PURPOSE
Glofitamab is a T-cell–engaging bispecific antibody possessing a novel 2:1 structure with bivalency for CD20 on B cells and monovalency for CD3 on T cells. This phase I study evaluated glofitamab in relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL). Data for single-agent glofitamab, with obinutuzumab pretreatment (Gpt) to reduce toxicity, are presented.
METHODS
Seven days before the first dose of glofitamab (0.005-30 mg), all patients received 1,000 mg Gpt. Dose-escalation steps were determined using a Bayesian continuous reassessment method with overdose control. Primary end points were safety, pharmacokinetics, and the maximum tolerated dose of glofitamab.
RESULTS
Following initial single-patient cohorts, 171 patients were treated within conventional multipatient cohorts and received at least one dose of glofitamab. This trial included heavily pretreated patients with R/R B-NHL; most were refractory to prior therapy (155; 90.6%) and had received a median of three prior therapies. One hundred and twenty-seven patients (74.3%) had diffuse large B-cell lymphoma, transformed follicular lymphoma, or other aggressive histology, and the remainder had indolent lymphoma subtypes. Five (2.9%) patients withdrew from treatment because of adverse events. Cytokine release syndrome occurred in 86 of 171 (50.3%) patients (grade 3 or 4: 3.5%); two (1.2%) patients experienced grade 3, transient immune effector cell–associated neurotoxicity syndrome-like symptoms. The overall response rate was 53.8% (complete response [CR], 36.8%) among all doses and 65.7% (CR, 57.1%) in those dosed at the recommended phase II dose. Of 63 patients with CR, 53 (84.1%) have ongoing CR with a maximum of 27.4 months observation.
CONCLUSION
In patients with predominantly refractory, aggressive B-NHL, glofitamab showed favorable activity with frequent and durable CRs and a predictable and manageable safety profile.
INTRODUCTION
Many patients with B-cell non-Hodgkin lymphoma (B-NHL) achieve complete response (CR) following first-line treatment with rituximab and chemotherapy. However, approximately 40% of patients with diffuse large B-cell lymphoma (DLBCL) will be refractory or relapse1,2; their prognosis is dismal. Although autologous stem-cell transplantation (ASCT) can cure a proportion of patients with relapsed or refractory (R/R) DLBCL, many patients cannot undergo this procedure because of toxicity or inadequate response to second-line chemotherapy.3 Before the development of chimeric antigen receptor T-cell (CAR-T) therapy, among patients with no response to chemo-immunotherapy or who relapse less than 1 year after ASCT, only 7% achieve CR following subsequent treatment.4 In refractory follicular lymphoma (FL), cure is rare, CR rates are low, and progression-free survival (PFS) is short.5
CONTEXT
Key Objective
The prognosis for patients with multiple relapsed or refractory B-cell lymphoma is poor, and there remains an unmet need for novel agents. This study evaluated safety, optimal dosing, and preliminary efficacy of glofitamab, a novel and uniquely designed CD20-targeted T-cell–engaging bispecific antibody.
Knowledge Generated
Cytokine release syndrome was the most common adverse event of interest. This was clinically predictable and manageable, and was rarely severe below the maximum tolerated dose. Complete and durable responses, which in the majority of cases are ongoing, were seen in a population with predominantly aggressive histology and across histological subtypes.
Relevance
Safety and preliminary efficacy data of glofitamab compare favorably with established third-line treatments. This treatment appears to be promising for aggressive and indolent B-cell lymphoma and is well-suited for planned evaluation in later-phase studies, both as a single agent and in combination.
CAR-T therapies are a significant advance,6-8 but require careful patient selection and extensive healthcare coordination,9,10 are limited by manufacturing timelines, and are complicated by serious adverse events (SAEs),7,11 mainly grade ≥ 3 cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS).12 Alternative approaches offering off-the-shelf availability, high response rates, durable remissions, and an improved tolerability are required.
Glofitamab (RO7082859) is a novel T-cell–engaging, bispecific, full-length antibody that has a longer half-life compared with non–Fc-bearing bispecific T-cell engagers.13 The 2:1 configuration enables bivalent binding to CD20 on B cells and monovalent binding to CD3 on T cells.14 Its CD3-binding region is fused to one of the CD20-binding regions in a head-to-tail manner via a flexible linker for improved target-effector cell binding.14 This endows glofitamab with superior in vitro potency versus other CD20-CD3 bispecific antibodies with a 1:1 configuration and leads to profound antitumor efficacy in preclinical DLBCL models.14 CD20 bivalency preserves this potency in the presence of competing anti-CD20 antibodies, providing the opportunity for pre- or co-treatment with these agents.14
Study NP30179 (ClinicalTrials.gov identifier: NCT03075696) is a first-in-human, phase I study, investigating the clinical activity of single-agent glofitamab after single-dose Gazyva (obinutuzumab; Genentech/Roche) pretreatment (Gpt) and glofitamab with ongoing, co-administered obinutuzumab. Here, we present data for glofitamab monotherapy with single-dose Gpt.
METHODS
Patients
Patients of age ≥ 18 years with histologically confirmed B-NHL expected to express CD20; who had ≥ 1 prior lymphoma treatment, with no available life-extending treatment options; and who had ≥ 1 measurable target lesion > 1.5 cm were included. Key exclusion criteria were a history of CNS lymphoma or other CNS pathology, anticancer therapy within 4 weeks or five half-lives of the drug or ASCT within 100 days before Gpt, or prior allogeneic stem-cell transplantation. Full eligibility criteria are available in the Data Supplement (online only).
Study Design
NP30179 is a phase I, multicenter, open-label, dose-escalation, and dose-expansion study comprising three parts. Herein, we describe part 1 (single-patient dose escalation) and part 2 (multiple-patient dose escalation; Data Supplement); part 3 (dose expansion) is ongoing.
Seven days before the first dose of glofitamab, all patients received 1,000 mg Gpt, to deplete peripheral and tissue-based B cells and mitigate serious CRS.14 Obinutuzumab was chosen as pretreatment because of its deeper clearance of peripheral and tissue-based B cells compared with rituximab.16 Glofitamab was given as an initial 4-hour intravenous (IV) infusion, reduced to 2 hours once a prior infusion had occurred without complications. Glofitamab was given in 14- or 21-day cycles. Details of premedication, infusion time, and scheduling are provided in the supplementary material. Dose escalation was guided by a Bayesian-modified continuous reassessment method with overdose control based on emerging toxicity data.15
The primary study end points were safety or tolerability, pharmacokinetics (PK), maximum tolerated dose, and dose-limiting toxicities. Secondary end points included CR and overall response rates (ORR) by Lugano classification,17 duration of response (DOR), duration of CR (DOCR), PFS, pharmacodynamic biomarkers, and incidence of antidrug antibodies.
Disease was documented by fluorodeoxyglucose positron emission tomography and computed tomography. Tumor evaluations were conducted at baseline, after two and five cycles, end of treatment, and every 6 months until disease progression. Adverse events (AEs) were evaluated according to National Cancer Institute–Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.18 Investigators graded CRS by consensus criteria of Lee et al19 and managed according to protocol guidance. On-site availability of tocilizumab was a requirement. As consensus criteria for ICANS were not available at the time of study initiation, these are described based on CTCAE terms of delirium, dysphasia, tremor, lethargy, difficulty in concentrating, agitation, confusional state, aphasia, depressed level of consciousness, encephalopathy, seizures, or cerebral edema.
All enrolled patients provided written informed consent. This study was approved by each center's ethics committee or institutional review board and conducted in conformance with the Declaration of Helsinki, International Conference on Harmonisation Guidelines for Good Clinical Practice, and appropriate laws and regulations.
Statistical Analysis
The planned sample size of 160 patients was based on dose-escalation stopping criteria and approximated using computational simulation across different scenarios. In multiple patient cohorts, a minimum of three patients were required for dose escalation; however, the size of individual cohorts was designed to be flexible and increased with dose to further establish the efficacy and safety of glofitamab at clinically effective doses and to determine the maximum tolerated dose for the first administration.
Analyses included all patients who received Gpt and were conducted by dose group and pooled for selected analysis. Patients who did not complete any response assessments were considered as nonresponders and censored at day 1 for time-to-event end points; if disease progression or death was reported, then patients were considered an event at this time. Exact 95% CI are provided for response rates. DOR (time from first response to disease progression or death), DOCR (time from first CR to disease progression or death), and PFS (time from Gpt to disease progression or death) were analyzed by Kaplan-Meier estimation; patients without disease progression or death were censored at the last disease assessment. Time to CR was analyzed using cumulative incidence, with disease progression or death considered competing risks. Preplanned subgroup analyses included prior therapy, time since last therapy, refractory status, tumor burden, and International Prognostic Index.2 Refractory was defined as no response to or relapse within 6 months of prior therapy.
Data were analyzed using SAS version 9.4. The clinical cutoff date was August 3, 2020.
RESULTS
Patients
Three patients were enrolled into single-patient (part 1) cohorts and dosed at 0.005 mg, 0.015 mg, and 0.045 mg; no responses were observed, and all withdrew due to progressive disease.
In part 2, the first dose was 0.015 mg and 171 patients were enrolled (Data Supplement). The median (range) duration of follow-up was 13.5 (0-30.3) months. Significant clinical activity was observed at doses from 0.6 mg; subsequent cohorts were expanded to provide additional clinical data. At a cycle (C) 1 day (D) 1 dose of 25 mg, CRS was reported in all patients (one grade 3 and one grade 4), and this was considered to exceed the maximum tolerated day-one dose. Based on safety data and PK or pharmacodynamic modeling, two step-up dosing (SUD) cohorts were subsequently tested with dosing of 2.5 mg (C1D1), 10 mg (C1D8), and 16 mg or 30 mg (C2D1), with the latter being selected as the recommended phase II dose (RP2D).21 Details can be found in Tables 1, 2 and 3.
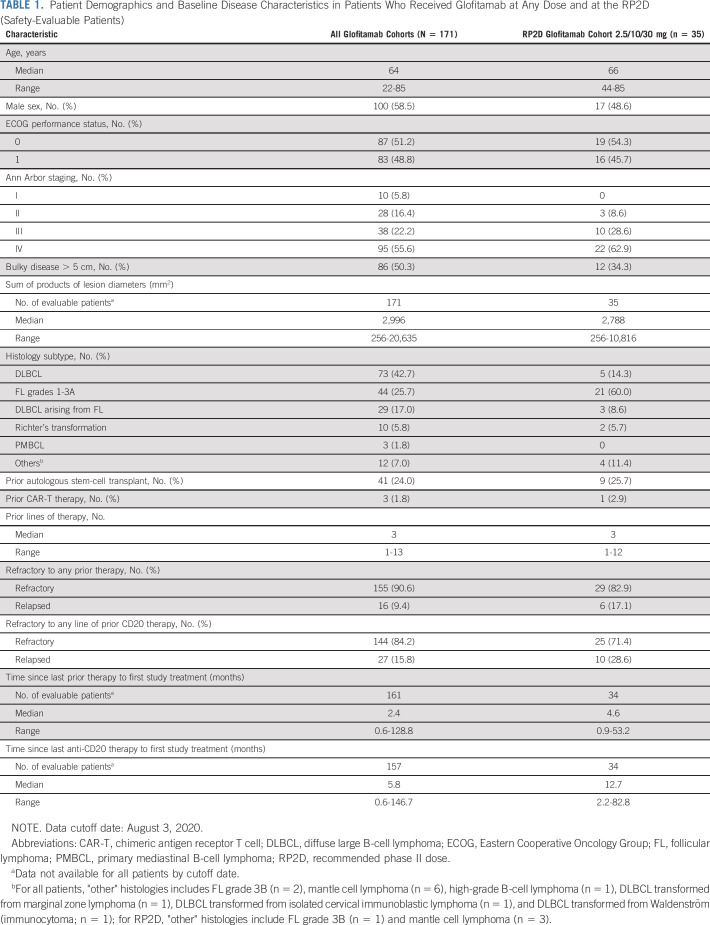
TABLE 1.
Patient Demographics and Baseline Disease Characteristics in Patients Who Received Glofitamab at Any Dose and at the RP2D (Safety-Evaluable Patients)
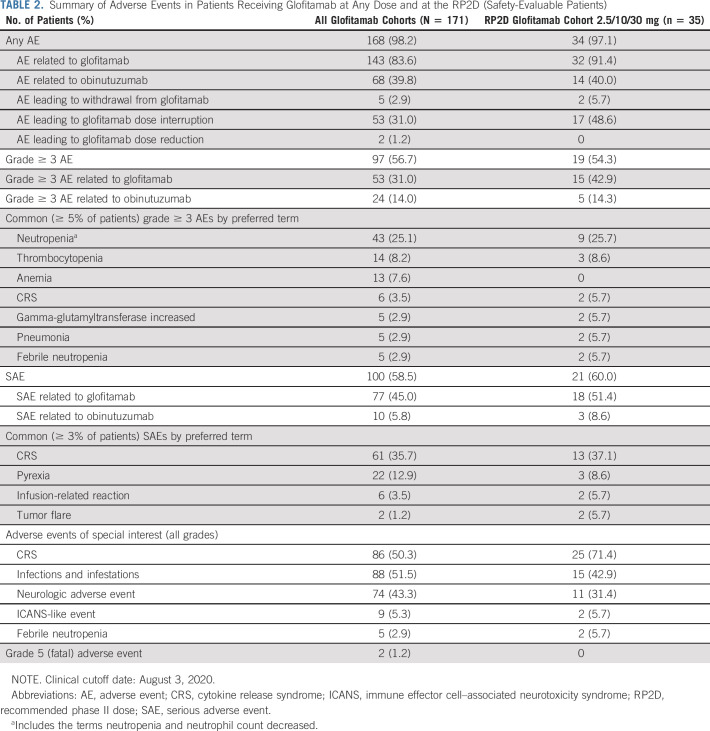
TABLE 2.
Summary of Adverse Events in Patients Receiving Glofitamab at Any Dose and at the RP2D (Safety-Evaluable Patients)
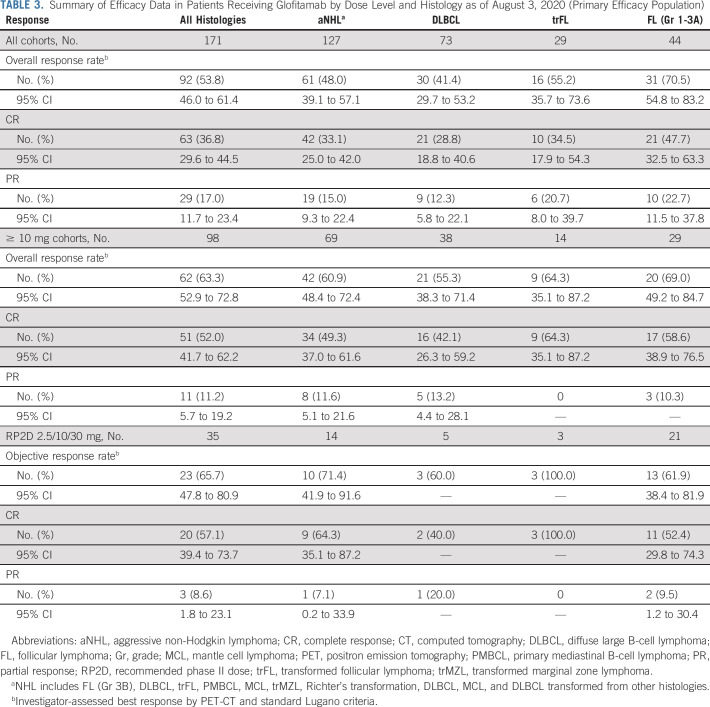
TABLE 3.
Summary of Efficacy Data in Patients Receiving Glofitamab by Dose Level and Histology as of August 3, 2020 (Primary Efficacy Population)
Patients had a median age of 64 (range, 22-85) years, with 62.0% (106 of 171) patients of age > 60 years, and 48.8% (83 of 171) had an Eastern Cooperative Oncology Group performance status of 1-2 (Table 1). Seventy-three patients (42.7%) had DLBCL, 29 (17.0%) had DLBCL arising from FL (transformed FL [trFL]), and 10 (5.8%) had Richter's transformation from chronic lymphocytic leukemia. Patients had a median of 3 (range, 1-13) prior lines of therapy; 155 (90.6%) were refractory to any prior therapy (Table 1). Median (range) times since last therapy and last anti-CD20 regimen were 2.4 (0.6-128.8) and 5.8 (0.6-146.7) months, respectively.
Safety
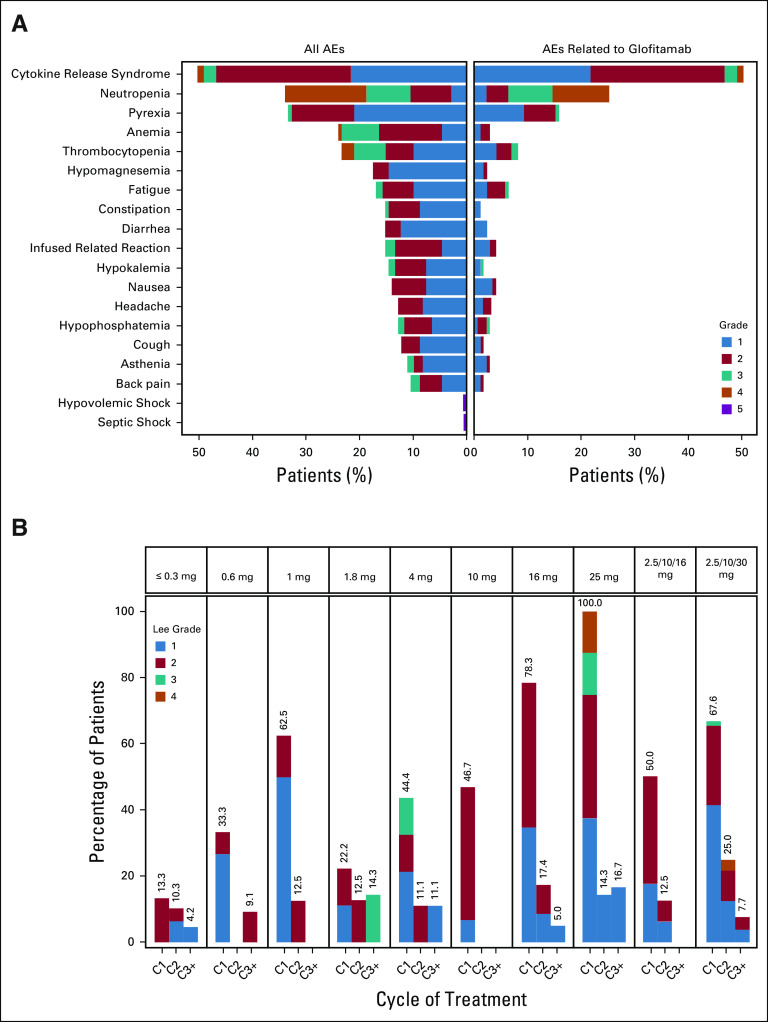
AEs were reported in 168 of 171 patients (98.2%) (Table 2); 143 (83.6%) had at least one AE considered glofitamab-related. The most common AE was CRS (Fig 1A), occurring in 86 of 171 patients (50.3%; grade 1, 21.6%; grade 2, 25.1%; grade 3, 2.3%; grade 4, 1.2%).19 Frequently (≥ 10%) associated symptoms of CRS were pyrexia (n = 79; 46.2%), hypotension (n = 42; 24.6%), tachycardia (n = 27; 15.8%), and chills (n = 21; 12.3%) (Data Supplement). Symptoms of ICANS during CRS were uncommon: confusional state in six patients (3.5%; grade 1-2, n = 4 [2.3%]; grade 3, n = 2 [1.2%]), aphasia in one (0.6%, grade 3), tremor in one (0.6%, grade 1), and depressed level of consciousness in one (0.6%, grade 2); all resolved within 3-72 hours. No seizures or increased intracranial pressure was reported. The median time to onset and duration of the earliest CRS event relative to the last prior glofitamab dose were 10.8 hours (range, 3.0-47) and 2.2 days (range, 0.0-31.0), respectively. Incidence of CRS increased with dose but declined considerably after the first administration: 21 of 160 patients (13.1%) experienced CRS at cycle 2 and 8 of 132 (6.1%) at cycle 3 or later (one grade 3) (Fig 1B).
FIG 1.
(A) Shows adverse events with an incidence of ≥ 10% or an NCI-CTCAE grade of 5 as of August 3, 2020. (B) Shows the incidence of CRS by cycle and dose (Lee grade).19 CRS events were predominantly confined to cycles 1 and 2. Step-up dosing of glofitamab allowed the administration of a high target dose (30 mg). AE, adverse event; C, cycle; CRS, cytokine release syndrome; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events.
At fixed doses of 10-25 mg, CRS occurred in 33 of 46 (71.7%) patients (grade 2, 43.5%; grade 3 and 4, 2.2% each); in SUD cohorts, in 33 of 52 (63.5%) patients (grade 2, 26.9%; grade 3 [after 2.5 mg] and 4 [after 30 mg], 1.9% each) and in 25 of 35 (71.4%) patients (grade 2, 22.9%; grade 3 and 4, 2.9% each) at the selected RP2D of 2.5/10/30 mg. At RP2D, tocilizumab, steroids, or both were used in 11.4%, 11.4%, and 8.6% of patients, respectively.
The Data Supplement summarizes subgroup analysis of CRS incidence.
CTCAE-defined neurological AEs were observed in 74 patients (43.3%), with ICANS-like events20 in nine patients (5.3%): confusional state (3; n = 1 grade 1, n = 2 grade 2), depressed level of consciousness (n = 2, grade 2), tremor (n = 3; grade 1), and agitation (n = 1; grade 1) (Data Supplement).
SAEs were reported in 100 patients (58.5%) and were considered glofitamab-related in 77 patients (45.0%); in 71 of 167 patients (42.5%), they occurred during cycle 1. SAEs in 61 patients (88 events) were due to CRS; in 46 of 88 (52.3%) and 31 of 88 (35.2%) of SAEs, seriousness was related to prolonged and new hospitalization, respectively. Grade 5 (fatal) AEs occurred in one patient at 25 mg (hypovolemic shock because of GI hemorrhage after CRS recovery) and in one patient at 0.015 mg (septic shock); both were considered by the investigator to be unrelated to glofitamab (Fig 1A).
Grade ≥ 3 neutropenia occurred in 43 patients (25.1%) (Table 2) and considered glofitamab-related in 34 patients (79.1%). Granulocyte colony–stimulating factor was given to 37 patients (21.6%). Median times to onset and duration of events were 21.5 and 7.9 days. Febrile neutropenia occurred in five patients (2.9%). Infections were observed in 88 patients (51.5%); 30 (17.5%) had grade ≥ 3 events, with the most common being pneumonia (n = 5). Five patients (2.9%) discontinued treatment because of AEs (Data Supplement): one acute myocardial infarction (at 0.22 mg), one grade 3 cytomegalovirus chorioretinitis (at 1 mg), one fatal event of GI bleeding (at 25 mg) after recovery from grade 4 CRS, one grade 4 neutropenia (at 2.5/10/30 mg), and one patient with grade 3 sepsis and grade 4 colitis (at 2.5/10/30 mg).
Efficacy
Clinical activity was observed at all doses, increasing substantially with dose escalation (Fig 2).
FIG 2.
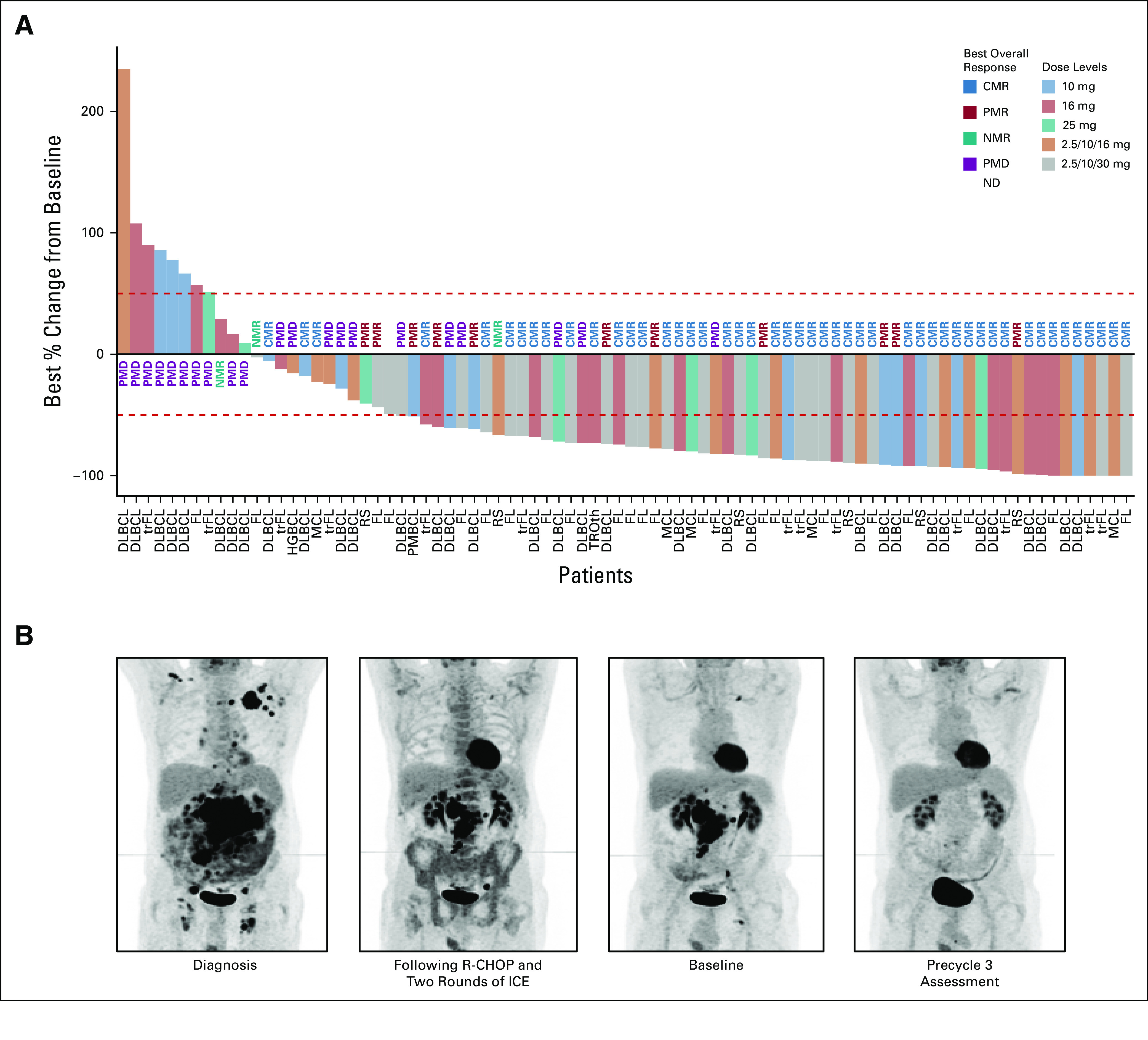
(A) Waterfall plot of the best overall change in the size of tumor target lesions. The percentage changes in the sum of the products of diameters of target lesions are shown. The columns represent the results from individual patients, color-coded according to the doses of glofitamab received. The dashed lines indicate 50% increase or decrease from baseline sum of the products of diameters. (B) PET scans of a 64-year-old patient with primary refractory transformed lymphoma who achieved complete response after two cycles of 10 mg glofitamab. The patient remains treatment-free and in complete response as of September 2020, 18 months after completion of glofitamab treatment. aNHL, aggressive non-Hodgkin lymphoma; CMR, complete metabolic response; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; ICE, ifosfamide, carboplatin, and etoposide; iNHL, indolent non-Hodgkin lymphoma; MCL, mantle cell lymphoma; ND, not determined; NMR, no metabolic response; PET, positron emission tomography; PFS, progression-free survival; PMBCL, primary mediastinal B-cell lymphoma; PMD, progressive metabolic disease; PMR, partial metabolic response; R-CHOP, rituximab, cyclophosphamide, adriamycin, vincristine, and prednisone; RS, Richter's transformation; trFL, transformed follicular lymphoma; trOth, transformed other.
Among patients with aggressive B-NHL (DLBCL, trFL, PMBCL, MCL, and Richter's transformation), ORR and CR were 48.0% (61 of 127) and 33.1% (42 of 127), respectively, including 41.1% (30 of 73) and 28.8% (21 of 73) in patients with DLBCL and 55.2% (16 of 29) and 34.5% (10 of 29) in patients with trFL (Table 3). At doses ≥ 10 mg, ORR and CR were 60.9% (42 of 69) and 49.3% (34 of 69), respectively, including 55.3% (21 of 38) and 42.1% (16 of 38) for DLBCL and 64.3% (9 of 14, all CRs) for trFL (Table 3). At the RP2D, ORR and CR were 71.4% (10 of 14) and 64.3% (9 of 14), respectively (Table 3).
Of 44 patients with grade 1-3A FL, 31 (70.5%) achieved response and 21 (47.7%) achieved CR. At doses ≥ 10 mg, ORR and CR rates were 69.0% (20 of 29) and 58.6% (17 of 29), and at the RP2D, 61.9% (13 of 21) and 52.4% (11 of 21), respectively.
Time to CR was short, with the majority occurring by cycle 3 (Fig 3A). Responses were observed across patient subgroups, including high-risk populations with ≥ 4 prior regimens and refractory disease (Data Supplement).
FIG 3.
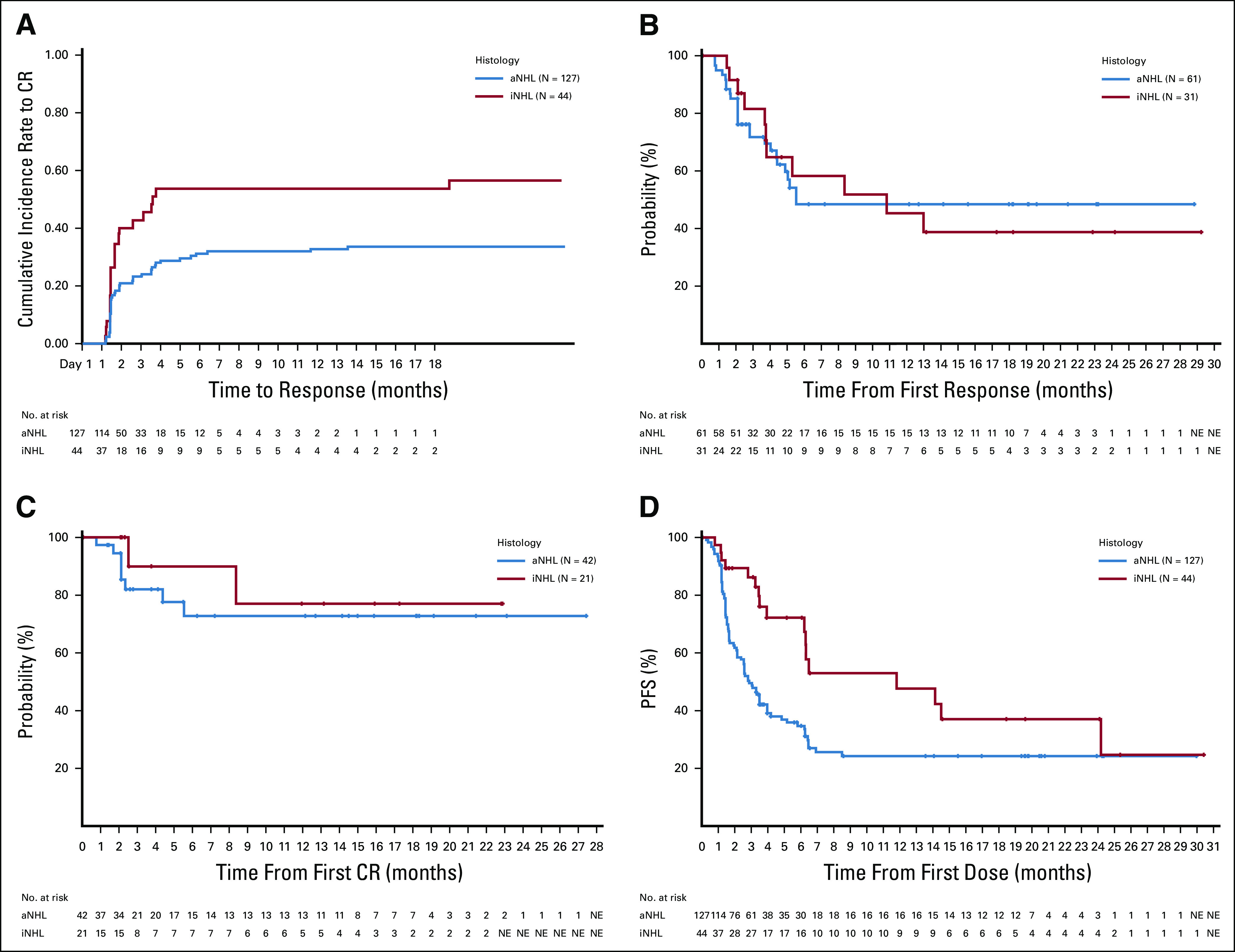
(A) Represents the cumulative incidence of time to CR. Kaplan-Meier curves for (B) DOR (PR and CR), (C) duration of CR, and (D) PFS. aNHL, aggressive non-Hodgkin lymphoma; CR, complete response; DOR, duration of response; iNHL, indolent non-Hodgkin lymphoma; NE, not estimable; PFS, progression-free survival; PR, partial response.
In aggressive NHL, the median DOR was 5.5 months (95% CI, 4.4 to not estimable; range, 0.8-28.8 months) and median DOCR was not reached (range, 0.0-27.4 months), with 48.6% (any response) and 72.8% (CR) of patients still responding at 12 months (Figs 3B and 3C). The median PFS was 2.9 (95% CI, 2.1 to 3.9) months, with a plateau of approximately 24% from 8 months onward (maximum follow-up of 30 months). In grade 1-3A FL, the median PFS was 11.8 months (95% CI, 6.3 to 24.2). Of 31 responders, the median DOR was 10.8 months (95% CI, 3.8 to not estimable). Median DOCR was not reached, and 19 of 21 (90.5%) patients remain in CR up to 22.9 months (Fig 3B).
Pharmacokinetics
Following IV infusion, glofitamab serum concentrations peak at the end of the infusion and decline in a biphasic manner thereafter (Data Supplement). Glofitamab appears to be eliminated with an apparent half-life of 6-11 days and demonstrated dose-linear PK across the 0.005-25 mg range. There was no evidence of either substantial accumulation or time dependence upon multiple dosing across treatment cycles. Overall, glofitamab PK showed moderate between-patient variability. The median (range) obinutuzumab serum concentration at baseline (before first glofitamab administration) was 249 (98.4-858) µg/mL. Anti-glofitamab antibodies were not detected in any patient.
Pharmacodynamics
Biomarker data were obtained from 158 patients dosed with glofitamab 0.005-30 mg. Glofitamab infusion resulted in a rapid and transient reduction in T cells in the peripheral circulation in all patients, with the nadir recorded 6 hours after the infusion (Data Supplement). This T-cell redistribution was associated with dose level and receptor occupancy.21 Following administration of glofitamab ≥ 0.6 mg in fixed dosing cohorts, responding patients showed long-term T-cell activation up to cycle 5 (Data Supplement). This was demonstrated by two-to-fourfold elevation of T-cell activation markers, such as Ki67, HLA-DR, PD-1, and Tim3.22 In line with the clinical activity, this effect was observed at doses at and above 0.6 mg. In the SUD cohorts (n = 40), T-cell activation, as measured by granzyme B expression, was higher after 30 mg compared with 16 mg in responding patients (Data Supplement).
DISCUSSION
This study demonstrated that the novel bispecific CD20 antibody T-cell engager glofitamab offers significant antitumor activity in patients with heavily pretreated B-NHL refractory to prior therapy (90.6%).
CRS was manageable with low rates of grade ≥ 3 and moderate use of steroids or tocilizumab, with no treatment withdrawals. Events fully resolved in all but one patient who died because of progressive disease before recovery. Time to onset was predictable and mostly confined to the first administration; only 13.1% and 6.1% of patients experienced CRS at cycle 2 or at cycle 3 or later, respectively. Factors associated with severe CRS included high disease burden (Ann Arbor stage) and bone marrow infiltration (data not shown). The use of Gpt allowed escalation of glofitamab with fixed doses up to 25 mg. Although overall CRS rates were similar between the highest fixed dosing and SUD, grade 2 or higher CRS was reduced with SUD and was therefore selected as RP2D (grade ≥ 2; 47.8% in ≥ 10 mg fixed dosing versus 28.6% in 2.5/10/30 mg).
ICANS-like AEs were rare, self-limiting, and considered qualitatively different from those seen with anti-CD19 CAR-T therapies and bispecifics where neurological toxicity is dose limiting.23-25 Treatment-emergent cytopenias did not lead to increased rates of serious infections. The discontinuation rate of 2.9% due to AEs suggests a favorable benefit–risk profile.
Response rates were high. At doses ≥ 10 mg, 49.3% of patients with aggressive B-NHL achieved CR (95% CI, 37.0 to 61.6), demonstrating substantial and clinically meaningful benefit. CRs were achieved rapidly in patients with high tumor burden, bulky disease, and refractoriness to multiple therapies. Duration of benefit was impaired by limited follow-up, but 34 of 42 (81.0%) CRs in patients with aggressive histologies are ongoing with follow-up to 27.4 months. In addition, SUD maintained the high ORR and CR rates observed in fixed dosing.
The pharmacokinetic results indicate that the half-life of glofitamab is approximately 10 days, enabling convenient 3-weekly dosing. As obinutuzumab and glofitamab bind to the same CD20 epitope, the concentration profiles, alongside biomarker and clinical data, support potent glofitamab activity despite CD20 receptor competition. The preservation of activity in the presence of residual or in combination with another anti-CD20 monoclonal antibody represents a unique benefit of glofitamab. The potency of glofitamab is further supported by population pharmacokinetic and exposure–response analyses, confirming efficacy at CD20 receptor occupancies by cycle 3 of < 1%.21 Based on this, SUD was introduced to decrease severity of CRS in the first cycle, and a weekly dosing schedule of 2.5 mg (C1D1), 10 mg (C1D8), 30 mg (C2D1) followed by 30 mg at subsequent cycles is considered safe, demonstrates high activity, and was taken forward as RP2D.
Glofitamab is an available and accessible “off-the-shelf” T-cell–engaging therapy. These properties contrast with those of current CAR-T cell therapies, which require manufacturing, may require bridging therapy, and may not be feasible in patients with rapidly progressive disease.26 So far, the clinical activity of glofitamab appears to exceed that of blinatumomab27 and to be in the range of registered CAR-T therapies,23,24 with possibly a more favorable and temporally predictable safety profile. The observation of rapidly achieved CRs lasting more than 18 months across a range of doses suggests that glofitamab is highly active in a difficult-to-treat patient group with few clinical treatment options. As a consequence, glofitamab is undergoing expanded evaluation in R/R and untreated B-NHL, alone and in combination with conventional chemotherapy and novel agents (ClinicalTrials.gov identifiers: NCT03467373, NCT03533283, NCT04313608, and NCT04408638).
In conclusion, this novel T-cell–engaging bispecific antibody has shown high levels of single-agent activity in R/R B-NHL. Glofitamab has demonstrated frequent, durable CRs and a manageable tolerability profile and allows off-the-shelf treatment for patients with refractory B-NHL in need of timely therapy.
ACKNOWLEDGMENT
The authors would like to thank the patients and their families, the study investigators, study coordinators, nurses, and representatives of the sponsor who were involved in data collection and analyses. Study NP30179 was sponsored by F. Hoffmann-La Roche Ltd. Third-party Medical Writing assistance, under the authors' direction, was provided by Khalida Rizi of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche Ltd.
Martin Hutchings
Consulting or Advisory Role: Takeda, Roche, Genmab
Research Funding: Celgene, Genmab, Roche, Takeda, Novartis
Franck Morschhauser
Consulting or Advisory Role: Roche/Genentech, Gilead Sciences, Celgene, Bristol Myers Squibb, AbbVie, Epizyme, Servier
Speakers' Bureau: Roche
Expert Testimony: Roche/Genentech
Gloria Iacoboni
Honoraria: Gilead Sciences, Novartis, Roche/Genentech, Celgene/Bristol Myers Squibb, Janssen
Consulting or Advisory Role: Novartis, Celgene/Bristol Myers Squibb, Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Novartis, Celgene/Bristol Myers Squibb
Carmelo Carlo-Stella
Honoraria: Bristol Myers Squibb, Merck Sharp & Dohme, Janssen Oncology, AstraZeneca, Celgene, Takeda, Incyte, Gilead Sciences
Consulting or Advisory Role: Sanofi, ADC Therapeutics, Roche, Karyopharm Therapeutics, Celgene/Bristol Myers Squibb, Incyte
Research Funding: ADC Therapeutics, Sanofi, Roche
Travel, Accommodations, Expenses: Roche, Janssen, Takeda, ADC Therapeutics
Anna Sureda
Honoraria: Takeda, Bristol Myers Squibb, Merck Sharp & Dohme, Celgene, Janssen, Sanofi, Roche, Novartis, Gilead Sciences, Janssen-Cilag
Consulting or Advisory Role: Takeda, Bristol Myers Squibb, Gilead Sciences, Celgene, Janssen, Novartis
Speakers' Bureau: Takeda
Other Relationship: Sanofi, Takeda, Roche, Celgene, Gilead Sciences
Gilles Salles
Honoraria: Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys
Consulting or Advisory Role: Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol Myers Squibb, BeiGene, Incyte, Miltenyi Biotec
Joaquín Martínez-Lopez
Speakers' Bureau: Roche, Janssen-Cilag, BMSi
Research Funding: Astellas Pharma, Bristol Myers Squibb
Michael Crump
Honoraria: Gilead Sciences, Servier/Pfizer
Consulting or Advisory Role: Servier, Gilead Sciences, Novartis Canada Pharmaceuticals Inc
Research Funding: Roche Canada
Denise N. Thomas
Employment: Roche TCRC, Genmab, Cellectis
Peter N. Morcos
Employment: Roche/Genentech, Bayer
Stock and Other Ownership Interests: Roche/Genentech, Bayer
Cristiano Ferlini
Employment: Roche/Genentech, AstraZeneca
Stock and Other Ownership Interests: AstraZeneca, Roche
Ann-Marie E. Bröske
Employment: Roche
Stock and Other Ownership Interests: Roche, BioNTech AG
Anton Belousov
Employment: Roche
Marina Bacac
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: Coinventor in Roche patents
Travel, Accommodations, Expenses: Roche
Natalie Dimier
Employment: Roche
Stock and Other Ownership Interests: Roche
Travel, Accommodations, Expenses: Roche
David J. Carlile
Employment: Roche, AstraZeneca
Stock and Other Ownership Interests: AstraZeneca, Roche
Linda Lundberg
Employment: F. Hoffmann LaRoche
Stock and Other Ownership Interests: F. Hoffmann LaRoche
David Perez-Callejo
Employment: Roche
Stock and Other Ownership Interests: Roche
Pablo Umaña
Employment: Roche
Leadership: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Co-inventor in Roche-owned patents on glofitamab and obinutuzumab
Travel, Accommodations, Expenses: Roche
Tom Moore
Employment: Roche
Stock and Other Ownership Interests: Roche
Travel, Accommodations, Expenses: Roche
Martin Weisser
Employment: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: I hold patents for biomarkers and drug combinations. These are not related to the present study. I do not receive royalties
Michael J. Dickinson
Honoraria: Roche, Amgen, MSD, Janssen, Bristol Myers Squibb, Novartis
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Gilead Sciences, Roche, Janssen
Speakers' Bureau: Novartis
Research Funding: Novartis, Roche, Takeda, Celgene, MSD
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported by F. Hoffmann-La Roche Ltd.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
AUTHOR CONTRIBUTIONS
Conception and design: Martin Hutchings, Carmelo Carlo-Stella, Fritz C. Offner, Anna Sureda, Joaquín Martínez-Lopez, Denise N. Thomas, Peter N. Morcos, Ann-Marie E. Bröske, Linda Lundberg, David Perez-Callejo, Pablo Umaña, Tom Moore, Martin Weisser, Michael J. Dickinson
Provision of study materials or patients: Martin Hutchings, Franck Morschhauser, Gloria Iacoboni, Anna Sureda, Gilles Salles, Joaquín Martínez-Lopez, Michael Crump
Collection and assembly of data: Martin Hutchings, Franck Morschhauser, Gloria Iacoboni, Carmelo Carlo-Stella, Fritz C. Offner, Anna Sureda, Gilles Salles, Michael Crump, Denise N. Thomas, Ann-Marie E. Bröske, Anton Belousov, Natalie Dimier, Linda Lundberg, David Perez-Callejo, Martin Weisser, Michael J. Dickinson
Data analysis and interpretation: Martin Hutchings, Franck Morschhauser, Gloria Iacoboni, Carmelo Carlo-Stella, Fritz C. Offner, Anna Sureda, Gilles Salles, Joaquín Martínez-Lopez, Denise N. Thomas, Peter N. Morcos, Cristiano Ferlini, Ann-Marie E. Bröske, Anton Belousov, Marina Bacac, Natalie Dimier, David J. Carlile, Linda Lundberg, David Perez-Callejo, Tom Moore, Martin Weisser, Michael J. Dickinson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell–Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Martin Hutchings
Consulting or Advisory Role: Takeda, Roche, Genmab
Research Funding: Celgene, Genmab, Roche, Takeda, Novartis
Franck Morschhauser
Consulting or Advisory Role: Roche/Genentech, Gilead Sciences, Celgene, Bristol Myers Squibb, AbbVie, Epizyme, Servier
Speakers' Bureau: Roche
Expert Testimony: Roche/Genentech
Gloria Iacoboni
Honoraria: Gilead Sciences, Novartis, Roche/Genentech, Celgene/Bristol Myers Squibb, Janssen
Consulting or Advisory Role: Novartis, Celgene/Bristol Myers Squibb, Gilead Sciences
Travel, Accommodations, Expenses: Gilead Sciences, Novartis, Celgene/Bristol Myers Squibb
Carmelo Carlo-Stella
Honoraria: Bristol Myers Squibb, Merck Sharp & Dohme, Janssen Oncology, AstraZeneca, Celgene, Takeda, Incyte, Gilead Sciences
Consulting or Advisory Role: Sanofi, ADC Therapeutics, Roche, Karyopharm Therapeutics, Celgene/Bristol Myers Squibb, Incyte
Research Funding: ADC Therapeutics, Sanofi, Roche
Travel, Accommodations, Expenses: Roche, Janssen, Takeda, ADC Therapeutics
Anna Sureda
Honoraria: Takeda, Bristol Myers Squibb, Merck Sharp & Dohme, Celgene, Janssen, Sanofi, Roche, Novartis, Gilead Sciences, Janssen-Cilag
Consulting or Advisory Role: Takeda, Bristol Myers Squibb, Gilead Sciences, Celgene, Janssen, Novartis
Speakers' Bureau: Takeda
Other Relationship: Sanofi, Takeda, Roche, Celgene, Gilead Sciences
Gilles Salles
Honoraria: Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys
Consulting or Advisory Role: Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol Myers Squibb, BeiGene, Incyte, Miltenyi Biotec
Joaquín Martínez-Lopez
Speakers' Bureau: Roche, Janssen-Cilag, BMSi
Research Funding: Astellas Pharma, Bristol Myers Squibb
Michael Crump
Honoraria: Gilead Sciences, Servier/Pfizer
Consulting or Advisory Role: Servier, Gilead Sciences, Novartis Canada Pharmaceuticals Inc
Research Funding: Roche Canada
Denise N. Thomas
Employment: Roche TCRC, Genmab, Cellectis
Peter N. Morcos
Employment: Roche/Genentech, Bayer
Stock and Other Ownership Interests: Roche/Genentech, Bayer
Cristiano Ferlini
Employment: Roche/Genentech, AstraZeneca
Stock and Other Ownership Interests: AstraZeneca, Roche
Ann-Marie E. Bröske
Employment: Roche
Stock and Other Ownership Interests: Roche, BioNTech AG
Anton Belousov
Employment: Roche
Marina Bacac
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Roche
Patents, Royalties, Other Intellectual Property: Coinventor in Roche patents
Travel, Accommodations, Expenses: Roche
Natalie Dimier
Employment: Roche
Stock and Other Ownership Interests: Roche
Travel, Accommodations, Expenses: Roche
David J. Carlile
Employment: Roche, AstraZeneca
Stock and Other Ownership Interests: AstraZeneca, Roche
Linda Lundberg
Employment: F. Hoffmann LaRoche
Stock and Other Ownership Interests: F. Hoffmann LaRoche
David Perez-Callejo
Employment: Roche
Stock and Other Ownership Interests: Roche
Pablo Umaña
Employment: Roche
Leadership: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: Co-inventor in Roche-owned patents on glofitamab and obinutuzumab
Travel, Accommodations, Expenses: Roche
Tom Moore
Employment: Roche
Stock and Other Ownership Interests: Roche
Travel, Accommodations, Expenses: Roche
Martin Weisser
Employment: Roche
Stock and Other Ownership Interests: Roche
Patents, Royalties, Other Intellectual Property: I hold patents for biomarkers and drug combinations. These are not related to the present study. I do not receive royalties
Michael J. Dickinson
Honoraria: Roche, Amgen, MSD, Janssen, Bristol Myers Squibb, Novartis
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Gilead Sciences, Roche, Janssen
Speakers' Bureau: Novartis
Research Funding: Novartis, Roche, Takeda, Celgene, MSD
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.International Non-Hodgkin's Lymphoma Prognostic Factors Project : A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329:987-994, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Sehn LH Berry B Chhanabhai M, et al. : The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109:1857-1861, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Gisselbrecht C, Van Den Neste E: How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol 182:633-643, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump M Neelapu SS Farooq U, et al. : Outcomes in refractory diffuse large B-cell lymphoma: Results from the International SCHOLAR-1 study. Blood 130:1800-1808, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hübel K Ghielmini M Ladetto M, et al. : Controversies in the treatment of follicular lymphoma. Hemasphere 4:e317, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locke FL Ghobadi A Jacobson CA, et al. : Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol 20:31-42, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster SJ Bishop MR Tam CS, et al. : Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 380:45-56, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Jacobson CA Chavez JC Sehgal AR, et al. : Interim analysis of ZUMA-5: A phase II study of axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed/refractory indolent non-Hodgkin lymphoma (R/R iNHL). J Clin Oncol 38:8008, 2020 [Google Scholar]
- 9.Chomienne C Sierra J Einsele H, et al. : EHA guidance document: The process of CAR-T cell therapy in Europe. Hemasphere 3:e280, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoudjafari Z Hawks KG Hsieh AA, et al. : American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group survey on chimeric antigen receptor t cell therapy administrative, logistic, and toxicity management practicesthe United States. Biol Blood Marrow Transplant 25:26-33, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Neelapu SS Locke FL Bartlett NL, et al. : Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531-2544, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skrabek P Assouline S Christofides A, et al. : Emerging therapies for the treatment of relapsed or refractory diffuse large B cell lymphoma. Curr Oncol 26:253-265, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu M Wu B Brandl C, et al. : Blinatumomab, a bispecific T-cell engager (BiTE®) for CD-19 targeted cancer immunotherapy: Clinical pharmacology and its implications. Clin Pharmacokinet 55:1271-1288, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Bacac M Colombetti S Herter S, et al. : CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res 24:4785-4797, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Yin G, Yuan Y: Bayesian data augmentation dose finding with continual reassessment method and delayed toxicity. Ann App Stat 7:1837-2457, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goede V Fischer K Busch R, et al. : Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370:1101-1110, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD Fisher RI Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano Classification. J Clin Oncol 32:3059-3068, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Common Terminology Criteria for Adverse Events (CTCAE), v4.03. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf, 2009 [Google Scholar]
- 19.Lee DW Gardner R Porter DL, et al. : Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW Santomasso BD Locke FL, et al. : ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 25:625-638, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Djebli N Morcos PN Jaminion F, et al. : Population pharmacokinetics and exposure-response analyses for glofitamab in relapsed/refractory B-cell non-Hodgkin lymphoma (R/R NHL): Confirmation of efficacy and CRS mitigation in patients with step-up dosing. Blood 136:1-2, 2020. (suppl 1)32430499 [Google Scholar]
- 22.Bröske A-ME James I Belousov A, et al. : CD20-TCB, a novel T-cell-engaging bispecific antibody, induces T-cell-mediated killing in relapsed or refractory non-Hodgkin lymphoma: Biomarker results from a phase I dose-escalation trial. Blood 134, 2019. (suppl; abstr 5319) [Google Scholar]
- 23.Yescarta Prescribing Information. https://www.fda.gov/media/108377/download, 2020 [Google Scholar]
- 24.Kymriah Prescribing Information. www.novartis.us/sites/www.novartis.us/files/kymriah.pdf, 2018 [Google Scholar]
- 25.Blincyto Prescribing Information. www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/blincyto/blincyto_pi_hcp_english.pdf, 2020 [Google Scholar]
- 26.Jain MD Jacobs MT Nastoupil LJ, et al. : Characteristics and outcomes of patients receiving bridging therapy while awaiting manufacture of standard of care axicabtagene ciloleucel CD19 chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory large B-cell lymphoma: Results from the US lymphoma CAR-T consortium. Blood 134, 2019. (suppl; abstr 245) [Google Scholar]
- 27.Viardot A, Bargou R: Bispecific antibodies in haematological malignancies. Cancer Treat Rev 65:87-95, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.