Abstract
Embryo polarization is critical for mouse development; however, neither the regulatory clock nor the molecular trigger that it activates is known. Here, we show that the embryo polarization clock reflects the onset of zygotic genome activation, and we identify three factors required to trigger polarization. Advancing the timing of transcription factor AP-2 gamma (Tfap2c) and TEA domain transcription factor 4 (Tead4) expression in the presence of activated Ras homolog family member A (RhoA) induces precocious polarization as well as subsequent cell fate specification and morphogenesis. Tfap2c and Tead4 induce expression of actin regulators that control the recruitment of apical proteins on the membrane, whereas RhoA regulates their lateral mobility, allowing the emergence of the apical domain. Thus, Tfap2c, Tead4, and RhoA are regulators for the onset of polarization and cell fate segregation in the mouse.
Graphical Abstract
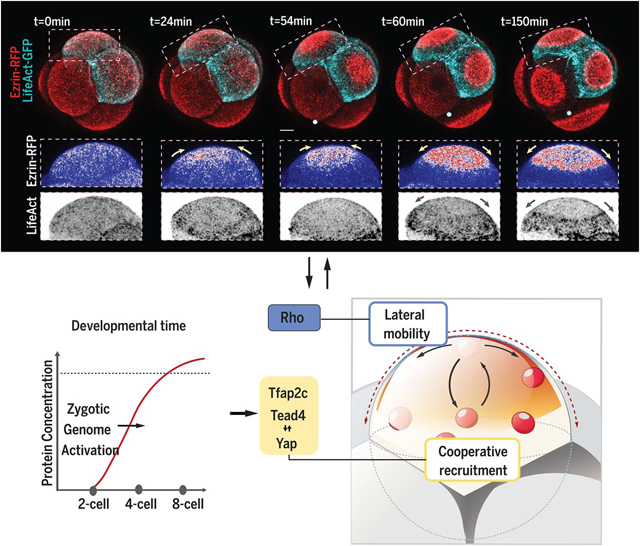
Molecular mechanism and temporal regulation for the apical domain formation. Zygotic genome activation enables the expression of Tfap2c and Tead4, which regulates the cooperative membrane recruitment of apical proteins. Cooperative recruitment interacts with Rho GTPase–regulated apical protein lateral mobility to establish the apical domain.
INTRODUCTION:
During preimplantation development, the establishment of apicobasal cell polarity is key for the transition from totipotency to pluripotency, which induces cell differentiation toward trophectoderm (TE). In the mouse embryo, this event is programmed to occur at the eight-cell stage, and this timing follows an intrinsic developmental clock that is independent of embryo size or cell cycle progression. Despite the importance of apical domain formation, the molecular mechanisms to establish cell polarization and the temporal regulation of this event in mouse and human embryos have remained largely elusive.
RATIONALE:
In different mammalian species, zygotic genome activation (ZGA) is evolutionarily conserved to occur before the establishment of cell polarization. We therefore hypothesized that zygotic transcription regulates the timing of polarization. To test this, we deployed assays to alter the cellular concentration of zygotic transcripts and to assess the consequences of these changes for the timing of embryo polarization. We also performed an RNA interference (RNAi) screen on 124 zygotically expressed transcripts to determine the molecular identity of zygotic transcripts crucial to cell polarization. Finally, we combined cutting-edge imaging methods with biophysical modeling to account for how the factors we identified regulate the de novo establishment of cell polarization.
RESULTS:
Cell polarity in the mouse embryo is marked by the appearance of a cap-shaped apical domain. Consistent with our hypothesis, an increase or decrease of zygotic transcripts respectively accelerated or inhibited apical domain formation. Our RNAi screen identified two transcription factors—transcription factor AP-2 gamma (Tfap2c) and TEA domain transcription factor 4 (Tead4), which play a redundant role in regulating cell polarization timing. Both Tfap2c and Tead4 proteins accumulate after ZGA, and elevation of their expression allows polarity proteins to anchor to the apical surface prematurely at the four-cell stage. However, these apical proteins failed to organize into an expanded apical domain, instead becoming hypercentralized to form membrane protrusions. This indicates that an additional condition is required for the apical domain formation. We have previously characterized that Rho guanosine triphosphatase (GTPase) signaling, which regulates the actomyosin apical localization and becomes activated around the eight-cell stage, is important for cell polarity. In this study, we found that premature activation of Rho GTPase with expression of Tfap2c and Tead4 allows a complete, precocious induction of the apical domain, leading to the premature expression of TE transcription factors and to morphogenesis events downstream of cell polarization. By combining quantitative imaging measurements and mathematical modeling, we show that apical domain formation is driven by the dynamic interplay between two key processes: (i) the cooperative recruitment of ezrin via the actin network and (ii) the lateral mobility of ezrin on the membrane. On the basis of the experimental evidence and biophysical simulations of these interactions, we show that Tfap2c and Tead4 control the cooperative recruitment of ezrin, whereas RhoA promotes membrane mobility.
CONCLUSION:
The timing and mechanisms for cell polarization have remained largely unknown. We now identify molecules that are necessary and sufficient for the de novo establishment of cell polarization in the mouse embryo. Our results indicate a direct role of ZGA in regulating the timing of cell polarization. Beyond identifying the key molecules sufficient to establish cell polarization, we also provide biophysical understanding of the mechanism by which these molecules act to build cell polarization in the mammalian embryo.
The totipotent mammalian zygote can produce any embryonic or extraembryonic tissue, but this ability becomes restricted in the first cell fate decision that generates distinct inner cell mass (ICM) and outer extraembryonic trophectoderm (TE). The ICM will form epiblast (EPI) and extraembryonic primitive endoderm (PE), generating the future fetus and the yolk sac, respectively. The TE will form the placenta. Formation of these three lineages by implantation is a prerequisite for successful pregnancy.
Embryo polarization is key to the segregation of the ICM and TE lineages (1, 2). In the mouse, this process happens at the eight-cell stage (2–4), when each blastomere acquires an apical domain, comprising the Par protein complex and ERM proteins (ezrin, radixin, and moesin) enclosed by an actomyosin ring (5, 6). The apical domain enables expression of transcription factors such as Cdx2 and Gata3, which drive differentiation into TE, whereas apolar cells maintain pluripotency to become ICM (7, 8).
Mammalian embryo development is regulative, yet the timing of embryo polarization remains unchanged even if embryos are split into individual blastomeres, when cells are aggregated together, or when cell divisions are prevented. Thus, the polarization seems set to a strict developmental clock that is independent of cell number (9, 10). Here, we show that this clock reflects activation of the zygotic genome, and we identify three factors whose convergent activity triggers self-organization of the apical domain.
Critical threshold of transcripts required for embryo polarization
Polarization timing varies between species, reflecting the onset of zygotic genome activation (ZGA) (11, 12). In mouse, the major wave of ZGA occurs at the two-cell stage, but an additional transcriptional wave also occurs at the early eight-cell stage, just before polarization (13). To determine whether the latter transcriptional wave is associated with polarization, we first treated embryos from the early eight-cell stage with two transcription inhibitors: 5,6-dichlorobenzimidazole 1-β-d-ribofuranoside (DRB) and triptolide. Each drug prevented apical localization of the polarity marker Pard6 (Fig. 1, A to D, and fig. S1, A to G). Polarization was restored by washing out the reversible transcription inhibitor DRB (fig. S1, H to J). Thus, transcription at the early eight-cell stage appears to be required for embryo polarization. As a second test, we reduced the cytoplasmic volume (Fig. 1E), shown by others to increase the concentration of newly synthesized mRNA (14, 15). We injected zygotes with an apical marker [ezrin–red fluorescent protein (RFP) mRNA] and resected 30 to 40% of the cytoplasm from a two- or four-cell-stage blastomere using a technique that does not compromise development (16) (Fig. 1F). Single-molecule fluorescence in situ hybridization confirmed that this reduction increased the concentration of newly transcribed mRNAs (17) (fig. S2, A to E). Such blastomere resection advanced embryo polarization by 2.1 hours when performed at the two-cell stage (Fig. 1, F to I; N = 62 pairs; movie S1) and by 3.3 hours at the four-cell stage (fig. S2, F to H, and Fig. 1J; N = 76 pairs; movie S2). Both experimental and control embryos established all three lineages as blastocysts (fig. S2, I to K). Inhibiting transcription with a 3-hour pulse of DRB led both resected and control blastomeres to polarize simultaneously (Fig. 1K and fig. S2, L to N). These results indicate that de novo synthesis of transcripts and their accumulation to a critical threshold is required for embryo polarization.
Fig. 1. The dependency of polarization on nascent transcripts.
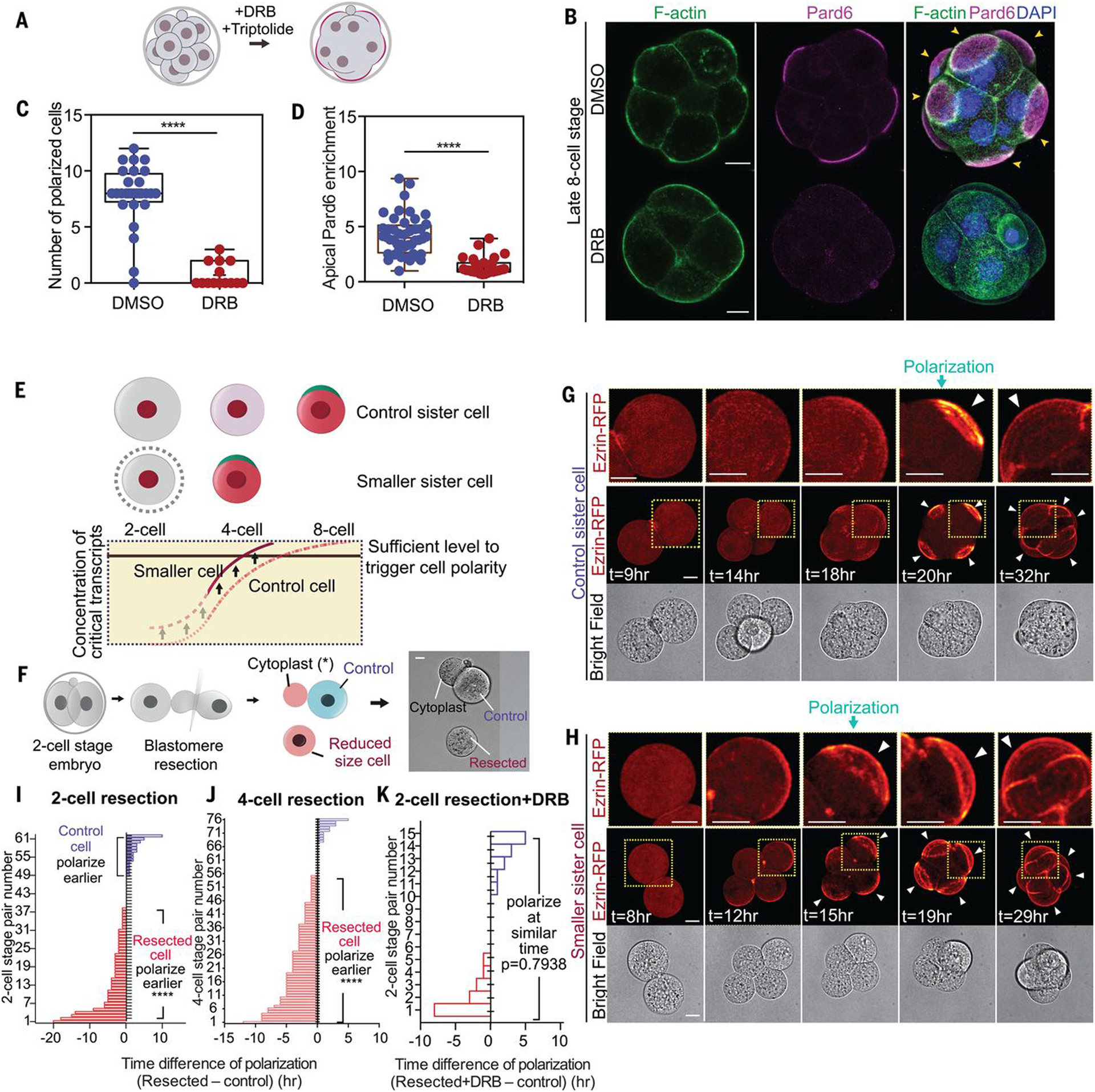
(A) Scheme indicating inhibitor treatments. (B) Dimethyl sulfoxide (DMSO)–treated (control) or DRB-treated 8- to 16-cell embryos were analyzed for the localization of F-actin, Pard6, and DNA. Arrowheads indicate the apical domain. (C) Polarized cell number in DMSO (control) or DRB-treated embryos. ****P < 0.0001, Mann-Whitney U test. N = 2 experiments. (D) Apical enrichment of Pard6 (see methods) in 8- to 16-cell-stage cells treated with DMSO (control) or DRB. ****P < 0.0001. Mann-Whitney U test. (E) Scheme of the hypothesis: Newly synthesized factors important for polarization accumulate up to a point at which polarization is induced at the eight-cell stage. Decreasing the cell size elevates the concentration of such factors, leading to an advance in polarization timing. (F) Scheme showing the blastomere resection procedure. (G and H) Time-lapse of control or smaller sister blastomeres from the experiment described in (F). Arrowheads indicate the apical domain. Dotted yellow squares indicate the magnified regions (top row). (I and J) Polarization time difference (see methods) between smaller and control sister blastomeres from (F) or fig. S2F; each bar represents one comparison. Smaller cells polarize earlier in the significant majority of cases. N = 13 experiments for (I), N = 6 experiments for (J). ****P < 0.0001, one-sample t test, hypothetical mean = 0. (K) Polarization time difference between control and smaller DRB-treated sister cells, from experiments in fig. S2L. Each bar represents one comparison. Pulsed transcription inhibition prevents the early polarization of smaller cells. N = 3 experiments. One-sample t test, hypothetical mean = 0. Arrowheads indicate the apical domain. Scale bars, 15 μm.
Tfap2c and Tead4 are required for embryo polarization
We hypothesized that the requirement for eight-cell-stage transcription could result from direct expression of cytoskeletal regulators of cell polarization or their indirect expression through a transcriptional hierarchy. We therefore interrogated published single-cell RNA sequencing (RNA-seq) data (18) and selected genes for 118 cytoskeletal polarity regulators and six transcription factors that show increased expression by the eight-cell stage and hence are likely to be active according to assay for transposase-accessible chromatin sequencing (ATAC-seq) (19) (fig. S3 and tables S1 and S2). We down-regulated each of these 124 genes by RNA interference (RNAi) (figs. S3 and S4, A and B) and scored the timing of embryo polarization from time-lapse imaging of the distribution of ezrin-RFP (Fig. 2, A to D). Only depletion of the transcription factors Tfap2c (transcription factor AP-2 gamma) or Tead4 (TEA domain transcription factor 4) prevented embryo polarization (Fig. 2, A to C, and E, and fig. S4, A to E). Individual depletion of Tfap2c and Tead4 delayed polarization from the 8- to 16-cell stage (Fig. 2, A to C, E, and F), whereas polarization was entirely abolished by their co-depletion (Fig. 2, D to H). Depletion of Tfap2c and Tead4 also prevented precocious polarization resulting from blastomere resection (fig. S4, F and G).
Fig. 2. Zygotic expression of Tfap2c and Tead4 is essential for polarization.
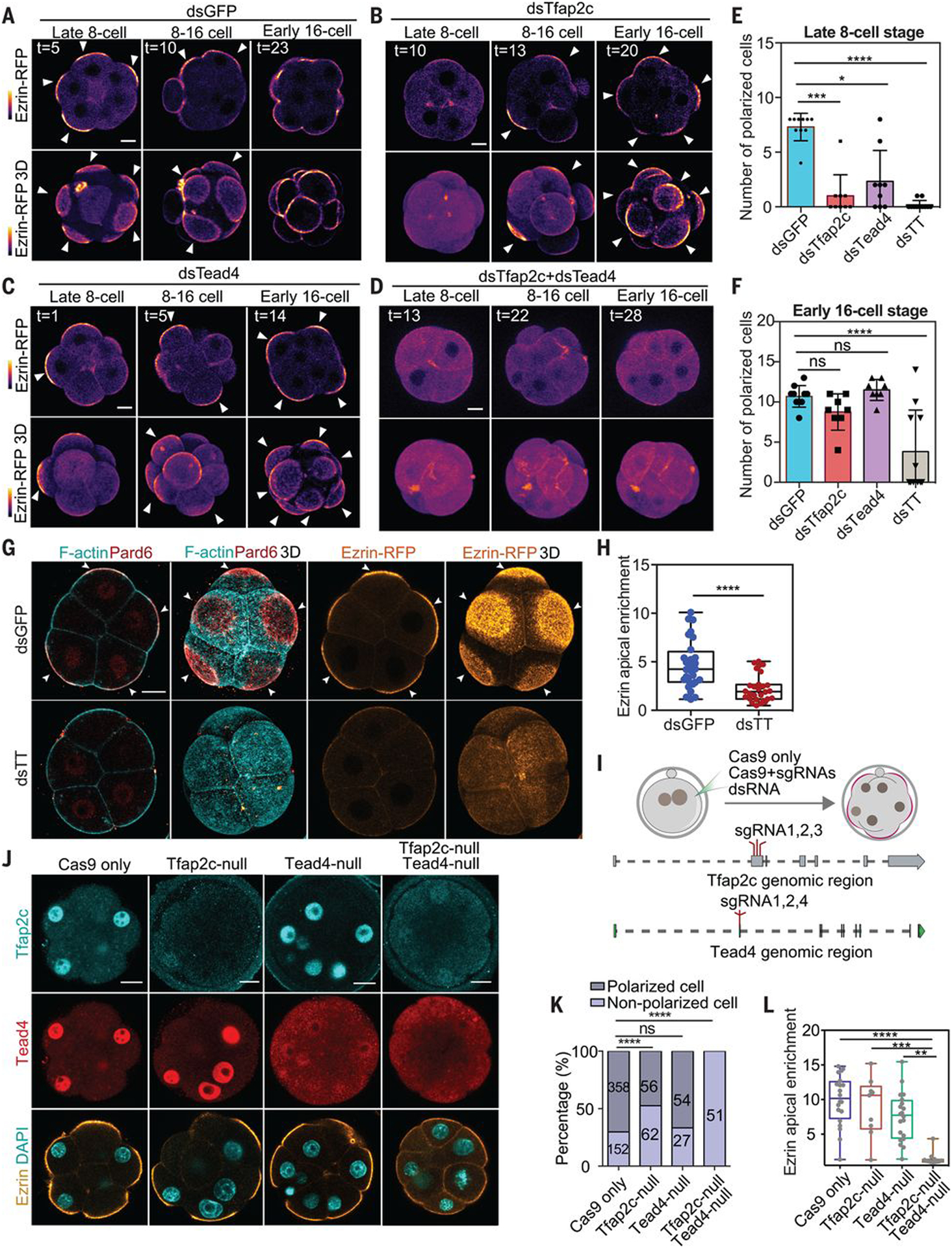
(A to D) Time lapse of ezrin-RFP localization in embryos with or without Tfap2c and/or Tead4. Tfap2c and Tead4 co-depletion causes polarization failure until the 16-cell stage. Arrowheads indicate the apical domain. (E and F) Polarized cell number in different conditions and stages. Each dot, square, or triangle represents an embryo. dsTT, dsTfap2c+dsTead4. ns, not significant; *P = 0.0306; ***P = 0.0006; ****P < 0.0001. Kruskal-Wallis test for (E), one-way analysis of variance (ANOVA) test for (F). (G) F-actin, Pard6, and ezrin localization in late eight-cell-stage embryos injected with double-strand RNA targeting GFP (dsGFP; control) or dsTT. (H) Quantification of ezrin apical enrichment. ****P < 0.0001, Student’s t test. (I) CRISPR-Cas9 strategy to deplete Tfap2c and Tead4. (J) Tfap2c and Tead4 protein levels in wild-type (Cas9 mRNA, control), Tfap2c-depleted (with Tfap2c sgRNAs), Tead4-depleted (with Tead4 sgRNAs), Tfap2c and Tead4–co-depleted (with sgRNAs targeting both Tfap2c and Tead4), and ezrin-RFP–expressing embryos. (K) Proportions of polarized cells in different genotypes presented in (J). The number of cells analyzed is shown within each bar. ****P < 0.0001, Fisher’s exact test. N = 2 experiments. Tfap2c and Tead4 co-depletion represses apical domain formation. (L) Quantifications of ezrin apical enrichment. Each dot represents a cell. **P = 0.0012, ***P = 0.0007, ****P < 0.0001, Kruskal-Wallis test. N = 4 experiments. Scale bars, 15 μm.
To confirm the requirement for Tfap2c and Tead4 in polarization, we deleted both genes by CRISPR-Cas9 mutagenesis. We designed three single-guide RNAs (sgRNAs) to target a single protein-coding exon of each gene (Fig. 2I) and injected them into the zygote together with Cas9 mRNA and ezrin-RFP mRNA as an apical marker. We categorized blastomeres on the basis of whether they had undetectable, moderate, or wild-type levels of Tfap2c or Tead4 proteins at the 8- to 16-cell stage (fig. S5, A and B) and confirmed by DNA sequencing that blastomeres with undetectable Tfap2c or Tead4 were homozygous mutants (Fig. 2J and fig. S5, C and D). Simultaneous deletion of Tfap2c and Tead4 completely abolished embryo polarization, in contrast to their individual deletions, which were less severe (Fig. 2, J to L, and fig. S5, E and F). Thus, zygotic expression of Tfap2c and Tead4 is required for embryo polarization at the eight-cell stage.
Tead4 had previously been shown to function only downstream of polarization, after nuclear relocalization of its transcriptional coactivator Yap, to induce TE transcription factor expression (7). To gain further insight into the earlier role of Tead4, we examined the localization of Yap. We found Yap localization in the nucleus before polarization at the eight-cell stage (20) (fig. S6, A to C) that was diminished by down-regulation (fig. S6, D and E) and enhanced by up-regulation of Tead4 (fig. S6, F and G). Thus, Tead4 affects the localization of Yap before polarization, indicating a previously undescribed, polarity-independent Tead4 function.
Advancing expression of Tfap2c, Tead4, and Rho GTPase advances polarization timing
Having found that Tfap2c and Tead4 are required for embryo polarization, we sought to determine whether advancing their expression would advance the timing of polarization. We therefore injected Tfap2c and Tead4 mRNAs into one blastomere at the late two-cell stage to elevate their expression at the four-cell stage (fig. S7, A to C). Advancing expression of Tead4 did not induce premature polarization (fig. S7, D, E, I, and J). Advancing expression of Tfap2c led to formation of cell protrusions enriched in apical polarity proteins, including Pard6 and ezrin at the four-cell stage (fig. S7, F to I). Advancing the expression of Tfap2c and Tead4 together also induced premature formation of protrusions (fig. S7, G to I; Fig. 3, B and C; and movies S3 and S4). In all cases, the induced protrusions were smaller than the natural apical domain at the eight-cell stage (Fig. 3B; movies S3 and S4; and fig. S7H). These results suggested that Tfap2c and Tead4 might be sufficient to trigger polarization of apical proteins but that other factors are required for proper apical domain formation.
Fig. 3. Premature expression of Tfap2c, Tead4, and activated RhoA is sufficient to advance polarization timing.
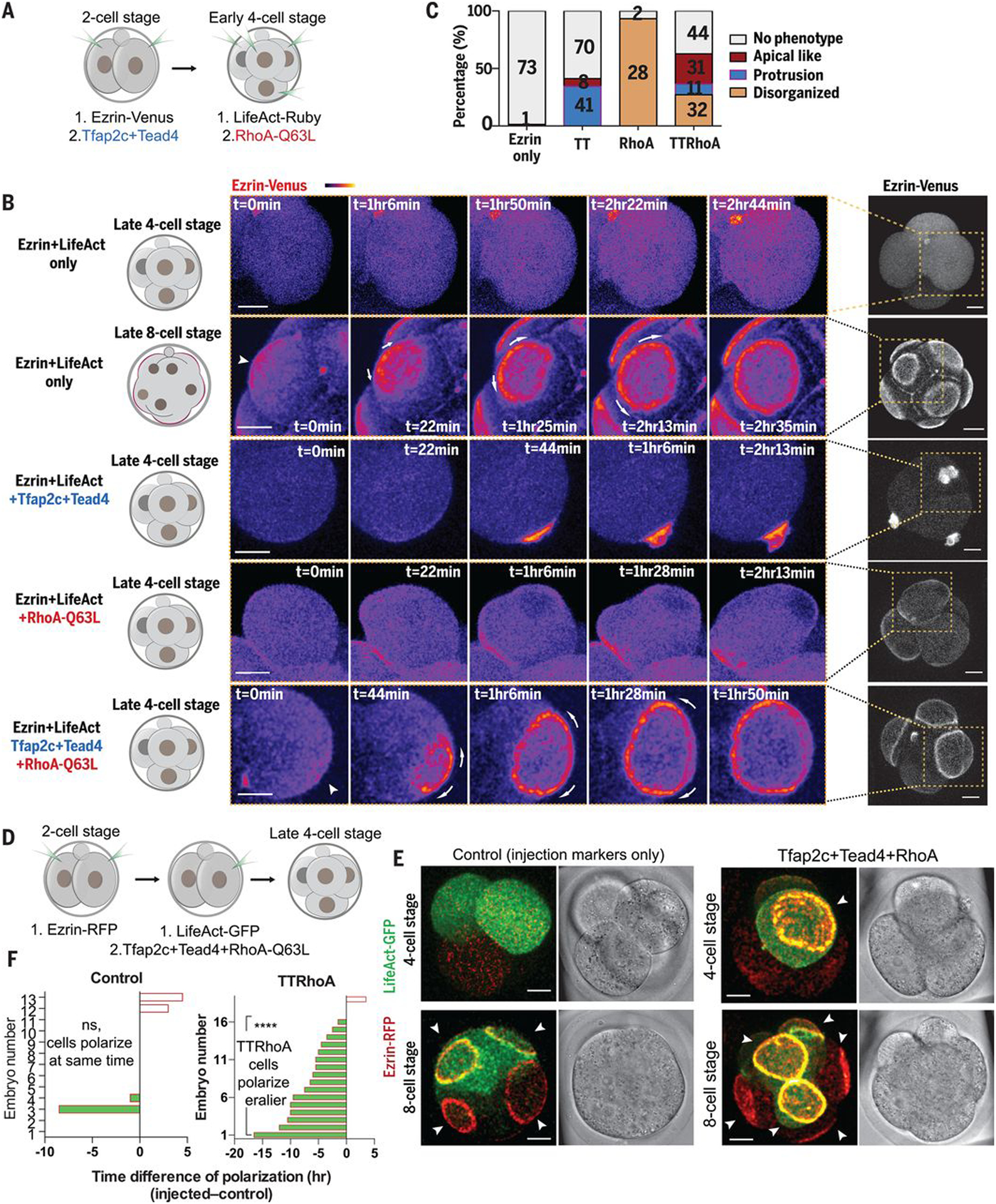
(A) Scheme of Tfap2c, Tead4, and RhoA-Q63L overexpression. (B) Ezrin-Venus dynamics in late four-cell or eight-cell stage control cells (ezrin-Venus only), or in late four-cell stage cells overexpressing (i) Tfap2c+Tead4 (TT), (ii) RhoA-Q63L (RhoA), or (iii) Tfap2c+Tead4+RhoA-Q63L (TTRhoA). TTRhoA overexpression induces a premature, full apical domain. In all conditions, cell divisions were not affected. Arrowheads indicate ezrin-Venus enrichment at the cell-contact free surface. Arrows indicate apical domain expansion. Dashed squares indicate the magnified regions. (C) Quantification of morphologies induced by the conditions in (B). The number of cells analyzed is shown within each bar. (D) Scheme of Tfap2c, Tead4, and RhoA-Q63L overexpression. (E) Representative images of embryos overexpressed with ezrin-RFP, LifeAct-GFP mRNA only (control), or with Tfap2c, Tead4, and RhoA-Q63L mRNA at the four- or eight-cell stage. The cells overexpressed with Tfap2c, Tead4, and RhoA-Q63L polarize significantly earlier than control cells in the same embryos, or cells in the control embryos. Arrowheads indicate the apical domain. (F) Polarization time difference between cells with or without LifeAct-GFP or TTRhoA overexpression in the same embryo, or in control embryos. ****P < 0.0001, one-sample t test, hypothetical mean = 0. Control group, N = 13 embryos ; TTRhoA group, N = 19 embryos. N = 2 experiments. Scale bars, 15 μm.
We previously found that actomyosin activation by protein kinase C–Rho guanosine triphosphatase (GTPase) signaling was necessary but not sufficient to trigger apical domain formation (Fig. 3, B and C, and movie S5) (21). We therefore hypothesized that Rho GTPase activation might be required in addition to Tfap2c and Tead4 to achieve complete polarization. To test this, we injected mRNAs for Tfap2c and Tead4 at the two-cell stage (with ezrin-RFP mRNA as apical marker) and mRNA for constitutively active Ras homolog family member A (RhoA)–Q63L (Gln63→Leu) at the four-cell stage [with LifeAct–green fluorescent protein (GFP) as injection marker] (Fig. 3A and fig. S7K). Expression of all three factors established complete apical domains at the four-cell stage (Fig. 3, B and C, and movie S6). These induced apical domains were enriched with ezrin and Pard6, strongly resembling apical domains that normally form only at the eight-cell stage (Fig. 3B and fig. S8, B and C).
To confirm these results, we overexpressed these factors in just half the embryo and found that the targeted blastomeres polarized earlier than controls in the other embryo half (Fig. 3, D to F, and movies S7 and S8). We observed no differences in division timing between blastomeres, suggesting that induced four-cell-stage polarization is not caused by a cytokinesis delay (fig. S8A). Our results indicate that a transcriptional program triggered by Tfap2c and Tead4 alongside activation of actomyosin downstream of Rho GTPase signaling triggers polarization at a specific stage of embryogenesis.
Advancing Tfap2c, Tead4, and Rho GTPase expression advances morphogenesis and differentiation
Embryo polarization at the eight-cell stage is followed by the zippering of adjacent apical domains, which expand and seal their boundaries at the late 16-cell stage to enable blastocyst formation (22). To determine whether premature polarization could also advance the zippering process, we induced expression of Tfap2c, Tead4, and RhoA-Q63L either in the whole embryo or in half of the embryo to trigger four-cell-stage polarization and followed subsequent development by time-lapse microscopy. The induced premature polarization resulted in zippered domains associated with the tight junction protein ZO-1, not at the 16-cell stage but at the eight-cell stage (fig. S8, B to F). Thus, embryo polarization is sufficient to advance the subsequent step of embryogenesis leading to blastocyst formation.
As polarization in the mouse embryo is followed by cell fate specification, we determined whether overexpressing Tfap2c, Tead4, and RhoA-Q63L to induce polarization at the four-cell stage would advance differentiation of cells inheriting an apical domain into TE. Premature polarization induced premature expression of the TE transcription factors Cdx2 and Gata3 in a cell-autonomous manner (fig. S8, G to K, and movies S9 and S10). Thus, the combined activities of Tfap2c, Tead4, and RhoA-Q63L are sufficient to advance the timing not only of polarization but also the differentiation program.
Tfap2c and Tead4 are required for apical protein centralization
To define the relative roles of RhoA, Tfap2c, and Tead4 in driving polarization, we visualized events leading to apical domain formation in living embryos expressing LifeAct-GFP and ezrin-RFP from the mid to late 8-cell stages. During apical protein polarization, ezrin-RFP first became concentrated at the center of the cell contact–free surface to form an apical patch concomitant with a local reduction of actin. We refer to this stage as centralization (Fig. 4, A and B, and movie S11). This apical patch of ezrin-GFP expanded, and actin became concentrated in a ring around it. We refer to this phase as expansion (Fig. 4, A and C, and movie S11). Down-regulation of Tfap2c and Tead4 diminished the initial centralization of ezrin-RFP (Figs. 2G and 4D), implying that centralization is required for apical domain formation.
Fig. 4. Tfap2c and Tead4 regulate apical domain centralization by regulating apical protein clustering.
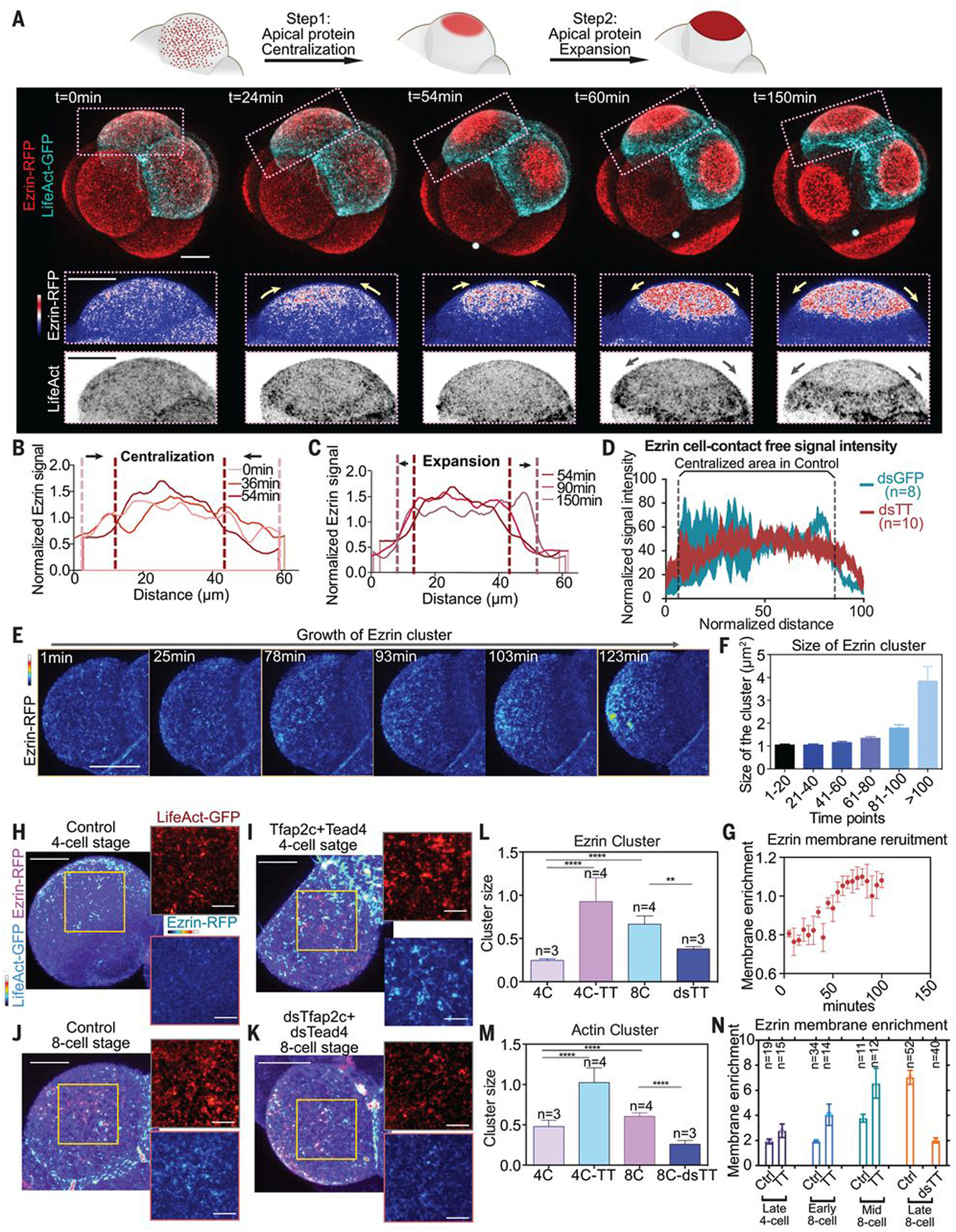
(A) LifeAct-GFP and ezrin-RFP dynamics during polarization. Squares denote magnified regions; yellow and gray arrows indicate apical protein or actin ring movements. The apical domain forms after centralization and expansion steps. N = 7 embryos, N = 4 experiments. (B and C) Ezrin-RFP signal at the cell-contact free surface during centralization or expansion steps in (A). Ezrin signal is normalized against average membrane signal intensity. (D) Ezrin-RFP distribution on cell-contact free surface at the late eight-cell stage in dsGFP (control) or dsTfap2c+dsTead4 injected cells. Numbers indicate examined cells. N = 2 experiments. (E) Ezrin-RFP distribution during apical centralization. (F) Ezrin cluster size during polarization. More than 1500 clusters were analyzed for each time point. Data shown as mean ± SD. N = 2 experiments. (G) Ezrin membrane enrichment during centralization. N = 4 cells from four embryos. Data shown as mean ± SEM. (H to K) Localization of LifeAct-GFP and ezrin-RFP in embryos injected with or without Tfap2c+Tead4 mRNA at late four-cell stage, or embryos injected with or without dsTfap2c+dsTead4 at eight-cell stage. Yellow squares indicate the magnified regions (right). (L and M) Size of actin (L) or ezrin (M) clusters in embryos shown in (H) to (K). Data shown as mean ± SEM. **P < 0.01, ****P < 0.0001, one-way ANOVA test. Numbers (n) indicate examined cells. More than 500 clusters were measured in each condition. (N) Ezrin membrane enrichment in different conditions and stages. TT, Tfap2c+Tead4 overexpression; dsTT, dsTfap2c+dsTead4 knockdown. Numbers indicate examined cells. Data presented as mean ± SEM. Scale bars for magnified images in (H) to (K), 5 μm. All other scale bars, 15 μm.
Tfap2c and Tead4 control polarized growth of apical protein clusters
Imaging of apical domain centralization in mid to late eight-cell-stage embryos with higher temporal-spatial resolution (movie S12) revealed that ezrin formed clusters that colocalized with actin clusters when the embryo had just compacted but was not yet polarized (Fig. 4, E and J). As polarization progressed, the ezrin clusters grew; the more-distant clusters grew faster than those near cell-cell contacts, resulting in ezrin enrichment toward the middle of the cell-contact free surface (Fig. 4, E and F). The amount of membrane-associated ezrin increased as the ezrin clusters grew (Fig. 4G), suggesting that cluster growth is driven by ezrin’s recruitment to the membrane.
Overexpression of Tfap2c and Tead4 led to an increase in membrane enrichment of ezrin and precocious growth of both ezrin and actin clusters at the late-four-cell stage (Fig. 4, H, I, and L to N). By contrast, Tfap2c and Tead4 depletion decreased ezrin’s membrane enrichment and prevented growth of ezrin and actin clusters at the mid-eight-cell stage (Fig. 4, J to N). Together, this suggests that Tfap2c and Tead4 are required for the growth of actin and apical protein clusters, recruitment of apical protein to the membrane, and centralization of apical protein.
Tfap2c and Tead4 regulate actin dynamics to promote apical protein cluster growth
As our results suggested that Tfap2c and Tead4 could regulate actin dynamics to direct the growth of apical protein clusters, we next examined the cortical movements generated by the contractile actomyosin network during apical domain formation (23) (movie S13). We first tested whether such cortical movements can drive asymmetric growth of apical protein clusters by tracking LifeAct and ezrin clusters using particle image velocimetry (PIV) (movie S14; and see methods section in the supplementary materials). In contrast to actin flows of postmitotic cells (22) (fig. S9C), PIV did not detect any obvious movement toward the center of the cell-contact free surface (fig. S9, A and B). Accordingly, we found that inhibiting actin flows with blebbistatin failed to prevent asymmetric ezrin cluster growth (fig. S9, D to G, and movie S15). Thus, asymmetric clustering of ezrin is not driven by the cortical flow mediated by actomyosin contractility.
Time-lapse observations revealed that ezrin cluster growth occurred during the merging and splitting of actin clusters and was unimpeded by blebbistatin (Fig. 5A), suggesting that cortical actin remodeling may allow ezrin’s recruitment to growing clusters. Consistently, perturbing actin depolymerization in eight-cell embryos with Jasplakinolide (JASP) prevented apical domain formation (fig. S9, H and K). Moreover, when we inhibited the Arp2/3 complex with CK666 to prevent actin nucleation, ezrin cluster growth (Fig. 5, B and C) and apical domain formation (fig. S9, I and K) were also blocked. By contrast, treatment of embryos with a formin inhibitor (SMiFH2) did not affect apical domain formation (fig. S9, J and L). These observations indicate that actin remodeling is required for ezrin clustering and apical domain formation. We also found that CK666 abolished apical protein polarization induced by Tfap2c and Tead4 overexpression in four-cell embryos (Fig. 5, D and E). Thus, Tfap2c- and Tead4-dependent regulation of actin dynamics is required for the growth of ezrin clusters and apical protein formation.
Fig. 5. Clustering of apical proteins is regulated by local actin dynamics.
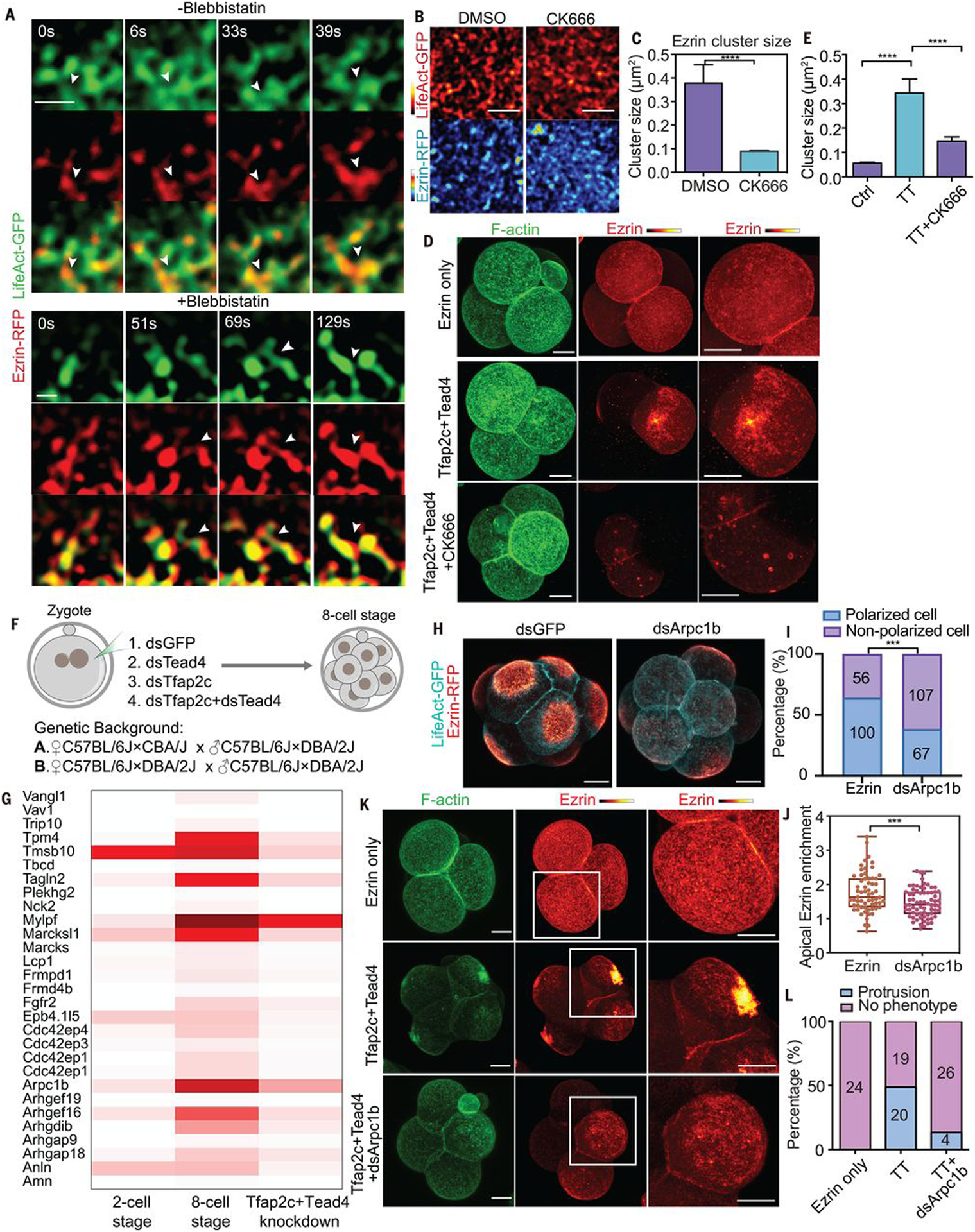
(A) LifeAct-GFP and ezrin-RFP dynamics with or without blebbistatin treatment during polarization. Arrowheads indicate the merging of ezrin clusters during actin polymerization. Scale bar, 1 μm. N = 5 cells for each condition. N = 3 experiments. Blebbistatin treatment did not prevent the clustering of actin or ezrin proteins. (B) LifeAct-GFP and ezrin-RFP localization in mid eight-cell stage embryos treated with DMSO (control) and CK666. Scale bars, 5 μm. (C) Ezrin cluster size in cells treated with DMSO or CK666 in (B). ****P < 0.0001, Mann-Whitney U test. More than 1500 clusters were measured in each condition, N = 2 experiments. (D) F-actin and ezrin-RFP localization in late four-cell-stage embryos expressing ezrin-RFP only, or with Tfap2c+Tead4 treated with or without CK666. (E) Ezrin cluster size in (D). ****P < 0.0001, Kruskal-Wallis test. More than 1100 clusters were calculated in each condition, N = 2 experiments. (F) Experimental strategy for RNA-seq. (G) Heatmap showing the expression of selective cytoskeleton regulators downstream of Tfap2c and Tead4. (H) LifeAct-GFP and ezrin-RFP localization in dsGFP or dsArpc1b injected embryos. (I) Polarized cell numbers in dsGFP and dsArpc1b groups. Numbers within bars represent cell number. ***P < 0.001, Fisher’s exact test. (J) Ezrin-RFP apical enrichment in dsGFP or dsArpc1b cells. Each dot represents a cell. ***P < 0.001, Student’s t test. (K) LifeAct-GFP and ezrin-RFP localization in different conditions. Squares indicate magnified regions (right). (L) Number of cells showing apical protrusions in (K). Scale bars, 15 μm.
Tfap2c and Tead4 control expression of actin regulators
To view events downstream of Tfap2c and Tead4, we carried out RNA-seq of eight-cell-stage embryos depleted of Tfap2c and/or Tead4. For each group of embryos (control GFP RNAi, Tfap2c RNAi, Tead4 RNAi, and Tfap2c/Tead4 co-RNAi), two biological replicates were collected with 10 embryos per sample, and experiments were performed on two strains to eliminate genetic background effects (Fig. 5F and methods). The effect of Tfap2c and Tead4 depletion was highly reproducible between biological replicates and between genetic backgrounds (fig. S10A). Depletion of Tfap2c led to the down-regulation of 749 or 929 genes (with a twofold differential cutoff), depending on the strain, whereas Tead4 depletion led to down-regulation of 242 or 314 genes (fig. S10, B and C). Their co-depletion led to an additional 135 or 95 genes being down-regulated compared with single knockdown embryos, depending on the strain (fig. S10, B and C).
A significant proportion of down-regulated genes in double-knockdown embryos had actin polymerization functions (Fig. 5G and table S3). These included known actin regulators for apical domain formation, such as Cdc42 effector protein family members (Borg) (24), and other actin regulators, including the Arp2/3 complex component Arpc1b, the tropomyosin protein Tpm4, Marcks and Marcksl1 proteins, and the FREM family member Ebp4.1l5, whose functions have not been explored in the mouse embryo. Expression of these actin regulators becomes up-regulated between the two- and eight-cell stages and correlates with the size increase of actin clusters during polarization (Fig. 5G). Depletion of Tfap2c and Tead4 eliminated expression of actin regulators and accordingly led to a decreased actin cluster size (Figs. 4, H and J to M, and 5G).
To test whether these actin regulators participate in apical domain formation, we depleted Arpc1b, Tpm4, Marcksl1, or Ebp4.1l5 individually from two-cell embryos and determined the apical domain formation efficiency at the late eight-cell stage (Fig. 5, H to J, and fig. S11, A to D). Consistent with the effects of CK666, depletion of Arpc1b led to defective apical domain formation (Fig. 5, H to J) in natural eight-cell embryos and prevented Tfap2c- and Tead4-induced apical protein polarization at the four-cell stage (Fig. 5, K and L). Together, these results suggest that Tfap2c and Tead4 control embryo polarization by activating expression of actin regulatory proteins.
RhoA signaling reorganizes the actin network during polarization
Knowing that not only Tfap2c and Tead4 but also RhoA-Q63L were required for apical protein clustering and apical domain formation in four-cell embryos, we aimed to determine how excess RhoA activity (by overexpressing RhoA-Q63L) or reduced RhoA activity (by treatment with RhoA inhibitor C3-transferase) at the mid eight-cell stage would affect ezrin’s membrane distribution (fig. S12A). Overexpression of RhoA-Q63L eliminated actin and ezrin cluster formation, resulting in the homogeneous distribution of actin and ezrin on the membrane (fig. S12, B and C). By contrast, C3-transferase treatment resulted in the ectopic clustering of actin and ezrin on the cell membrane (fig. S12, D and E), reminiscent of four-cell embryos overexpressing Tfap2c and Tead4 but lacking RhoA activity (Fig. 3B and fig. S7, G and H). Thus, RhoA signaling is required to reorganize the actin network in the embryo and thereby the clustering of apical proteins induced by Tfap2c and Tead4.
Positive feedback and lateral mobility govern apical domain formation
To gain biophysical understanding of how Tfap2c, Tead4, and RhoA regulate the timing and pattern of apical domain formation, we measured the growth of ezrin cluster size during apical protein centralization and found that it increases exponentially (Fig. 4F), suggesting involvement of a positive feedback mechanism. To gain understanding of this mechanism, we tagged ezrin with green to red photoactivatable Dendra2 fluorescent protein to track Dendra2-ezrin movement after blue light conversion. RFP signal dynamically dissipated within seconds when Dendra2 was photoconverted at the mid eight-cell stage either within or outside the nascent apical domain, suggesting rapid ezrin membrane turnover (Fig. 6, A to D). Irrespective of the site of photoconversion, ezrin-Dendra2 relocated to the nascent domain in proportion to the concentration of ezrin in this area, indicating positive feedback of ezrin on its own accumulation. This correlates with previous measurements of cooperative recruitment of ezrin to phosphatidylinositol 4,5-bisphosphate (PIP2) membranes (25, 26).
Fig. 6. Tfap2c, Tead4, and RhoA regulate apical domain formation through a positive feedback and mobility system.
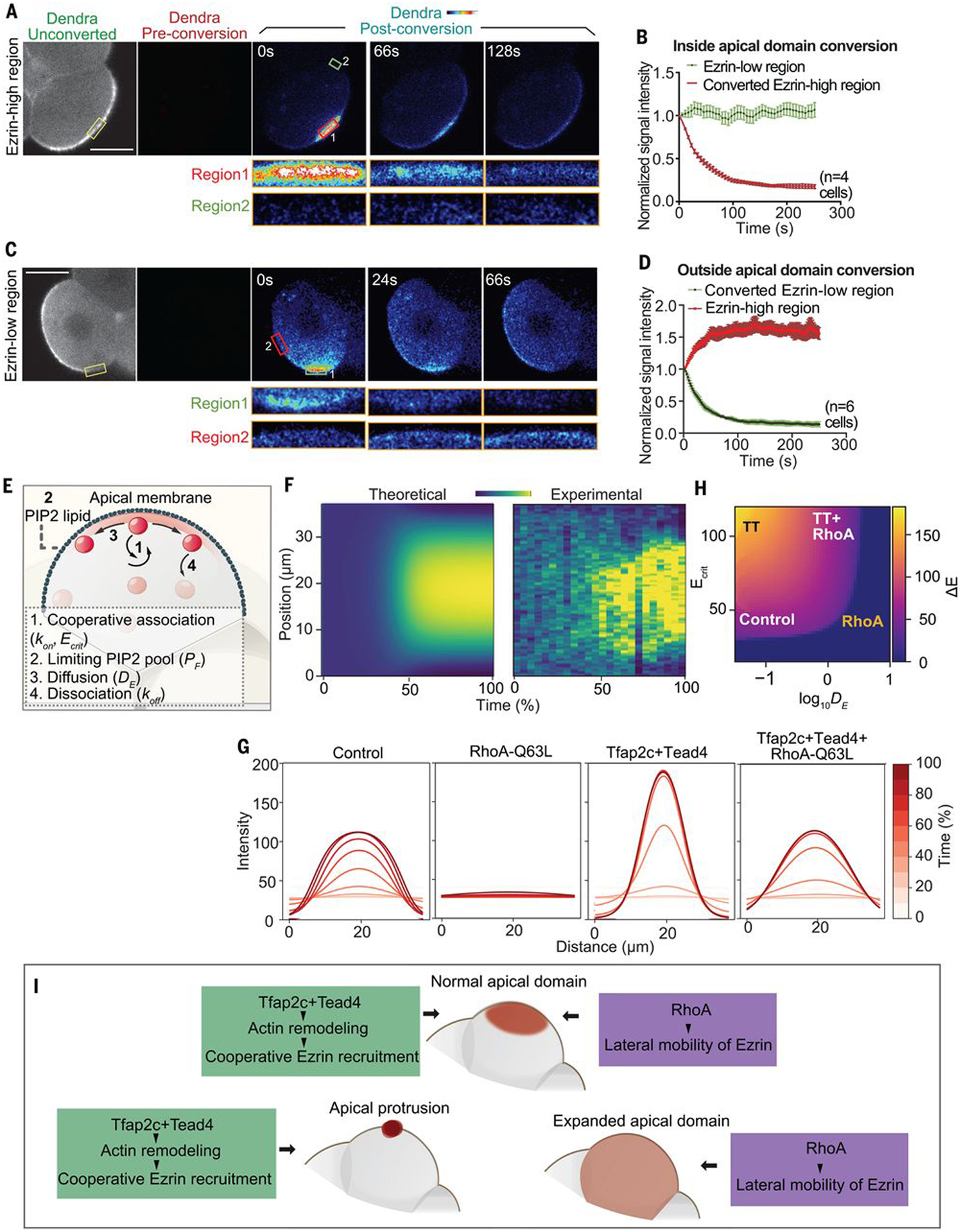
(A and C) Ezrin-Dendra2 localization during and after photoconversion. Yellow squares indicate converted region, red and green squares indicate ezrin-high or ezrin-low regions, respectively, measured in (B) and (D). (B and D) Signal intensity of converted ezrin-Dendra2 [experimental setting shown in (A) and (C)] in ezrin-low and ezrin-high regions within 6 min after conversion. Scale bars, 15 μm. (E) Structure of the biophysical model. (F) Kymographs comparing in silico and in vivo polarization dynamics. Same color corresponds to the same ezrin intensity. (G) Time courses of simulated ezrin apical distribution at different regions of parameter space. Colors corresponding to the elapsed simulation time. (H) Phase space of polarization shape within the monopolar regime. (I) Summary of the results.
To determine whether ezrin dynamics can account for apical protein centralization, we constructed a model (see methods) based on four empirically grounded assumptions (Fig. 6E): (i) a cooperative increase in ezrin binding rate (kon) with increasing ezrin concentration saturating above a critical concentration (Ecrit) suggested by the positive feedback above (Fig. 6, B and D) and known cooperative binding of ezrin to membranes (25, 26); (ii) a limit to ezrin membrane loading by the finite pool of PIP2 (Ptot) based on colocalization of ezrin with PIP2 in the apical domain (fig. S12F) and prevention of apical domain formation by reduced PIP2 (21); (iii) lateral motility of ezrin along the membrane, as observed (Fig. 6, A to D, and fig. S12G), which can be modeled as effective diffusion with diffusivity (DE); and (iv) dissociation of ezrin at a uniform rate from the membrane (koff) (Fig. 6E).
We simulated ezrin dynamics in one dimension and estimated parameter values for simulations best fitting our experimental measurements (see methods and “Supplementary Modeling” section in the supplementary materials). Our simulations reproduced the dynamic changes of ezrin distribution during centralization in vivo (Fig. 6F). The model also recapitulated the ability of single cells to form a centralized ezrin domain in the absence of cell-cell contacts (fig. S12H and methods) and the ability of cell-cell contacts to constrain apical ezrin to the center of cell-contact free surface (fig. S12I). Thus, experimental observations and computational simulation together reveal that positive feedback, lateral mobility, and competition for limiting PIP2 are sufficient to explain the apical protein centralization step.
Tfap2c, Tead4, and RhoA control cooperative recruitment and lateral mobility of apical proteins
Our model predicts that elevating the saturation threshold (Ecrit) for cooperative recruitment of ezrin would change the steady-state distribution of membrane-bound ezrin, resulting in a narrowed peak resembling the Tfap2c and Tead4 overexpression phenotype (Fig. 6G and “Supplementary Modeling” section of the supplementary materials). This suggests that Tfap2c and Tead4 regulate the kinetics of the cooperative ezrin recruitment. To test this, we computed the rate of change in membrane-bound ezrin concentration (ΔE/ΔT) as a function of the ezrin concentration during apical centralization, for both control and Tfpa2c and Tead4 overexpressing embryos (fig. S12J). This rate of change revealed that when the ezrin concentration falls below a threshold value, local ezrin subsequently decreases, but when the local concentration exceeds this value, more ezrin gets recruited to the membrane (fig. S12J).
Myosin motor activity within the actin cortex can affect the lateral mobility of membrane proteins that can negatively regulate the formation of membrane clusters (27), suggesting that RhoA might regulate the lateral mobility of ezrin. To test this, we quantified the “spread” of photoconverted signal (see methods) and compared the difference between control and RhoA-Q63L–overexpressing cells (fig. S12K). Elevating RhoA activity increased the spreading effect of photoconverted ezrin on the membrane, suggesting that RhoA positively regulates the lateral mobility of ezrin (fig. S12K). In line with this, our simulations predict that increasing lateral mobility leads to homogeneously distributed ezrin (Fig. 6G) and that insufficient lateral mobility results in multiple peaks of apical protein (fig. S12L). These predictions are concordant with the phenotypes resulting from overexpression of RhoA-Q63L and depletion of RhoA in the eight-cell embryo (fig. S12, B to E).
Our model suggests that lateral mobility and the cooperative recruitment threshold have opposing effects on the shape of the apical patch (Fig. 6H and fig. S12M). Specifically, a normal apical domain will form only when both processes are activated at an appropriate level. This prediction provides a qualitative explanation for the concurrent requirements for Tfap2c, Tead4, and RhoA signaling in regulating apical domain formation (Figs. 3B and 6I).
Discussion
The importance of the first appearance of apical-basal cell polarity in mammalian development is evident from its requirement for triggering the first cell fate diversification event. Here, we show that zygotic expression of Tfap2c and Tead4 is a prerequisite for such polarization. We have been able to induce precocious embryo polarization and thereby advance subsequent embryogenesis by driving the ectopic expression of Tfap2c, Tead4, and activated Rho GTPase. Our findings help account for the temporal relationship between zygotic genome activation and the establishment of embryo polarization across multiple mammalian species (11, 12).
Embryo polarization at the eight-cell stage has been viewed as a model for epithelial polarization. However, formation of the apical domain is distinct from that of many other cell types, as it can occur in the absence of external cues, such as the extracellular matrix or cell adhesion. The mechanisms behind such distinctive spontaneous symmetry-breaking properties have remained elusive. Here, we show that the initial step for symmetry breaking is the centralization of apical proteins through their two types of behavior on the membrane: actin-mediated cooperative recruitment and lateral mobility. These two processes act as opposing forces to regulate the shape of the apical domain; a cooperative recruitment mechanism enables symmetry breaking and concentration of the apical proteins, whereas lateral mobility allows apical proteins to diffuse, thereby establishing a crescent-shaped patch (Fig. 6, G and H, and fig. S12M). The balanced activity between the two processes ensures the proper shape of the apical domain because an excessive cooperative recruitment force would lead to small and often multiple domains, whereas excessive lateral mobility would lead to the uniform distribution of apical proteins and thereby inhibit symmetry breaking (Fig. 6G and fig. S12L).
Our results suggest that cooperative recruitment is regulated by actin remodeling controlled by Tfap2c and Tead4. Although the detailed mechanism is beyond the scope of this work, it is possible that ezrin is preferentially recruited to the actin structure promoted by the Arp2/3 complex in a process similar to protein condensation (28). In such a case, the density of the branched actin network would positively affect the saturation level of ezrin in the cooperative recruitment process. It has been observed in vitro that the actin clusters formed by Arp2/3 activity are degraded by high levels of cortical myosin (29), which could explain the opposing effects between the two transcription factors and RhoA in regulating the apical protein clustering and accordingly the apical domain shape.
The regulatory regime we describe is based on the behavior of ezrin, and it is likely to apply to other apical proteins, such as the Par complex, whose polarization dynamics are highly similar and also require the actin network and membrane binding, the key conditions of the process we describe (21). Our work illustrates how the embryo establishes cell polarization at a specific developmental stage under the regulation of stage-dependent pathways. Our results also provide a biophysical explanation for how polarization is established, indicating that positive feedback combined with lateral mobility are sufficient to drive this self-organization process. These results therefore provide insight into both the timing and mechanism of the establishment of de novo polarization in the mouse embryo, the critical event for the first cell fate specification.
Methods summary
This work followed regulations of the Animals (Scientific Procedures) Act 1986 Amendment Regulations 2012 reviewed by the University of Cambridge Animal Welfare and Ethical Review. Embryos were collected from superovulated F1 females (C57BI6xCBA) crossed with F1 males. For embryo culture and inhibitor treatment, embryos were recovered at the zygote or two-cell stage in M2 medium and transferred to KSOM medium for long-term culture. The inhibitors—or the same amount of vehicles (for control conditions)—were applied to the culture. The microinjection procedure, immunostaining, static imaging and image processing, and real-time quantitative polymerase chain reaction were carried out as previously described (21). For the photoconversion experiment, the region of interest (ROI) covered a rectangular area of ~5 μm length and ~2 μm width on the membrane of cells expressing ezrin-Dendra2, using the midplane of the blastomeres as a reference. The ROI was illuminated at 405 nm for 5 s, after which converted proteins were imaged with a 568-nm laser at an emission wavelength between 580 and 620 nm every 2 s per frame for 5 min. The converted scanning speed is 200 Hz, and the normal scanning speed is 700 Hz. For all imaging settings, the images have been recorded using the 1024 pixel by 1024 pixel format. PIV analysis was performed using the PIVlab MATLAB algorithm (https://pivlab.blogspot.com/). For statistics, the sample distribution as well as statistical tests were performed using Prism software (www.graphpad.com/). Details of the materials and methods, as well as details for RNA-seq and modeling methods, can be found in the supplementary materials.
Supplementary Material
ACKNOWLEDGMENTS
We thank E. Munro, D. Glover, A. Andersen, and M. Shahbazi for helpful discussion; S. Shadkhoo for comments on the model; and S. Malas for the Gata3-GFP transgenic line. Some of the computations were conducted on the Caltech High Performance Cluster, supported by a Gordon and Betty Moore Foundation grant.
Funding:
This work was supported by grants from the Wellcome Trust (098287/Z/12/Z), ERC (669198), Leverhulme Trust (RPG-2018-085), Open Philanthropy/Silicon Valley, Weston Havens Foundations and NIH R01 HD100456-01A1 to M.Z.-G; Packard Foundation, Heritage Medical Research Institute, NIH U01CA244109 to M.T.; and the National Key R&D Program of China grants 2017YFA0102802 and 2019YFA0110001 to J.N.
Footnotes
Competing interests: The authors declare no competing interests.
Data and materials availability: All raw data are available upon request from the corresponding author. The RNA-seq data have been deposited in the Gene Expression Omnibus database (accession number GSE124755). The code for computation simulation has been deposited at https://jakesorel.github.io/Apical_Domain_2020/.
SUPPLEMENTARY MATERIALS
science.sciencemag.org/content/370/6522/eabd2703/suppl/DC1
Materials and Methods
Supplementary Text
Figs. S1 to S12
Tables S1 to S4
References (30–69)
MDAR Reproducibility Checklist
Movies S1 to S15
REFERENCES AND NOTES
- 1.Korotkevich E et al. , The apical domain is required and sufficient for the first lineage segregation in the mouse embryo. Dev. Cell 40, 235–247.e7 (2017). doi: 10.1016/j.devcel.2017.01.006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson MH, Ziomek CA, The foundation of two distinct cell lineages within the mouse morula. Cell 24, 71–80 (1981). doi: 10.1016/0092-8674(81)90502-X; [DOI] [PubMed] [Google Scholar]
- 3.Johnson MH, Ziomek CA, Induction of polarity in mouse 8-cell blastomeres: Specificity, geometry, and stability. J. Cell Biol 91, 303–308 (1981). doi: 10.1083/jcb.91.1.303; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleming TP, Cannon PM, Pickering SJ, The cytoskeleton, endocytosis and cell polarity in the mouse preimplantation embryo. Dev. Biol 113, 406–419 (1986). doi: 10.1016/0012-1606(86)90175-2; [DOI] [PubMed] [Google Scholar]
- 5.Louvet S, Aghion J, Santa-Maria A, Mangeat P, Maro B, Ezrin becomes restricted to outer cells following asymmetrical division in the preimplantation mouse embryo. Dev. Biol 177, 568–579 (1996). doi: 10.1006/dbio.1996.0186; [DOI] [PubMed] [Google Scholar]
- 6.Plusa B et al. , Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J. Cell Sci 118, 505–515 (2005). doi: 10.1242/jcs.01666; [DOI] [PubMed] [Google Scholar]
- 7.Nishioka N et al. , The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 (2009). doi: 10.1016/j.devcel.2009.02.003; [DOI] [PubMed] [Google Scholar]
- 8.Ralston A et al. , Gata3 regulates trophoblast development downstream of Tead4 and in parallel to Cdx2. Development 137, 395–403 (2010). doi: 10.1242/dev.038828; [DOI] [PubMed] [Google Scholar]
- 9.Johnson MH, McConnell J, Van Blerkom J, Programmed development in the mouse embryo. J. Embryol. Exp. Morphol 83 (suppl.), 197–231 (1984). [PubMed] [Google Scholar]
- 10.Morris SA, Guo Y, Zernicka-Goetz M, Developmental plasticity is bound by pluripotency and the Fgf and Wnt signaling pathways. Cell Rep. 2, 756–765 (2012). doi: 10.1016/j.celrep.2012.08.029; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koyama H, Suzuki H, Yang X, Jiang S, Foote RH, Analysis of polarity of bovine and rabbit embryos by scanning electron microscopy. Biol. Reprod 50, 163–170 (1994). doi: 10.1095/biolreprod50.1.163; [DOI] [PubMed] [Google Scholar]
- 12.Nikas G, Ao A, Winston RM, Handyside AH, Compaction and surface polarity in the human embryo in vitro. Biol. Reprod 55, 32–37 (1996). doi: 10.1095/biolreprod55.1.32; [DOI] [PubMed] [Google Scholar]
- 13.Hamatani T, Carter MG, Sharov AA, Ko MS, Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell 6, 117–131 (2004). doi: 10.1016/S1534-5807(03)00373-3; [DOI] [PubMed] [Google Scholar]
- 14.Padovan-Merhar O et al. , Single mammalian cells compensate for differences in cellular volume and DNA copy number through independent global transcriptional mechanisms. Mol. Cell 58, 339–352 (2015). doi: 10.1016/j.molcel.2015.03.005; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao M, Xie J, Piruska A, Huck WTS, 3D microniches reveal the importance of cell size and shape. Nat. Commun 8, 1962 (2017). doi: 10.1038/s41467-017-02163-2; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zernicka-Goetz M, Fertile offspring derived from mammalian eggs lacking either animal or vegetal poles. Development 125, 4803–4808 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Wang F et al. , RNAscope: A novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn 14, 22–29 (2012). doi: 10.1016/j.jmoldx.2011.08.002; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng Q, Ramsköld D, Reinius B, Sandberg R, Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193–196 (2014). doi: 10.1126/science.1245316; [DOI] [PubMed] [Google Scholar]
- 19.Wu J et al. , The landscape of accessible chromatin in mammalian preimplantation embryos. Nature 534, 652–657 (2016). doi: 10.1038/nature18606; [DOI] [PubMed] [Google Scholar]
- 20.Hirate Y et al. , Par-aPKC-dependent and -independent mechanisms cooperatively control cell polarity, Hippo signaling, and cell positioning in 16-cell stage mouse embryos. Dev. Growth Differ 57, 544–556 (2015). doi: 10.1111/dgd.12235; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M, Leung CY, Shahbazi MN, Zernicka-Goetz M, Actomyosin polarisation through PLC-PKC triggers symmetry breaking of the mouse embryo. Nat. Commun 8, 921 (2017). doi: 10.1038/s41467-017-00977-8; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zenker J et al. , Expanding actin rings zipper the mouse embryo for blastocyst formation. Cell 173, 776–791.e17 (2018). doi: 10.1016/j.cell.2018.02.035; [DOI] [PubMed] [Google Scholar]
- 23.Maître JL, Niwayama R, Turlier H, Nédélec F, Hiiragi T, Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nat. Cell Biol 17, 849–855 (2015). doi: 10.1038/ncb3185; [DOI] [PubMed] [Google Scholar]
- 24.Vong QP et al. , A role for Borg5 during trophectoderm differentiation. Stem Cells 28, 1030–1038 (2010). doi: 10.1002/stem.428; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herrig A et al. , Cooperative adsorption of ezrin on PIP2-containing membranes. Biochemistry 45, 13025–13034 (2006). doi: 10.1021/bi061064a; [DOI] [PubMed] [Google Scholar]
- 26.Jayasundar JJ et al. , Open conformation of ezrin bound to phosphatidylinositol 4,5-bisphosphate and to F-actin revealed by neutron scattering. J. Biol. Chem 287, 37119–37133 (2012). doi: 10.1074/jbc.M112.380972; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slaughter BD et al. , Non-uniform membrane diffusion enables steady-state cell polarization via vesicular trafficking. Nat. Commun 4, 1380 (2013). doi: 10.1038/ncomms2370; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banani SF, Lee HO, Hyman AA, Rosen MK, Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol 18, 285–298 (2017). doi: 10.1038/nrm.2017.7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganzinger KA, Vogel SK, Mücksch J, Blumhardt P, Schwille P, Myosin-II activity generates a dynamic steady state with continuous actin turnover in a minimal actin cortex. J. Cell Sci 132, jcs219899 (2019). doi: 10.1242/jcs.219899 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


