Abstract
Context:
Increased high-density lipoprotein cholesterol (HDL-C) is common in type 1 diabetes (T1D) and is associated both with hyperadiponectinemia and with elevated lipoprotein lipase activity (LPL). Because adiponectin has been shown to increase LPL expression, elevated LPL may link the hyperadiponectinemia in T1D with increased HDL.
Objective:
The purpose of this study was to determine whether LPL activity accounts for the association between adiponectin and HDL in T1D.
Design, Participants, and Setting:
A cohort of 127 patients with T1D attending the Diabetes Clinic at the University of Miami and 103 healthy control subjects were recruited.
Main Outcome Measure:
HDL-C and adiponectin were measured in the full cohort and in a subgroup, HDL subfractions were obtained by ultracentrifugation, and LPL and hepatic lipase were measured in postheparin plasma.
Results:
Total HDL-C and the lowest density HDL subfraction, apolipoprotein A-I, LPL activity, and adiponectin levels were higher in subjects with T1D than in control subjects (P < .05). Both adiponectin and LPL activity were directly associated with total HDL-C and its lowest density subfraction, but adiponectin and LPL were not correlated (P = 0.13). Adiponectin alone explained 11.6% and adiponectin plus LPL explained 23.8% of the HDL-C variance. In a multivariate model, adiponectin remained an independent predictor of HDL-C along with LPL and serum creatinine, explaining together 27% of HDL-C variance.
Conclusions:
Adiponectin was strongly associated with HDL-C in T1D, suggesting that hyperadiponectinemia is linked to the elevated HDL-C in this population. However, this relationship is independent of the association between LPL and HDL-C. Thus, elevated adiponectin and LPL activity are independently related to increased HDL-C in T1D.
Although cardiovascular disease (CVD) is well recognized as the leading cause of morbidity and mortality in both type 1 diabetes (T1D) (1) and type 2 diabetes (2), important differences in the determinants of CVD have been described in the 2 forms of the disease. Among these are the characteristic lipid profiles in types 1 and 2 diabetes, in which a distinctive difference is the high-density lipoprotein cholesterol (HDL-C) value. HDL-C is typically reduced in those with type 2 diabetes, and this finding has been associated with the increased CVD risk in these subjects (3). In contrast, HDL-C levels in T1D are usually reported as being normal (4) or elevated (5), which has raised questions concerning the relationship between HDL-C and CVD risk in this form of diabetes (6). Elevated HDL-C in T1D has been proposed to result from increased activity of lipoprotein lipase (LPL) due to peripheral hyperinsulinemia resulting from systemic insulin administration (5), which enriches HDL with cholesterol as a consequence of the hydrolysis of triglyceride-rich lipoproteins (7).
Another important difference between the 2 forms of diabetes in this regard that may be related to the HDL-C difference is the adiponectin level. Adiponectin levels are usually reduced in type 2 diabetes (8), whereas they are typically increased in T1D relative to those of control subjects (9). Adiponectin is an adipokine with insulin-sensitizing and anti-inflammatory activity that has been shown to have protective effects on endothelium and on development of atherosclerosis in animal models (10). Cohort studies of the association of adiponectin with CVD have varied, with reports indicating that adiponectin is inversely related to CVD in type 2 diabetes (10), whereas studies in T1D have reported both positive and inverse associations (11, 12). Adiponectin has been demonstrated to associate strongly with HDL-C levels both in the general population (13) and in cohorts with diabetes (14, 15). The basis for this association is not understood although adiponectin has been shown to increase lipoprotein lipase (LPL) production experimentally (16) and to associate with LPL activity and inversely with hepatic lipase (HL) activity levels in clinical studies (17, 18). Lipases play a key role in regulating HDL levels (7), which could account for the relationship between adiponectin and HDL-C.
To further our understanding of the nature of elevated HDL-C in T1D, we have examined the associations between adiponectin, HDL-C, and postheparin lipase activities in a cohort of subjects with T1D and control subjects to test the hypothesis that the association between hyperadiponectinemia and elevated HDL-C in these subjects is principally dependent on increased LPL activity.
Subjects and Methods
Research subjects
A convenience sample that included 127 patients with T1D who attended the Diabetes Clinic at the Diabetes Research Institute/University of Miami Miller School of Medicine volunteered for the study. Exclusion criteria included age of <18 years, pregnancy, the presence of chronic kidney disease, a recent cardiovascular event or other systemic disease, or therapy with fibrate or niacin. A further 103 otherwise healthy, nondiabetic subjects were recruited as control subjects by advertisement. They denied a history of diabetes or prediabetes or fasting glucose elevation. The study was approved by the Human Subjects Research Office of the University of Miami, and written consent was obtained from all individuals.
Clinical and biochemical variables
Clinical characteristics, which included body mass index (BMI), medications, and duration of disease, were assessed on the day of the study visit. Blood samples were obtained from all subjects after an overnight fast, and random urine samples for albumin/creatinine ratio (ACR) measurement were obtained from individuals with T1D. Glycosylated hemoglobin (HbA1c), total cholesterol, HDL-C, triglyceride, HDL-C, urinary ACR, apolipoprotein A-I (apo A-I), and serum creatinine were measured by standard laboratory assays, and low-density lipoprotein cholesterol (LDL-C) and the estimated glomerular filtration rate (eGFR) were calculated. Total adiponectin levels were quantitated in duplicate by ELISA (Mercodia, Inc.). Both the intra- and interassay coefficients of variation were <5%. A subgroup of participants with T1D and control subjects consented to measurement of postheparin lipolytic activity and HDL subfractionation by ultracentrifugation (69 patients with T1D and 43 control subjects). Although fasting glucose levels were not available in the full control group, the mean ± SEM fasting glucose level for control subjects in the subset was 92.6 ± 1.4 mg/dL. There were no significant differences in age (42.5 ± 1.28 vs 44.9 ± 1.7 years, P = .26), BMI (26.3 ± 0.39 vs 26.1 ± 0.53 kg/m2, P = .85), sex distribution, and HbA1c levels (7.77 ± 0.12 vs 7.88 ± 0.17%, P = .56) between the full cohort and the subset of subjects with T1D, respectively (Table 1), although the duration of the disease was somewhat longer in the full cohort than in the subset (25.46 ± 1.15 vs 20.66 ± 1.66 years, P ≤ .05)
Table 1.
Baseline Characteristics
| T1D Group | Control Group | |||
|---|---|---|---|---|
| Men (n = 60; a n = 30) | Women (n = 67; a n = 39) | Men (n = 49; a n = 20) | Women (n = 54; a n = 23) | |
| Age, y | 40.3 ± 1.7 | 44.5 ± 1.8 | 39.5 ± 1.4 | 39.7 ± 1.6 |
| BMI, kg/m2 | 27 ± 0.4b | 25.6 ± 0.6 | 25.6 ± 0.4 | 25 ± 0.5 |
| Diabetes duration, y | 25.9 ± 2.1 | 26.6 ± 1.6 | NA | NA |
| HbA1c, % (mmol/mol) | 7.6 ± 0.1 (60 ± 1) | 7.9 ± 0.1 (63 ± 1) | NA | NA |
| Serum creatinine, mg/dL (μmol/L) | 0.9 ± 0.2 (79.56 ± 17)c | 0.6 ± 0.01 (53.04 ± 0.8)b | 0.9 ± 0.02 (79.56 ± 1.7)c | 0.7 ± 0.01 (61.88 ± 0.8) |
| eGFR, mL/min/1.73 m2 | 112.9 ± 6.2 | 119.2 ± 5.4 | 91.8 ± 4.5 | 101.8 ± 4.1 |
| Adiponectin, μg/mL | 11.2 ± 0.8b , c | 16.5 ± 0.9b | 8.9 ± 0.4c | 11.4 ± 0.6 |
| Apo A-I, mg/dL (g/L) | 151.2 ± 3 (1.51 ± 0.03)b , c | 181.5 ± 4.1 (1.81 ± 0.04)b | 137.9 ± 2.6 (1.37 ± 0.02)c | 163.2 ± 3.7 (1.63 ± 0.03) |
| Triglyceride, mg/dL (mmol/L) | 93.5 ± 6.6 (1.06 ± 0.07)b | 83.3 ± 5.6 (0.94 ± 0.06) | 133.9 ± 11.8 (1.51 ± 0.13)c | 99.3 ± 8.1 (1.12 ± 0.09) |
| LDL-C, mg/dL (mmol/L) | 99.2 ± 4.7 (2.57 ± 0.12)b | 95.6 ± 3.7 (2.48 ± 0.09)b | 123.3 ± 4.9 (3.19 ± 0.12) | 116.4 ± 4 (3.01 ± 0.10) |
| HDL-C, mg/dL (mmol/L) | 55.9 ± 1.8 (1.45 ± 0.04)b , c | 72.6 ± 2.4 (1.88 ± 0.06)b | 45.9 ± 1.4 (1.19 ± 0.04)c | 61.1 ± 2.1 (1.58 ± 0.05) |
| HDL-T (DGU), mg/dL (mmol/L)c | 53 ± 1.8 (1.37 ± 0.04)b , c | 68.6 ± 3.1 (1.78 ± 0.08) | 44.5 ± 2.6 (1.15 ± 0.06)c | 60.6 ± 3.7 (1.57 ± 0.09) |
| HDL-L, mg/dL (mmol/L)c | 20.1 ± 1.7 (0.52 ± 0.09)b , c | 32.9 ± 2.7 (0.85 ± 0.06)b | 12.1 ± 1.1 (0.31 ± 0.02)c | 21.1 ± 2.6 (0.54 ± 0.06) |
| HDL-M, mg/dL (mmol/L)c | 25.8 ± 0.9 (0.66 ± 0.02)c | 29.5 ± 1.0 (0.76 ± 0.03) | 24.1 ± 1.5 (0.62 ± 0.03)c | 31.1 ± 1.4 (0.81 ± 0.03) |
| HDL-D, mg/dL (mmol/L)c | 6.5 ± 0.3 (0.17 ± 0.01)b | 6.2 ± 0.3 (0.16 ± 0.01)b | 8.3 ± 0.3 (0.21 ± 0.01) | 8.4 ± 0.4 (0.22 ± 0.01) |
| LPL, μmol FFA/mL/hc | 3.7 ± 0.5c’ | 5.6 ± 0.6c | 3.7 ± 0.4 | 2.7 ± 0.3 |
| HL, μmol FFA/mL/hc | 7.6 ± 1.1b , c | 2.0 ± 0.2b | 5 ± 0.6 | 4.1 ± 0.6 |
| LPL/HL | 0.7 ± 0.1c | 4.7 ± 1.0b | 1.2 ± 0.4 | 0.9 ± 0.2 |
Abbreviations: DGU, density gradient ultracentrifugation; NA, not applicable. Data are means ± SEM.
Number of subjects in the subgroup with HDL subfractions and postheparin lipase activity measurements.
P < .05, diabetes vs control by sex.
P < .05, men vs women within the diabetes or control groups.
Ultracentrifugal separation and quantification of HDL and its subfractions
Fasting EDTA-plasma refrigerated at 4°C was shipped on ice within 1 to 3 days of collection for density gradient ultracentrifugation in the laboratory of Dr Tom Hughes at the University of Tennessee Health Sciences Center to separate HDL subfractions as described previously (19). In brief, 9 mL of EDTA-plasma was centrifuged at 70 000 rpm in a 70 Ti rotor (Beckman) through a potassium bromide density gradient for 3 hours and 15 minutes and then fractionated into very low-density lipoprotein, intermediate-density lipoprotein, LDL, and low-density (L), medium-density (M), and dense (D) HDL subfractions (corresponding approximately to standard HDL2b, HDL2a+3a, and HDL3b+3c subfractions on electrophoresis, respectively). HDL subfractions were assayed for cholesterol content, and total (T) HDL-C was calculated by summation (designated as HDL-T, HDL-L, HDL M, and HDL-D). The correlation coefficient between standard and ultracentrifugal measurements of HDL-C in these subjects was 0.92. Ultracentrifugal HDL subfraction data are reported only for the subgroup with lipase measurements.
Measurement of LPL and HL activities
LPL and HL activities were measured in postheparin plasma as described earlier in a subsample of T1D and control subjects who volunteered for a heparin injection (T1D: men, n = 30; women, n = 39; control subjects: men, n = 20; women, n = 23) (20). After an overnight fast, venous blood samples were collected into EDTA tubes 15 minutes after an iv injection of 60 IU of heparin/kg of body weight. The samples were immediately centrifuged and stored at −80°C until assayed. Total postheparin lipolytic activity was measured by incubation of postheparin plasma for 45 minutes at 37°C in a buffered [14C]triolein-lecithin-albumin emulsion with measurement of generated [14C]oleic acid by scintillation counting and is expressed as micromoles of free fatty acid (FFA) released per milliliter of plasma per hour. HL activity was measured in the presence of 1 M NaCl, and LPL activity was calculated by the difference from total postheparin lipolytic activity.
Statistical analyses
Statistical analyses were performed using SPSS (version 19; IBM). The distribution of variables was assessed for normality and where necessary was logarithmically transformed. Data are expressed as means ± SEM. Two-tailed Student t tests were applied to assess differences. Pearson and Spearman correlation coefficients were used to describe the association between continuous and categorical variables, respectively. Linear stepwise regression analysis was used to assess the relationships between adiponectin, LPL, and HL activities and HDL-C, with HDL-C as the dependent variable, controlling for potentially confounding variables.
Results
Clinical and biochemical characteristics in T1D vs control groups
The clinical characteristics of the diabetes and control groups stratified by sex are shown in Table 1. The groups were similar in age and BMI, although men with diabetes had higher BMIs than control men, and the LDL-C and serum creatinine levels were lower than those for control subjects in both sexes. HDL-C, apo A-I, and adiponectin levels were higher in both men and women with diabetes than in their respective control subjects and were higher in women vs men within each group. In the subgroup in which HDL subfractions and lipase activities were measured, HDL-T was higher than that in control subjects for both men and women with diabetes although this did not reach significance in the women. Both men and women with diabetes had higher HDL-L and lower HDL-D subfraction levels than control subjects, and the HDL-L and HDL-M subfractions were higher in women from both groups than in men. Overall, LPL activity was higher (4.67 ± 0.44 vs 3.17 ± 0.26 μmol FFA/mL/h, P = .012), but HL activity was no different (4.42 ± 0.59 vs 4.53 ± 0.41 μmol FFA/mL/h, P = .88) in subjects with diabetes than in control subjects. Women with diabetes had higher LPL, lower HL, and higher LPL/HL activity ratios than men with diabetes and control women. Men with diabetes had higher HL activity than control men.
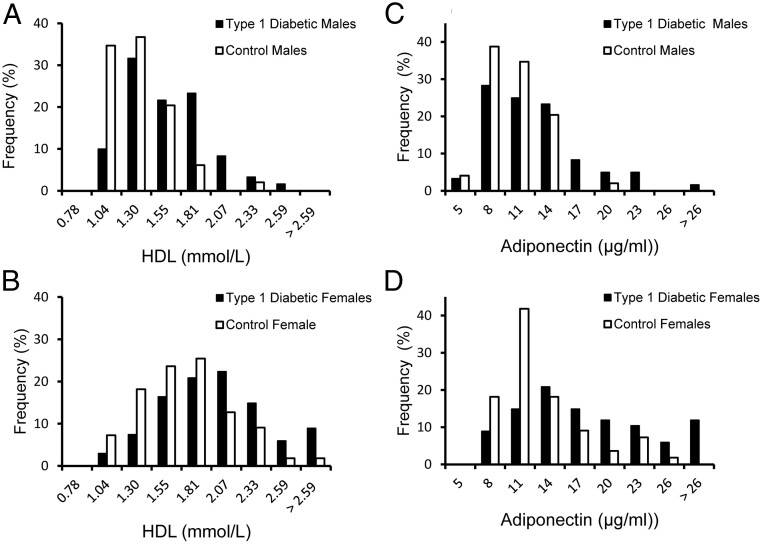
Figure 1 illustrates the distribution of HDL-C and adiponectin values in the 2 groups by sex. As shown in Figure 1, A and B, the HDL-C values in the group with diabetes were distributed toward higher values compared with those for the control group. By using >85th percentile of the HDL-C levels in the control group as an arbitrary cutpoint to define elevated HDL-C (54 mg/dL for men and 76 mg/dL for women), 52% of men and 46% of women with diabetes had HDL-C levels above these cutoff values. Similarly, adiponectin values (Figure 1, C and D) in the group with diabetes were shifted toward higher values in both sexes. For comparative purposes, when hyperadiponectinemia was defined as adiponectin levels of >85th percentile of the control group (13 μg/mL for men and 16.4 μg/mL for women), 30% of men and 46.2% of women in the diabetes group were found to have elevated adiponectin levels.
Figure 1.
Frequency distribution of HDL-C and adiponectin values in men (A and C) and women (B and D) expressed as percentages.
There were 49 men and 54 women in the control group (□) and 60 men and 67 women with T1D (■).
Associations between HDL-C and other variables in the T1D cohort
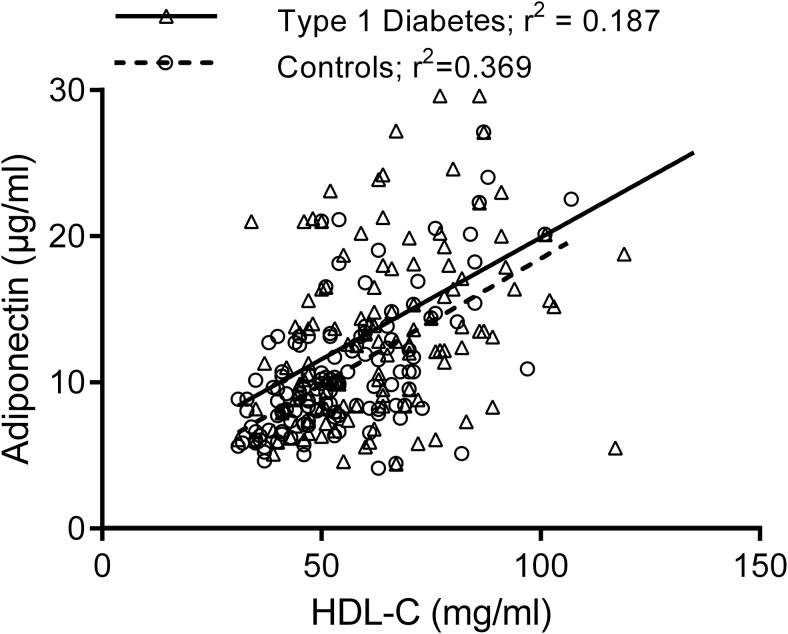
HDL-C was positively correlated with age (r = 0.23; P < .01), apo A-I (r = 0.87, P < .01), and adiponectin (r = 0.43, P < .01) and negatively with BMI (r = −0.31, P < .01), triglyceride (r = −0.44, P < .01), LDL-C (r = −0.22, P < .05), and serum creatinine (r = −0.24, P < .05) (Table 2). There was no relationship with HbA1c, urine ACR, eGFR, statin use, or diabetes duration. Figure 2 shows the regression of HDL-C on adiponectin among subjects with T1D and control subjects in the full cohort. The regression lines are almost parallel and not significantly different from one another although there was more variation around the regression line in those with diabetes, possibly reflecting the greater spread of adiponectin levels in this group.
Table 2.
Correlation Coefficients for HDL-C in Subjects With T1D
| Men | Women | All | |
|---|---|---|---|
| n | 60 | 67 | 127 |
| Age, y | 0.14 | 0.21 | 0.23b |
| BMI, kg/m2 | −0.14 | −0.33a | −0.31b |
| Ethnicity | −0.06 | −0.22 | 0.16 |
| Diabetes duration, y | 0.05 | 0.07 | 0.02 |
| HbA1c, % | −0.22 | −0.11 | −0.09 |
| Triglyceride, mg/dL | −0.58b | −0.35a | −0.44b |
| LDL-C, mg/dL | −0.15 | −0.27a | −0.22a |
| Apo A-I, mg/dL | 0.84b | 0.83b | 0.87b |
| Serum creatinine, mg/dL | −0.08 | 0.14 | −0.24a |
| eGFR, mL/min/1.73 m2 | 0.074 | −0.13 | 0.01 |
| Urine ACR, μg/mg | −0.13 | −0.09 | 0.07 |
| Statin use | 0.08 | 0.21 | 0.01 |
| Adiponectin, μg/mL | 0.36a | 0.29a | 0.43b |
Values are r coefficients. Pearson (continuous variables) and Spearman (categorical variables) correlations were determined.
P < .05.
P < .01.
Figure 2.
Regression of adiponectin concentration on HDL-C values in subjects with T1D and control subjects.
The regression equation for the T1D group is y = 0.1524x + 2.1076; r 2 = 0.3387 (P < .001), and for the control group the equation is y = 0.1665x + 3.2655; r 2 = 0.1858 (P < .001).
Associations between adiponectin, LPL, HL, and HDL components
Table 3 shows the associations between adiponectin, lipases, apo A-I, and the cholesterol content of HDL and its subfractions in the subgroup of subjects with these measurements. Adiponectin was correlated with HDL-T, apo A-I, and HDL-L in both diabetes and control groups but not with HDL-D nor with either of the lipases. LPL activity was directly correlated with apo A-I, HDL-T, and HDL-L and inversely associated with HDL-D subfractions in the group with diabetes only, and this was largely due to its associations with these parameters in women, except for apo A-I in both sexes. HL activity correlated inversely with apo A-I, HDL-T, and HDL-M in the diabetes group and with HDL-T and HDL-L in control men but not women.
Table 3.
Correlations Between Plasma Adiponectin, Lipase Activities, and HDL components
| T1D Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Men | Women | All | Men | Women | All | |
| Adiponectin | ||||||
| Apo A-I | −0.20 | 0.17 | 0.32a | −0.04 | 0.54b | 0.45b |
| LPL | −0.03 | 0.12 | 0.13 | 0.24 | −0.10 | −0.09 |
| HL | −0.16 | 0.08 | −0.21 | 0.08 | 0.12 | 0.02 |
| LPL/HL | −0.04 | 0.00 | 0.10 | 0.11 | −0.23 | −0.08 |
| HDL-T | 0.34 | 0.28 | 0.36a | 0.00 | 0.70b | 0.59b |
| HDL-L | 0.29 | 0.36a | 0.39b | 0.15 | 0.71b | 0.66b |
| HDL-M | 0.18 | 0.01 | 0.14 | −0.02 | 0.57b | 0.46b |
| HDL-D | −0.13 | −0.18 | −0.17 | −0.35 | 0.00 | −0.09 |
| LPL | ||||||
| Apo A-1 | 0.44a | 0.48b | 0.52b | 0.24 | −0.20 | −0.12 |
| Adiponectin | −0.03 | 0.12 | 0.13 | 0.24 | −0.10 | −0.09 |
| HL | 0.29 | 0.23 | 0.01 | 0.33 | 0.27 | 0.33a |
| HDL-T | 0.07 | 0.40a | 0.39b | 0.24 | −0.27 | −0.19 |
| HDL-L | 0.07 | 0.52b | 0.47b | 0.18 | −0.26 | −0.23 |
| HDL-M | 0.08 | −0.02 | 0.09 | 0.22 | −0.33 | −0.16 |
| HDL-D | −0.20 | −0.32a | −0.29a | 0.26 | 0.21 | 0.21 |
| HL | ||||||
| Apo A-I | −0.03 | −0.12 | −0.29a | 0.26 | 0.04 | 0.04 |
| Adiponectin | −0.16 | 0.08 | −0.21 | 0.08 | 0.12 | 0.02 |
| HDL-T | 0.12 | −0.14 | −0.24a | 0.48a | 0.05 | 0.09 |
| HDL-L | 0.28 | −0.08 | −0.16 | 0.50a | 0.01 | 0.03 |
| HDL-M | −0.12 | −0.27 | −0.28a | 0.43 | 0.01 | 0.09 |
| HDL-D | −0.27 | 0.15 | −0.06 | 0.26 | 0.30 | 0.27 |
P < .05.
P < .01.
Multivariate analysis
The association between HDL-T and adiponectin was evaluated in a multivariate analysis in the diabetes subset that included age, triglyceride, LDL-C, BMI, adiponectin, serum creatinine, and the lipases as covariates. Adiponectin alone accounted for 11.6% of the HDL-C variance (β = 0.359, P = .002) (Table 4, model 1). When the covariates without LPL and HL activities were included in the model, adiponectin remained an independent predictor (β = 0.316, P = .001) along with triglyceride (β = −0.248, P = .023) and serum creatinine (β = −0.268, P = .015), collectively accounting for 23.8% of the HDL-C variance (Table 4, model 2). When LPL and HL activities were also included in the analysis, adiponectin remained an independent predictor of HDL-C (β = 0.289, P = .007) along with serum creatinine (β = −0.225, P = .036) and LPL activity (β = 0.267, P = .015) (Table 4, model 3), together accounting for 29.5% of the HDL-C variance with adiponectin and LPL alone accounting for 22.8% of the variance. In multivariate analysis testing of associations between adiponectin and the lipases against HDL-C in a combined model in the control group, adiponectin remained associated with HDL-C, whereas the lipases were not associated.
Table 4.
Multiple Regression Models Predicting HDL-C in the T1D Group
| Variable | Adiponectin | Adiponectin + Lipase | |||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| β | P | β | P | Variable | β | P | |
| Adiponectin | 0.359 | .002 | 0.316 | .001 | Adiponectin | 0.289 | .007 |
| Age | 0.143 | .187 | Age | 0.076 | .488 | ||
| Triglyceride | −0.248 | .023 | Triglyceride | −0.190 | .074 | ||
| LDL-C | −0.042 | .728 | LDL-C | 0.092 | .432 | ||
| BMI | −0.140 | .239 | BMI | −0.172 | .132 | ||
| Creatinine | −0.268 | .015 | Creatinine | −0.225 | .036 | ||
| LPL | 0.267 | .015 | |||||
| HL | −0.199 | .065 | |||||
| r 2 = 0.116; P = .002 | r 2 = 0.238; P = .015 | r 2 = 0.295; P = .015 | |||||
Model 1 is an unadjusted regression model of adiponectin on HDL-C in the subgroup that had lipase measurements performed. Model 2 is model 1 plus adjustments for age, triglyceride, LDL-C, BMI, and serum creatinine. Model 3 is model 2 plus adjustments for LPL and HL.
Discussion
As noted in a previous report of elevated HDL-C in T1D (5), we found that our subjects with T1D had higher HDL-C levels than control subjects, with almost one half having elevated HDL-C using the 85th percentile cutoff values in our control population. We have reported similar findings in a larger population using the 85th percentile cutoff values from a representative US population (21). In confirmation of the findings in a reported study of determinants of adiponectin in a large cohort with T1D (15), we found a robust association between adiponectin and HDL-C levels in both subjects with T1D and control subjects. We also observed a similar relationship between adiponectin and the major HDL apolipoprotein, apo A-I. This relationship was largely due to the association between adiponectin and the lowest density HDL subfraction, which comprised the HDL subfraction that was increased in the group with diabetes, leading to the high HDL-C anomaly in these subjects. In the control subjects, adiponectin was also associated with the low- and the medium-density HDL subfractions, and, unlike in the group with diabetes, this was due to a strong association in the women. Common to both groups was the absence of an association between adiponectin and the most dense HDL subfraction. Previous publications have reported an association between adiponectin and HDL size (22) as well as with the number of large but not small HDL particles (23), including one study in which adiponectin levels were associated with a prospective rise in HDL size after 6.4 years of follow-up (24). Collectively, these findings suggest that adiponectin is linked to processes associated with increased HDL size and lipid content and with the number of large HDL particles.
Because adiponectin is strongly associated with HDL-C and because we found that similar proportions of subjects with T1D have both hyperadiponectinemia and elevated HDL-C based on the 85th percentile of the control group values, one explanation for the frequent presence of high HDL-C in this form of diabetes is that it results from hyperadiponectinemia. We recently reported in a large, prediabetic population, 57% of whom had low HDL-C and who received intensive lifestyle change treatment to prevent type 2 diabetes, that adiponectin was a stronger determinant of the lifestyle-associated increase in HDL-C than BMI or insulin resistance (25). Thus, the association between adiponectin and HDL-C is strong in both types of diabetes even though the typical adiponectin and HDL-C values in these 2 forms of the disease lie at the opposite ends of their concentration ranges. The available evidence supports the theory that adiponectin regulates HDL-C levels by its action on LPL through cholesterol enrichment of HDL as a result of triglyceride-rich lipoprotein hydrolysis. The 2 major sites of release of LPL into the blood after a heparin injection are adipose tissue and muscle. Adiponectin overexpression in a mouse model was shown to increase LPL expression and production from skeletal muscle but not adipose tissue sufficient to increase postheparin lipolytic activity and to lower plasma triglyceride (16). Several clinical reports have also linked adiponectin levels with postheparin LPL activity in both nondiabetic subjects and in those with type 2 diabetes (17, 26). In addition, adiponectin was found to be an important inverse determinant of the apo A-I catabolic rate in nonobese subjects (27), consistent with a stimulatory effect on LPL activity causing HDL remodeling to produce larger and less dense cholesterol-enriched HDL particles with slower clearance.
LPL activity has been shown to be increased in T1D, possibly because of hyperinsulinemia consequent upon systemic insulin administration, although this has not been proven. Based on the foregoing, an alternative hypothesis for the development of elevated HDL-C in T1D diabetes is that hyperadiponectinemia increases LPL activity, leading to a rise in HDL-C. In addition, adiponectin has also been inversely associated with HL activity in some clinical studies (18, 25), which would also favor a positive relationship between adiponectin and HDL-C levels. However, we found that despite its association with HDL-C, adiponectin was not related to either LPL or HL activities in our T1D cohort, even though LPL activity correlated directly and HL activity inversely with HDL-C and apo A-I. Furthermore, in multivariate analysis, adiponectin, LPL activity, and serum creatinine all remained significantly associated with HDL-C. Adiponectin and LPL activity together accounted for 22.8% of the variance of HDL-C, approximately twice the variance explained by adiponectin alone. LPL activity also accounted for the triglyceride association with HDL-C. These findings indicate that in this cohort with T1D, adiponectin and LPL activity are both related to HDL-C independently of each other. In addition, both adiponectin and LPL activity associated with the low-density HDL subfraction. Thus, despite the similarities in their associations with HDL, our data do not support the theory that adiponectin regulates HDL-C through an action on LPL activity to explain the association between hyperadiponectinemia and elevated HDL-C in T1D. However, if the major effect of adiponectin on LPL is on the skeletal muscle enzyme whereas that of hyperinsulinemia is expected to be on adipose tissue LPL (28), it is possible that their combined effects could obscure a relationship in T1D between adiponectin and postheparin LPL activity, which reflects both skeletal and adipose LPL activities. In that case, both hyperadiponectinemia and hyperinsulinemia could contribute to the elevated HDL-C. It is worth pointing out that the relationship between adiponectin and HDL-C did not differ between those with diabetes and control subjects. If hyperadiponectinemia contributes to increased HDL-C in T1D, there was no evidence for adiponectin resistance in relation to HDL-C such as that found in subjects with T1D compared to control subjects for the relationship between adiponectin levels and glucose uptake (29).
In keeping with a previous report, LPL activity was elevated in subjects with T1D, whereas HL activity was not different compared with control values (5). However, we observed that the overall LPL activity increase was due to higher values in women only because men with diabetes had values similar to those of the control subjects. This sex difference in T1D was not noted in the earlier study, although the investigators found that LPL activity tended to be higher in women than in men (5). LPL activity has also been reported to be higher in women than in men in a large Caucasian cohort (30). The basis for the difference in these 2 studies of T1D is unclear, although it is possible that the relatively small group sizes in our study and that by Nikkilä et al (5) may not be large enough to be sufficiently representative of the sex distribution of these lipase activities. We also found that HL activity was significantly lower in women and higher in men with T1D than in control subjects, a finding that has not previously been reported, although HL activity is higher in normal men than in women (30). The expected findings in these subjects of a positive association between LPL activity and HDL-C and its low-density subfraction and the inverse association between HL activity and HDL-C and its low- and medium-density HDL subfractions are consistent with the known actions of these enzymes on HDL metabolism. If both adiponectin and elevated lipase activities are important determinants of HDL-C values in T1D, there is a possibility that the etiology of elevated HDL-C in T1D differs between the sexes. The only factor that was common to both men and women in this regard in our study was elevated adiponectin levels.
There are several limitations to this study. First, this was a cross-sectional study, and thus associations between variables do not indicate cause and effect relationships. Although the available evidence fits best with the theory that adiponectin has an action on HDL metabolism, it is possible that the reverse is true (31). Second, the sizes of the subgroups used for lipase and HDL subfraction analyses, particularly when stratified by sex, were relatively small. Third, we did not measure the high molecular weight (HMWt) form of adiponectin, which is the most biologically active circulating adiponectin subfraction. However, total and HMWt adiponectin values associate very similarly with HDL-C (15). Furthermore, because previous studies demonstrating an association between adiponectin and LPL or HL used total adiponectin measurements (17–23), it is unlikely that the use of a HMWt adiponectin assay instead of total adiponectin would have produced a different result. Last, although glucose levels were not tested in all control subjects, it is possible that some individuals may have had subclinical dysglycemia. However, this would not influence the principal findings in the subjects with T1D in this study.
In summary, we show that the association between hyperadiponectinemia and high HDL-C in T1D is not accounted for by LPL activity and that adiponectin and LPL are independent determinants of the elevated HDL-C levels in T1D.
Acknowledgments
Parts of this study were presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 2012, and the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 2013.
R.M.C., A.S., T.A.H., A.J.M., and R.B.G. contributed to the conceptualization of the study, the development of the analytic plan, the interpretation, performance, and analysis of the results, and the writing of the manuscript. R.M.C., S.D., J.A.L., and T.A.H. performed clinical assessments and sample collection and maintained the database. R.B.G. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- ACR
albumin/creatinine ratio
- BMI
body mass index
- CVD
cardiovascular disease
- D
dense
- eGFR
estimated glomerular filtration rate
- HbA1c
glycosylated hemoglobin
- HDL-C
high-density lipoprotein cholesterol
- HL
hepatic lipase
- L
low density
- HMWt
high molecular weight
- LDL-C
low-density lipoprotein cholesterol
- LPL
lipoprotein lipase activity
- M
medium density
- T
total
- T1D
type 1 diabetes.
References
- 1. Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–1550. [DOI] [PubMed] [Google Scholar]
- 2. Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. [DOI] [PubMed] [Google Scholar]
- 3. Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998;316:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The DCCT Research Group. Lipid and lipoprotein levels in patients with IDDM. Diabetes Control and Complications trial experience. Diabetes Care. 1992;15:886–894. [DOI] [PubMed] [Google Scholar]
- 5. Nikkilä EA, Hormila P. Serum lipids and lipoproteins in insulin-treated diabetes. Demonstration of increased high density lipoprotein concentrations. Diabetes. 1978;27:1078–1086. [DOI] [PubMed] [Google Scholar]
- 6. Costacou T, Evans RW, Orchard TJ. High density lipoprotein cholesterol in diabetes: is higher always better? J Clin Lipid. 2011;5:387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tall AR. Metabolic and genetic control of HDL cholesterol levels. J Intern Med. 1992;231:661–668. [DOI] [PubMed] [Google Scholar]
- 8. Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. [DOI] [PubMed] [Google Scholar]
- 9. Imagawa A, Funahashi T, Nakamura T, et al. Elevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetes. Diabetes Care. 2002;25:1665–1666. [DOI] [PubMed] [Google Scholar]
- 10. Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. [DOI] [PubMed] [Google Scholar]
- 11. Forsblom C, Thomas MC, Moran J, et al. Serum adiponectin concentration is a positive predictor of all-cause and cardiovascular mortality in type 1 diabetes. J Intern Med. 2011;270:346–355. [DOI] [PubMed] [Google Scholar]
- 12. Costacou T, Zgibor JC, Evans RW, et al. The prospective association between adiponectin and coronary artery disease among individuals with type 1 diabetes. The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2005;48:41–48. [DOI] [PubMed] [Google Scholar]
- 13. Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–469. [DOI] [PubMed] [Google Scholar]
- 14. Zietz B, Herfarth H, Paul G, et al. Adiponectin represents an independent cardiovascular risk factor predicting serum HDL-cholesterol levels in type 2 diabetes. FEBS Lett. 2003;545:103–104. [DOI] [PubMed] [Google Scholar]
- 15. Maahs DM, Ogden LG, Snell-Bergeon JK, et al. Determinants of serum adiponectin in persons with and without type 1 diabetes. Am J Epidemiol. 2007;166:731–740. [DOI] [PubMed] [Google Scholar]
- 16. Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57:1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Eynatten M, Schneider JG, Humpert PM, et al. Decreased plasma lipoprotein lipase in hypoadiponectinemia: an association independent of systemic inflammation and insulin resistance. Diabetes Care. 2004;27:2925–2929. [DOI] [PubMed] [Google Scholar]
- 18. Schneider JG, von Eynatten M, Schiekofer S, Nawroth PP, Dugi KA. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care. 2005;28:2181–2186. [DOI] [PubMed] [Google Scholar]
- 19. Hughes TA, Moore MA, Neame P, Medley MF, Chung BH. Rapid quantitative apolipoprotein analysis by gradient ultracentrifugation and reversed-phase high performance liquid chromatography. J Lipid Res. 1988;29:363–376. [PubMed] [Google Scholar]
- 20. Shepard TY, Jensen DR, Blotner S, et al. Orlistat fails to alter postprandial plasma lipid excursions or plasma lipases in normal-weight male volunteers. Int J Obes Relat Metab Disord. 2000;24:187–194. [DOI] [PubMed] [Google Scholar]
- 21. Alessa T, Szeto A, Chacra W, Mendez A, Goldberg RB. High HDL-C prevalence is common in type 1 diabetes and increases with age but is lower in Hispanic individuals. J Diabetes Complications. 2015;29:105–107. [DOI] [PubMed] [Google Scholar]
- 22. Tsubakio-Yamamoto K, Sugimoto T, Nishida M, et al. Serum adiponectin level is correlated with the size of HDL and LDL particles determined by high performance liquid chromatography. Metabolism. 2012;61:1763–1770. [DOI] [PubMed] [Google Scholar]
- 23. Weiss R, Otvos JD, Flyvbjerg A, et al. Adiponectin and lipoprotein particle size. Diabetes Care. 2009;32:1317–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vanhala M, Kumpula LS, Soininen P, et al. High serum adiponectin is associated with favorable lipoprotein subclass profile in 6.4-year follow-up. Eur J Endocrinol. 2011;164:549–552. [DOI] [PubMed] [Google Scholar]
- 25. Goldberg R, Temprosa M, Otvos J, et al. Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2013;98:3989–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Vries R, Wolffenbuttel BH, Sluiter WJ, van Tol A, Dullaart RP. Post-heparin plasma lipoprotein lipase, but not hepatic lipase activity, is related to plasma adiponectin in type 2 diabetic patients and healthy subjects. Clin Lab. 2005;51:403–409. [PubMed] [Google Scholar]
- 27. Vergès B, Petit JM, Duvillard L, et al. Adiponectin is an important determinant of apo A-1 catabolism. Arterioscler Thromb Vasc Biol. 2006;26:1364–1369. [DOI] [PubMed] [Google Scholar]
- 28. Farese RV Jr, Yost TJ, Eckel RH. Tissue-specific regulation of lipoprotein lipase activity by insulin/glucose in normal-weight humans. Metabolism. 1991;40:214–216. [DOI] [PubMed] [Google Scholar]
- 29. Pereira RI, Snell-Bergeon JK, Erickson C, et al. Adiponectin dysregulation and insulin resistance in type 1 diabetes. J Clin Endocrinol Metab. 2012;97:E642–E647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Després JP, Couillard C, Gagnon J, et al. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938. [DOI] [PubMed] [Google Scholar]
- 31. Van Linthout S, Foryst-Ludwig A, Spillmann F, et al. Impact of HDL on adipose tissue metabolism and adiponectin expression. Atherosclerosis. 2010;210:438–444. [DOI] [PubMed] [Google Scholar]