ABSTRACT
Invasive fungal sinusitis can lead to dramatic complications in immunocompromised patients and requires prompt diagnosis. Here we report three cases with ophthalmological complications secondary to invasive fungal sinusitis in immunocompromised patients. From an ophthalmological point of view, these cases illustrate different clinical presentations, evolutions, complications, treatments, prognoses, and highlight different pathophysiological mechanisms. Diagnoses were delayed in all cases. In none of the cases did patients recover better vision than counting fingers at 24 months follow up, and two patients died. This case series highlights key points useful for quickly recognising this highly morbid infection in immunocompromised patients.
KEYWORDS: Fungal sinusitis, optic neuropathy, immunocompromised patients, orbital apex syndrome, ct scan, magnetic resonance imaging
Introduction
The incidence of invasive fungal infection has dramatically increased in recent years, mostly because of the development of immunosuppressive treatments and powerful diagnostic tools such as computed tomography (CT) and later, magnetic resonance imaging (MRI).1 The first case of invasive fungal infection was reported in 1953 concomitantly with the introduction of highly cytotoxic chemotherapy and corticosteroid therapies.2
Usually, the port of entry of infection is the colonisation of the nasal mucosa from where the fungi can reach the sinuses, leading to sinusitis. Fungal rhinosinusitis has been categorised into invasive and non-invasive types based on histopathological examination.2,3 Non-invasive sinusitis, such as a fungal ball or allergic sinusitis does not involve other tissues. Conversely, invasive sinusitis spreads to adjacent structures, usually through bone erosion or via blood vessels.3,4
Due to the narrow anatomical relationship between the paranasal sinuses and the orbit, fungal sinusitis frequently invades the orbit resulting in severe orbital apex syndrome. Typically, fungal sinusitis causes painful loss of vision and ophthalmoplegia through synergistic mechanisms such as compression, ischaemia, inflammation, and direct toxicity.4
We present three patients with different patterns of orbital fungal invasion secondary to paranasal sinusitis. We highlight differences in clinical presentation and prognosis depending on the type of fungus, medical histories, and delays in diagnosis. We also review mechanisms of vision loss associated with orbital fungal infection.
Case reports
Case 1
A 43-year-old female patient sought emergency care two weeks after a second renal transplant due to sudden loss of vision in her right eye associated with headaches. She had a history of neuroblastoma from childhood. Her immunosuppressive treatment consisted of methylprednisolone 4 mg daily, mycophenolate mofetil, and tacrolimus.
She had no perception of light in the right eye but could see 10/10 at 30 cm with the left eye. Bedside examination of the right eye revealed slight proptosis, incomplete ptosis, and exotropia. She had 15° to 10° limitation of eye movements in all gaze directions in the right eye. The pupillary light reflex was absent. Eye motility was normal in the left eye. Slit-lamp exam and fundus examination were unremarkable in both eyes, except for slight chemosis of the right eye.
Twenty-four hours later, her clinical condition and conscious level deteriorated to a Glasgow Coma Score (GCS) 10/15 requiring her admission to the intensive care unit (ICU). Brain MRI revealed a pansinusitis without any orbital or optic canal involvement, or brain parenchymal damage (not shown). A CT scan performed the same day failed to reveal any bone disruption of the orbit or the base of the skull (Figure 1). Treatment combining piperacillin/tazobactam, intravenous (IV) methylprednisolone 125 mg and prednisolone nasal spray was started.
Figure 1.
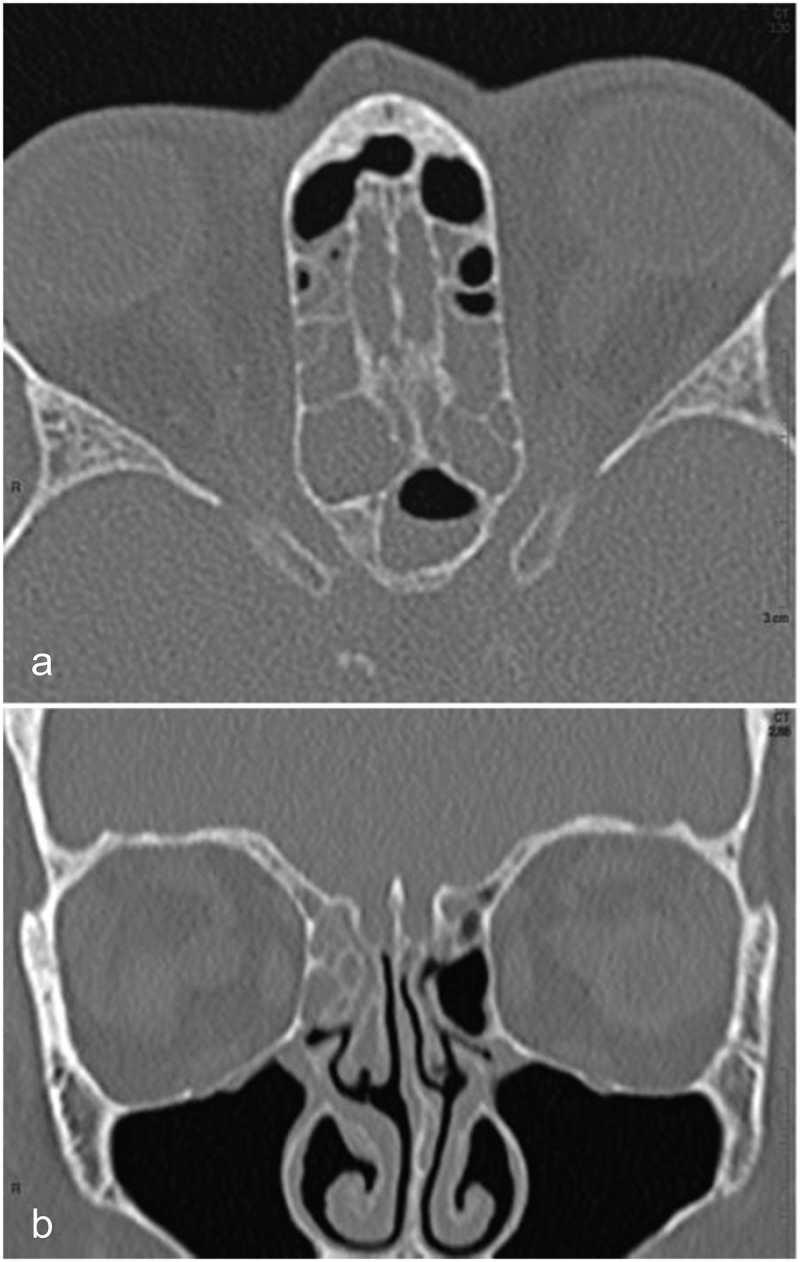
CT scan of case 1
(a) Transverse reformat from a helical acquisition of the ethmoid and sphenoid sinuses showing unruptured bone. Most ethmoidal cells are obliterated with mucosal thickening and a fluid/air level is seen in the left sphenoid cell; (b) Coronal reformat showing unremarkable mucosal thickening without bone erosion.
Forty-eight hours later, she developed severe loss of vision in the left eye. She now had complete bilateral ophthalmoplegia, chemosis, and proptosis in both eyes. She had a fixed mydriasis in the right eye (6 mm in the light) and a slightly reactive (1.5 mm) left pupil. A lumbar puncture revealed high cerebrospinal fluid (CSF) protein and glucose levels (1 white blood cell, 122 mg/dL protein, 2.7 mg/dL lactic acid, and 170 mg/dL glucose [70% of serum level] with negative CSF culture). A second MRI confirmed an acute posterior ethmoidal and sphenoidal sinusitis with orbital invasion, bilateral orbital apex syndrome, and bilateral optic neuritis together with cytotoxic oedema of the basal parenchyma of the frontal lobes (Figure 2).
Figure 2.
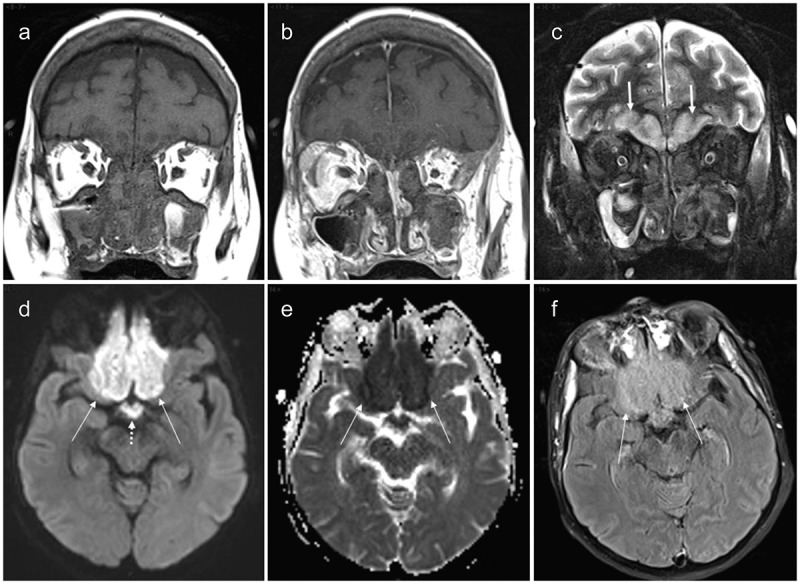
MRI of case 1. (a) Unenhanced coronal T1-weighted view showing almost complete naso-ethmoido-maxillary filling with overall low signal intensity; (b) Gadolinium-enhanced coronal T1-weighted view showing mucosal enhancement but without enhancement of the sinus contents; (c) Coronal short tau inversion recovery image showing low signal intensity of the sinus contents. High signal is becoming visible in the baso-frontal areas of the brain (arrows); (d) Axial diffusion-weighted image (b value: 1000) showing high signal within the baso-frontal areas (arrows) also involving the optic chiasm (dotted arrow); (e) Apparent diffusion coefficient map of the equivalent slice to 2D showing strongly decreased ADC values confirming restricted diffusion due to cytotoxic oedema (arrows); (f) Axial fluid attenuated inversion recovery image in similar slice location as 2D and 2E showing moderate-high signal within the injured parenchyma (arrows)
(a) Unenhanced coronal T1-weighted view showing almost complete naso-ethmoido-maxillary filling with overall low signal intensity; (b) Gadolinium-enhanced coronal T1-weighted view showing mucosal enhancement but without enhancement of the sinus contents; (c) Coronal short tau inversion recovery image showing low signal intensity of the sinus contents. High signal is becoming visible on the baso-frontal areas of the brain (arrows); (d) Axial diffusion-weighted image (b value: 1000) showing high signal within the baso-frontal areas (arrows) also involving the optic chiasm (dotted arrow); (e) Apparent diffusion coefficient map of the equivalent slice to 2D showing strongly decreased ADC values in the injured parenchyma (arrows); (f) Axial fluid attenuated inversion recovery image in similar slice location as 2D and 2E showing moderate-high signal within the injured parenchyma (arrows).
A nasal endoscopy found “hairy” material. Because of the high suspicion of mycosis, a large surgical drainage was performed and IV liposomal amphotericin B was commenced. Pathological examination disclosed septate hyphae with 90° branching, confirming invasive mucormycosis (Figure 3).
Figure 3.
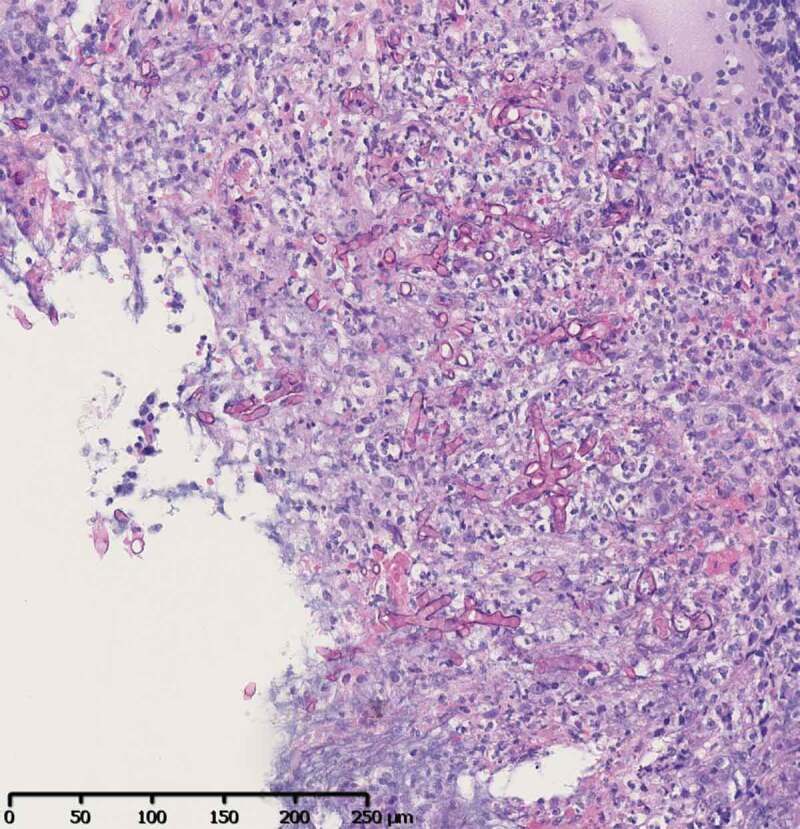
Sinus biopsy of case 1
Zygomycosis – PAS staining. Typical hyphae invading the necrotic tissue. Hyphae are broad (mean 12 µm), infrequently septate, appear empty, have focal bulbous dilatations, and show non-dichotomous, irregular branching that may sometimes be at right angles.
In order to discontinue immunosuppressive therapy, the renal graft was removed but her conscious level continued to deteriorate to GCS 3/15. A third MRI found an “explosive” pansinusitis and frontal cerebritis extending to the temporal lobes (not shown). She died two days later from central nervous system (CNS) infection and subarachnoid haemorrhage thought to be secondary to a ruptured mycotic aneurysm, although no autopsy occurred to confirm this.
Case 2
An 88-year-old female with a history of myelodysplasia and polymyalgia rheumatica presented to the emergency room with painful diplopia with temporal pain waking her up at night. Her medication consisted of hydrocortisone 8 mg per day and darbepoetin alpha. The clinical examination revealed a right sixth nerve palsy without any loss of vision, normal visual fields, and fundi.
A temporal artery ultrasound and a C-reactive protein (CRP) blood test for suspicion of giant cell arteritis (GCA) were performed. The ultrasound was normal, but because the CRP was raised at 150 mg/L, 500 mg of IV methylprednisolone was started. The full blood count test revealed evidence for myelodysplasia with 7.7 g/dL haemoglobin, 2.89 × 103/μL red blood cells, 5.82 × 103/μL white blood cells and 138 × 103/μL platelets.
The next day, her pain disappeared but her vision and the visual field slowly worsened (Figure 4) over the following two days to no perception of light in the right eye. In addition, the eye motility worsened as she developed additional right 3rd and 4th nerve palsies with fundus excyclotorsion of the right eye (Figure 5).
Figure 4.
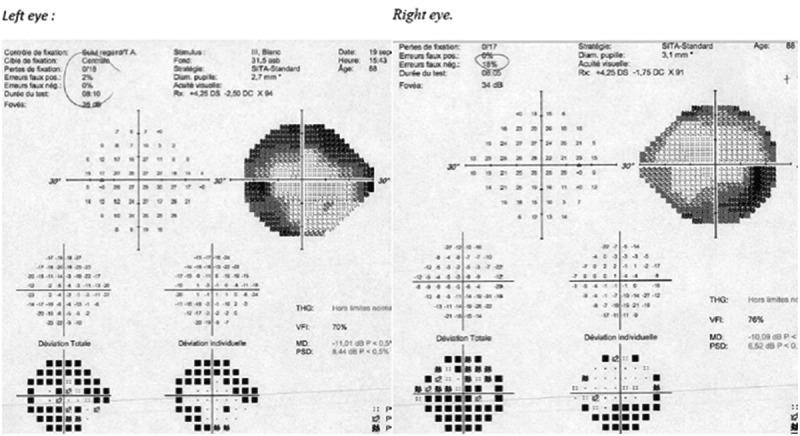
24–2 Humphrey visual field of both eyes of case 2
Large and diffuse defects before complete vision loss occurred in the right eye.
Figure 5.
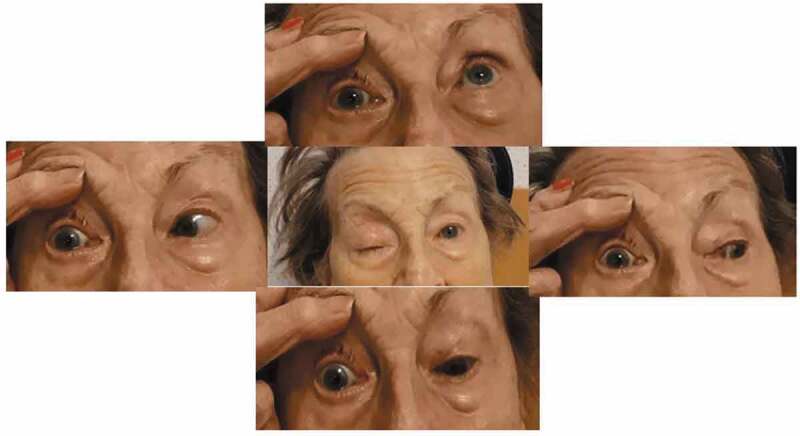
Extra-ocular movements of case 2
Complete ophthalmoplegia of the right eye.
An orbital CT scan revealed a right orbital apex syndrome with bone erosion and posterior sphenoidal sinusitis (Figure 6). The corticosteroids were discontinued and an endoscopic surgical debridement and biopsies of the sinus were promptly performed. Pathological examination found septate hyphae without 90° branching, confirming the presence of Aspergillus (Figure 7). Cultures also revealed methicillin-resistant Staphylococcus aureus (MRSA).
Figure 6.
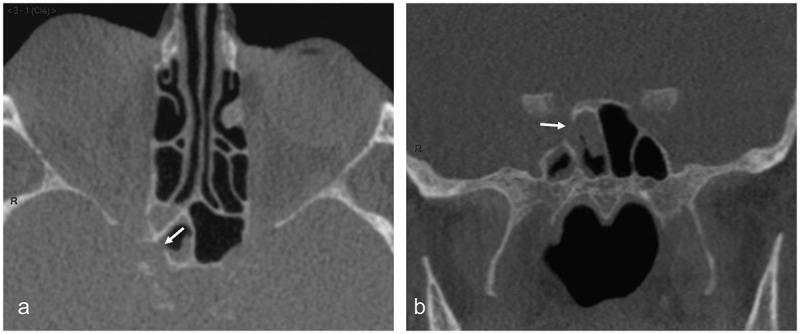
CT scan of case 2
Obvious focal bone disruption (arrow) of the lateral wall of the right sphenoid sinus at the level of the optic canal (arrows) on both axial (a) and coronal reformats (b). Focal mucosal thickening is present at the level of the bone disruption.
Figure 7.
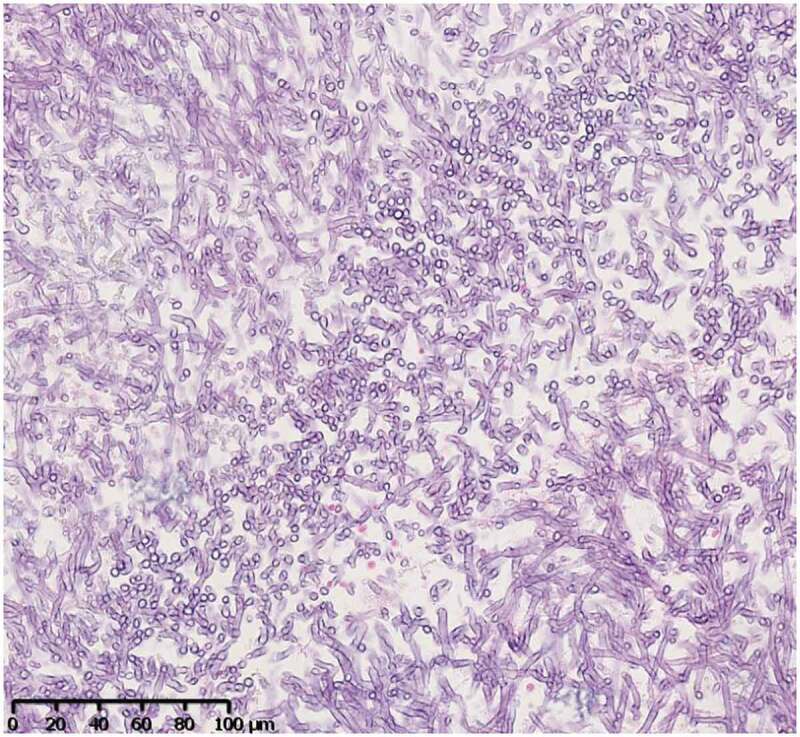
Sinus biopsy of case 2
PAS staining. The compact hyphae have parallel walls and are septate with relatively uniform width (3–6 µm). They have dichotomous branches with acute (45°) angles.
A combination of voriconazole and vancomycin led to a rapid improvement in the patient’s condition. Two weeks later the right 3rd nerve palsy had resolved, but the vision in the right eye only reached light perception.
Three weeks later she developed otitis media with tympanic perforation and a complete right facial nerve palsy due to maxillary sinusitis invading the pterygopalatine fossa and finally affecting the lateropharyngeal region (Figure 8).
Figure 8.
MRI of case 2
(a) Gadolinium-enhanced coronal T1-weighted image with fat suppression showing acute inflammation within the right sphenoid sinus cell and orbital apex (arrow); (b) Gadolinium-enhanced axial T1-weighted image with fat suppression showing similar findings as in 8a; (c) Gadolinium-enhanced coronal T1-weighted image with fat suppression showing parapharyngeal extension of inflammatory changes (arrow); (d) Gadolinium-enhanced axial T1-weighted image with fat suppression showing similar findings as in 8c.
She underwent a complete maxillectomy. Cultures taken at operation revealed MRSA without Aspergillus. Antibiotic treatment (ciprofloxacin and doxycycline) allowed a subjective clinical recovery from the mixed sinusitis, otitis, and peripheral facial nerve palsy, however, since she chose palliative care we did not perform additional testing. A month later, she developed MRSA pneumonia and died from a pulmonary embolism.
Case 3
A 63-year-old immunosuppressed male who had had a renal transplant 20 years previously for autosomal dominant polycystic kidney disease presented with loss of vision in the left eye associated with severe orbital and peri-orbital pain. His ophthalmologist referred him for suspicion of GCA. His daily treatment consisted of cyclosporin and prednisolone.
Two months earlier, a left sphenoidal aspergilloma had been resected, but pathological examination did not reveal any sign of invasion in the tested mucosa. A pre-operative CT scan showed a hyperdense mass within the sphenoid sinus but no bone disruption (Figure 9A).
Figure 9.
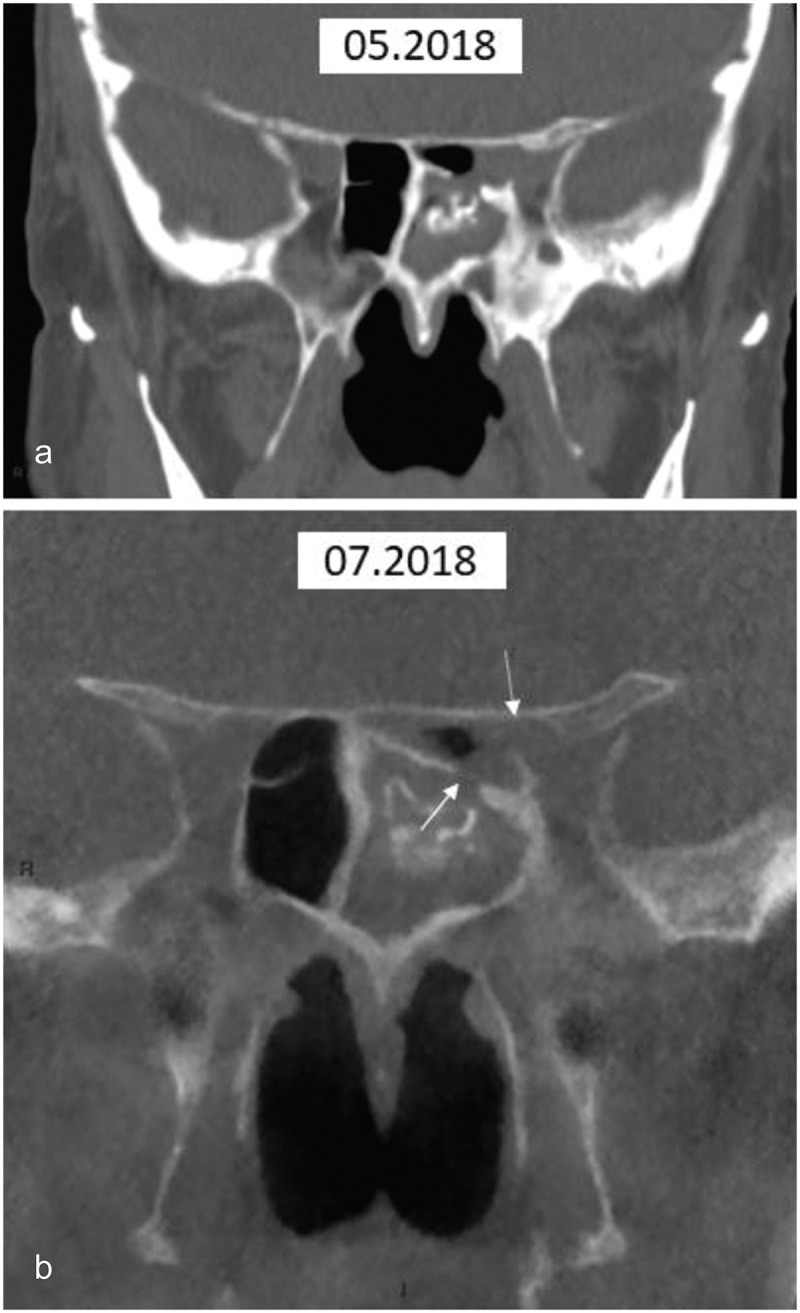
CT of case 3
(a) Coronal multi-slice CT. The initial examination demonstrates bone thickening of the left ethmoid sinus and mucosal thickening embedding mineralised sinus contents. Disruption of bone is not seen; (b) Coronal cone beam CT. This confirms the findings seen in 9A but also demonstrates disruption of bone communicating with the optic canal (arrows), which were retrospectively present in 9A.
One week after the surgery, he developed painful loss of vision in the left eye to light perception with a left relative afferent pupillary defect, left ptosis, limitation of left eye abduction and a pale optic disc. He had a high CRP (400 mg/L). A fluorescein angiogram performed in an outside institution revealed mild hyperfluorescence of the optic disc but no delayed retinal or choroidal perfusion.
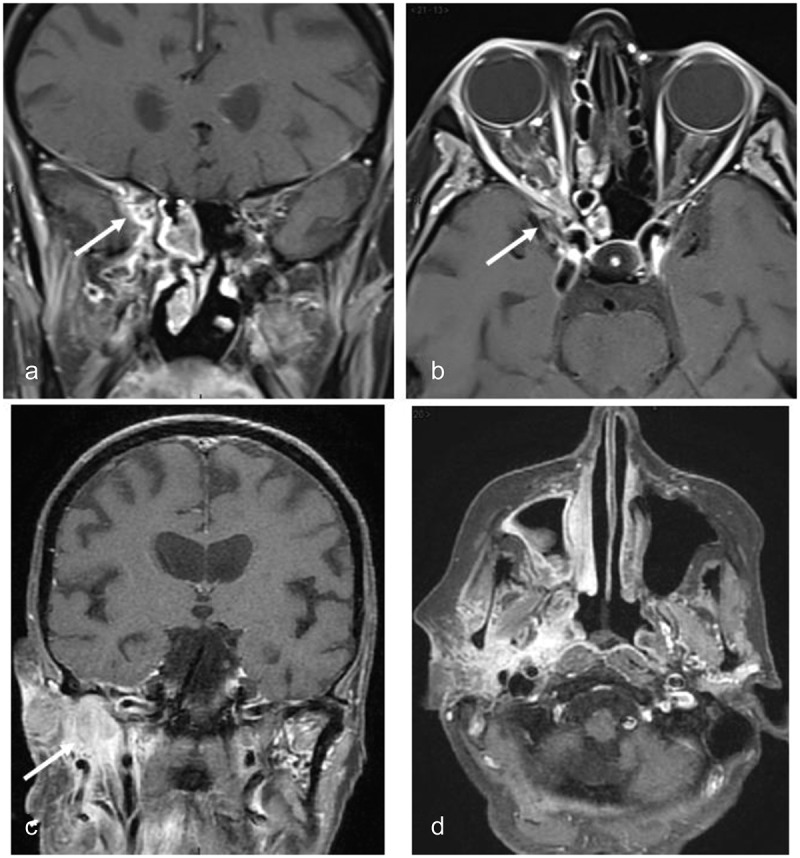
A cone-beam CT scan confirmed the bone invasion of the sphenoid sinus, optic canal, and the inferior orbital fissure, which had been missed two months previously (Figure 9B). An additional orbital contrast-enhanced MRI confirmed posterior ethmoidal sinusitis extending into the orbital apex with peri-optic neuritis (Figure 10). Treatment with voriconazole and methylprednisolone, started 48 hours after antifungal treatment, quickly improved the headaches and ocular motility his vision only recovered to counting fingers at 10 cm. One year later, the MRI showed complete resolution of the sino-orbital infiltration (Figure 11) but there was still no improvement in vision. Voriconazole treatment has been continued for the last one-and-a-half years.
Figure 10.
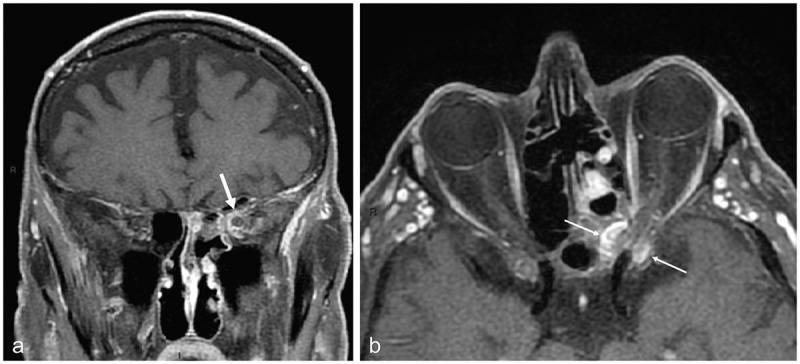
MRI of case 3
(a) Gadolinium-enhanced coronal T1-weighted image with fat suppression showing acute inflammation within the left orbital apex with encasement of the optic nerve within the inflammatory process (arrow); (b) Gadolinium-enhanced axial T1-weighted image with fat suppression showing inflammation on both sides of the optic canal, the sphenoid sinus medially and the anterior clinoid process laterally (arrows).
Figure 11.
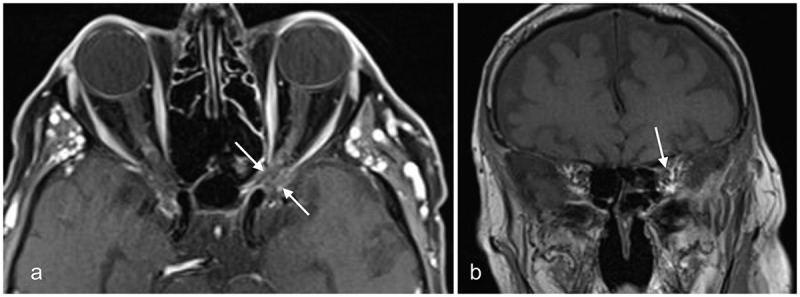
MRI of case 3 after 1 year of follow-up
(a) Gadolinium-enhanced axial T1-weighted image with fat suppression showing the absence of aspergilloma or sinusitis. Secondary optic nerve atrophy is present (arrows); (b) Gadolinium-enhanced coronal T1-weighted image with fat suppression showing the absence of inflammation and secondary optic nerve atrophy (arrow).
Discussion
Since the development of immunosuppressive treatments and chemotherapies, risk factors for invasive fungal infection continue to expand and are now well defined. The underlying condition and site of infection are important prognostic factors.5 All immunosuppressive conditions including old age, solid-organ transplantations, haematological malignancies, use of immunosuppressive regimens for autoimmune diseases, HIV, and intensive chemotherapy have been reported as the most important risk factors for invasive fungal infection.5
The first site of invasion with Aspergillus is the lung, then the sinuses and then the brain.5 Case fatality rates are dramatic and sinus infection is the third most devastating after cerebral and pulmonary aspergillosis. An older study, with a large pool of patients, reviewed 1223 cases of invasive aspergillosis with pulmonary, sinus, and cerebral aspergillosis in immunocompromised patients. The crude mortality rates were 86%, 66%, and 99%, respectively.6
Pathophysiology of infection
Case 1
Considering the acute loss of vision, proptosis, headaches, chemosis, and almost complete ophthalmoplegia, we hypothesised different diagnoses. First, a cavernous sinus thrombosis or a cavernous sinus fistula were suspected but quickly ruled out by the brain imaging. Then, the rapid spread to the other eye together with pain and general clinical deterioration suggested an infectious process affecting both orbits. The first CT scan revealed pansinusitis, and despite the absence of invasive signs, we, therefore, considered a complication of sphenoidal and ethmoidal sinusitis with an orbital apex syndrome secondary to mucormycosis.
The most common mucormycosis genera are Rhizopus (47%) and Mucor (18%).7 The most typical clinical manifestations are rhino-cerebral, maxillo-facial, and pulmonary.
Nithyanandam et al. described three clinical stages of mucormycosis infection: limited sino-nasal disease (clinical stage I); patients with limited rhino-orbital disease (clinical stage II); and patients with rhino-orbital-cerebral disease (clinical stage III).8 This study also revealed that in stage I of the disease the treatment success rate, defined as no evidence of disease at follow-up for up to 8 years, was 91% and the only death was related to secondary amphotericin B-induced renal failure. In stage II with sino-nasal debridement alone, success rate reached 100%, but it was only 11.1% in stage II with sino-nasal debridement paired with orbital exenteration; treatment failure and uncontrolled disease occurred in 88.9% of cases. Stage III disease was associated with treatment failure in all cases. The follow-up did not include imaging studies though.8
Our patient went through the three clinical stages. Considering her severe immunosuppression and the large extension of the infection orbital exenteration was not considered.
Mucormycosis is known to be more aggressive than aspergillosis.9 Some studies postulate that a strong pro-inflammatory response, as well as extensive angioinvasion, could explain this difference.10 A retrospective study conducted over 20 years revealed that patients with mucormycosis had a higher mortality rate (71%) than patients with aspergillosis (29%) but without any statistically significant difference (p = .16). Nonetheless, it also showed that orbital involvement had a higher mortality and was associated with a higher likelihood of mucormycosis infection compared with those with sinus-only disease (78.6% vs 20%, p = .01; 86% vs 30%, p = .01, respectively).11
CT scanning and MRI usually show non-specific changes in the early stages hence the delays we encountered. More specific findings may become visible as the infection progresses, particularly bony changes including sclerotic thickening, erosion, or remodelling. A central area of high density within the sinus cavity on CT may also suggest fungal disease.12 In later stages, MRI can reveal a black turbinate sign if necrosis is present and an intracranial mass in cases of cerebral invasion.13
Despite different prognoses, the identification of the species does not guide antifungal therapy since treatments are limited. Both liposomal amphotericin B and voriconazole (or itraconazole or fluconazole) are used in all fungal infections.
The pterygopalatine fossa is considered to be the main reservoir for rhino-cerebral mucormycosis after proliferation in the nasal fossa.14 The involvement of the brain and cavernous sinus occurs by way of the orbital apex leading to thrombosis and fatal brain haemorrhage, explaining the high casualty rate of this disease. Therefore, spheno-ethmoidectomy with debridement of the pterygopalatine fossa, with or without maxillectomy, is considered the only definitive method to eradicate the infection.14
Case 2
Aspergillus fumigatus and Aspergillus flavus are the most common fungi in the sinuses. Although invasive sinusitis can happen in normal individuals, immunocompromised hosts are more prone to both, especially chronic neutropenic patients and patients with uncontrolled diabetes mellitus with ketoacidosis.7,15,16
Aspergillus is less angio-invasive, therefore infection has a higher survival rate and a better visual outcome compared with mucormycosis. It can be divided into invasive and non-invasive types.10
Case 2 emphasised the importance of appreciation of the history in order to prevent secondary harm; especially in older patients where age is cause immunocompromise, even without any other co-morbidities.17 Pain and diplopia lead to a misdiagnosis of GCA. This scenario has already been described in literature; however, pain that causes awakening, usually worse during the night should be a red flag, since GCA does not typically cause such severe pain. The pain is due to the spread of fungi along the trigeminal nerve.18,19
Case 3
Aspergilloma is one of the two forms of non-invasive fungal rhino-sinusitis.20 It usually carries a good prognosis, since it is self-contained, but in immunocompromised patients Aspergillus can disseminate quickly resulting in invasive disease.21 CT scans typically reveal the so-called ‘fungus ball’ sign consisting of a round-shaped mineralised hyperdense area within a sinus cavity.
In immunocompetent patients, sinus surgery for aspergilloma is usually curative and no further treatment is required, but in immunocompromised patients, the follow-up has to be strict and the histopathological specimen needs to be carefully examined.22
Case 3 improved with long-term antifungal therapy (voriconazole). Small doses of corticosteroid were added 48 hours after voriconazole was started to reduce the associated inflammatory process at the orbital apex. He recovered from the motility disorder but the optic nerve function did not.
Vision impairment mechanism
Invasive fungi sinusitis infection is life-threatening, mostly because of rapid invasion by hyphae of blood vessels inducing luminal thrombosis and finally causing tissue necrosis.23,24 From the paranasal sinuses, the fungal infection spreads directly to close structures such as the orbital apex and optic nerves, the cavernous sinus, the carotid artery, and ultimately the brain. In the brain carotid occlusion, cerebral infarction, and ruptured mycotic aneurysms with subsequent subarachnoid haemorrhage have been reported.12,22 Loss of vision can be secondary to compression, para-inflammation, ischaemic lesions, or direct invasion.
Acute posterior ischaemic optic neuropathy has been described from fungal angio-invasion of small meningeal arteries supplying the optic nerve in and around the optic foramen and we suggest the same mechanism occurred in cases 2 and 3.25 Moreover, direct neural invasion of the optic nerve and chiasm by fungus can happen too, such as in case 1 (Figure 2D).
Congestion of the extraocular muscles (EOM), intracavernous perineural invasion leading to de-afferentation, or invasion of the meningeal arteries near the orbital apex causing EOM ischaemia can explain the development of ophthalmoplegia.19,25 Since case 2 had no muscle swelling on the MRI, we suggest either meningeal arterial or perineural invasion had occurred, since the ophthalmoplegia was complete (Figure 5). Fungi can also invade through the pterygopalatine fossa and lead to facial nerve palsy as also occurred in case 2.19,26 When there is an orbital invasion, visual prognosis is usually poor.27
Diagnosis
Early diagnosis can be difficult since blood cultures take time and have a significant false-negative rate. Fungal cultures are negative in approximately 30% of cases of acute fungal invasion.24 Pain is a good indicator of fungal invasive sinusitis versus GCA, since it is always described as a real lasting pain causing insomnia and requiring a high dose of painkillers, whereas GCA is described more like discomfort and almost never needs painkillers. Fungal perineural invasion explains this pain.22,27,28
Frontal headaches, retro-orbital pain, and lasting/awakening unilateral facial pain, especially in old or immunocompromised patients, should be considered as a red flag in any patient with ophthalmoplegia and should be investigated until a clinical reason is found and sinusitis ruled out. Imaging is critical for early diagnosis and should be performed as soon as possible. CT commonly reveals unilateral sinus filling or mucosal thickening, and perimaxillary fat blurring due to direct fungal tissue infiltration or vascular congestion.
Bone erosion should be ruled out although it is not mandatory for orbital invasion since fungus can spread through blood vessels.12 Definitive diagnosis relies on positive fungal culture and histopathology, which is usually performed during nasal endoscopy or debridement surgery. Frozen sections are mandatory to provide immediate results to guide treatment.1,15,24
Non-culture-based blood tests can aid diagnosis including serological testing and polymerase chain reaction (PCR). PCR has been reported to have a positive predictive value of 83.8% and a negative predictive value of 98.1% for Aspergillus. The presence of galactomannan, which is a specific cell wall component of Aspergillus, is also a useful marker for early diagnosis in Aspergillus infection.14
However, histopathological examination is considered to be the most reliable means of diagnosis.29 The presence of hyphae with specific characteristic features such as regular septa, uniform shape, and 3–6 μm hyphae are typical. Branching of the septa can discriminate mucormycosis from aspergillosis since Aspergillus does not have 90° branch septa; these are specific to mucormycosis.8
Clinical differential diagnosis is challenging. In our series, two who had painful, severe, and acute visual loss were initially suspected to have GCA and were treated accordingly with corticosteroids. Numerous studies highlight how difficult it is to make a prompt and adequate diagnosis, GCA and orbital pseudotumour are the most common confounding differential diagnoses described so far.21 Corticosteroid therapy is the first-line treatment in these cases but can be fatal when an underlying fungal invasion exists, as the literature describes an increase in the growth rate of Aspergillus in vivo when corticosteroids are used.30
Additional differential diagnoses include allergic fungal sinusitis, Tolosa-Hunt syndrome, benign and malignant neoplasms of the orbit, lymphoma, thyroid-associated orbitopathy with optic nerve compression, granulomatosis with polyangiitis, sarcoidosis, and other infectious diseases such as syphilis, tuberculosis, and parasites.
Treatment
The treatment of choice is maximal surgical debridement of fungi and necrotic tissue as early as possible combined with prompt intensive anti-fungal therapy. Extensive debridement surgery consisting of spheno-ethmoidectomy, with or without maxillectomy, was the first-line treatment applied in our cases.
Surgical optic nerve decompression has been successfully described in one patient who recovered vision after surgery in combination with methylprednisolone administration.31
Exenteration has been largely reported in the literature but no guidelines exist at the moment for this. Vision loss alone does not seem to be a good indication for performing this debilitating surgery without proven orbital invasion.25 At the moment, amphotericin B and itraconazole are the two most effective drug treatments.
A recent study compared treatment with amphotericin B alone to amphotericin B followed by itraconazole or itraconazole alone. Death rates reduced were 65% on amphotericin B alone, 36% on amphotericin B followed by itraconazole, and 25% with itraconazole on alone; however, sole treatment with amphotericin B was reserved for the most severely immunosuppressed patients.32
Initial treatment should be at high doses for at least two weeks, although first-line therapy has been reported to fail in at least 50% of patients.5 Treatment with amphotericin B or voriconazole should be started if aspergilloma surgery is performed in immunocompromised patients, to reduce the chance of secondary invasion. Blood levels of voriconazole must be monitored weekly.
New drugs such as isavuconazole, a second-generation triazole, are being tested. It has broad-spectrum antifungal activity and does not require blood monitoring. Compared with voriconazole and liposomal amphotericin B, it seems to offer some advantages, including good tolerability, a lower side effect profile, excellent bioavailability (switch from IV to oral treatment), and reduced drug interactions. It is also better tolerated when reduced renal function is present.33
Recent guidelines recommend isavuconazole, in combination with voriconazole, as the gold standard treatment for invasive aspergillosis in patients with haematological malignancies but these guidelines do not include neuro-ophthalmological involvement.26
Larger studies are needed to define the most effective treatment to recover vision when and if possible. Immunosuppressed patients should be carefully followed when sinusitis is present and high suspicion should be maintained when significant pain or ophthalmological signs appear.
Declaration of interest statement
The authors report no conflict of interest.
References
- 1.Krennmair G, Lenglinger F.. Maxillary sinus aspergillosis: diagnosis and differentiation of the pathogenesis based on computed tomography densitometry of sinus concretions. J Oral Maxillofac Surg. 1995;53(6):657–663. doi: 10.1016/0278-2391(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 2.Rankin NE. Disseminated aspergillosis and moniliasis associated with agranulocytosis and antibiotic therapy. Br Med J. 1953;1(4816):918–919. doi: 10.1136/bmj.1.4816.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivak-Callcott JA, Livesley N, Nugent RA, Rasmussen SL, Saeed P, Rootman J. Localised invasive sino-orbital aspergillosis: characteristic features. Br J Ophthalmol. 2004;88(5):681–687. doi: 10.1136/bjo.2003.021725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marple BF, Gibbs SR, Newcomer MT, Mabry RL. Allergic fungal sinusitis-induced visual loss. Am J Rhinol. 1999;13(3):191–195. doi: 10.2500/105065899781389740. [DOI] [PubMed] [Google Scholar]
- 5.Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 6.Denning DW. Therapeutic outcome in invasive aspergillosis. Clin Infect Dis. 1996;23(3):608–615. doi: 10.1093/clinids/23.3.608. [DOI] [PubMed] [Google Scholar]
- 7.Bellazreg F, Hattab Z, Meksi S, et al. Outcome of mucormycosis after treatment: report of five cases. New Microbes New Infect. 2015;6:49–52. doi: 10.1016/j.nmni.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D’Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol. 2003;51:231–236. [PubMed] [Google Scholar]
- 9.Lin S, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. 2001;60612. Clin Infect Dis. 2001. February 1;32(3):358–366. Epub 2001 Jan 26. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 10.Katragkou A, Walsh TJ, Roilides E. Why is mucormycosis more difficult to cure than more common mycoses? Clin Microbiol Infect. 2014;20(s6):74–81. doi: 10.1111/1469-0691.12466. [DOI] [PubMed] [Google Scholar]
- 11.Trief D, Gray ST, Jakobiec FA, et al. Invasive fungal disease of the sinus and orbit: a comparison between mucormycosis and Aspergillus. Br J Ophthalmol. 2016;100(2):184–188.doi: 10.1136/bjophthalmol-2015-306945. [DOI] [PubMed] [Google Scholar]
- 12.Hurst RW, Judkins A, Bolger W, Chu A, Loevner LA. Mycotic aneurysm and cerebral infarction resulting from fungal sinusitis: imaging and pathologic correlation. Am J Neuroradiol. 2001;22:858–863. [PMC free article] [PubMed] [Google Scholar]
- 13.Koc Z, Koc F, Yerdelen D, Ozdogu H. Rhino-orbital-cerebral mucormycosis with different cerebral involvements: infarct, hemorrhage, and ophthalmoplegia. Int J Neurosci. 2007;117(12):1677–1690. doi: 10.1080/00207450601050238. [DOI] [PubMed] [Google Scholar]
- 14.Rallis G, Gkinis G, Dais P, Stathopoulos P. Visual loss due to paranasal sinus invasive aspergillosis in a diabetic patient. Ann Maxillofac Surg. 2014;4(2):247–250. doi: 10.4103/2231-0746.147169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson TF, Thompson GR, Denning DW, et al. Executive summary: practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):433–442.doi: 10.1093/cid/ciw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latge JP. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 2001;9(8):382–389. doi: 10.1016/S0966-842X(01)02104-7. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar P, Price C, Fish M, et al. Skull base aspergillosis in an immunocompetent elderly man with early response to steroid. BMJ Case Rep CP. 2018;11:e226998. doi: 10.1136/bcr-2018-226998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JK, Lin S-Y, Lai P-C, Jou J-R. Compressive optic neuropathy secondary to sphenoid sinus aspergillosis. Neuro-Ophthalmology. 2005;29(2):77–80. doi: 10.1080/01658100590933460. [DOI] [Google Scholar]
- 19.McLean FM, Ginsberg LE, Stanton CA. Perineural spread of rhinocerebral mucormycosis. Am J Neuroradiol. 1996;17:114–116. [PMC free article] [PubMed] [Google Scholar]
- 20.deShazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. JAMA Otolaryngol Neck Surg. 1997;123(11):1181–1188. doi: 10.1001/archotol.1997.01900110031005. [DOI] [PubMed] [Google Scholar]
- 21.Leyngold I, Olivi A, Ishii M, et al. Acute chiasmal abscess resulting from perineural extension of invasive sino-orbital aspergillosis in an immunocompetent patient. World Neurosurg. 2014;81(1):203.e1-203.e2036.doi: 10.1016/j.wneu.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurtell MJ, Chiu ALS, Goold LA, et al. Neuro-ophthalmology of invasive fungal sinusitis: 14 consecutive patients and a review of the literature. Clin Exp Ophthalmol. 2013;41(6):567–576.doi: 10.1111/ceo.12055. [DOI] [PubMed] [Google Scholar]
- 23.Brandwein M. Histopathology of sinonasal fungal disease. Otolaryngol Clin North Am. 1993;26:949–981. [PubMed] [Google Scholar]
- 24.Montone KT. Pathology of fungal rhinosinusitis: a review. Head Neck Pathol. 2016;10(1):40–46. doi: 10.1007/s12105-016-0690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghabrial R, Ananda A, van Hal SJ, et al. Invasive fungal sinusitis presenting as acute posterior ischemic optic neuropathy. Neuro-Ophthalmology. 2018;42(4):209–214.doi: 10.1080/01658107.2017.1392581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102(3):433–444.doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Y-M, Chang Y-H, Chien K-H, et al. Orbital apex syndrome secondary to aspergilloma masquerading as a paranasal sinus tumor: a case report and literature review. Medicine (Baltimore). 2018;97(30):e11650.doi: 10.1097/MD.0000000000011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho SH, Jin BJ, Lee YS, Paik SS, Ko MK, Yi H-J. Orbital apex syndrome in a patient with sphenoid fungal balls. Clin Exp Otorhinolaryngol. 2009;2(1):52–54. doi: 10.3342/ceo.2009.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kontoyiannis DP, Bodey GP. Invasive aspergillosis in 2002: an update. Eur J Clin Microbiol Infect Dis. 2002;21(3):161–172. doi: 10.1007/s10096-002-0699-z. [DOI] [PubMed] [Google Scholar]
- 30.Levin LA, Avery R, Shore JW, Woog JJ, Sullivan Baker A. The spectrum of orbital aspergillosis: a clinicopathological review. Surv Ophthalmol. 1996;41(2):142–154. doi: 10.1016/S0039-6257(96)80004-X. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida K, Shirata A, Sato T, Kishida Y, Saito K, Yamane K. A case of orbital apex syndrome due to aspergillus infection that avoided loss of visual acuity by optic canal decompression. Rinsho Shinkeigaku. 2016;56(1):1–6. doi: 10.5692/clinicalneurol.cn-000741. [DOI] [PubMed] [Google Scholar]
- 32.Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore). 2000;79(4):250–260.doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Jenks JD, Salzer HJ, Prattes J, Krause R, Buchheidt D, Hoenigl M. Spotlight on isavuconazole in the treatment of invasive aspergillosis and mucormycosis: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:1033–1044. doi: 10.2147/DDDT.S145545. [DOI] [PMC free article] [PubMed] [Google Scholar]


