Abstract
Polypharmacy, or the daily use of five or more medications, is well documented in older adults and linked to negative outcomes such as medication errors, adverse drug reactions, and increased healthcare utilization. Like older adults, people with multiple sclerosis (PwMS) are susceptible to polypharmacy, owing to the variety of treatments used to address individual multiple sclerosis (MS) symptoms and other comorbidities. Between 15–65% of PwMS meet criteria for polypharmacy; in this population, polypharmacy is associated with increased reports of fatigue, subjective cognitive impairment, and reduced quality of life. Despite evidence of adverse outcomes, polypharmacy among PwMS remains a neglected area of research. This article examines the current literature regarding polypharmacy in MS, as well as implications for clinical practice and directions for future research.
What is Multiple Sclerosis and Who Gets it?
Multiple sclerosis (MS) is an inflammatory autoimmune disease affecting the brain and spinal cord (i.e., the central nervous system, or CNS). Characterized by a demyelinating neurodegenerative process, lesions (or ‘scleroses’) form throughout the CNS, thereby slowing or interrupting the conduction of nerve impulses that lead to a variety of diverse symptoms. Patients typically experience acute inflammatory episodes, also referred to as flare-ups, which occur at unpredictable intervals and can last from days to months.1 MS affects 2.3 million people worldwide, with higher prevalence in Europe and North America.2 MS is the leading cause of non-traumatic neurologic disability among young adults and disproportionately affects women.3
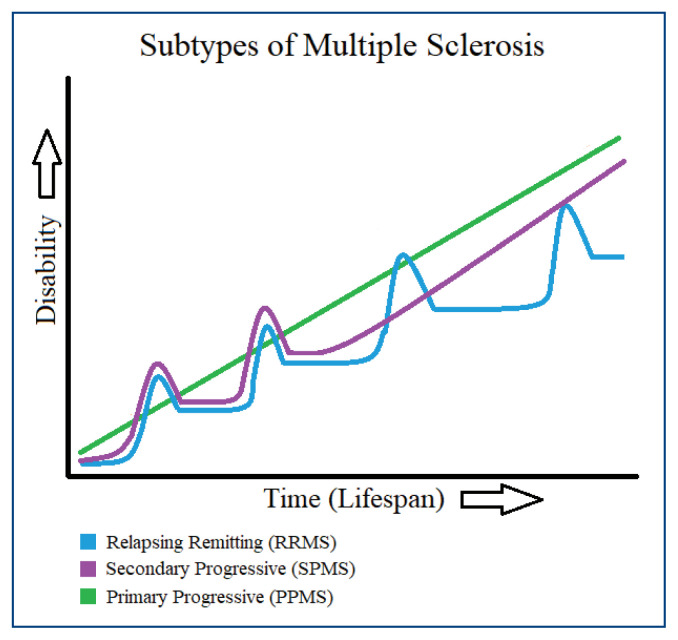
While the pathogenesis of MS is unclear, it likely involves a complex interplay of susceptible gene variants and environmental risk factors, such as limited sun exposure, obesity, Epstein-Barr virus infection, and smoking.4 Genome wide association studies have implicated genetic loci in alleles of the major histocompatibility complex, most notably involving the human leukocyte antigen class II locus.5 Symptom expression in MS is highly variable; the clinical subtypes of MS are characterized by the rate and pattern of symptom exacerbations and disability accumulation over time (Figure 1).1 While there is no cure for MS, a variety of medications and lifestyle modifications can aid in symptom management, and in some cases, reduce the rate of relapses and accumulation of disability.6,7
Figure 1.
Disability Progression Across Subtypes of Multiple Sclerosis 63
Symptomatic Treatment in MS
Treatments for each patient are highly individualized according to the specific symptoms expressed. Clinical manifestations of MS comprise a wide range of physical, cognitive, and emotional symptoms, which vary according to the location of damage in the CNS. Typical physical sequelae include muscle weakness, spasticity, pain, tingling, numbness, impaired balance, visual disturbance, difficulties with speech and swallowing, bladder and bowel symptoms, and sexual dysfunction.8 Cognitive and emotional symptoms typically include slowed processing speed, impaired working memory, attentional deficits, anxiety, depression, fatigue, and sleep disturbance.9 The following section provides a brief review of common MS symptoms and their respective treatments, highlighting those with CNS-active properties.
Spasticity occurs in 40–84% of people with multiple sclerosis (PwMS), causing painful muscle contractions that can even lead to sleep disturbances.10 The most common treatments for spasticity are baclofen, tizanidine, and dantrolene (which are muscle relaxants), diazepam (a sedative in the benzodiazepine class) and botulinum toxin (administered via intramuscular injections).10 Additionally, many PwMS report that spasticity is relieved with cannabis use; a systematic review by the American Academy of Neurology provided supportive evidence for this indication.11,12 It is worth noting that, with the exception of botulinum toxin, all of these treatments permeate the blood-brain-barrier and are thus more likely to cause adverse effects like somnolence, dizziness, and asthenia.13
Approximately 50% of PwMS may experience disabling pain caused by a variety of pathways, such as nociceptive pain, neuropathic pain, psychogenic pain, and mixed pain.14 Treatments for pain account for nearly 30% of all medications used for management of MS symptoms.6 Typical medications prescribed for pain in MS include anticonvulsants, antidepressants, sodium-channel blockers, opiate analgesics, and cannabinoids.8,14 The majority of these pharmacotherapies have the potential for adverse CNS effects.15
Bladder dysfunction affects up to 88% of PwMS.16,17 The first line therapy for bladder symptoms in MS are anticholinergics such as oxybutynin and tolterodine.16 A recent investigation suggests that anticholinergic medications, including those used to manage bladder symptoms, may negatively impact cognition in PwMS.17 This finding is particularly relevant because 40–70% of PwMS experience cognitive problems as a direct consequence of MS disease pathology.18
Cognitive dysfunction is frequently reported in MS, most commonly in the domains of information processing speed, working memory, attention, visual processing, and verbal fluency.18 Evidence of effective pharmacological treatment options for cognitive symptoms is limited.19 While some isolated small studies have reported benefit with CNS-active medications (such as memantine or methylphenidate), larger studies have frequently failed to confirm these findings.19,20 Mood disorders, including depression and anxiety, affect at least 50% of PwMS and are key predictors of morbidity, quality of life, medication adherence, and suicide risk.9,21 Treatment of mood disorders in MS typically involves antidepressant medication.8
Fatigue is ubiquitous among PwMS; in fact, many regard it as their most disabling symptom.8 Despite its prevalence, fatigue is notoriously difficult to measure and treat. There is little data to support pharmacological treatments for MS fatigue, perhaps with the exception of amantadine.22 Though evidence is limited and no medications are FDA approved for the treatment of fatigue in MS, patients with MS are frequently prescribed CNS-active medications for this purpose.22 Fatigue, along with mood disorders and cognitive difficulties, significantly contributes to problems in employment, social relationships, and poorer health-related quality of life (HRQoL) among PwMS.8
Disease-Modifying Therapies in MS
Beyond managing individual symptoms, people with relapsing-remitting forms of MS are typically encouraged to use a disease modifying therapy (DMT).2 DMTs are a group of medications that modulate activity of the immune system. Each medication has its own mechanism of action, thus resulting in different side effects. Because of the various medications and their associated side effects, DMTs should be tailored to each patient with careful consideration of the drug’s safety and tolerability. Current recommendations indicate that DMTs should be initiated as soon as possible following diagnosis of relapsing-remitting MS in order to slow disease progression and reduce overall lesion load, thus potentially minimizing preventable disability.7
What is Polypharmacy?
Polypharmacy is commonly defined as the daily use of five or more medications, and represents a known independent risk factor for adverse drug reactions (ADRs), particularly among older adults.23 The prevalence of polypharmacy continues to rise in older adults as the risk of developing multiple chronic health conditions increases with age.23 Although the benefit-to-risk ratios of individual medications are well understood, polypharmacy leads to dramatically increased complexity, contributing to increased risk for ADRs, falls, hospitalization, and mortality, especially among older adults.
Although the elderly represent approximately 15% of the U.S. population, they take 33% of all prescription medications, and this number is expected to increase to 50% by 2040.24 Further, estimates suggest that up to 10% of all hospital admissions among older adults are due to ADRs; indeed, the presence of polypharmacy at the time of hospitalization is one of the few significant predictors of ADRs in the inpatient setting.25 Given the increasing prevalence and additional medical burdens posed by polypharmacy, it represents a significant topic of public health concern.
Though life expectancies in PwMS are similar to those seen in the general population, little is known about the effects of polypharmacy in older PwMS. It is common for studies on aging to cite neurological disorders as exclusion criteria, thereby precluding PwMS from study eligibility and participation.26 In a recent presentation, our research group compared a cohort of older PwMS (≥55 years) with age-matched non-neurologic controls. PwMS reported significantly more daily medications than their counterparts without MS (Table 1).27 Further, the number of daily medications in PwMS was significantly correlated with fatigue, pain, and depression, while no such relationship emerged in the non-neurologic controls (Table 2).27 These findings underscore the need for further study of polypharmacy in the aging MS population.
Table 1.
Number of Daily Medications in Older Adults with and without Multiple Sclerosis27
| Group | n | Daily Medications (M ± SD) |
|---|---|---|
| PwMS | 50 | 11.6 ± 5.9* |
| PwoMS | 55 | 8.5 ± 6.0 |
Patients with MS reported significantly more daily medications than their age-matched, non-neurologic peers, t(104) = −2.68, p < .01.
Abbreviations: PwMS = people with multiple sclerosis; PwoMS = people without multiple sclerosis.
Table 2.
Spearman’s ρ Correlations: Number of Medications, Fatigue, Pain, and Mood in Older Adults with and without Multiple Sclerosis27
p < .01
p < .001
MFIS = Modified Fatigue Impact Scale; Pain = MOS Pain Effects Scale; HADS-D = Hospital Anxiety and Depression Scale - Depression subscale; HADS-A = Hospital Anxiety and Depression Scale - Anxiety subscale; PwMS = people with multiple sclerosis; PwoMS = people without multiple sclerosis.
Clinical Aspects of Appropriate and Inappropriate Polypharmacy
Polypharmacy is associated with obvious benefits afforded by multiple pharmaceuticals for treating ongoing illnesses but also with a potentially complex set of adverse effects. These adverse effects can result from interactions between two or more drugs, accidental duplicate drug therapy, or additive therapeutic effects that exceed the upper therapeutic window.23,24 Altogether, the negative effects of polypharmacy lead to significant direct costs for patients, payors, and providers due to the increased rates of hospitalization, increased healthcare utilization, and high prescription drug costs.28
Beyond the direct impacts on outcomes like risk of hospitalization, falls, and ADRs, polypharmacy contributes to more nuanced effects that broadly influence quality of life.29,30 For example, patients with polypharmacy may endorse feeling burdensome to others or experience role limitations at home or work.29 The negative effects of polypharmacy are underscored by the fact that functional declines correspond with drug burden increases.31 Indeed, the burden of taking five or more daily medications can be significant for patients, such as requiring trips to multiple pharmacies and difficulty managing complex drug regimens, which can increase risk for non-adherence.32 The risk of improper dosing and other forms of non-adherence may be especially magnified among PwMS at increased risk of depression and cognitive decline.21,33
Polypharmacy in MS
Older age, comorbidities, MS disease duration, inpatient treatment, and increased disability are significant risk factors for polypharmacy in PwMS.34–36 We found prevalence rates for polypharmacy in MS ranging from 33–90% in our prior study cohorts.27,37 Notably, those who met criteria for polypharmacy reported significantly more fatigue and subjective cognitive difficulties than their non-polypharmacy counterparts, even after controlling for age, disease duration, and level of disability.37 This investigation was the first of seven original research studies included in a qualitative systematic review by Frahm and colleagues in 2020.36 The review highlighted the hazards of polypharmacy in MS, including reduced HRQoL and increased fatigue, particularly among those on CNS-active medications.30,36,38
In a separate report, Frahm et al. explored variations in polypharmacy in MS by sex; results suggested that women and men with MS present similar rates of polypharmacy, though the use of certain drug types can vary by sex.34 For instance, women were more likely to take thyroid medications and contraceptives compared to men. This finding is suggestive of the fact that the use of one medication can sometimes necessitate another: certain DMTs are contraindicated for pregnancy, thus warranting women of childbearing age with MS to use contraception, such as birth control pills.36 Additionally, DMTs can cause adverse side effects that necessitate pharmacological treatment such as antibiotic treatment for urinary tract infections associated with mitoxantrone or pre-treatment to mitigate flu-like symptoms associated with interferon beta.39
Comorbidities among PwMS
Beyond the symptoms directly related to MS, PwMS commonly have additional comorbid health conditions.40 Typical comorbidities in PwMS include hyperlipidemia, hypertension, gastrointestinal disease, chronic lung disease, thyroid disease, obesity, anxiety, and depression.27,41,42 Certain comorbidities (i.e., diabetes, obesity, cardiovascular disease, and chronic obstructive pulmonary disease) increase the likelihood of disability progression in PwMS.43 Additionally, comorbidities in this population can decrease quality of life and even cause death: indeed, mortality is increased among PwMS with psychiatric, cerebrovascular, and cardiovascular comorbidities, as well as diabetes, cancer, and Parkinson’s disease.42,44
Of particular concern, comorbidities may alter the clinical presentation of MS and even delay the prompt and accurate diagnosis of MS; these effects can detrimentally influence decisions regarding the patient’s MS treatment, adherence, and outcomes.32,40,41,45 For instance, the likelihood of initiating a DMT for MS decreases as the patients’ number of comorbidities increases.40
Contribution of Herbal Supplements, Recreational Drugs, or Other Substances
Outside of the prescription therapeutics used to manage chronic diseases, the use of herbal supplements, recreational drugs, or other substances may also contribute to the burden of polypharmacy. A growing literature indicates that these compounds can interfere with other medical treatments by affecting compliance and pharmacokinetics; such impacts range from reducing therapeutic outcomes to producing toxic adverse effects.46,47
Within the general population, moderate alcohol consumption increases the risk of ADRs; indeed, the incidence of emergency department visits for alcohol-related ADRs increased as the patients’ number of prescriptions increased.48,49 Similarly, individuals with polypharmacy who consume caffeinated beverages may be at an increased risk of experiencing adverse effects such as jolts, jitters, or tachycardia.50 Cannabis was the third most common cause of drug-related ER visits between 2004 and 2011,51 and mounting evidence suggests a link between cannabis use and adverse events related to cardiovascular disease.52 Furthermore, regular cannabis use is associated with short- and long-term effects, including respiratory, cardiovascular, cognitive, and mood symptoms.52
Such risks must be considered when examining the potential therapeutic value of cannabis and cannabinoid formulations when polypharmacy is a concern. This is even more relevant in neurologic populations like MS, which are predisposed to experience anxiety, depression, and impaired cognition.12 Further information on the use of herbal supplements, recreational drugs, or other substances as related to MS development and progression are detailed elsewhere.53,54 The specific mechanisms by which these substances contribute to polypharmacy effects in MS remain unexplored. Of particular concern, it is estimated that 50–75% of PwMS do not disclose their use of complementary and/or recreational substances to their treating physician.55,56
Integration of Behavioral Health
Individuals with MS frequently suffer from comorbidities that can be addressed with empirically supported behavioral health treatments.8 Among others, empirically supported behavioral health treatments are available for fatigue, depression, anxiety, sleep disturbance, pain, obesity and spasticity.57,58 Regular use of behavioral treatment as a first line therapeutic should be considered among PwMS at risk of polypharmacy.36 Behavioral treatments have few side effects and can be equally efficacious, if not more so, compared to symptomatic pharmacologic interventions.8
Unfortunately, several barriers to proper behavioral treatment exist. The integration of health psychologists into neurology departments as part of standard care would address many of these obstacles by reducing stigma and increasing access to behavioral health. Further research and development of computer-assisted behavioral health programs and behavioral telehealth is also indicated. Along with regular medication reviews, stronger integration of behavioral health has the potential to significantly reduce the negative effects of polypharmacy in MS.58
Patients are rarely given information about the effects of polypharmacy and are instead told about the possible adverse effects of individual medications.24 As a result, providers should employ shared decision-making approaches that provide education about polypharmacy and, when possible, offer alternative behavioral approaches with reduced risk.36
Future Directions for Research
Further research is needed to develop effective tools for measuring and mitigating polypharmacy effects in the MS population. One such direction may include the use of clinical decision-support knowledge bases.59 For instance, our current research utilizes an online calculator that estimates the anticholinergic and sedative burden of an individual’s medication regimen, a known contributor to polypharmacy effects.60 Vulnerability to adverse effects of polypharmacy can vary considerably across individuals, and the identification of biomarkers, including genetic and epigenetic susceptibilities, which may predict adverse outcomes remains an area of active research.61 Additionally, future investigations are needed to fine-tune the methods of assessing polypharmacy, such as accounting for varying medication dosages and PRN medications (i.e., those used on an ‘as needed’ basis).
We emphasize that causality cannot be inferred from results currently described in the literature. In other words, it is possible that PwMS take more medications specifically for the treatment of symptoms like fatigue, pain, depression, etc., so one cannot state with certainty that polypharmacy causes or contributes to these effects. Longitudinal study designs may elucidate the causal relationships between polypharmacy and patient health outcomes. While ethical considerations limit the use of certain research methodologies (e.g., PwMS cannot be randomized to polypharmacy or non-polypharmacy conditions), one viable alternative could involve randomization of patients to medication review and adjustment versus treatment as usual.
A deeper examination of lifestyle factors may clarify the impact of substances and behaviors for which physicians do not routinely assess in the context of polypharmacy, such as the use of nutritional supplements, herbal remedies, cannabis, alcohol or caffeinated beverages.53 Given the prevalence of factors that are neither assessed by physicians nor disclosed by patients, innovative approaches are needed to facilitate open communication and shared decision-making between providers and PwMS.62 Finally, future research should involve the development of medication review interventions aimed at reducing polypharmacy in PwMS.36 Armed with the right information, providers for PwMS with polypharmacy can collaborate with pharmacists to carefully evaluate the full scope of their patients’ medications, supplements and lifestyle factors to reduce adverse outcomes and, ultimately, enhance quality of life.36
Footnotes
Joanie Thelen, MA, is in the Department of Psychology; Valeriy Zvonarev, MD, is in the Department of Psychiatry; Sarah Lam, BA, is a Medical Student, School of Medicine (SOM); all at the University of Missouri – Kansas City, Kansas City, Missouri (UMKC KCMO). Crystal Burkhardt, PharmD, is in the Department of Pharmacy Practice, School of Pharmacy, University of Kansas, Lawrence, Kansas. Sharon Lynch, MD, is in the Department of Neurology, University of Kansas Medical Center, Kansas City, Kansas. Jared Bruce, PhD, (above), is in the Department of Biomedical and Health Informatics, the UMKC-KCMO.
Disclosure
None reported.
References
- 1.Goodin DS. The epidemiology of multiple sclerosis: insights to disease pathogenesis. Handb Clin Neurol. 2014;122:231–266. doi: 10.1016/B978-0-444-52001-2.00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med (Lond) 2016;16(Suppl 6):s53–s59. doi: 10.7861/clinmedicine.16-6s-s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 4.Zheleznyakova GY, Piket E, Marabita F, et al. Epigenetic research in multiple sclerosis: progress challenges, and opportunities. Physiol Genomics. 2017;49(9):447–461. doi: 10.1152/physiolgenomics.00060.2017. [DOI] [PubMed] [Google Scholar]
- 5.Chan VS. Epigenetics in Multiple Sclerosis. Adv Exp Med Biol. 2020;1253:309–374. doi: 10.1007/978-981-15-3449-2_12. [DOI] [PubMed] [Google Scholar]
- 6.Brichetto G, Messmer Uccelli M, Mancardi GL, Solaro C. Symptomatic medication use in multiple sclerosis. Mult Scler. 2003;9(5):458–460. doi: 10.1191/1352458503ms957oa. [DOI] [PubMed] [Google Scholar]
- 7.Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):789–800. doi: 10.1212/WNL.0000000000005345. [DOI] [PubMed] [Google Scholar]
- 8.Rommer PS, Eichstadt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: Results from a nationwide MS registry. Mult Scler. 2019;25(12):1641–1652. doi: 10.1177/1352458518799580. [DOI] [PubMed] [Google Scholar]
- 9.Paparrigopoulos T, Ferentinos P, Kouzoupis A, Koutsis G, Papadimitriou GN. The neuropsychiatry of multiple sclerosis: focus on disorders of mood, affect and behaviour. Int Rev Psychiatry. 2010;22(1):14–21. doi: 10.3109/09540261003589323. [DOI] [PubMed] [Google Scholar]
- 10.Fu X, Wang Y, Wang C, et al. A mixed treatment comparison on efficacy and safety of treatments for spasticity caused by multiple sclerosis: a systematic review and network meta-analysis. Clin Rehabil. 2018;32(6):713–721. doi: 10.1177/0269215517745348. [DOI] [PubMed] [Google Scholar]
- 11.Rice J, Hugos C, Hildebrand A, Cameron M. Cannabis use in people with multiple sclerosis and spasticity: A cross-sectional analysis. Mult Scler Relat Disord. 2020;2009;41:10. doi: 10.1016/j.msard.2020.102009. [DOI] [PubMed] [Google Scholar]
- 12.Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–1563. doi: 10.1212/WNL.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox C, Smith T, Maidment I, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing. 2014;43(5):604–615. doi: 10.1093/ageing/afu096. [DOI] [PubMed] [Google Scholar]
- 14.Duffy SS, Lees JG, Perera CJ, Moalem-Taylor G. Managing Neuropathic Pain in Multiple Sclerosis: Pharmacological Interventions. Med Chem. 2018;14(2):106–119. doi: 10.2174/1573406413666170906122508. [DOI] [PubMed] [Google Scholar]
- 15.Meador KJ. Cognitive side effects of medications. Neurol Clin. 1998;16(1):141–155. doi: 10.1016/s0733-8619(05)70371-6. [DOI] [PubMed] [Google Scholar]
- 16.DasGupta R, Fowler CJ. Bladder, bowel and sexual dysfunction in multiple sclerosis: management strategies. Drugs. 2003;63(2):153–166. doi: 10.2165/00003495-200363020-00003. [DOI] [PubMed] [Google Scholar]
- 17.Morrow SA, Rosehart H, Sener A, Welk B. Anti-cholinergic medications for bladder dysfunction worsen cognition in persons with multiple sclerosis. J Neurol Sci. 2018;385:39–44. doi: 10.1016/j.jns.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41(5):685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 19.Miller E, Morel A, Redlicka J, Miller I, Saluk J. Pharmacological and Non-pharmacological Therapies of Cognitive Impairment in Multiple Sclerosis. Curr Neuropharmacol. 2018;16(4):475–483. doi: 10.2174/1570159X15666171109132650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzegorski T, Losy J. Cognitive impairment in multiple sclerosis - a review of current knowledge and recent research. Rev Neurosci. 2017;28(8):845–860. doi: 10.1515/revneuro-2017-0011. [DOI] [PubMed] [Google Scholar]
- 21.Bruce JM, Hancock LM, Arnett P, Lynch S. Treatment adherence in multiple sclerosis: association with emotional status, personality, and cognition. J Behav Med. 2010;33(3):219–227. doi: 10.1007/s10865-010-9247-y. [DOI] [PubMed] [Google Scholar]
- 22.Yang TT, Wang L, Deng XY, Yu G. Pharmacological treatments for fatigue in patients with multiple sclerosis: A systematic review and meta-analysis. J Neurol Sci. 2017;380:256–261. doi: 10.1016/j.jns.2017.07.042. [DOI] [PubMed] [Google Scholar]
- 23.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Parish AL. Polypharmacy and Medication Management in Older Adults. Nurs Clin North Am. 2017;52(3):457–468. doi: 10.1016/j.cnur.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol. 2017;73(6):759–770. doi: 10.1007/s00228-017-2225-3. [DOI] [PubMed] [Google Scholar]
- 26.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075–1084. doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 27.Thelen J, Baker S, Bruce J, Thuringer A, Nashatizadeh M, Lynch S. Polypharmacy in MS: Correlations with pain, fatigue, and mood. MSVirtual2020, the 8th Joint ACTRIMS-ECTRIMS Meeting; September 11–13, 2020; 2020. [Google Scholar]
- 28.Kojima G, Bell C, Tamura B, et al. Reducing cost by reducing polypharmacy: the polypharmacy outcomes project. J Am Med Dir Assoc. 2012;13(9):818 e811–815. doi: 10.1016/j.jamda.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng HM, Lee CH, Chen YJ, Hsu HH, Huang LY, Huang JL. Developing a measure of medication-related quality of life for people with polypharmacy. Qual Life Res. 2016;25(5):1295–1302. doi: 10.1007/s11136-015-1177-2. [DOI] [PubMed] [Google Scholar]
- 30.Jelinek GA, Weiland TJ, Hadgkiss EJ, Marck CH, Pereira N, van der Meer DM. Medication use in a large international sample of people with multiple sclerosis: associations with quality of life, relapse rate and disability. Neurol Res. 2015;37(8):662–673. doi: 10.1179/1743132815Y.0000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilmer SN, Mager DE, Simonsick EM, et al. Drug burden index score and functional decline in older people. Am J Med. 2009;122(12):1142–1149e1141–1142. doi: 10.1016/j.amjmed.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruce JM, Bruce AS, Lynch S, et al. Probability discounting of treatment decisions in multiple sclerosis: associations with disease knowledge, neuropsychiatric status, and adherence. Psychopharmacology (Berl) 2018;235(11):3303–3313. doi: 10.1007/s00213-018-5037-y. [DOI] [PubMed] [Google Scholar]
- 33.McKay KA, Tremlett H, Patten SB, et al. Determinants of non-adherence to disease-modifying therapies in multiple sclerosis: A cross-Canada prospective study. Mult Scler. 2017;23(4):588–596. doi: 10.1177/1352458516657440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frahm N, Hecker M, Zettl UK. Polypharmacy in patients with multiple sclerosis: a gender-specific analysis. Biol Sex Differ. 2019;10(1):27. doi: 10.1186/s13293-019-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frahm N, Hecker M, Zettl UK. Polypharmacy in outpatients with relapsing-remitting multiple sclerosis: A single-center study. PLoS One. 2019;14(1):e0211120. doi: 10.1371/journal.pone.0211120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frahm N, Hecker M, Zettl UK. Polypharmacy among patients with multiple sclerosis: a qualitative systematic review. Expert Opin Drug Saf. 2020;19(2):139–145. doi: 10.1080/14740338.2020.1720646. [DOI] [PubMed] [Google Scholar]
- 37.Thelen JM, Lynch SG, Bruce AS, Hancock LM, Bruce JM. Polypharmacy in multiple sclerosis: relationship with fatigue, perceived cognition, and objective cognitive performance. J Psychosom Res. 2014;76(5):400–404. doi: 10.1016/j.jpsychores.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Oken BS, Flegal K, Zajdel D, et al. Cognition and fatigue in multiple sclerosis: Potential effects of medications with central nervous system activity. J Rehabil Res Dev. 2006;43(1):83–90. doi: 10.1682/jrrd.2004.11.0148. [DOI] [PubMed] [Google Scholar]
- 39.Langer-Gould A, Moses HH, Murray TJ. Strategies for managing the side effects of treatments for multiple sclerosis. Neurology. 2004;63(11 Suppl 5):S35–41. doi: 10.1212/wnl.63.11_suppl_5.s35. [DOI] [PubMed] [Google Scholar]
- 40.Marrie RA. Comorbidity in multiple sclerosis: Past, present and future. Clin Invest Med. 2019;42(1):E5–E12. doi: 10.25011/cim.v42i1.32383. [DOI] [PubMed] [Google Scholar]
- 41.Stampanoni Bassi M, Iezzi E, Buttari F, et al. Obesity worsens central inflammation and disability in multiple sclerosis. Mult Scler. 2020;26(10):1237–1246. doi: 10.1177/1352458519853473. [DOI] [PubMed] [Google Scholar]
- 42.Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. 2018;9:97–102. doi: 10.2147/PROM.S148387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thormann A, Sorensen PS, Koch-Henriksen N, Laursen B, Magyari M. Comorbidity in multiple sclerosis is associated with diagnostic delays and increased mortality. Neurology. 2017;89(16):1668–1675. doi: 10.1212/WNL.0000000000004508. [DOI] [PubMed] [Google Scholar]
- 45.Bruce JM, Jarmolowicz DP, Lynch S, et al. How patients with multiple sclerosis weigh treatment risks and benefits. Health Psychol. 2018;37(7):680–690. doi: 10.1037/hea0000626. [DOI] [PubMed] [Google Scholar]
- 46.Rahman H, Kim M, Leung G, Green JA, Katz S. Drug-Herb Interactions in the Elderly Patient with IBD: a Growing Concern. Curr Treat Options Gastroenterol. 2017;15(4):618–636. doi: 10.1007/s11938-017-0154-y. [DOI] [PubMed] [Google Scholar]
- 47.Sweet E, Dowd F, Zhou M, Standish LJ, Andersen MR. The Use of Complementary and Alternative Medicine Supplements of Potential Concern during Breast Cancer Chemotherapy. Evid Based Complement Alternat Med. 2016;2016:4382687. doi: 10.1155/2016/4382687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castle IJ, Dong C, Haughwout SP, White AM. Emergency Department Visits for Adverse Drug Reactions Involving Alcohol: United States 2005 to 2011. Alcohol Clin Exp Res. 2016;40(9):1913–1925. doi: 10.1111/acer.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onder G, Landi F, Della Vedova C, et al. Moderate alcohol consumption and adverse drug reactions among older adults. Pharmacoepidemiol Drug Saf. 2002;11(5):385–392. doi: 10.1002/pds.721. [DOI] [PubMed] [Google Scholar]
- 50.Hammond D, Reid JL, Zukowski S. Adverse effects of caffeinated energy drinks among youth and young adults in Canada: a Web-based survey. CMAJ Open. 2018;6(1):E19–E25. doi: 10.9778/cmajo.20160154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NIDA. Drug-Related Hospital Emergency Room Visits. 2011. https://www.drugabuse.gov/publications/drugfacts/drug-related-hospital-emergency-room-visits.
- 52.Subramaniam VN, Menezes AR, DeSchutter A, Lavie CJ. The Cardiovascular Effects of Marijuana: Are the Potential Adverse Effects Worth the High? Mo Med. 2019;116(2):146–153. [PMC free article] [PubMed] [Google Scholar]
- 53.Abdollahpour I, Nedjat S, Mansournia MA, Sahraian MA, van der Mei I. Lifestyle factors and multiple sclerosis: A population-based incident case-control study. Mult Scler Relat Disord. 2018;22:128–133. doi: 10.1016/j.msard.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Salzer J, Hallmans G, Nystrom M, Stenlund H, Wadell G, Sundstrom P. Smoking as a risk factor for multiple sclerosis. Mult Scler. 2013;19(8):1022–1027. doi: 10.1177/1352458512470862. [DOI] [PubMed] [Google Scholar]
- 55.Shariff EM, Al-Shammrani FJ, Nazish S, et al. Is non-traditional therapy for multiple sclerosis overwhelming in Saudi Arabia. Neurosciences (Riyadh) 2019;24(3):192–198. doi: 10.17712/nsj.2019.3.20180010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gotta M, Mayer CA, Huebner J. Use of complementary and alternative medicine in patients with multiple sclerosis in Germany. Complement Ther Med. 2018;36:113–117. doi: 10.1016/j.ctim.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017;17(1):185. doi: 10.1186/s12883-017-0960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennison L, Moss-Morris R. Cognitive-behavioral therapy: what benefits can it offer people with multiple sclerosis? Expert Rev Neurother. 2010;10(9):1383–1390. doi: 10.1586/ern.10.111. [DOI] [PubMed] [Google Scholar]
- 59.Monteiro L, Maricoto T, Solha I, Ribeiro-Vaz I, Martins C, Monteiro-Soares M. Reducing Potentially Inappropriate Prescriptions for Older Patients Using Computerized Decision Support Tools: Systematic Review. J Med Internet Res. 2019;21(11):e15385. doi: 10.2196/15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villalba-Moreno AM, Alfaro-Lara ER, Perez-Guerrero MC, Nieto-Martin MD, Santos-Ramos B. Systematic review on the use of anticholinergic scales in poly pathological patients. Arch Gerontol Geriatr. 2016;62:1–8. doi: 10.1016/j.archger.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Brockmoller J, Stingl JC. Multimorbidity, polypharmacy and pharmacogenomics in old age. Pharmacogenomics. 2017;18(6):515–517. doi: 10.2217/pgs-2017-0026. [DOI] [PubMed] [Google Scholar]
- 62.Yeandle D, Rieckmann P, Giovannoni G, Alexandri N, Langdon D. Patient Power Revolution in Multiple Sclerosis: Navigating the New Frontier. Neurol Ther. 2018;7(2):179–187. doi: 10.1007/s40120-018-0118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]