Summary
Background
Hospitals are sources for acquisition of carbapenem-resistant Enterobacteriaceae (CRE), and it is believed that the contamination of healthcare personnel (HCP) hands and clothing play a major role in patient-to-patient transmission of antibiotic-resistant bacteria.
Aim
The aim of this study was to determine which HCP types, HCP-patient interactions, and patient characteristics are associated with greater transmission of CRE to HCP gloves and gowns in the hospital.
Methods
This was a prospective observational cohort study that enrolled patients with recent surveillance or clinical cultures positive for CRE at five hospitals in four states in the United States. HCP gloves and gown were cultured after patient care. Samples were also obtained from patients’ stool, perianal area, and skin of the chest and arm to assess bacterial burden.
Results
Among 313 CRE-colonized patients and 3070 glove and gown cultures obtained after patient care, we found that HCP gloves and gowns were contaminated with CRE 7.9% and 4.3% of the time, respectively. Contamination of either gloves or gowns occurred in 10.0% of interactions. Contamination was highest (15.3%) among respiratory therapists (OR: 3.79, 95% CI: 1.61- 8.94) and when any HCP touched the patient (OR: 1.52, 95% CI: 1.10-2.12). We also found associations between being in the ICU, having a positive clinical culture, and increasing bacterial burden on the patient and CRE transmission to HCP gloves or gown.
Conclusion
CRE transmission to HCP gloves and gown occurred frequently. These findings may inform evidence-based policies about what situations and for which patients contact precautions are most critical.
Keywords: antimicrobial resistance, healthcare-acquired infections, epidemiology
Introduction
At the World Health Assembly in 2015, a global action plan was endorsed to tackle the urgent trend of antimicrobial resistance.1 Despite this, there are more than 2.8 million antibiotic-resistant infections in the United States each year, and more than 35,000 people die as a result of these infections.2 The World Health Organization and the U.S. Centers for Disease Control and Prevention (CDC) both classified carbapenem-resistant Enterobacteriaceae (CRE) as a “critical priority”3 and “urgent threat”.2 Hospitals are hotspots for acquisition of CRE, and it is believed that the contamination of healthcare personnel (HCP) hands and clothing play a major role in patient-to-patient transmission of antibiotic-resistant bacteria. This has led the CDC and the World Health Organization to recommend that HCP use appropriate personal protective equipment to decrease the spread of antibiotic-resistant bacteria such as CRE in acute care settings. Contact precautions (gloves and gown) are known to decrease the spread of antibiotic-resistant bacteria because HCP discard the contaminated gloves and gowns on room exit, prior to seeing the next patient.
As the world continues to grapple with the rapid spread of SARS-CoV-2 and hospitals respond to the influx of patients with COVID-19, transmission of antibiotic-resistant bacteria also continues. Limited data from case series suggests that it is reasonable to anticipate that some patients with severe COVID-19 will develop superinfections, many due to healthcare-associated bacteria such as CRE.4 Furthermore, personal protective equipment, including gloves and gowns, are in short supply in many regions and are being re-allocated to units caring for confirmed or suspected COVID-19 patients. Given this scarcity, it is especially critical now more than ever to better understand risk factors for transmission of high-consequence organisms such as CRE where morbidity and mortality are high. The aim of this study was to determine which HCP types and which HCP-patient interactions lead to greater transmission of CRE in the hospital.
Methods
Data collection
This prospective multi-center observational cohort study sought to identify which HCP types and patient care interactions are the greatest risk factors for CRE transmission to HCP’ gloves or gowns. Contamination of HCP gloves and gown is a surrogate for potential transmission to other patients in the hospital. CRE colonized patients admitted at two hospitals in Baltimore, MD, one in Pittsburgh, PA, one in Torrance, CA, and one in New York, NY were enrolled in the study between May 2017-August 2018. Eligible patients had a surveillance or clinical culture positive for CRE within seven days of enrollment. Research staff stationed themselves outside the patient room and recorded all items touched by the HCP while in the room using a standardized data collection form also used in previous studies.5–7 After patient care, but prior to removal, research staff cultured the HCP gloves and gown. For each HCP, a BBL dual Culturette swab (BD, Franklin Lakes, NJ) was rubbed in a twirling motion along the dorsum of each gloved finger and the palm of both hands. HCP gowns were sampled with a separate swab along each forearm and then in a W pattern along the beltline. All study sites used disposable gowns. We observed 10 HCP interactions per patient. Interactions were categorized into two domains: interactions with the patient domain or the environmental domain. We collected up to 4 patient samples (stool and perianal, arm skin, and chest skin swabs) to assess bacterial burden on a subset of patients. The Institutional Review Board at each facility granted approval for waived consent of participants.
Laboratory methods
HCP gloves and gowns:
For the gown and glove samples we enriched in BHI broth (Becton Dickenson, Sparks, MD), incubated the overnight and then plated the broths to MacConkey and streaked for isolation. Vitek MS was used for bacterial identification and antimicrobial susceptibility testing was used using disk diffusion following CLSI guidelines.8
Patient samples:
Samples from patients were serially diluted into Butterfield’s Buffer and the original sample and dilutions were plated in triplicate on Klebsiella pneumoniae carbapenemase (KPC) agar. Vitek MS was used for bacterial identification and antimicrobial susceptibility testing was used using disk diffusion following CLSI guidelines.9
Statistical analyses
We estimated associations between HCP glove or gown contamination and the following variables: 1) HCP type; 2) touching the patient or environmental domain; 3) specific patient-care interactions; and 4) patient bacterial burden. Risk factors significant at α ≤0.05 in the bivariate analyses were considered candidate predictors for the multivariable model. All models were built using logistic regression models fit by generalized estimating equations with an exchangeable correlation matrix to take into account within-patient correlation. Patient bacterial burden (x+1) was log transformed and is expressed in log10 colony forming units per milliliter or centimeter squared (CFU/mL or CFU/cm2). All analyses were conducted using SAS version 9.4 (The SAS Institute, Cary, NC).
Results
We enrolled a total of 313 patients with CRE and obtained at least one patient culture for quantification from 223 of these patients. We documented 3,070 HCP-patient interactions with a mean of 9.77 observations per patient. Most patients were enrolled in Maryland at Hospital A (n=148, 47%), followed by New York (n=56, 18%), Pennsylvania (n=53, 17%), Maryland Hospital B (n=37, 12%), and California (n=19, 6%). The most common CRE organism at the time of enrollment was Klebsiella pneumoniae (n=171, 53%) followed by Enterobacter cloacae (n=36, 11%), Enterobacter aerogenes (n=32, 10%), and Escherichia coli (n=33, 10%). Notably, 28/313 patients (8.9%) were co-colonized with at least 2 different CRE species and this patient subset is described in detail elsewhere.10 Just over half of the 313 patients were in the intensive care unit (ICU) at the time of enrolment (n=177, 57%). Most patients enrolled in the study were identified as a result of a positive clinical culture (n=237, 76%). Of the 237 clinical cultures, 69 (29%) were urine cultures, 61 (26%) were classified as from ‘other’ sources, 51 (22%) were from sputum, 30 (13%) were blood, and 26 (11%) were wound cultures. A large proportion of patients had a wound (n=238, 76%), a central/peripherally inserted central catheter (n=189, 60%), or an endotracheal tube (n=155, 50%) (Table I).
Table I.
Description of study patients with CRE (N=313)
| Study site | N (%) |
|---|---|
| Maryland- Hospital A | 147 (47.3) |
| New York | 56 (17.9 |
| Pennsylvania | 53 (16.9) |
| Maryland-Hospital B | 37 (11.8) |
| California | 19 (6.1) |
| ICU | N (%) |
| Yes | 177 (56.5) |
| No | 136 (43.5) |
| Culture source | |
| Clinical | 237 (75.5) |
| Surveillance | 77 (24.5) |
| Clinical characteristics of patients | N (%) |
| Wound | 238 (76.0) |
| Central/peripherally inserted central catheter | 189 (60.4) |
| Endotracheal tube | 155 (49.5) |
| Nasogastric tube | 138 (44.1) |
| Foley catheter | 128 (40.9) |
| Diarrhea | 105 (33.5) |
| Surgical drain | 94 (30.0) |
| Rectal tube | 53 (16.9) |
| Chest tube | 26 (8.3) |
| CRE organism* | N (%) |
| Klebsiella pneumoniae | 171 (53.2) |
| Enterobacter cloacae | 36 (11.2) |
| Enterobacter aerogenes | 32 (10.0) |
| Escherichia coli | 33 (10.3) |
| Citrobacter freundii | 18 (5.6) |
| Klebsiella oxytoca | 12 (3.7) |
| Proteus mirabilis | 11 (3.4) |
| Serratia marcescens | 7 (2.2) |
| Pantoea sp. | 1 (0.3) |
Denominator is 321 because some patients had more than one CRE at the time of enrollment
Overall, we found that 242/3070 HCP-patient interactions (7.9%) led to transmission to gloves, 132/3070 interactions (4.3%) led to transmission to gowns, and transmission to gloves or gowns occurred in 308/3070 interactions (10.0%).
ICU status and culture source
Patients who were in the ICU at the time of enrollment transmitted CRE to HCP gloves or gown 12.0% of the time whereas those not in the ICU transmitted 7.4% of the time. When adjusted for culture source and HCP type, those in the ICU still had higher odds of CRE transmission (OR: 1.71, 95% CI: 1.10-2.66). Patients who were enrolled with a positive clinical culture had increased odds of transmission when compared to patients enrolled with a positive surveillance culture when adjusted for ICU status and HCP type (OR: 1.93, 95% CI: 1.12-3.31).
Healthcare personnel type
The odds of CRE transmission to gloves or gown differed by HCP type (Table II). Respiratory therapists had the highest odds of glove or gown contamination with 15.3% of all interactions resulting in CRE contamination (adjusted odds ratio OR: 3.79 [95% confidence interval [CI]: 1.61- 8.94]). The second highest odds of contamination were observed among occupational and physical therapists (OR: 2.82 [95% CI: 0.96- 9.32]) followed by environmental service employees (OR: 2.68 [95% CI: 1.13-6.37]) when compared to HCP in the “Other” category (e.g., social workers, nutritionists, etc.).
Table II.
Association between healthcare personnel type and contamination of gloves or gown with CRE
| Type of healthcare personnel (N=3,070) | Number of observations n (%) | Interactions resulting in contamination (%) | aOR (95% CI) | p-value |
|---|---|---|---|---|
| Respiratory therapist | 157 (5.1) | 15.3 | 3.79 (1.61-8.94) | 0.002 |
| Occupational/physical therapist | 97 (3.2) | 9.3 | 2.82 (0.96-9.32) | 0.06 |
| Environmental services | 174 (5.7) | 10.3 | 2.68 (1.13-6.37) | 0.03 |
| Nurse | 1605 (52.3) | 11.6 | 2.50 (1.28-4.86) | 0.007 |
| Patient care technician | 329 (10.7) | 7.9 | 1.56 (0.70-3.46) | 0.28 |
| Medical doctor/nurse practitioner | 462 (15.0) | 6.5 | 1.09 (0.50-2.37) | 0.83 |
| Other* | 246 (8.0) | 5.7 | Ref | Ref |
Includes: social workers, nutritionists, researchers, etc.
Adjusted for: culture source and ICU status
Abbreviations: CI, confidence interval; OR, odds ratio
Touching the patient and the environment
Among interactions that involved any patient contact (when the HCP touched patient only or both patient and environment), we observed a 52% increase in transmission of CRE to HCP gloves or gown (OR: 1.52, 95% CI: 1.10-2.12) (Table III and Figure 1) as compared to interactions that did not include any patient contact. HCP in the study touched a median of 2 different items in the patient domain and 2 different environmental items. The odds of transmission increased with the number of different patient touches (OR: 1.31, 95% CI: 1.20-1.42) for each additional touch and with the number of different items touched in the environment (OR: 1.09, 95% CI: 1.01-1.18) when adjusted for culture source, ICU status, and HCP type.
Table III.
Association between touching patient and environmental domains and contamination of gloves or gown with CRE
| Domain touched (N=3070) | Number of observations n (%) | Interactions resulting in contamination (%) | aOR (95% CI) | p-value |
|---|---|---|---|---|
| Patient only | 522 (17.0) | 8.8 | 0.85 (0.58-1.27) | 0.43 |
| Environment only | 405 (13.2) | 5.9 | 0.61 (0.39-0.95) | 0.03 |
| Any patient contact | 2073 (67.5) | 11.4 | 1.52 (1.10-2.12) | 0.01 |
| Nothing | 70 (2.3) | 2.9 | 0.28 (0.07-1.12) | 0.07 |
Any patient contact: patient only or both patient and environment
Adjusted for: culture source, ICU status, and healthcare personnel type
Figure 1.
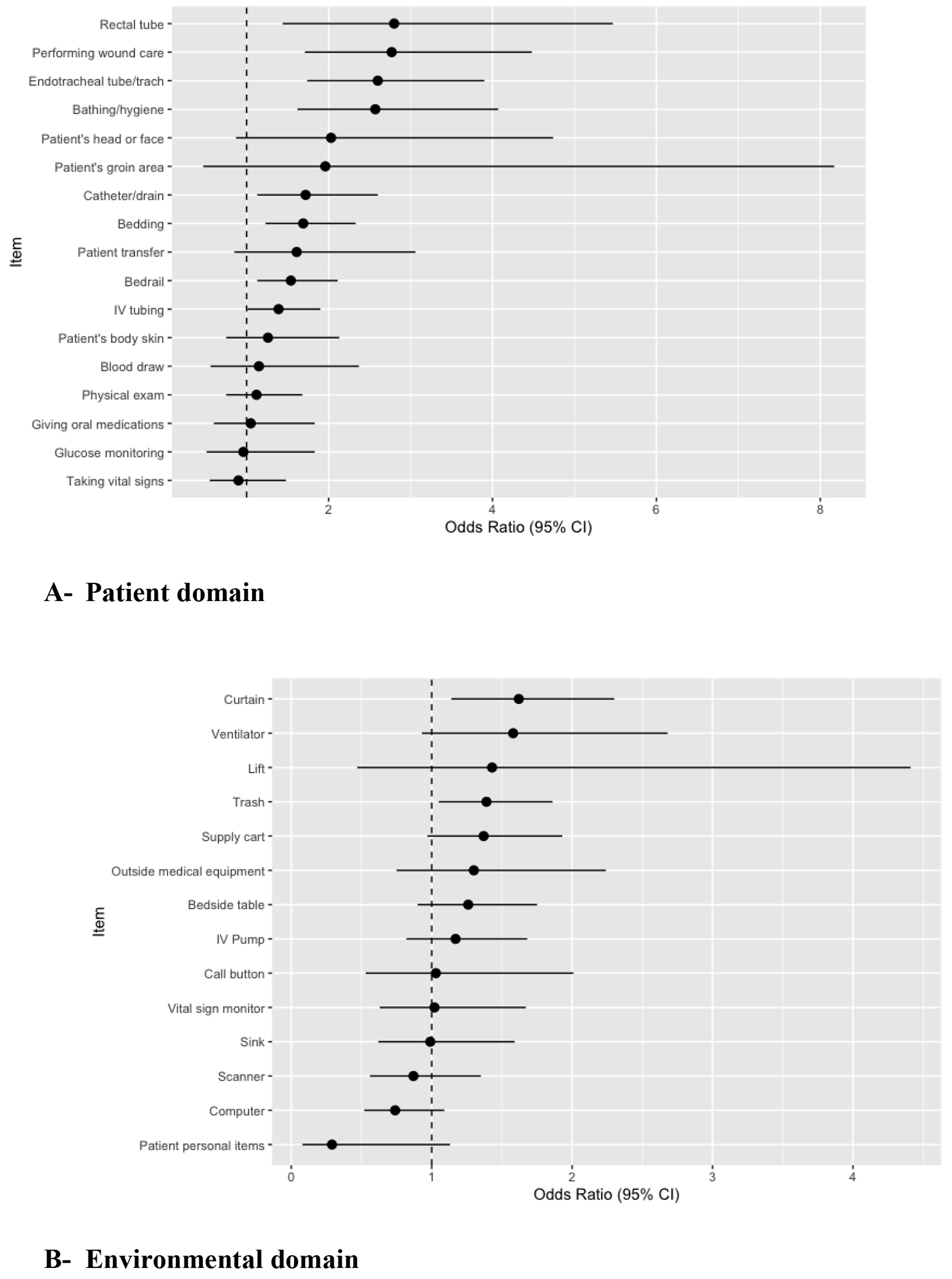
Adjusted Odds Ratios and 95% confidence intervals of healthcare personnel glove or gown contamination with CRE for each individual patient care activity
*Adjusted for culture type, ICU status, and healthcare worker type
Figures 1A and 1B show the adjusted odds ratios and 95% confidence intervals for the outcome of CRE transmission to HCP gloves or gown for each individual patient care interaction. In the patient domain (Figure 1A), touching the rectal tube (OR: 2.84, 95% CI: 1.44-5.58), providing wound care (OR: 2.76, 95% CI: 1.71-4.46), touching the endotracheal tube (OR: 2.50, 95% CI: 1.68-3.65), assisting the patient with bathing or hygiene activities (OR: 2.48, 95% CI: 1.57-3.90), touching the catheter or drain (OR: 1.68, 95% CI: 1.09-2.57), touching the bedding (OR: 1.67, 95% CI: 1.22-2.29), and touching the bedrail (OR: 1.51, 95% CI: 1.12-2.05) were all associated with increased odds of transmission when compared to not touching these individual items and when adjusted for HCP type, culture source, and ICU status. In the environmental domain (Figure 1B), touching the curtain (OR: 1.61, 95% CI: 1.1-2.20) and touching the trash (OR: 1.40, 95% CI: 1.06-1.87) were associated with increased odds of transmission when compared to not touching these items and adjusting for HCP type, culture source, and ICU status.
CRE bacterial burden
Among the subset of patients from whom we were able to obtain additional patient samples for quantitative analysis, CRE was identified in 83/133 (62%) of stool samples, in 84/176 (48%) of perirectal samples, 21/223 (9%) of arm skin samples, and 25/223 (11%) of chest skin samples. For all four sample types, we found an association between increasing bacterial burden on the patient (per log10 increase) and CRE transmission to HCP gloves or gown; for stool, OR: 1.23, 95% CI: 1.14-1.33, for the perianal, OR: 1.22, 95% CI: 1.12-1.32, for the arm skin, OR: 1.46, 95% CI: 1.28-1.66, and for the chest skin OR: 1.31, 95% CI: 1.15-1.49 (Table IV). When we dichotomized the exposure variable to compare those patients with any detectable CRE in their stool to those with none, we found that there was a 4.33 fold increase in HCP glove and gown contamination (OR: 4.33, 95% CI: 2.22-8.42). Similarly, contamination increased for patients with any detectable CRE on the perianal sample (OR: 3.44, 95% CI: 1.93-6.12), by 412% for patients with any CRE on the arm (OR: 5.12, 95% CI: 2.93-8.95), and for patients with any CRE on the chest (OR: 4.07, 95% CI: 2.38-6.95).
Table IV.
Associations between CRE bacterial burden and contamination of gloves or gown with CRE by body site
| Body site samples | OR (95% CI) | p-value |
|---|---|---|
| Stool (log10 CFU/mL) | 1.23 (1.14-1.33) | <0.0001 |
| Perianal (log10 CFU/mL) | 1.22 (1.12-1.32) | <0.0001 |
| Arm skin (log10 CFU/cm2) | 1.46 (1.28-1.66) | <0.0001 |
| Chest skin (log10 CFU/ cm2) | 1.31 (1.15-1.49) | <0.0001 |
Discussion
The major mechanism of patient-to-patient transmission in the healthcare setting is the contamination of HCP hands and clothing with antibiotic-resistant bacteria. HCP become contaminated with antibiotic-resistant bacteria from one patient and can then transmit the bacteria to other patients.11–14 Contact precautions decrease transmission of antibiotic-resistant bacteria because HCP discard the contaminated gloves and gowns on room exit, prior to seeing the next patient. This multicenter cohort study demonstrates that healthcare workers’ gloves and gowns are frequently contaminated with CRE after caring for patients with CRE both in the ICU and non-ICU setting. We also found that certain healthcare workers more frequently became contaminated than others and that certain activities such as touching the patient led to higher rates of contamination.
The study design was very similar to what our group has previously used to study Methicillin-resistant Staphylococcus aureus (MRSA) transmission risks5. The similarity of the findings despite there being a number of differences between the biology of MRSA and the different species in this study that comprise CRE is striking. Both studies found that physical therapists, occupational therapists and respiratory therapists were at increased risk glove and gown contamination compared to other professions including doctors and nurses. Both studies found that touching the patient led to increased glove and gown contamination. And both studies found that increased bacterial colonization on the skin, stool, peri-rectal area and nose for MRSA, and stool and skin for CRE led to increased glove and gown contamination. Since this study enrolled patients from all areas of the hospital, we were able to assess the effect of being in the ICU and found that the gloves and gowns of HCP caring for ICU patients were more frequently contaminated with CRE when compared to patients who were not in the ICU. This suggests that it may be appropriate to consider implementation of some interventions to decrease spread of antibiotic resistant bacteria only in the ICU if resources are limited.
The finding that certain healthcare workers are at increased risk for CRE and MRSA glove and gown contamination warrants further studies. This may be because some HCP, such as respiratory therapists, have extensive physical contact with patients or routine exposure to secretions, but more work is needed on which individual activities are leading to these healthcare workers being at greater risk. It is also possible that allied health professions (such as respiratory therapists, physical and occupational therapists) may not receive as much infection prevention and control training as nurses and physicians. It also suggests that these healthcare workers should be prioritized to continue using contact precautions when caring for patients with CRE and MRSA if there are personal protective equipment shortages. In times of severe shortages such as during a pandemic, hospitals may consider continuing to recommend use of gloves and gowns for the highest risk HCP, activities, and patients and not requiring them for low risk HCP, activities, and patients.
The finding that bacterial burden leads to increased glove and gown contamination is interesting and suggests that certain interventions need to be studied to see if they can affect transmission. Selective gut decontamination is not widely used in the United States but is more widely used in Europe.15,16 Future studies should be performed to assess whether selective gut decontamination could lead to decreases in glove and gown contamination and thus potentially subsequent spread of antibiotic-resistant bacteria. In addition, it will be interesting to see if future work on probiotics that may lower concentrations of antibiotic-resistant bacteria in the intestinal flora could lead to not only lower infection rates for the patient taking the probiotic but also other patients due to decreased patient to patient transmission.
To our knowledge, our study is the largest and first multi-center study of CRE analyzing risk factors for glove and gown contamination. Despite this strength, limitations of our study are as follows. We did not culture items in the patient’s environment and did not collect any information regarding quality and timing of room cleaning. Second, we did not culture the entire surface of the HCP gloves and gowns and thus might have underestimated the percentage of gloves and gowns that became contaminated. However, we did use a standardized technique thought to maximize recovery of CRE without interfering with patient care.5,17–20 We also acknowledge that high rates of glove and gown contamination do not necessarily lead to high rates of subsequent patient transmission and that this last step in transmission was not part of this study.
Conclusions
The pros and cons of contact precautions have been widely debated.21–27 We believe that the work we and others are performing will hopefully lead to the identification of which patients are at high risk of transmission and need to be placed on contact precautions and when and which patients may not require contact precautions. This could lead to precision public health to be brought into the field of antibiotic resistance: the right type of precautions, for the right type of encounter at the right time. This issue has become particularly important during the SARS-CoV-2 pandemic. Many institutions have been forced to make difficult decisions about how to use limited supplies of gloves, gowns and masks. This has led to many institutions abandoning the use of contact precautions for patients with antibiotic-resistant bacteria. The results of this study may contribute to more evidence-based policies about what situations and for which patients contact precautions for CRE are most critical, particularly during times of limited supplies of personal protective equipment such as gloves and gowns.
Acknowledgements
The authors thank Georgia Papaminas, Shirley Goodman, Evelyn Flores, Bryn Launer, Erin Rieger, Katharine Robb, Barbara Kuklinksa, and Timileyin Adediran for help with data collection, specimen shipping, and review of the literature.
Funding source
This work was supported by the CDC Prevention Epicenter Program (U43CK000450-01 to A.D.H.) and the NIH National Institute of Allergy and Infectious Diseases (R01 AI121146-01 to A.D.H.).
Footnotes
Conflict of interest statement
The authors have no conflicts of interest.
References
- 1.Global action plan on antimicrobial resistance. Accessed July 8, 2020. https://www.who.int/publications-detail-redirect/global-action-plan-on-antimicrobial-resistance
- 2.CDC. The biggest antibiotic-resistant threats in the U.S. Centers for Disease Control and Prevention. Published May 7, 2019. Accessed August 7, 2019. https://www.cdc.gov/drugresistance/biggest_threats.html [Google Scholar]
- 3.Tacconelli E, Magrini N, Kahlmeter G, Singh N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization. 2017. February 27;27:318–27. [Google Scholar]
- 4.Clancy CJ, Nguyen MH. COVID-19, superinfections and antimicrobial development: What can we expect? Clin Infect Dis. Published online May 1, 2020. doi: 10.1093/cid/ciaa524 [DOI] [PMC free article] [PubMed]
- 5.O’Hara LM, Calfee DP, Miller LG, et al. Optimizing Contact Precautions to Curb the Spread of Antibiotic-resistant Bacteria in Hospitals: A Multicenter Cohort Study to Identify Patient Characteristics and Healthcare Personnel Interactions Associated With Transmission of Methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2019;69(Supplement_3):S171–S177. doi: 10.1093/cid/ciz621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson SS, Harris AD, Magder LS, et al. Bacterial burden is associated with increased transmission to health care workers from patients colonized with vancomycin-resistant Enterococcus. Am J Infect Control. 2019;47(1):13–17. doi: 10.1016/j.ajic.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadimpalli G, O’Hara LM, Pineles L, et al. Patient to Healthcare Personnel Transmission of MRSA in the Non-Intensive Care Unit Setting. Infect Control Hosp Epidemiol. Published online February 10, 2020. doi: 10.1017/ice.2020.10 [DOI] [PMC free article] [PubMed]
- 8.CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement. Vol CLSI Document M100-S21. Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- 9.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. Vol 27th. Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 10.Adedrian T, Harris AD, Johnson JK, et al. Epidemiologic and microbiologic characteristics of hospitalized patients co-colonized with multiple species of carbapenem-resistant Enterobacteriaceae in the United States. Open Forum Infectious Diseases. Published online In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan DJ, Rogawski E, Thom KA, et al. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med. 2012;40(4):1045–1051. doi: 10.1097/CCM.0b013e31823bc7c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popovich KJ, Green SJ, Okamoto K, et al. MRSA transmission in ICUs: Genomic analysis of patients, their environments and healthcare workers. Clin Infect Dis. Published online June 6, 2020. doi: 10.1093/cid/ciaa731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4(148):148ra116. doi: 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer MD, Winglee K, Passaretti C, et al. Whole Genome Sequencing detects Inter-Facility Transmission of Carbapenem-resistant Klebsiella pneumoniae. J Infect. 2019;78(3):187–199. doi: 10.1016/j.jinf.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvestri L, van Saene HKF. Selective decontamination of the digestive tract: an update of the evidence. HSR Proc Intensive Care Cardiovasc Anesth. 2012;4(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- 16.Walden AP, Bonten MJ, Wise MP. Should selective digestive decontamination be used in critically ill patients? BMJ. 2012;345:e6697. doi: 10.1136/bmj.e6697 [DOI] [PubMed] [Google Scholar]
- 17.Jackson SS, Thom KA, Magder LS, et al. Patient contact is the main risk factor for vancomycin-resistant Enterococcus contamination of healthcare workers’ gloves and gowns in the intensive care unit. Infect Control Hosp Epidemiol. 2018;39(9):1063–1067. doi: 10.1017/ice.2018.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder GM, Thom KA, Furuno JP, et al. Detection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on the gowns and gloves of healthcare workers. Infect Control Hosp Epidemiol. 2008;29(7):583–589. doi: 10.1086/588701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineles L, Morgan DJ, Lydecker A, et al. Transmission of methicillin-resistant Staphylococcus aureus to health care worker gowns and gloves during care of residents in Veterans Affairs nursing homes. Am J Infect Control. 2017;45(9):947–953. doi: 10.1016/j.ajic.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roghmann M-C, Johnson JK, Sorkin JD, et al. Transmission of Methicillin-Resistant Staphylococcus aureus (MRSA) to Healthcare Worker Gowns and Gloves During Care of Nursing Home Residents. Infect Control Hosp Epidemiol. 2015;36(9):1050–1057. doi: 10.1017/ice.2015.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan DJ, Wenzel RP, Bearman G. Contact Precautions for Endemic MRSA and VRE: Time to Retire Legal Mandates. JAMA. 2017;318(4):329–330. doi: 10.1001/jama.2017.7419 [DOI] [PubMed] [Google Scholar]
- 22.Dhar S, Marchaim D, Tansek R, et al. Contact precautions: more is not necessarily better. Infect Control Hosp Epidemiol. 2014;35(3):213–221. doi: 10.1086/675294 [DOI] [PubMed] [Google Scholar]
- 23.Anderson DJ, Weber DJ, Sickbert-Bennett E. On contact precautions: the good, the bad, and the ugly. Infect Control Hosp Epidemiol. 2014;35(3):222–224. doi: 10.1086/675295 [DOI] [PubMed] [Google Scholar]
- 24.Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163–1172. doi: 10.1017/ice.2015.156 [DOI] [PubMed] [Google Scholar]
- 25.Rubin MA, Samore MH, Harris AD. The Importance of Contact Precautions for Endemic Methicillin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococci. JAMA. 2018;319(9):863–864. doi: 10.1001/jama.2017.21122 [DOI] [PubMed] [Google Scholar]
- 26.Maragakis LL, Jernigan JA. Things We Do For Good Reasons: Contact Precautions for Multidrug-resistant Organisms, Including MRSA and VRE. J Hosp Med. 2019;14(3):194–196. doi: 10.12788/jhm.3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young K, Doernberg SB, Snedecor RF, Mallin E. Things We Do For No Reason: Contact Precautions for MRSA and VRE. J Hosp Med. 2019;14(3):178–180. doi: 10.12788/jhm.3126 [DOI] [PubMed] [Google Scholar]


