ABSTRACT
Dysbiosis of gut microbiota has been retrospectively linked to autism spectrum disorders but the temporal association between gut microbiota and early neurodevelopment in healthy infants is largely unknown. We undertook this study to determine associations between gut microbiota at two critical periods during infancy and neurodevelopment in a general population birth cohort.
Here, we analyzed data from 405 infants (199 females) from the CHILD (Canadian Healthy Infant Longitudinal Development) Cohort Study. Neurodevelopmental outcomes were objectively assessed using the Bayley Scale of Infant Development (BSID-III) at 1 and 2 years of age. Microbiota profiling with 16S rRNA gene sequencing was conducted on fecal samples obtained at a mean age of 4 and 12 months.
Using clustering methods, we identified three groups of infants based on relative abundance of gut microbiota at 12 months: Proteobacteria-dominant cluster (22.4% higher abundance at 12 months), Firmicutes-dominant cluster (46.0% higher abundance at 12 months) and Bacteroidetes-dominant cluster (31.6% higher abundance at 12 months). Relative to the Proteobacteria-dominant cluster, the Bacteroidetes-dominant cluster was associated with higher scores for cognitive (4.8 points; FDRp = .02), language (4.2 points; FDRp≤0.001), and motor (3.1 points; FDRp = .03) development at age 2 in models adjusted for covariates. When stratified by sex, only male infants with a Bacteroidetes-dominant microbiota had more favorable cognitive (5.9 points, FDRp = .06) and language (7.9 points; FDRp≤0.001) development. Genus Bacteroides abundance in gut microbiota was positively correlated with cognitive and language scores at age 2. Fully adjusted linear mixed model analysis revealed a positive association between Bacteroidetes-dominant cluster and change in cognitive and language performance from 1 to 2 years, predominantly among males. No associations were evident between 4-month microbiota clusters and BSID-II scores. Noteworthy is that enhanced sphingolipid synthesis and metabolism, and antagonism or competition between Bacteroides and Streptococcus were characteristic of a Bacteroidetes-dominant gut microbiota.
This study found strong evidence of positive associations between Bacteroidetes gut microbiota in late infancy and subsequent neurodevelopment, most prominently among males but not females.
KEYWORDS: Infant, gut microbiota, neurodevelopment, cognition, bacteroidetes, early colonization, birth cohort
Introduction
Neurodevelopmental disorders (e.g. autism, attention-deficit/hyperactivity disorder, learning disabilities) manifest early in development and result in lifelong deficits in cognitive, social, emotional, academic, and adaptive functioning.1 The number of children affected by a developmental disorder now represents 13.4% of children aged 6 to 172–4 and 20.1% of children aged 1 to 75 years old worldwide. Environmental factors are the primary drivers of neurodevelopment in very early childhood6 and genetic variations in brain signaling pathways can be modified by early life environments.7 The gut microbiome is altered in children with well-defined phenotypes of developmental delay such as autism,8–11 or risk factors for developmental delay, such as preterm birth.12,13 It is becoming increasingly clear that gut microbiota influences brain function and behavior through signaling pathways of the microbiome-gut-brain axis.14
Starting with colonization by facultative anaerobes, such as the lactic acid bacteria, then followed by an expansion of strict anaerobes within the Firmicutes (e.g. genus Ruminococcus, Veillonella) and Bacteroidetes (e.g. genus Bacteroides) phyla,15,16 the trajectory of the gut microbiome across infancy coincides with key neurodevelopmental periods.17 Peak abundance of lactic acid bacteria and bifidobacteria in the infant gut post birth coincides with the time period for aspects of neuronal development related to synaptogenesis and myelination.15,18 Since microbial signals are hypothesized to be critical for establishing the gut-brain axis,17 early life exposures that shape gut microbiota such as cesarean delivery,19 formula-feeding,20 and antibiotic treatment,21 are not inconsequential. Indeed, several first-colonizing microbiota, as well as members of the Bacteroidetes, are depleted for several weeks after birth by cesarean section,15,21,22 which is a risk factor for developmental delay.23
Whereas changes in the infant gut microbiome coincide with a critical period in early brain development, little is known about their relation to developmental and behavior outcomes. In a cross-sectional study of 77 toddlers at age 2, Christian et al.24 found greater gut microbial diversity and abundance of taxa in the Bacteroidetes phylum (i.e. Parabacteroides) to be associated with infant temperament based on parent report. Longitudinal studies have shown other taxa in the Bacteroidetes phylum (i.e. Prevotella), when depleted in late infancy, to be associated with internalizing behaviors at age 225 but found few associations between Bacteroides-dominant microbiota at age 2 months and temperament in 6-month-old infants.26 Similarly, the Carlson et al.27 cohort study of 89 infants found enrichment of gut microbiota with genus Bacteroides at 12 months to be associated increased cognitive development on the Mullen scale at 2-years old. On the other hand, Sordillo et al. 201928 reported that a Bacteroides-dominant gut microbiota at 3–6 months was associated with increased odds for delayed fine motor skills in 309 preschool children assessed by the Ages and Stages Questionnaire. Further, these small-scale studies did not all report on gut microbial function or sex differences in neurodevelopmental outcomes. Sex differences in brain development are well established29,30 and likely account for sex-specific skill acquisition in domains like verbal communication.31,32 Finally, there is very little information regarding the function of infant gut microbiota underlying healthy neurodevelopment.
Using data from the large population-based CHILD (Canadian Healthy Infant Longitudinal Development) Cohort Study, we examined temporal associations between gut microbiota composition and function during infancy and neurodevelopmental outcomes at 1 and 2 years of age. We aimed to identify microbial clusters and their relation to three objectively-assessed neurodevelopmental domains (cognitive, language, motor). We hypothesized that genus Bacteroides would be associated with enhanced neurodevelopmental scores in a sex-specific manner.
Results
Participant characteristics
Among the 405 study infants with complete data, 50.9% were males, 53.8% had an older sibling, and 26.4% were born by cesarean delivery. At 6 months of age, a large proportion (73.6%) of infants were partially or exclusively breastfed. Other than siblingship, these characteristics did not differ significantly from infants without fecal samples that were not available for study (Table S1). Study infants were from higher income families and their mothers were more highly educated than infants excluded from analysis. Among infants with neurodevelopmental outcomes assessed using the Bayley Scale of Infant Development (BSID-III) at 2 years of age, the mean and standard deviation (SD) for the cognitive composite was 105.6 (SD = 14.2), language composite was 100.7 (SD = 11.9), and motor composite was 99.1 (SD = 9.6), shown in Table 1. Females had a higher mean cognitive score (Mean = 108.4 vs 102.9, p ≤ .0001), and mean language score (Mean = 103.9 vs. 97.7, p ≤ .0001) than males. No sex differences were observed for motor development (p = .17). At 1 year of age, the mean and SD for the cognitive composite was 109.9 (SD = 9.9), language composite was 107.7 (SD = 13.1), and motor composite was 102.6 (SD = 14.4), respectfully. Only performance on the language composite score at aged 1 year significantly differed, with females demonstrating a higher mean language score (Mean = 110.1 vs 105.5, p ≤ .0001) relative to males. There were positive correlations between cognitive scores (r = 0.14; p ≤ 0.001), and between language scores (r = 0.33, p ≤ 0.001) assessed at age 1 and 2 years.
Table 1.
Covariates and their associations with infant neurodevelopment to 2 years of age (n = 405)
| Outcome |
|
BISD-III Cognitive Score |
BISD-III Language Score |
BISD-III Motor Score |
|||
|---|---|---|---|---|---|---|---|
| Variable | No.a (%) | Mean (SD) or, β (95%CI) |
p-value | Mean (SD) | p-value | Mean (SD) | p-value |
| Categorical factors – Mean (SD)a | |||||||
| Gender | |||||||
| Female | 199 (49.1) | 108.4 (14.8) | ≤0.001 | 103.9 (11.6) | ≤0.001 | 99.7 (9.4) | 0.17 |
| Male | 206 (50.9) | 102.9 (13.2) | 97.7 (11.3) | 98.4 (9.7) | |||
| Maternal ethnicity | |||||||
| White | 324 (80.6) | 106.4 (14.2) | 0.14 | 101.6 (11.4) | ≤0.001 | 98.8 (9.4) | 0.40 |
| Asian | 37 (9.2) | 102.6 (14.9) | 95.5 (13.0)c | 98.7 (10.5) | |||
| Other | 41 (10.2) | 103.1 (13.6) | 99.4 (13.2) | 101.0 (10.4) | |||
| Family income | |||||||
| ≥ $60,000 | 376 (94.5) | 106.0 (14.0) | 0.25 | 102.3 (11.6) | 0.22 | 99.3 (9.4) | 0.32 |
| < $60,000 | 22 (5.5) | 102.5 (17.2) | 98.0 (13.3) | 97.2 (12.3) | |||
| Birth Mode | |||||||
| Vaginal, No IAP | 208 (51.7) | 107.4 (15.5) | 0.04 | 101.6 (11.5) | 0.05 | 99.8 (9.1) | 0.29 |
| Vaginal, IAP | 88 (21.9) | 104.1 (12.3) | 101.6 (11.5)c | 97.8 (9.6) | |||
| Scheduled CS | 49 (12.2) | 101.3 (11.8) | 96.9 (12.4) | 97.7 (11.1) | |||
| Emergence CS | 57 (14.2) | 105.2 (13.6) | 99.1 (12.8) | 99.2 (9.6) | |||
| Direct Antibiotic Exposure | |||||||
| Yes | 72 (17.8) | 104.4 (13.1) | 0.20 | 99.8 (10.9) | 0.44 | 99.2 (8.7) | 0.92 |
| No | 333 (82.2) | 105.9 (14.5) | 100.9 (12.1) | 99.0 (9.7) | |||
| Older Sibling | |||||||
| Yes | 218 (53.8) | 105.1 (13.8) | 100.3 (12.0) | 99.8 (9.8) | 0.07 | ||
| No | 187 (46,2) | 106.3 (14.7) | 0.40 | 101.3 (11.7) | 0.40 | 98.1 (9.2) | |
| Ear Infection (0–12 months) | |||||||
| Yes | 42 (11.4) | 104.2 (12.8) | 0.43 | 98.1 (9.5) | 0.11 | 98.0 (9.3) | 0.43 |
| No | 328 (88.7) | 106.6 (14.5) | 101.2 (12.2) | 99.2 (9.3) | |||
| Breastfeeding, 6 months | |||||||
| None | 107 (26.4) | 103.8 (11.9) | 0.25 | 98.6 (10.8) | 0.03 | 98.9 (10.4) | 0.95 |
| Partial | 229 (56.5) | 106.0 (14.9) | 100.8 (11.4) | 99.1 (9.5) | |||
| Exclusive | 69 (17.1) | 107.3 (15.0) | 103.6 (14.2)c | 99.4 (8.4) | |||
| Pre-pregnancy weight | |||||||
| Overweight | 173 (44.4) | 104.6 (13.8) | 0.16 | 99.8 (11.5) | 0.14 | 98.3 (10.2) | 0.29 |
| Normal weight | 217 (56.6) | 106.6 (14.7) | 101.6 (12.1) | 99.3 (9.0) | |||
| Continuous factors – β (95% CI)c | |||||||
| Gestational age at delivery | 405 (100) | 1.1 (0.1, 2.2) | 0.03 | 1.2 (0.3, 2.1) | ≤0.001 | 0.9 (0.2, 1.6) | 0.01 |
| Maternal prenatal fruit intaked | 383 (94.6) | 0.35 (−0.51, 1.2) | 0.21 | 1.2 (0.5, 1.9) | ≤0.001 | −0.10 (−0.7, 0.5) | 0.75 |
| Age at microbiota sampling, in months | 405 (100) | 0.7 (−0.4, 1.8) | 0.43 | −0.07 (−0.9, 0.8) | 0.88 | 0.22 (−0.5, 1.0) | 0.55 |
Abbreviations: BISD-III = Bayley Infant Scales of Development Third Edition; SD = standard deviation; β = Coefficient.
Note Neurodevelopment was assessed in three domains: cognitive, language, and motor. The standardized population mean is 100 (standard deviation of 15). Higher scores indicate better abilities. Total sample (N) represents those participants with neurodevelopmental data collected at the 2-year study visit and 12-month microbiome sampling (mean = 12.5 months).
aAnalyzed by ttest or One-Way Analysis of Variance (ANOVA).
bAnalyzed by linear regression.
cSignificant group comparisons using Tukey post hoc test.
dTotal fruit intake indicates the “5-a-day” method (calculated as sum of servings of fruit, not including juices, plus servings of juice per day).
Identifying the infant gut microbiota clusters
4-month microbiota clusters
Separation of the bacteria populations by Partitioning around medoids (PAM) clustering identified three microbiota clusters at aged 4 months as follows (Figure 1): Proteobacteria and Firmicutes-dominant cluster (35.8%), Firmicutes-dominant cluster (24.7%) and Bacteroidetes-dominant cluster (39.5%). Since the 4-month clusters were not associated with BSID-III composite scores at 1 and 2 years of age they were not further characterized (presented later).
Figure 1.
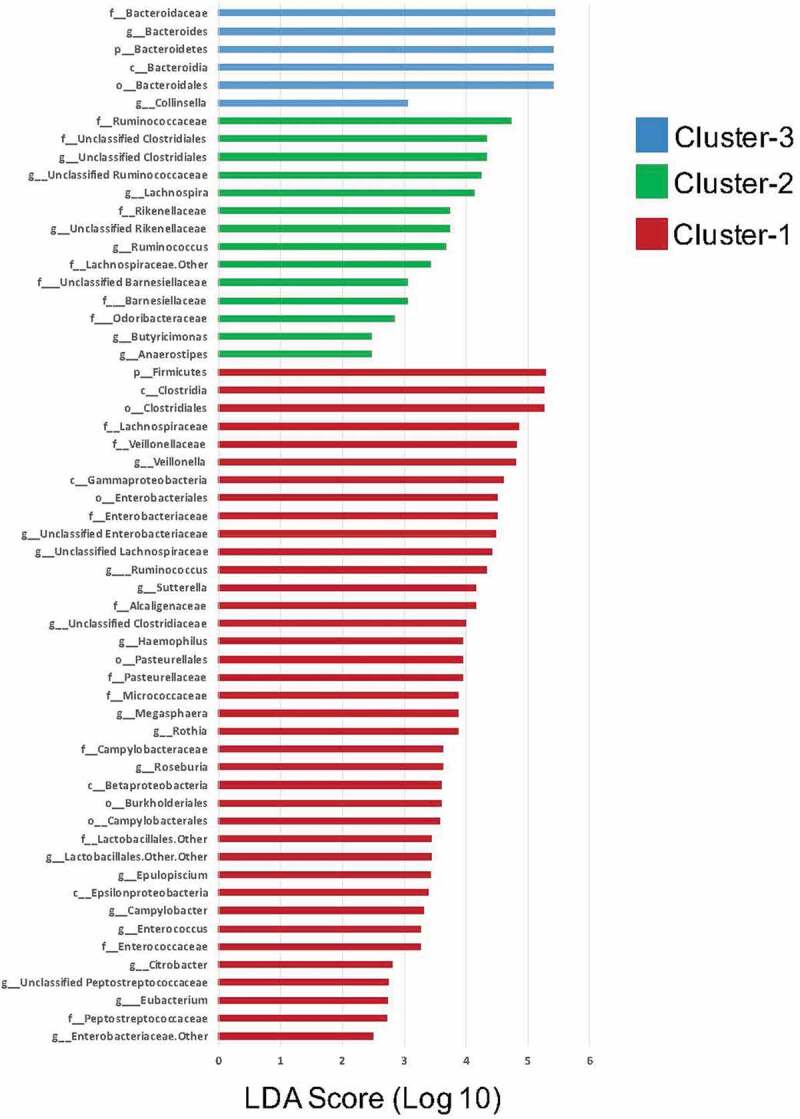
Linear discriminant analysis (LDA) scores for bacterial taxa differentially abundant in infant gut microbiota according to PAM Clusters at 12 months (LDA >2)
12-month microbiota clusters
PAM clustering yielded three distinct, mutually-exclusive gut microbiota clusters shown in Figure S2. At 12 months, the LEfSe analysis (Figure S2) revealed a higher abundance of several Proteobacteria within Cluster 1 (22.4%, n = 91; Proteobacteria-dominant Cluster), a higher abundance of Firmicutes microbiota within Cluster 2 (46.0%, n = 186; Firmicutes-dominant Cluster), and a higher abundance of Bacteroidetes species within Cluster 3 (31.6%, n = 128; Bacteroidetes-dominant Cluster).
The distribution of covariates across the three microbiota clusters are shown in Table 2.
Table 2.
Distribution of infant and maternal characteristics according to each infant gut microbiota cluster group
| |
Microbiota at 1 year |
|
||
|---|---|---|---|---|
| Descriptive Variable | Cluster 1 (n = 91/405) % (n) |
Cluster 2 (n = 186/405) % (n) |
Cluster 3 (n = 128/405) % (n) |
p-value |
| Categorical | ||||
| Sex | ||||
| Male | 47.3 (43/91) | 55.9 (104/186) | 46.1 (59/128) | 0.17 |
| Female | 52.7 (48/91) | 44.1 (82/186) | 53.9 (69/128) | |
| Maternal ethnicity | ||||
| White | 74.7 (68/91) | 86.3 (158/186) | 76.6 (98/128) | |
| Asian | 16.5 (15/91) | 3.8 (7/186) | 11.7 (15/128) | |
| Other | 8.8 (8/91) | 9.8 (18/186) | 11.7 (15/128) | |
| Family income | ||||
| High | 95.5 (84/91) | 92.3 (167/186) | 88.7 (110/128) | 0.71 |
| Low | 4.5 (4/91) | 7.7 (14/186) | 11.3 (14/128) | |
| Birth Mode | ||||
| Vaginal, NO IAP | 42.9 (39/91) | 54.3 (100/186) | 54.3 (69/128) | ≤0.001 |
| Vaginal, IAP | 22.0 (20/91) | 17.9 (33/186) | 27.6 (35/128) | |
| Scheduled CS | 9.9 (9/91) | 14.7 (27/186) | 10.2 (13/128) | |
| Emergency CS | 25.3 (23/91) | 13.0 (24/186) | 7.9 (10/128) | |
| Direct Antibiotic exposure 0–12 months | ||||
| Yes | 19.8 (18/91) | 16.1 (30/186) | 18.8 (24/128) | 0.71 |
| No | 80.2 (73) | 83.9 (156) | 81.3 (104) | |
| Older sibling | ||||
| Yes | 37.4 (34) | 67.2 (125) | 45.7 (58) | ≤0.001 |
| No | 62.6 (57) | 32.8 (61) | 54.3 (69) | |
| Ear infection (0–12 month) | ||||
| Yes | 7.7 (7) | 15.2 (28) | 11.7 (15) | 0.20 |
| No | 92.3 (84) | 84.8 (156) | 88.3 (113) | |
| Breastfeeding status at 6 months | ||||
| None | 25.3 (23) | 24.2 (45) | 30.5 (39) | 0.07 |
| Partial | 49.5 (45) | 58.6 (109) | 58.6 (75) | |
| Exclusive | 25.3 (23) | 17.2 (32) | 10.9 (14) | |
| Maternal pre-pregnancy weight | ||||
| Overweight | 38.6 (34) | 45.6 (83) | 46.7 (56) | 0.46 |
| Normal | 61.4 (54) | 54.4 (99) | 53.3 (64) | |
| Continuous | Mean (SD) | Mean (SD) | Mean (SD) | |
| Gestational age at delivery | 39.49 (1.37) | 39.51 (1.27) | 39.44 (1.38) | 0.88 |
| Age at microbiota sampling | 12.21 (0.89) | 12.67 (1.53) | 12.33 (1.09) | ≤0.001 |
| Maternal prenatal fruit intakea | 3.02 (1.71) | 2.84 (1.49) | 3.10 (1.86) | 0.40 |
Abbreviations: PMCs = Proteobacteria; FMCs = Fimicutes; BMCs = Bacteroidetes; SD = standard deviation; IAP = intrapartum antibiotics; CS = cesarean section.
aTotal fruit intake was defined as the “5-a-day” method calculated as sum of servings of fruit, not including juices, plus servings of juice per day.
Significantly more infants in Firmicutes-dominant Cluster 2 were born to a Caucasian mother relative to the other two cluster groups and they were likely to have an older sibling. Delivery by emergency cesarean and breastfeeding exclusivity was highest in Firmicutes-dominant Cluster 1. Other factors (antibiotic exposure, ear infection, maternal pre-pregnancy overweight, gestational age) known to influence infant gut microbiota did not differ among the three clusters. Notably, there were no sex differences by microbiota cluster. Next, we assessed the relationship between the same covariates and neurodevelopmental outcomes (cognitive, language, motor) shown in Table 1. Gender and birth mode were significantly associated with cognitive and language composite scores, while breastfeeding at 6-months, ethnicity, and maternal prenatal fruit intake were significantly associated with language compositive score (p’s <0.05). We found significant associations between gestational age and all three outcome measures; other more subtle differences in scores by study covariates are shown in Table 1.
Infant microbiome and neurodevelopmental outcomes
No associations between microbiota clusters in early infancy and neurodevelopmental outcomes
We tested associations between the microbiota clusters analyzed at a mean age of 4 months with cognitive development (primary outcome). We found no associations between 4-month microbiota cluster membership and BSID-III cognitive composite scores at age 1 year (q = 0.18) and age 2 years (q = 0.99, Figure 2a and b) in univariate analysis. Similarly, there were no other significant associations found between the 4-month microbiota clusters membership and the other BSID-III language and motor composite scores (not presented).
Figure 2.
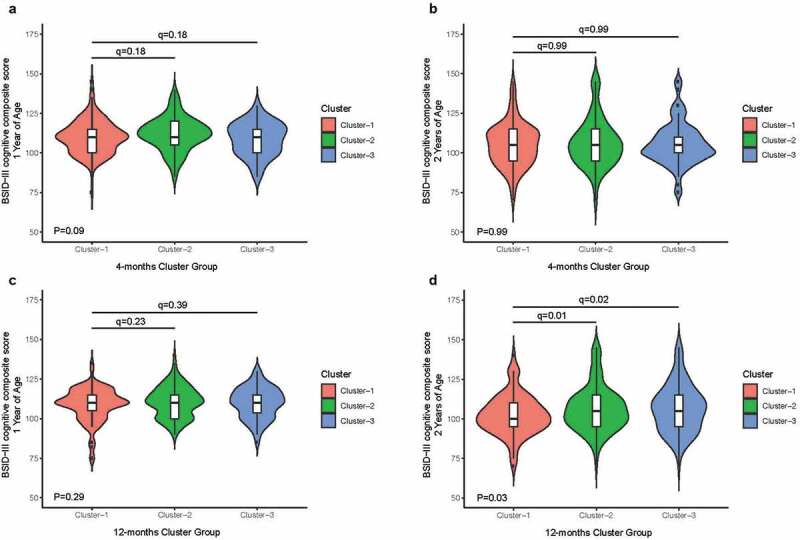
Associations between cognitive composite scores and microbiota cluster membership
Evidence of associations between microbiota clusters in late infancy and neurodevelopmental outcomes
Microbial diversity and richness differ among 12-month clusters
Total microbial species richness and diversity of infant gut microbiota differed among the three clusters (all FDRp’s<0.001; Table S2). In brief, Proteobacteria-dominant Cluster 1 had the lowest total species richness and diversity, but highest richness within the Proteobacteria phylum. Firmicutes-dominant Cluster 2 had the highest total species richness and diversity, especially within the Fimicutes phylum. In Bacteroidetes-dominant Cluster 3, richness within the Bacteroidetes phylum was highest without observed differences in total phylogenetic diversity.
Bacteroidetes and Firmicutes microbiota clusters in late infancy are associated with enhanced neurodevelopmental outcomes at age 2 in all infants
We assessed the relationship between microbiota clusters analyzed at a mean age of 12 months and the primary outcome cognitive development using the Proteobacteria-dominant cluster as the reference group. We found no significant associations between the 12-month cluster membership and concurrent BSID-III cognitive composite scores at 1 year of age in multivariate analysis (Table S3 and Figure 2c). Next, we tested the relationship between 12-month microbiota clusters and the BSID-II cognitive composite score at 2 years of age. Relative to the Proteobacteria-dominant Cluster 1, infants in microbiota Bacteroidetes-dominant Cluster 3 and Firmicutes-dominant Cluster 2 exhibited higher scores for cognitive development at 2 years indicating better abilities (4.8 points, 95%CI: 0.8–8.7, FDRp = .02; and 5.3 points, 95%CI: 1.4–9.1, FDRp = .01, respectively; Figure 2d and Table 3).
Table 3.
Crude and adjusted effects of microbiota at 12 months and neurodevelopment to 2 years of age
| Microbiota Cluster Group | BSID-III composite scores at 2 years of age (n = 405) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive |
Language |
Motor |
||||||||||||
| Crude |
Adjusteda |
Crude |
Adjusteda |
Crude |
Adjusteda |
|||||||||
| Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | |||
| Cluster 1 | Reference | Reference | Reference | |||||||||||
| Cluster 2 | 4.0 (0.5, 7.5) |
0.03 | 5.3 (1.4, 9.1) |
0.01 | 2.2 (−0.8, 5.2) |
0.14 | 4.1 (1.1, 7.2) |
≤0.001 | 2.6 (0.2, 4.9) |
0.04 | 3.0 (0.4, 5.6) |
0.03 | ||
| Cluster 3 | 4.2 (0.4, 8.0) |
0.03 | 4.8 (0.8, 8.7) |
0.02 | 3.2 (0.1, 6.4) |
0.10 | 4.2 (1.1, 7.4) |
≤0.001 | 2.8 (0.3, 5.4) |
0.04 | 3.1 (0.4, 5.9) |
0.03 | ||
| BSID-III composite scores 1–2 years (n = 401) | ||||||||||||||
| Cluster 1 | Reference | Reference | Reference | |||||||||||
| Cluster 2 | 3.2 (0.8, 5.7) |
0.02 | 3.8 (1.3, 6.4) |
≤0.001 | 1.9 (−0.7, 4.5) |
0.15 | 2.8 (0.3, 5.4) |
0.03 | 2.4 (0.0, 4.9) |
0.10 | 2.0 (−0.4, 4.5) |
0.14 | ||
| Cluster 3 | 3.2 (0.4, 5.6) |
0.03 | 3.4 (0.8, 6.0) |
0.02 | 2.8 (0.0, 5.6) |
0.10 | 3.2 (0.5, 5.8) |
0.03 | 1.8 (−0.7, 4.4) |
0.17 | 2.0 (−0.6, 4.5) |
0.14 | ||
Abbreviations: BSID-III: Bayley Infant Scales of Development Third Edition.
Note Analyzed by separate Generalized Linear Models and separate Linear Mixed Models with adjustments for the same covariates. The standardized population mean is 100 (standard deviation of 15). Higher scores indicate better abilities.
aCovariates include birth mode, sex, maternal ethnicity, older sibling, breastfeeding status at 6 months, family income, maternal overweight, and age at sampling.
We further determined independence of associations between the 12-month microbiota clusters and the BSID-III language and motor composite scores shown in Table 3. Here, we found that the Bacteroidetes-dominant and Firmicutes-dominant microbiota clusters were independently associated with higher scores indicating better language and motor abilities at age 2. Relative to the Proteobacteria-dominant Cluster 1, the Bacteroidetes-dominant Cluster 3 group had a 4.2-point increase in language composite score (94%CI: 1.1, 7.4, FDRp≤0.001), while infants in Firmicutes-dominant Cluster 2 showed a 4.1-point increase in language composite score (95%CI: 1.1, 7.2 FDRp≤0.001) in fully adjusted models. Similarly, infants in Firmicutes-dominant and Bacteroidetes-dominant microbiota clusters also had significantly higher scores relative to the Proteobacteria-dominant Cluster 1 in the motor domain of the BSID-III in adjusted analyses (all FDRp’s<0.05; shown in Table 3). These associations were independent of family income, maternal ethnicity, birth mode, breastfeeding status, direct antibiotic exposure, older sibling, gestational fruit intake, maternal overweight, and age at sampling.
We then tested associations between the microbiome cluster group membership and change in performance on the BSID-III scales from 1 to 2 years using linear mixed model (LMM) analysis and Proteobacteria-dominant cluster as the reference group. After adjustment for confounders, we found that the Bacteroidetes-dominant and Firmicutes-dominant microbiota clusters were independently and positively associated with cognitive and language performance change between 1 and 2 years old shown in Table 3. Bacteroidetes-dominant Cluster 3 was associated with a 3.4-point (95%CI: 0.8, 6.0, FDRp = .02) increase in cognitive performance between 1 and 2 years. Bacteroidetes-dominant Cluster 3 group was also associated with a 3.2-point (95%CI: 0.5, 5.8, FDRp = .03) increase in language performance but not for motor performance (FDRp>.05; shown in Table 3). Similarly, Firmicutes-dominant Cluster 2 group was positively associated with an increase in cognitive (3.8-points; 95%CI: 1.3, 6.4, FDRp≤0.001) and language performance (2.8-points; 95%CI: 0.3, 5.4, FDRp = .03) from 1 to 2 years but not for motor performance (FDRp>.05; shown in Table 3).
Sex specific associations between the Bacteroidetes microbiota cluster in late infancy and neurodevelopmental outcomes
Next, we ascertained whether associations observed between microbiota cluster membership and neurodevelopmental domains occurred in a sex-specific manner. In a stratified analysis and relative to the Proteobacteria-dominant Cluster 1, male infants within Bacteroidetes-dominant Cluster 3 exhibited a 5.9-point increase to cognitive development score at age 2 (95%CI: 0.6, 11.1, FDRp = .06; Table 4). Bacteroidetes-dominant Cluster 3 was also associated with enhanced language development in males at this age (7.9 points; 95%CI: 3.4, 12.3, FDR p ≤ 0.001; Table 4). Male infants in the Firmicutes-dominant Cluster 2 group scored higher on language development (5.1 points; 95%CI: 0.9, 9.3, FDRp = .02; Table 4) but not cognitive development at age 2 (FDRp>.05; Table 4). These associations remained statistically significant after adjustment for several covariates including birth mode, maternal prenatal fruit intake, maternal overweight, and breastfeeding status. However, we found that microbiota cluster type was unrelated to motor development among male infants.
Table 4.
Crude and adjusted effects of microbiota at 12 months and infant neurodevelopment, stratified by sex
| Microbiota Cluster Group | BSID-III composite scores at 2 years of age (n = 405) by sex |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive |
Language |
Motor |
||||||||||
| Crude |
Adjusted |
Crude |
Adjusted |
Crude |
Adjusted |
|||||||
| Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | Beta (95%CI) | q-value | |
| Cluster 1 | Reference | Reference | Reference | |||||||||
|
Cluster 2 Females |
4.8 (−0.5,10.0) |
0.15 | 5.0 (−0.8,10.8) |
0.18 | 2.6 (−1.5, 6.7) |
0.44 | 2.6 (−1.6, 6.8) |
0.46 | 4.3 (1.0, 7.5) |
0.02 | 3.9 (0.2, 7.7) |
0.08 |
| Males | 4.3 (−0.3, 8.9) |
0.07 | 4.8 (−0.13, 9.8) |
0.16 | 3.2 (−0.7, 7.1) |
0.11 | 5.1 (0.9, 9.3) |
0.02 | 1.1 (−2.4, 4.5) |
0.55 | 1.5 (−2.2, 5.1) |
0.42 |
|
Cluster 3 Females |
3.4 (−2.0, 8.8) |
0.22 | 3.4 (−2.4, 9.2) |
0.25 | 0.5 (−3.8, 4.7) |
0.83 | 0.3 (−3.9, 4.6) |
0.88 | 4.1 (0.7, 7.5) |
0.02 | 3.3 (−0.5, 7.1) |
0.09 |
| Males | 5.0 (−0.1, 10.1) |
0.07 | 5.9 (0.6, 11.1) |
0.06 | 6.2 (1.9, 10.6) |
0.01 | 7.9 (3.4, 12.3) |
≤0.001 | 1.3 (−2.5, 5.1) |
0.55 | 2.8 (−1.1, 6.7) |
0.34 |
| BSID-III composite scores 1–2 years (n = 401) by sex | ||||||||||||
| Cluster 1 | Reference | Reference | Reference | |||||||||
|
Cluster 2 Females |
3.5 (0.0, 7.0) |
0.10 | 3.2 (−0.05, 6.8) |
0.20 | 1.4 (−2.1, 4.9) |
0.86 | 0.8 (−2.8,4.3) |
0.99 | 3.3 (0.0, 6.7) |
0.06 | 2.1 (−1.4, 5.6) |
0.52 |
| Males | 3.9 (0.5, 7.2) |
0.02 | 4.1 (0.6, 7.6) |
0.03 | 4.0 (0.5, 7.6) |
0.03 | 4.7 (1.0, 8.3) |
0.02 | 2.0 (−1.5, 5.4) |
0.26 | 1.8 (−1.8, 5.3) |
0.35 |
|
Cluster 3 Females |
1.4 (−2.3, 5.0) |
0.46 | 1.4 (−2.2, 5.1) |
0.46 | 0.3 (−3.4, 3.9) |
0.89 | 0.0 (−3.6, 3.5) |
0.99 | 1.1 (−2.4, 4.7) |
0.53 | 1.2 (−2.3, 4.7) |
0.52 |
| Males | 4.9 (1.3, 8.6) |
0.02 | 4.9 (1.1, 8.7) |
0.03 | 6.1 (2.2, 9.9) |
≤0.001 | 6.2 (2.3, 10.1) |
≤0.001 | 2.6 (−1.1, 6.3) |
0.26 | 2.8 (−1.1, 6.6) |
0.34 |
Among female infants, both Firmicutes-dominant and Bacteroidetes-dominant microbiota clusters were significantly associated with enhanced motor development at age 2, but these associations did not survive FDR correction following adjustment for covariates. We also observed no significant associations among females between the microbiota clusters and cognitive or language development.
In a stratified LMM analysis, male infants in the Bacteroidetes-dominant Cluster 3 showed an increase in cognitive composite score (4.9-point; 95%CI: 1.1, 8.7, FDRp = .03; Table 4) and language composite score (6.2-point; 95%CI: 2.3, 10.1, FDR≤0.001; Table 4) from 1 to 2 years, but not females. Similarly, male infants classified into the Firmicutes-dominant group showed an increase in cognitive composite score (4.1-point; 95%CI: 0.6, 7.6, FDRp = .03) and language composite score (4.7-point; 95%CI: 1.0, 8.3, FDRp = .02) from 1 to 2 years, but not females. No sex-dependent differences were observed for motor performance from 1 to 2 years (Table 4).
Correlation between abundances of microbiota keystone species at age 1 year and neurodevelopmental outcomes
Based on GLM models, different species of Bacteroides including B. fragilis, B. uniformis, and unclassified Bacteroides were positively associated with increased cognitive development (all FDRp’s<0.05). Similarly, B. uniformis and unclassified Bacteroides were associated with improved language development, while unclassified Prevotella were positively associated with motor development (all FDRp’s<0.05). Shown in Tables S4, S5, and S6.
Microbial interaction networks associated with 12-month clusters
A total of 37 microbial families were involved in the co-occurrence network of Proteobacteria-dominant Cluster 1, while this number was 25 and 23 families in Firmicutes-dominant Cluster 2 and Bacteroidetes-dominant Cluster 3, respectively. A unique feature of the microbial network of Cluster 1 was the lack of Bacterioidaceae’s involvement. However, the abundance of Bacterioidaceae was inversely associated with that of Streptococcaceae in the other two clusters. Shown in Figure 3a–c.
Figure 3.
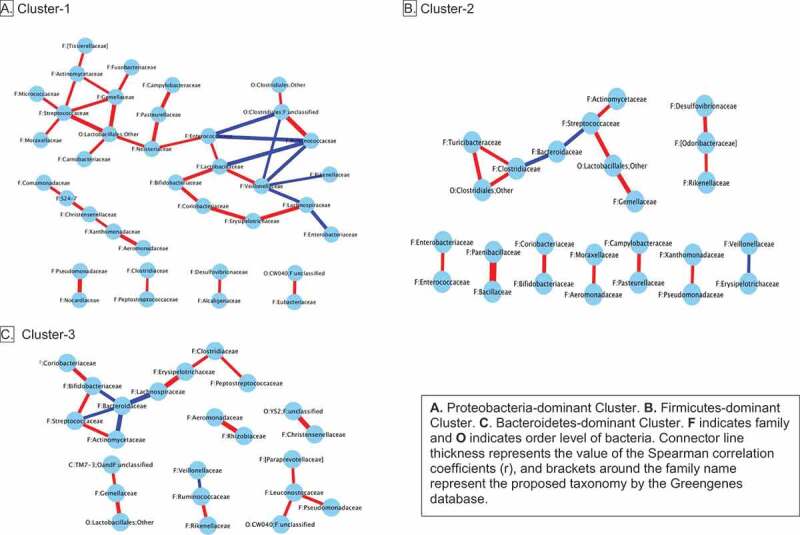
Microbiota interaction networks for microbiota clusters at 12 months
Microbiota metabolic function differs among 12-month clusters
Microbiota clusters differed by level-3 metabolic functional categories of KEGG pathways (Figure 4a–c). Among the three clusters, Bacteroidetes-dominant Cluster 3 was enriched with multiple metabolic functions including sphingolipid metabolism and glycosphingolipid biosynthesis. Moreover, genes involved in metabolism of folate, biotin, pyruvate, vitamin B6, lipoic acid and fatty acid biosynthesis were enriched in this cluster.
Figure 4.
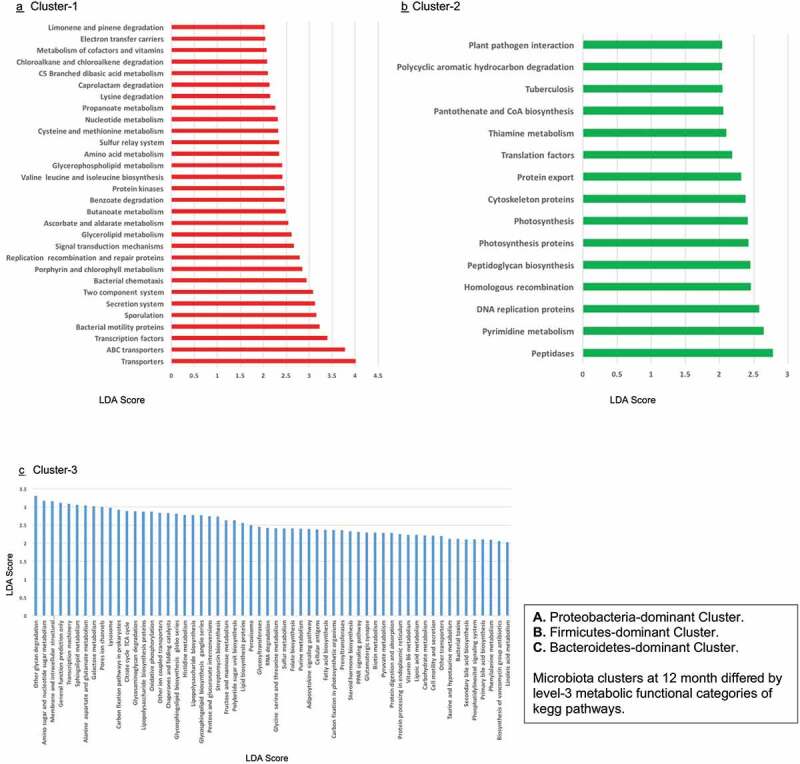
Microbiota metabolic function at 12 months according to cluster group membership
Discussion
In this general population birth cohort of 405 infants, a beneficial impact of gut microbiota during infancy was documented for early neurodevelopmental outcomes. We observed that infants with an enriched abundance of Bacteroides in their gut microbiota at 1 year had more favorable BSID-III cognitive and language development at 2 years. In fact, infants with a Bacteroidetes-dominant microbial composition achieved 4.8-point and 4.2-point higher cognitive and language development scores, respectively, which represents close to half a standard deviation difference in performance. These observed effect sizes, and especially those in boys (7.9-point higher score for language) are similar to ones found for breastfeeding33 and infant sleep duration.34 Depletion of Bacteroides in gut microbiota is also characteristic of children diagnosed with autism spectrum disorder at age 2–3 years.35 Moreover, our findings were driven by male infants since statistical significance was not found for Bacteroidetes-dominant gut microbiota and advanced cognitive or language development in female infants. The gut-brain axis of boys seems to be more susceptible to disruptions in the gut microbiome,36 which has been linked to alterations to the brain’s serotonergic system in germ-free animal models.37 Hence, our study contributes to growing evidence that neurodevelopmental outcomes are shaped by the gut microbial composition of infants in a sex-dependent manner. Importantly, we found statistical significance with the Bacteroidetes cluster in male infants for change to BSID-III cognition and language scores between 1 and 2 years of age, adding stronger evidence for a causal association.
Bacteroidetes species may play a key role in promoting neurodevelopment during a critical time in late infancy when myelination and expanded connectivity of neuronal networks normally occur; when these processes are slowed, pervasive developmental delay can result.38,39 With successive increases to gut microbial species richness in both young and older infants, greater connectivity in brain areas that support cognitive development and language acquisition has been reported.40,41 We observed that presence of the Bacteroidetes-cluster (with the highest species richness of Bacteroidetes), as well as higher relative abundance of genus Bacteroides at 1 year, were associated with improved neurodevelopmental outcomes. Our results are quite similar to the 12-month Bacteroides association with neurodevelopmental outcomes at age 2 in the Carlson et al.27 study, and they are consistent with critical periods of postnatal brain development. Their study27 also identified Bacteroides-dominance to be characteristic of 1 years olds with large gray-matter volumes in the superior occipital gyrus, the back region of the brain also shown to have reduced integration with other brain networks in preschool children with autism versus controls.42 Contrary to the VDAART study28 (Ages and Stages questionnaire), we did not observe associations between 4-month microbial composition and neurodevelopmental outcomes in infancy. Our findings are supported by the microbiota gut-brain-axis concept, which suggests that the presence of the gut microbiota plays a key role in the gut-brain-axis and in neurodevelopment. This is evident in germ-free mice models whereby the absence of a gut microbiota causes alterations in cognition and memory,43 and social development, a core deficit in autism.44 Murine models of autism point to the potential role of Bacteroides fragilis in ameliorating defects in communicative and sensorimotor behaviors, potentially through a modulatory effect on serum metabolites, including sphingolipids.11
Our findings also have biological plausibility in terms of the actions of microbial short-chain fatty acid (SCFA) metabolite pathways on the developing brain in the infant. The SCFA propionate, is chiefly produced by the Bacteroidetes species which become more prominent in the gut of later infancy.45–47 A tendency for lower fecal propionate levels has been reported in school children with pervasive developmental and language disorders.48 Through its conversion to odd-chain fatty acids such as pentadecanoic acid, propionate has been posited to play a role in ganglioside production and myelination of neurons.49–51 Indeed, we found sphingolipid metabolism, especially the biosynthesis of glycosphingolipid (ganglio series), to be enriched in the Bacteroidetes-dominant cluster. The absence of ganglio-series gangliosides (sialylated glycosphingolipids) in mutant mice leads to early developmental deficits in reflexes, strength, coordination, and balance,52 as well as to progressive motor and sensory dysfunction, and deterioration in spatial learning and memory.52,53 In humans, the expression of gangliosides undergoes significant change during the development of the brain, largely attributed to the normal functioning and maintenance of the brain.49 When sphingomyelin levels are higher, be it in infant serum or supplemented formula, or through breastfeeding, preschool children perform better on neurocognitive scales and exhibit improved myelination of the brain.54,55 Further, similar to the study by Carlson et al.27 we also observed greater abundance of functional genes related to the production of vitamins and cofactors such biotin, lipoic acid, folate and vitamin B6 in the cluster dominated by Bacteroidetes.
Gut microbiome pathways to neurodevelopment may also operate through the interactions of genus Bacteroides with other gut microbiota. Multiple associations between the abundance of microbial families were apparent within the Bacteroidetes-dominant microbiota cluster, including an inverse correlation between the abundance of Bacteroidaceae and Streptococcaceae. Group B Streptococcus (GBS: S. agalactiae) is a leading cause of newborn sepsis and meningitis, and the main indication for maternal intrapartum prophylaxis during vaginal delivery.56 A recent meta-analysis57 confirmed neurodevelopmental impairment to develop in 20% of infants with neonatal GBS infection. Others too have found higher propionate levels, the main metabolite produced by Bacteroidetes species, with lower abundance of Streptococcus in term infants.58 Unable to compete with Bacteroidetes and other microbes in an increasing anaerobic environment, Streptococcus abundance declines to very low levels in gut microbiota as infants grow older; yet, when Bacteroidetes are depleted in the gut following emergency cesarean or maternal intrapartum prophylaxis, some streptococcal species continue to thrive in older infants who had been exclusively breastfed for 3–4 months.46 In our study, 28% of infants in Bacteroidetes-dominant microbiota cluster were born following maternal intrapartum prophylaxis for GBS, 18% had been delivered by cesarean and 70% had been breastfed for 6-months. A short duration of breastfeeding may explain why the streptococcal interaction was not found with the Bacteroides cluster in the Carlson et al. study,27 where half of the infants in this cluster had received formula by age 1.
Study strengths and limitations
There are several strengths of our study: i) high-throughput deep sequencing to profile gut microbiota at two critical periods of microbiota over the first year of life in relation to brain development between 1 and 2 years, ii) objective assessment of neurodevelopment by experts using a well-validated and widely-used gold standard measure, and iii) a large sample size that enabled adjustment for ethnicity and early life covariates. An important limitation of this work is that the PICRUSt analysis of function can only infer potential mechanisms since it predicts metagenomic function according to the 16S sequences of reference genomes. Future studies should also consider a shotgun metagenomic approach to examine microbial function with more depth to further assess causality and mechanisms. We were also unable to examine infants’ high risk for neurodevelopmental morbidity as the CHILD Cohort Study excluded preterm birth below 35 weeks. Other high-risk groups excluded from our study were families of low socioeconomic status. Further studies are required to investigate the generalizability of our findings to other populations.
Conclusions
In a general population, we found evidence for the influence of gut microbiota, namely the Bacteroides species, and associated sphingolipid synthesis in late infancy on subsequent neurodevelopmental outcomes. Our study suggests a greater effect size among male infants, particularly for cognitive and language abilities. Future studies are needed to confirm these findings and examine the impact of the infant gut microbiome on more complex tasks at later neurodevelopmental stages.
Methods
Study design and population
This was a microbiome study of 577 infants with neurodevelopmental outcomes at ages 1 and 2 years old, as part of a substudy at the Edmonton site of the CHILD Cohort Study.59,60 This sample included a subset of infants with fecal samples collected at approximately 4 months (from 414 infants) and/or 1 year follow-up (from 405 infants). Enrollment methods for their expectant mothers in the general population and their predominantly term newborns (35+ weeks gestation) have been described in detail elsewhere (www.childstudy.ca).59 Mothers of studied infants were enrolled during pregnancy between January 2009 and December 2012. Their infants were seen at a planned 3–4 months, 1 year, and 2 years study visits.59,60 Informed consent was obtained from all mothers and the study was approved by the Human Research Ethics Board at the University of Alberta (Pro00002099).
Neurodevelopmental assessments
Infant neurodevelopmental assessments, using the Bayley Scale of Infant Development Third Edition (BSID-III),61 were completed at 1 year and 2 years of age, during the day at a time when parents felt their infant was most alert (i.e. not during a scheduled naptime). The BSID-III is a validated objective measure of cognitive, language, motor development for infants aged 1 to 42 months. The BSID-III cognitive scale (91-items) assesses visual preference, attention, memory, exploration, manipulation, and concept formation; the language scale assesses receptive communication (49-items) and expressive communication (48-items); and the motor scale assess gross motor (72-items) and fine motor (66-items) skills. It’s cognitive (0.91), language (0.93), and motor (0.92) subscales have high reliability coefficients, and good test-retest stability with coefficients around 0.80.61,62 A registered educational psychologist trained research staff to administer the BSID-III instrument and conducted semiannual assessments. Testing of participants was completed during a single session by two research staff. All scores were obtained based on the child’s chronological age at the time of testing. Raw scores were converted to scaled scores, then to composite scores. The standardized population mean for the composite score is 100 (standard deviation of 15). A higher score on the BSID-III scales indicates better abilities.
Confounding variables
Data from study questionnaires or hospital birth records were obtained to create covariates as follows: infant sex, maternal ethnicity (Caucasian, Asian or other), family income (<, ≥60,000 USD), maternal pre-pregnancy overweight, birth mode (vaginal, elective or emergency cesarean section), maternal intrapartum antibiotic prophylaxis (IAP), any infant oral antibiotic treatment until age 1, any infant ear infections, breastfeeding status (exclusively, partially, or not breastfeed) and older siblingship. Maternal prenatal fruit intake (“5-a-day” method) the sum of “servings of fruit, not including juices, “plus servings of juice” per day,63 which we previously found to be associated with infant cognition,64 was based on the 5-day method from a modified 174-item, self-reported Food Frequency Questionnaire.63
Fecal microbiota analysis
Gut microbiota were profiled by 16S rRNA gene sequencing in fecal samples collected from 414 infants at a mean age of 4.2 months (SD = 1.24) and from 405 infants at a mean age of 12.5 months (SD = 1.29) during planned study visits. Within the funding scope of the CHILD Cohort Study, these collection points strategically represented a sample during the peak of breastfeeding in Canadian infants and a post-weaning sample at the end of infancy. Sample collection, DNA extraction and amplification methods have been previously described in detail21,65 (see online supplementary content for details).
Statistical analysis
Study sample characteristics were compared to those of infants missing neurodevelopment and microbiome data using Chi-square or ANOVA tests. OTU relative abundances were summarized at the phylum, family and genus levels of taxonomy with QIIME software. Microbial alpha-diversity was calculated with four standard indices (Chao1, Shannon, Simpson, and Faith Phylogenetic Diversity).
(i) Clustering analysis
To identify microbiota clusters according to genus abundance, all samples were clustered using the partitioning around medoids (PAM) clustering algorithm, described by Arumugan et al.66 and tested in infants;67 the optimal number of clusters was determined by the Calinski-Harabasz index and Silhouette width. The linear discriminant analysis effect size (LEfSE) with an LDA log cutoff of 2 was applied to identify unique taxa that differentiated the microbiota cluster groups and could be used to name clusters by dominant microbiota. Thereafter, clusters were characterized and compared according to microbial diversity (non-parametric Kruskal-Wallis and post hoc Dunn tests with a false discovery rate (FDR) correction), co-occurrence taxon abundance networks and PICRUSt metabolic function.68
(i) General linear modeling (GLM)
Our units of analysis were cognitive (primary outcome), and language and motor (secondary outcomes) BSID-III composite scores. Univariate analysis (t-test, ANOVA, Pearson correlations) identified covariates that differed (p < .05) among microbiota clusters and BSID-III scores. Using GLM, associations between microbiota clusters with cluster group 1 as the reference and BSID-III composite scores at aged 1 and 2 were tested separately in fully adjusted models. Adjusted for covariates, associations between BSID-III composite scores and taxon relative abundance were determined by GLM.
(i) Linear mixed model (LMM)
To explore the influence of microbiome group membership on neurodevelopmental scores, we used linear effects models with BSID-III composite scores from the repeated aged 1 and aged 2 visit as outcomes. Sampling age, neurodevelopmental assessment visit age, and covariates were entered into the model as random terms. Statistical significance was defined as a two-sided q-value <0.05, after FDR correction of the p-value for multiple comparison. GLM and LMM analyses were conducted in SAS Software version 9.4 (SAS).
Supplementary Material
Acknowledgments
The authors thank all the families who took part in this study, and the whole CHILD Study team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Funding Statement
This research was specifically funded by grant number 108028 from the Canadian Institutes of Health Research Canadian Microbiome Initiative (Dr Kozyrskyj). The Edmonton-site Sleep and Neurodevelopment substudy was supported by grant number 211722 from The Canadian Institutes of Health Research and by the Women and Children’s Health Research Institute. The Canadian Institutes of Health Research and the Allergy, Genes, and Environment (AllerGen) Network of Centres of Excellence provided core support for the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study.The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Data availability statement
The data that supports the findings of this study are available from the corresponding author and CHILD Cohort Study coordinators upon reasonable request. These data, including study data, are securely stored in the https://childdb.ca database.
Author contributions
Dr Kozyrskyj had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Chari, Field, Turvey, Subbarao, Sears, Scott, Mandhane, Kozyrskyj conceptualized and designed the study. Drs. Tun, Tamana, Field, Guttman, Becker, Mandhane, Moraes, Turvey, Subbarao, Pei, Scott, Kozyrskyj contributed to data acquisition, analysis, and/or interpretation of data. Drs. Tamana, Tun, Kozyrskyj, Mandhane contributed to the drafting of the manuscript. Drs. Tamana, Tun, Konya, Chari, Field, Guttman, Mandhane, Becker, Mandhane, Moraes, Turvey, Subbarao, Sears, Pei, Scott, Kozyrskyj contributed to critical revision of the manuscript for important intellectual content. Drs. Tun, Scott, Tamana contributed to statistical data analysis. Drs. Chari, Field, Becker, Mandhane, Moraes, Turvey, Subbarao, Sears, Scott, Kozyrskyj obtained funding. Drs. Konya, Guttman, Pei, Becker, Mandhane, Moraes, Turvey, Subbarao, Scott, Kozyrskyj contributed to administrative, technical, or material support. Drs. Mandhane and Kozyrskyj provided study supervision.
Supplementary material
Supplemental data for this article can be accessed on thepublisher’s website.
References
- 1.Thapar A, Cooper M, Rutter M.. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4(4):339–17. doi: 10.1016/S2215-0366(16)30376-5. [DOI] [PubMed] [Google Scholar]
- 2.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry. 2015;56(3):345–365. doi: 10.1111/jcpp.12381. [DOI] [PubMed] [Google Scholar]
- 3.Prasad A, Burneo J, Corbett B. A national profile of neurodevelopmental disabilities in Canadian children: data from the national longitudinal study of children and youth. J Neurol Sci. 2015;357:e207–e208. doi: 10.1016/j.jns.2015.08.715. [DOI] [Google Scholar]
- 4.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, Visser S, Kogan MD. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127(6):1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 5.Vasileva M, Graf RK, Reinelt T, Petermann U, Petermann F. Research review: a meta-analysis of the international prevalence and comorbidity of mental disorders in children between 1 and 7 years. J Child Psychol Psychiatry. 2020;62:372–381. doi: 10.1111/jcpp.13261. [DOI] [PubMed] [Google Scholar]
- 6.Tucker-Drob EM, Briley DA. Continuity of genetic and environmental influences on cognition across the life span: a meta-analysis of longitudinal twin and adoption studies. Psychol Bull. 2014;140(4):949–979. doi: 10.1037/a0035893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miguel PM, Pereira LO, Silveira PP, Meaney MJ. Early environmental influences on the development of children’s brain structure and function. Dev Med Child Neurol. 2019;61(10):1127–1133. doi: 10.1111/dmcn.14182. [DOI] [PubMed] [Google Scholar]
- 8.Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4(1):42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde E, McCue T, Codelli J, Chow J, Reisman S, Petrosino J, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauchi H, Yahagi K, Yamauchi T, Hara T, Yamaoka R, Tsukuda N, Watanabe Y, Tajima S, Ochi F, Iwata H, et al. Gut microbiota development of preterm infants hospitalised in intensive care unitsGut microbiota development of preterm infants hospitalised in intensive care units. Benef Microbes. 2019; 10(6):1–12. [DOI] [PubMed] [Google Scholar]
- 13.Cong X, Xu W, Janton S, Henderson WA, Matson A, McGrath JM, Maas K, Graf J. Gut microbiome developmental patterns in early life of preterm infants: impacts of feeding and gender. PLoS One. 2016;11(4):e0152751. doi: 10.1371/journal.pone.0152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TF, Boehme M, MG Codagnone, Cussotto S, Fulling C, Golubeva AV, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 15.Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E, Kubota H, Swinkels S, Sakai T, Oishi K, et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One. 2016;11(6):e0158498. doi: 10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(6):852. doi: 10.1016/j.chom.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Cowan CSM, Dinan TG, Cryan JF. Annual research review: critical windows - the microbiota-gut-brain axis in neurocognitive development. J Child Psychol Psychiatry. 2019;61:353–371. doi: 10.1111/jcpp.13156. [DOI] [PubMed] [Google Scholar]
- 18.Lebovitz Y, Ringel-Scaia VM, Allen IC, Theus MH. Emerging developments in microbiome and microglia research: implications for neurodevelopmental disorders. Front Immunol. 2018;9:1993. doi: 10.3389/fimmu.2018.01993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR, et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018;172(4):368–377. doi: 10.1001/jamapediatrics.2017.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho NT, Li F, Lee-Sarwar KA, Tun HM, Brown BP, Pannaraj PS, Bender JM, Azad MB, Thompson AL, Weiss ST, et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9(1):4169. doi: 10.1038/s41467-018-06473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, Sears MR, Mandhane PJ, Turvey SE, Subbarao P, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983–993. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Cai W, Feng Y. Development of intestinal bifidobacteria and lactobacilli in breast-fed neonates. Clin Nutr. 2007;26(5):559–566. doi: 10.1016/j.clnu.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Sidorchuk A, Sevilla-Cermeno L, Vilaplana-Pérez A, Chang Z, Larsson H, Mataix-Cols D, Fernández de la Cruz L. Association of cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e1910236. doi: 10.1001/jamanetworkopen.2019.10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christian LM, Galley JD, Hade EM, Schoppe-Sullivan S, Kamp Dush C, Bailey MT. Gut microbiome composition is associated with temperament during early childhood. Brain Behav Immun. 2015;45:118–127. doi: 10.1016/j.bbi.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loughman A, Ponsonby A-L, O’Hely M, Symeonides C, Collier F, Tang MLK, Carlin J, Ranganathan S, Allen K, Pezic A, et al. Gut microbiota composition during infancy and subsequent behavioural outcomes. EBioMedicine. 2020;52:102640. doi: 10.1016/j.ebiom.2020.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aatsinki A-K, Lahti L, Uusitupa H-M, Munukka E, Keskitalo A, Nolvi S, O’Mahony S, Pietilä S, Elo LL, Eerola E, et al. Gut microbiota composition is associated with temperament traits in infants. Brain Behav Immun. 2019;80:849–858. doi: 10.1016/j.bbi.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, Thompson AL, Geng X, Gilmore JH, Knickmeyer RC, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry. 2018;83(2):148–159. doi: 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sordillo JE, Korrick S, Laranjo N, Carey V, GM Weinstock, DR Gold, O’Connor G, Sandel M, Bacharier LB, Beigelman A, Zeiger R. Association of the infant gut microbiome with early childhood neurodevelopmental outcomes: an ancillary study to the VDAART randomized clinical trial. JAMA Netw Open. 2019;2(3):e190905. doi: 10.1001/jamanetworkopen.2019.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmithorst VJ, Holland SK, Dardzinski BJ; Schmithorst VJ, Holland SK, Dardzinski BJ . Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakstad PH. Gender differences in the human brain. Acta Radiol. 2015;56(2):131–132. doi: 10.1177/0284185114562993. [DOI] [PubMed] [Google Scholar]
- 32.Michael G, Ginette D, Philip D, Robert P. Sex differences in early verbal and non‐verbal cognitive development. Dev Sci. 2000;3(2):206–215. doi: 10.1111/1467-7687.00114. [DOI] [Google Scholar]
- 33.Horta BL, Loret de Mola C, CG Victora. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):14–19. doi: 10.1111/apa.13139. [DOI] [PubMed] [Google Scholar]
- 34.Smithson L, Baird T, Tamana SK, Lau A, Mariasine J, Chikuma J, Lefebvre DL, Subbarao P, Becker AB, Turvey SE, et al. CHILD Study Investigators, Beal DS, Pei J, Mandhane PJ. Shorter sleep duration is associated with reduced cognitive development at two years of age. Sleep Med. 2018 Aug;48:131–139. doi: 10.1016/j.sleep.2018.04.005. Epub 2018 Apr 30. PMID: 29906629. [DOI] [PubMed] [Google Scholar]
- 35.Dan Z, Mao X, Liu Q, Guo M, Zhuang Y, Liu Z, Chen K, Chen J, Xu R, Tang J, et al. Altered gut microbial profile is associated with abnormal metabolism activity of autism spectrum disorder. Gut Microbes. 2020;11(5):1246–1267. doi: 10.1080/19490976.2020.1747329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jasarevic E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150122. doi: 10.1098/rstb.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 38.Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19(3):123–137. doi: 10.1038/nrn.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jena A, Montoya CA, Mullaney JA, Dilger RN, Young W, McNabb WC, Roy NC. Gut-brain axis in the early postnatal years of life: a developmental perspective. Front Integr Neurosci. 2020;14:44. doi: 10.3389/fnint.2020.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelsey CM, Prescott S, McCulloch JA, Trinchieri G, TL Valladares, Dreisbach C, Alhusen J, Grossmann T.. Gut microbiota composition is associated with newborn functional brain connectivity and behavioral temperament. Brain Behav Immun. 2021 Jan;91:472–486. doi: 10.1016/j.bbi.2020.11.003. Epub 2020 Nov 4. PMID: 33157257. [DOI] [PubMed] [Google Scholar]
- 41.Gao W, Salzwedel AP, Carlson AL, Xia K, Azcarate-Peril MA, Styner MA, Thompson AL, Geng X, Goldman BD, Gilmore JH, et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology (Berl). 2019;236(5):1641–1651. doi: 10.1007/s00213-018-5161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Fu K, Chen L, Duan X, Guo X, Chen H, Wu Q, Xia W, Wu L, Chen H, et al. Increased gray matter volume and resting-state functional connectivity in somatosensory cortex and their relationship with autistic symptoms in young boys with autism spectrum disorder. Front Physiol. 2017;8:588. doi: 10.3389/fphys.2017.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, MacQueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- 44.Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry. 2014;19(2):146–148. doi: 10.1038/mp.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 46.Yasmin F, Tun HM, Konya TB, Guttman DS, Chari RS, Field CJ, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, et al. Cesarean section, formula feeding, and infant antibiotic exposure: separate and combined impacts on gut microbial changes in later infancy. Front Pediatr. 2017;5:200. doi: 10.3389/fped.2017.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev. 2017;18(1):18–31. doi: 10.1111/obr.12484. [DOI] [PubMed] [Google Scholar]
- 48.Bojovic K, Ignjatovic Eth I, Sokovic Bajic S, Vojnović Milutinović D, Tomić M, Golić N, Tolinački M. Gut microbiota dysbiosis associated with altered production of short chain fatty acids in children with neurodevelopmental disorders. Front Cell Infect Microbiol. 2020;10:223. doi: 10.3389/fcimb.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmano K, Rowan A, Guillermo R, Guan J, McJarrow P. The role of gangliosides in neurodevelopment. Nutrients. 2015;7(5):3891–3913. doi: 10.3390/nu7053891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weitkunat K, Schumann S, Nickel D, Hornemann S, Petzke KJ, Schulze MB, Pfeiffer AF, Klaus S. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr. 2017;105(6):1544–1551. doi: 10.3945/ajcn.117.152702. [DOI] [PubMed] [Google Scholar]
- 51.Pfeuffer M, Jaudszus JA. Pentadecanoic and heptadecanoic acids: multifaceted odd-chain fatty acids. Adv Nutr. 2016;7(4):730–734. doi: 10.3945/an.115.011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tajima O, Egashira N, Ohmi Y, Fukue Y, Mishima K, Iwasaki K, Fujiwara M, Inokuchi J, Sugiura Y, Furukawa K, et al. Reduced motor and sensory functions and emotional response in GM3-only mice: emergence from early stage of life and exacerbation with aging. Behav Brain Res. 2009;198(1):74–82. doi: 10.1016/j.bbr.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 53.Tajima O, Egashira N, Ohmi Y, Fukue Y, Mishima K, Iwasaki K, Fujiwara M, Sugiura Y, Furukawa K, Furukawa K, et al. Dysfunction of muscarinic acetylcholine receptors as a substantial basis for progressive neurological deterioration in GM3-only mice. Behav Brain Res. 2010;206(1):101–108. doi: 10.1016/j.bbr.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Moreau GB, Ramakrishnan G, Cook HL, Fox TE, Nayak U, Ma JZ, Colgate ER, Kirkpatrick BD, Haque R, Petri WA, et al. Childhood growth and neurocognition are associated with distinct sets of metabolites. EBioMedicine. 2019;44:597–606. doi: 10.1016/j.ebiom.2019.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deoni S, Dean D 3rd, Joelson S, O’Regan J, Schneider N. Early nutrition influences developmental myelination and cognition in infants and young children. Neuroimage. 2018;178:649–659. doi: 10.1016/j.neuroimage.2017.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Persaud RR, Azad MB, Chari RS, Sears MR, Becker AB, Kozyrskyj AL. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: a population-based study. J Matern Fetal Neonatal Med. 2015;28(10):1190–1195. doi: 10.3109/14767058.2014.947578. [DOI] [PubMed] [Google Scholar]
- 57.Kohli-Lynch M, Russell NJ, Seale AC, Dangor Z, Tann CJ, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, et al. Neurodevelopmental impairment in children after group B streptococcal disease worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S190–S199. doi: 10.1093/cid/cix663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pham VT, Lacroix C, Braegger CP, Chassard C. Early colonization of functional groups of microbes in the infant gut. Environ Microbiol. 2016;18(7):2246–2258. doi: 10.1111/1462-2920.13316. [DOI] [PubMed] [Google Scholar]
- 59.Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, Denburg JA, HayGlass KT, Kobor MS, Kollmann TR, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015;70(10):998–1000. doi: 10.1136/thoraxjnl-2015-207246. [DOI] [PubMed] [Google Scholar]
- 60.Takaro TK, Scott JA, Allen RW, Anand SS, Becker AB, Befus AD, Brauer M, Duncan J, Lefebvre DL, Lou W, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort study: assessment of environmental exposures. J Expo Sci Environ Epidemiol. 2015;25(6):580–592. doi: 10.1038/jes.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayley N. Bayley scales of infant and toddler development. San Antonio (Texas): The Psychological Corporation; 2006. [Google Scholar]
- 62.Albers CA, Grieve AJ. Test Review: bayley, N. (2006). Bayley scales of infant and toddler development– third edition. San Antonio, TX: harcourt Assessment. J Psychoeduc Assess. 2007;25(2):180–190. doi: 10.1177/0734282906297199. [DOI] [Google Scholar]
- 63.Kristal AR, Vizenor NC, Patterson RE, Neuhouser ML, Shattuck AL, McLerran D. Precision and bias of food frequency-based measures of fruit and vegetable intakes. Cancer Epidemiol Biomarkers Prev. 2000;9:939–944. [PubMed] [Google Scholar]
- 64.Bolduc FV, Lau A, Rosenfelt CS, Langer S, Wang N, Smithson L, Lefebvre D, Alexander RT, Dickson CT, Li L, et al. Cognitive enhancement in infants associated with increased maternal fruit intake during pregnancy: results from a birth cohort study with validation in an animal model. EBioMedicine. 2016;8:331–340. doi: 10.1016/j.ebiom.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, Mandhane PJ, Turvey SE, Subbarao P, Becker AB, et al. Infant gut microbiota and food sensitization: associations in the first year of life. Clin Exp Allergy. 2015;45(3):632–643. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 66.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, Schoos AMM, Kunøe A, Fink NR, Chawes BL, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9(1):141. doi: 10.1038/s41467-017-02573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author and CHILD Cohort Study coordinators upon reasonable request. These data, including study data, are securely stored in the https://childdb.ca database.


