanoma. The common presence of keratins. Lab Invest. 1989;61:623–8. [PubMed] [Google Scholar]
- 40.Hendrix MJ, Seftor EA, Chu YW, et al. Coexpression of vimentin and keratins by human melanoma tumor cells: correlation with invasive and metastatic potential. J Natl Cancer Inst. 1992;84:165–74. [DOI] [PubMed] [Google Scholar]
- 41.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. [DOI] [PubMed] [Google Scholar]
- 42.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawryluk EB, Tsao H. Melanoma: clinical features and genomic insights. Cold Spring Harb Perspect Med. 2014;4:a015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366–75. [DOI] [PubMed] [Google Scholar]
- 45.Jakob JA, Bassett RL Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer . 2012;118:4014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther. 2012;11:909–20. [DOI] [PubMed] [Google Scholar]
- 47.Menezes J, Acquadro F, Wiseman M, et al. Exome sequencing reveals novel and recurrent mutations with clinical impact in blastic plasmacytoid dendritic cell neoplasm. Leukemia. 2014;28:823–9. [DOI] [PubMed] [Google Scholar]
- 48.Neumann M, Vosberg S, Schlee C, et al. Mutational spectrum of adult T-ALL. Oncotarget. 2015;6:2754–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Smith AJ, Ojha J, Francis SS, et al. Clonal and microclonal mutational heterogeneity in high hyperdiploid acute lymphoblastic leukemia. Oncotarget. 2016;7:72733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lennartsson J, Jelacic T, Linnekin D, et al. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23:16–43. [DOI] [PubMed] [Google Scholar]
- 51.Handolias D, Salemi R, Murray W, et al. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment Cell Melanoma Res. 2010;23:210–5. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Abed S, Pennell N, Petrella T, et al. KIT gene mutations and patterns of protein expression in mucosal and acral melanoma. J Cutan Med Surg. 2012;16:135–42. [DOI] [PubMed] [Google Scholar]
- 53.Woodman SE, Davies MA. Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol. 2010;80:568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shtivelman E, Davies MQ, Hwu P, et al. Pathways and therapeutic targets in melanoma. Oncotarget. 2014;5:1701–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montone KT, van Belle P, Elenitsas R, et al. Proto-oncogene c-kit expression in malignant melanoma: protein loss with tumor progression. Mod Pathol. 1997;10:939–44. [PubMed] [Google Scholar]
- 56.Hodi FS, Friedlander P, Corless CL, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26:2046–51. [DOI] [PubMed] [Google Scholar]
- 57.Quintás-Cardama A, Lazar AJ, Woodman SE, et al. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat Clin Pract Oncol. 2008;5:737–40. [DOI] [PubMed] [Google Scholar]
- 58.Kim KB, Alrwas A. Treatment of KIT-mutated metastatic mucosal melanoma. Chin Clin Oncol. 2014;3:35. [DOI] [PubMed] [Google Scholar]
- 59.Harbour JW, Roberson ED, Anbunathan H, et al. Recurrent mutations at codon 625 of the splicing factor SF3B1 in uveal melanoma. Nat Genet. 2013;45:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong Y, Krauthammer M, Halaban R. Rare SF3B1 R625 mutations in cutaneous melanoma. Melanoma Res. 2014;24:332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong Y, Si L, Li Y, et al. Analysis of mTOR Gene aberrations in melanoma patients and evaluation of their sensitivity to PI3K-AKT-mTOR pathway inhibitors. Clin Cancer Res. 2016;22:1018–27. [DOI] [PubMed] [Google Scholar]
- 62.Villar VH, Nguyen TL, Terés S, et al. Escaping mTOR inhibition for cancer therapy: tumor suppressor functions of mTOR. Mol Cell Oncol. 2017;4:e1297284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aydin IT, Melamed RD, Adams SJ, et al. FBXW7 mutations in melanoma and a new therapeutic paradigm. J Natl Cancer Inst. 2014;106:dju107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jardim DL, Wheler JJ, Hess K, et al. FBXW7 mutations in patients with advanced cancers: clinical and molecular characteristics and outcomes with mTOR inhibitors. PLoS ONE. 2014;9: e89388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma M, Dai J, Xu T, et al. Analysis of TSC1 mutation spectrum in mucosal melanoma. J Cancer Res Clin Oncol. 2018;144: 257–67. [DOI] [PubMed] [Google Scholar]
- 66.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao T, Kong FW, Wang H, et al. A long-term survivor with esophageal melanoma and pulmonary metastasis after single-stage esophagectomy and lobectomy: case report and literature review. Medicine (Baltimore). 2017;96:e7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


