Abstract
Chimeric antigen receptor (CAR) T-cell therapy has significantly advanced the treatment of patients with relapsed and refractory hematologic malignancies and is increasingly investigated as therapeutic option of other malignancies. The main adverse effect of CAR T-cell therapy is potentially life-threatening cytokine release syndrome (CRS). Clinical cardiovascular (CV) manifestations of CRS include tachycardia, hypotension, troponin elevation, reduced left ventricular ejection fraction (LVEF), pulmonary edema and cardiogenic shock. Although insults related to CRS toxicity might be transient and reversible in most instances in patients with adequate CV reserve, they can be particularly challenging in higher-risk, often elderly patients with pre-existing CV disease. As the use of CAR T-cell therapy expands to include wider patient population, careful patient selection, pre-treatment cardiac evaluation and CV risk stratification should be considered within the CAR T-cell treatment protocol. Early diagnosis and management of CV complications in patients with CRS require awareness and multidisciplinary collaboration.
Keywords: Chimeric antigen receptor T-cell therapy, CAR T cell therapy, cytokine release syndrome, CRS, cardiotoxicity
Condensed Abstract:
The main adverse effect of CAR T-cell therapy is CRS. Clinical CV manifestations of CRS include tachycardia, hypotension, troponin elevation, reduced LVEF, pulmonary edema and cardiogenic shock. Pre- and during treatment CV assessment may identify patients with diminished CV reserve, allow early diagnosis of cardiac injury and high mortality risk. Management of CRS includes hemodynamic support and consideration of tocilizumab, a monoclonal anti-IL-6 receptor antibody, often in the critical care setting with multidisciplinary collaboration.
Introduction
Chimeric-antigen receptor (CAR) T-cell therapy has emerged as a promising new therapy for refractory hematologic malignanicies, offering a treatment option where none had existed. Since the breakthrough cure of Emily Whitehead in 2012 that captured the world’s attention, the first pediatric patient to be treated with CAR T-cell therapy after two relapses of acute lymphoblastic leukemia (ALL),(1) CAR T-cell therapy has been approved for treatment of children with ALL and adults with advanced B-cell lymphoma.(2) In the recent years, the number of clinical trials testing CAR T-cells has increased dramatically with over 180 clinical trials worldwide and various CAR T-cell products in development, mostly for treatment of blood cancers but also in patients with solid tumors.(3) However, like other cancer therapies, CAR T-cells can cause severe, and sometimes fatal, side effects. One of the most common is the cytokine release syndrome (CRS) which can lead to hemodynamic instability and cardiogenic shock.(4) In this review, we summarize the current indications for CAR T-cell therapy and its mechanism of action as an anti-cancer immunomodulatory agent. We describe the cardiovascular (CV) manifestions of CRS and present a practical approach to CV assessment and management during CAR T-cell therapy.
Clinical Vignette
A 54-year-old woman with history of non-Hodgkin’s follicular lymphoma since 1999, with later transformation to diffuse large B-cell lymphoma (DLBCL), had received multiple chemotherapeutic regimens including rituximab, anthracycline-based combination chemotherapy, ibrutinib and most recently idelalisib, a phosphoinositide 3-kinase delta inhibitor. On further disease progression she received infusion of anti-CD-19 CAR T-cells and immediately following the first infusion, developed high-grade fever, tachycardia, tachypnea and mild hypotension. She developed hypoxic respiratory failure requiring mechanical ventilation and severe hypotension, despite intravenous fluid rescusitation, requiring vasopressor support. Echocardiogram demonstrated decreased left ventricular ejection fraction (LVEF) of 25% and laboratory testing showed elevated markers of inflammation and evidence of acute renal failure. This case illustrates clinical manifestations of high grade CRS with hemodynamic instability and cardiac failure. Diagnosis and management strategies for CRS and CV complications are discussed in the review.
Mechanism of CAR T-cell Therapy
Antitumor immunity comprises complementary innate and adaptive immune responses. Adaptive immunity is antigen-specific and mediated by B and T-lymphocytes involving antigen-presenting cells such as dendritic cells.(5) The pivotal obstacle in the successful development of anti-neoplastic immunotherapy is that most tumor antigens are also expressed on normal tissues (self antigens) which makes antitumor responses often transient or ineffective, owing to host responses that evolved to prevent autoimmunity.(6) Advancement in T-cell engineering has helped overcome immune tolerance.(6)
CAR are engineered receptors that graft tumor specificity to T-cells and augment T-cell function. (7) The CAR is a synthetic fusion protein consisting of the variable portion of an antibody, or single-chain variable fragment that can target an antigen displayed on the surface of a tumor cell. In CAR T-cell immunotherapy, the patient’s own T-cells are extracted, genetically engineered to target tumor-associated antigens, expanded, and infused back into the patient’s body where they continue to multiply, recognize and destroy cancer cells (Figure 1). Once infused into the patient, CAR T-cells engraft and can undergo extensive proliferation. Each CAR T-cell can kill many tumor cells, and also promote immune surveillance to prevent tumor recurrence through antigen release, by assisting tumor-infiltrating lymphocytes to attack tumors, or by their own persistence. The first two approved CAR T-cell therapies targeted the CD-19 protein, which is broadly expressed on most B-cell malignancies and has limited expression beyond B-cell lineage.(2) More recently, other CAR T-cell therapies have shown encouraging results with targeting B-cell maturation antigen in multiple myeloma (8) or targeting CD-22, in patients with B-cell precursor ALL who have relapsed following treatment with CD-19 CAR T-cell therapy.(9)
Figure 1. Mechanism of CAR T-cell therapy and Cytokine Release Syndrome.
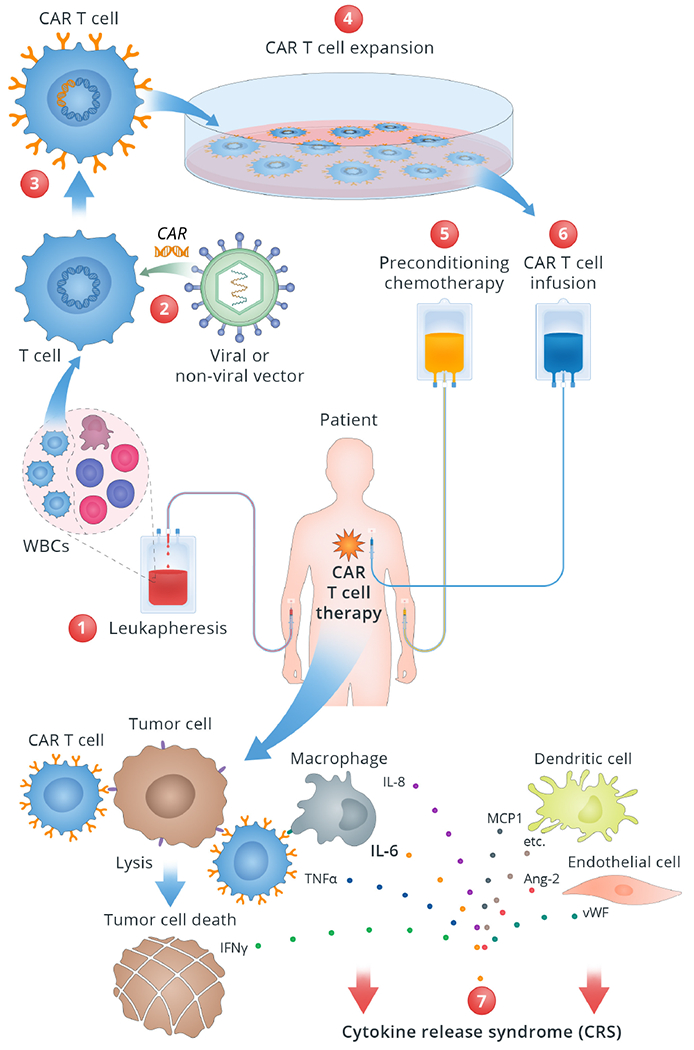
This figure illustrates stepwise mechanism of CAR T-cell therapy and related CRS.
1. White blood cells are drawn from the patient via leukapheresis. T cells are then separated in the Lab. 2. T cells are genetically modified or “engineered” with viral or nonviral vector inserting a gene encoding a chimeric antigen receptor into the T cells. 3. Engineered T cells, now known as CAR T cells, can recognize and attach to the specific antigen on the cancer cells. 4. CAR T cells are grown and multiplied in the bioreactor to create millions of copies. 5. Before the CAR T cells are administered, the patient receives preconditioning chemotherapy to lower the cell count to allow space for the incoming CAR T cells. 6. CAR T cells are infused back into the patient’s blood where they proliferate, detect and destroy the tumor cells. 7. CRS occurs as a result of the supraphysiologic levels of inflammatory cytokines released by the activated CAR T-cells and other immune cells such as macrophages.
Abbreviations: Ang-2: Angiopoetin 2; CAR: chimeric antigen receptor; IL: interleukin; IFN-γ: interferon gamma; MCP1: Monocyte chemoattractant protein 1; TNF-α: tumor necrosis factor alpha; vWF: von Willebrand factor.
Overview of Clinical Trials and Efficacy
In 2017, the U.S. FDA approved the first genetically-modified autologous T-cell immunotherapeutic agents that target CD-19, tisagenlecleucel (Kymriah®, Novartis) and axicabtagene ciloleucel (Yescarta®, Kite, Gilead), marking a milestone in the development of this novel therapeutic strategy. (2,10–12)
Tisagenlecleucel was initially approved for children and young adults up to 25 years of age with relapsed or refractory B-cell precursor ALL. In the phase 2 trial, the complete remission (CR) rate defined by hematologic remission within three months of treatment was 60%, and the overall clinical remission was 81%.(10) Approval for tisagenlecleucel treatment was expanded in May 2018 to include adult patients with large B-cell lymphoma relapsed/refractory after two or more lines of systemic therapy, including DLBCL not otherwise specified, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma.(12) Axicabtagene ciloleucel was approved in October 2017 and is indicated in adult patients with large B-cell lymphoma relapsed/refractory after two or more lines of systemic therapy, including DLBCL not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. Fifty one of the 101 patients treated on the pivotal trial reached CR.(11) It is noteworthy that the reported CAR T-cell therapy efficacy was not based on the intention-to-treat analysis as patients who discontinued participation before the infusion of CAR T-cells, either due to disease progression or death, were not included in the primary analysis.(11)
CD-19 CAR T-cell therapy appears to be less effective for non-Hodgkin’s lymphoma and chronic lymphocytic leukemia as compared to ALL. In adult patients with DLBCL, various CD-19 CAR T-cells have reported CR rates of 40-50% and partial response rates of 12-30%.(11,12) While the CR rates are lower in DLBCL compared to ALL, responses appear more durable with 70-80% of responding patients achieving long-term remission beyond 12 months.(10–13) A recent phase-I clinical trial of CAR T-cells targeting B cell maturation antigen demonstrated highly encouraging results (response rate 85%) in relapsed/refractory multiple myeloma.(8)
In addition to oncology applications, there is growing enthusiasm to explore the utility of genetically engineered T-cells in the treatment of autoimmune disease, infection, inflammation and fibrosis.(14) CAR T-cell products under development for cancer treatment differ from one another in several ways, including the antigen they are engineered to target, cellular switches, and the viral vector used. As of January 2018, the Cancer Research Institute reported 291 different CAR T-cell therapies, with 162 of them being tested clinically.(3)
Adverse Effects of CAR T-Cell Therapy
Cytokine Release Syndrome
CRS is the most known toxicity of CAR T-cells and consists of a constellation of signs and symptoms caused by supraphysiologic levels of inflammatory cytokines released by the activated CAR T-cells and other immune cells such as macrophages (Figure 1).(15,16) CRS has previously been associated with immunomodulatory therapies such as high dose IL-2, used in the early 1990s for metastatic renal carcinoma, and infusion of monoclonal antibodies, including anti-CD3 (OKT3), anti-CD52 (alemtuzumab) and anti-CD20 (rituximab).(17)
The hallmarks of CRS are fever and tachycardia that may be associated with hypotension and hypoxia. It is a systemic inflammatory response that can affect multiple organs, ranging in severity from mild to severe, with life-threatening conditions including cardiac dysfunction, adult respiratory distress syndrome, neurologic toxicity, coagulopathy, liver and renal failure (Table 1). (18) CRS is frequent with CAR T-cell infusion and has been reported in 70-90% of patients in recent studies.(4) Although toxic effects mediated by the cytokine release largely remain mild in severity, as many as 50% of patients can develop life-threatening complications such as vascular leak syndrome with circulatory collapse and multiorgan failure.(19) The severity of CRS toxicity correlates with the disease burden at the time of treatment and higher infused CAR T-cell dose.(20,21) Symptom onset can occur within minutes to hours or days after infusion begins, coinciding with maximal T-cell expansion. Fever and elevated plasma cytokine levels within 36 hours of CAR T-cell infusion have been shown to predict CRS development. IL-6 has been implicated as a key mediator of the systemic adverse effects.(22) The IL-6 signaling pathway is activated via the interaction of IL-6 with its specific receptor (IL-6R), and monocyte-lineage antigen presenting-cells (APCs).(23) There is no evidence to date that T-cells or CAR T-cells may be a significant source of IL-6. Although IL-6 production by APCs occurs in response to CAR T-cell mediated recognition of malignant cells, it is independent of direct contact between CAR T-cell and APCs. IL-6 signaling may occur either through the higher-affinity membrane-bound receptor (classic IL-6 signaling) or via a soluble IL-6 receptor (sIL-6R; trans-IL-6 signaling).(23) Recent evidence also points to activated endothelial cells as an important source of IL-6 production and with a significant modulatory role in CRS severity.(16) For example, endothelial dysregulation contributes to many of the key symptoms of CRS such as vascular leak syndrome and neurotoxicity due to disruption of blood brain barrier.
Table 1.
Clinical Manifestation of CRS related toxicities*
| Organ System | Manifestation |
|---|---|
| Constitutional | Fever, malaise, fatigue, anorexia, arthralgias |
| Cardiovascular | Tachycardia, widened pulse pressure, hypotension/shock, arrhythmias, pulmonary edema, decreased LV ejection fraction, troponinemia, QT prolongation |
| Renal | Acute kidney injury, tumor lysis syndrome |
| Pulmonary | Hypoxia, pulmonary edema |
| Hepatic | Transaminitis |
| Hematologic | Anemia, thrombocytopenia, coagulopathy |
Some of the toxicities may in part be attributed to the lymphodepletion regimen used prior to CAR T-cell infusion and to acute volume changes
There are currently no studies directly comparing the safety or efficacy of the approved CAR T-cell products and the comparisons are further limited by the use of different CRS grading systems in reported clinical trials. Data from the early studies identified earlier onset of CRS (median day 2 vs. day 3) and a higher incidence of all grade CRS (93% vs. 58%) with axicabtagene ciloleucel compared to tisagenlecleucel, respectively.(11,12) In patients receiving axicabtagene ciloleucel, CRS treatment was also associated with higher use of anti-IL6 antibody, tocilizumab, and corticosteroids, as compared to patients who received tisagenlecleucel. Whether these differences in CRS may translate into similar differences in cardiac events and/or outcomes will require additional research and comparisons between the two products.
The grading of CRS has varied among different centers using commercially approved and investigational T-cell therapy, making toxicity and management comparisons between studies difficult. Several CRS grading systems (CTCAE v5.0, Lee, Penn, MSKCC, CARTOX criteria) with different grading criteria have been used. (4,13,19) Recently, the American Society for Transplantation and Cellular Therapy assembled a multidisciplinary expert group and published a consensus CRS grading scale using fever, hypotension and hypoxia as principal determinants and grading of severity based on the need for vasopressor support and supplemental oxygen requirement.(24) The intent of the consensus grading system is to provide a simple, objective and practical clinical algorithm that may be applied across different trials and provide consistent categories of the severity of CRS toxicities.
CRS-related Cardiovascular Effects
CAR T-cell therapy associated CV effects have been mostly reported in the context of CRS limited to early clinical trials and case reports. (25) Clinical CV manifestations of CRS include a spectrum of adverse effects. Tachycardia occurs often with fever. With more severe CRS, troponin elevation, hypotension, reduced LVEF, and cardiogenic shock requiring vasopressor inotropic support can occur. Hypotension requiring inotropic support with or without LV systolic dysfunction has been reported in 24% of children with ALL receiving CAR T-cell therapy. (25) In one trial of CD19 CAR T-cells for the treatment of lymphoma, 13.6% of patients required vasopressor support for hypotension.(26) Predictors of hypotension requiring inotropic support included pre-existing systolic dysfunction, diastolic dysfunction, and ECG abnormalities, as well as greater hematologic disease burden before treatment.(25) QT prolongation and arrhythmias such as atrial fibrillation have also been reported.(18) In the pivotal studies, myocardial infarction was not observed, (10–12) cardiac arrest was reported in 4 patients with death occurring in one patient, and cardiac failure in 2 patients following CAR T-cell therapy. (10) (Table 2)
Table 2.
Incidence of CRS and CV complications in Pivotal Trials
| Clinical Trial | Type of cancer | Type of CAR T-cell therapy | CRS (%) | MI | Cardiac arrest | Cardiac failure | Death due to CRS |
|---|---|---|---|---|---|---|---|
| No. of patients (percent) | |||||||
| JULIET(12) | Relapsed or refractory diffuse large B-cell lymphoma | Tisagenlecleucel | 64/111 (58%) | None | None | No report | None |
| ELIANA(10) | Relapsed or refractory B-cell lymphoblastic leukemia | Tisagenlecleucel | 58/75 (77%) | None | 3 (4%) | 2 (2.7%) | None |
| ZUMA-1(11) | Refractory large B-cell lymphoma | Axicabtagene ciloleucel | 94/101 (93%) | None | 1 (1%) | No report | 1 (1%, same patient as cardiac arrest) |
The pathophysiology of cardiac dysfunction during CRS is unclear, but resembles cardiomyopathy associated with sepsis and stress, likely associated with IL-6, which has been implicated as a mediator of myocardial depression in infectious and inflammatory states.(22) While the onset of the cardiac dysfunction can be acute and severe, it is generally reversible.
Cardiovascular Evaluation and Management Before CAR T-cell Therapy
Patients receiving CAR T-cell therapy may be particularly susceptible to CV injury as many have exposure to prior cardiotoxic treatment, and may also have underlying CV comorbidities. While CV risk factors and diminished CV reserve may increase the risk of adverse outcomes with CRS, this question has not yet been studied. In many of the CAR T-cell clinical trials, patients were required to have normal LVEF and no history of myocardial infarction or cardiac arrhythmias including atrial fibrillation. (18) “Pre-CAR-T” cardiac evaluation is likely to vary between institutions, however assessment often includes exclusion of coronary ischemia and structural heart disease (Central Illustration). Although CRS-related hemodynamic instability and CV injury are often transient and reversible in most instances, they can be particularly challenging in higher-risk, often elderly patients with pre-existing CVD.
Central Illustration. Cardiovascular Evaluation of Patients Planned for CAR T-cell Therapy.
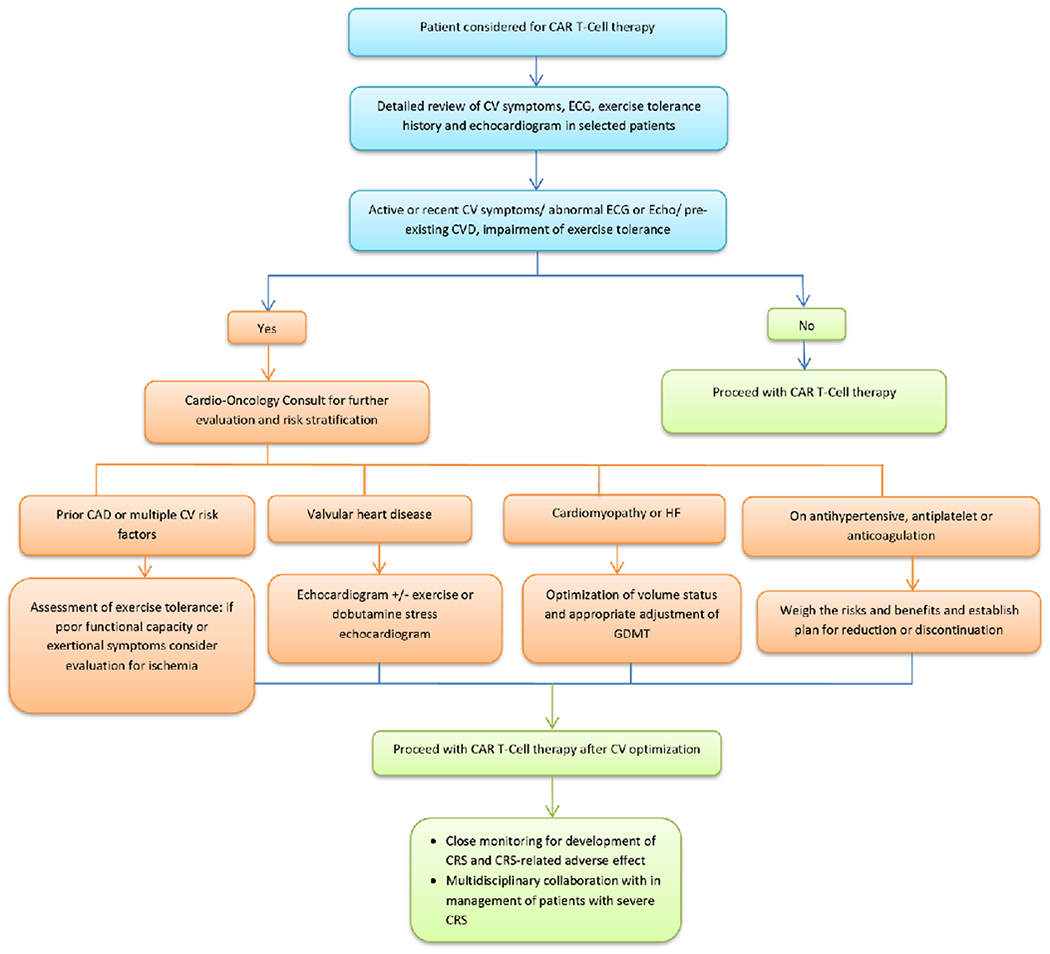
Proposed algorithm for cardiovascular evaluation prior to CAR T-cell therapy.
Abbreviations: CAR: chimeric antigen receptor; CAD: coronary artery disease; CRS: cytokine release syndrome; CV: cardiovascular; CVD: cardiovascular disease; ECG: electrocardiogram; GDMT: guideline directed medical therapy; HF: heart failure
In the absence of CAR-T therapy specific recommendations, it is reasonable to consider the American Society of Clinical Oncology Guidelines (ASCO) for prevention and monitoring of cardiac dysfunction (31) in particular for patients with a history of anthracycline therapy and/or chest radiation. In those patients, detailed cardiovascular history and physical examination are recommended before initiation of treatment.(27) In addition, patients with pre-existing CVD, multiple CV risk factors or active CV symptoms will likely benefit from further risk stratification and optimization of CV status. An echocardiogram prior to the initiation of CAR T-cell therapy is not mandated, however, it is often performed to assess biventricular systolic function and exclude significant valvular disease. Echocardiographic evaluation is of particular relevance among heavily pre-treated patients, particularly those with significant exposure to anthracyclines. In patients with pre-existing coronary artery disease (CAD) or multiple CV risk factors, exercise tolerance should be assessed and, for patients with poor exercise capacity or exertional symptoms, it is reasonable to consider imaging stress test to rule out occult obstructive CAD. Patients with obstructive CAD or significant aortic stenosis may be at increased risk for major cardiovascular events such as myocardial infarction, ventricular arrhythmia, heart failure (HF), cardiogenic shock and even death in the setting of CRS induced hypotension. Such cases require multidisciplinary discussion with patient involvement to weigh the risk and benefits of the CAR T-cell therapy, based on the status/prognosis of their hematologic malignancy and CVD burden. Patients with preexisting HF regardless of the cause are likely to be at risk for HF exacerbation due to volume shifts and recommendations for volume status monitoring and HF management are commonly provided prior to initiating CAR T-cell therapy. Current practices and the above considerations are largely based on clinical experience and proposed pathophysiology of CV injury in the setting of systemic inflammatory response. While evidence-based guidance is lacking at the present time, it is likely to evolve in the coming years with rapidly growing number of CAR T-cell trials and inclusion of wider patient population at risk.
Several patient and therapy related predictors of CRS development during treatment have been identified. Factors that increase in-vivo CAR T-cell numbers, such as high disease burden, higher infused CAR T-cell dose, or high intensity lymphodepletion regimen, may increase the risk of CRS.(16,28,29)
Cardiac Monitoring and Management During Therapy
Management of CRS requires a high level of clinical surveillance and cardiac monitoring algorithms have included telemetry, 12-lead ECG, echocardiogram and cardiac biomarkers including cardiac troponin and brain natriuretic peptide, triggered most often by clinical signs and symptoms of high-grade CRS.(19,29) Close BP monitoring during CAR T-cell therapy is important for prompt recognition and treatment of hypotension. Intravenous fluids are used in patients with intravascular volume depletion while monitoring signs of vascular leak and pulmonary congestion. Other causes of hypotension such as septic shock with initiation of empiric antibiotics since infection often mimics or co-presents after onset of CRS, as well as pulmonary embolism or primary cardiac events need to be considered. Transfer to the intensive care unit for hemodynamically unstable patients or ones requiring mechanical ventilation (Table 3). While shock in most patients with CRS is vasodilatory in nature, in patients not responding to volume resuscitation and vasopressors, mixed vasodilatory and cardiogenic, or purely cardiogenic shock should be considered. These patients often benefit from continued invasive hemodynamic monitoring to guide volume and pressor requirements.
Table 3.
Cardiovascular Preventative and Supportive Care Interventions for CAR T-Cell Therapy
| Pre T cell Infusion |
| 1. Comprehensive assessment that includes a detailed cardiovascular (CV) history and physical examination including estimation of exercise tolerance, screening for CV disease risk factors (hypertension, diabetes, hyperlipidemia, obesity, smoking) and baseline 12 lead electrocardiography (ECG), analogous to assessment prior to cardiotoxic treatment. Cardio-oncology consult for further evaluation and risk stratification as per Figure 1 |
| 2. Baseline echocardiogram to evaluate cardiac structure and function especially in older patients, those with impaired exercise tolerance, known structural heart disease, abnormal baseline ECGs, suggestive symptoms or multiple CV risk factors |
| 3. Consider evaluation for ischemia in patients with poor exercise tolerance or any exertional symptoms |
| 4. Consider tapering antihypertensive medications prior to infusion |
| During Therapy |
| 1. Continue low dose aspirin in patients with known coronary artery disease / percutaneous coronary intervention until platelets <30,000(35) |
| 2. Monitor vital signs every 4 hours with attention to fevers, hypotension and tachycardia; every 2 hours in patients with fever and tachycardia. Telemetry monitoring for patients found to have persistent tachycardia or arrhythmia |
| 3. Maintenance of adequate hydration. Initiate replacement IV fluids for patients with poor oral intake or high insensible losses to maintain euvolemia |
| 4. Initiate volume resuscitation with IV fluid for sustained hypotension |
| 5. Consider intensive care monitoring if hypotension recurs after 1st fluid bolus or HR persistently >125 bpm. |
| 6. ECG, troponin, and echocardiogram for persistent hypotension not responsive to intravenous fluid boluses. Consider intensive care unit transfer for hemodynamic management |
| 7. Initiate vasopressor support if BP unresponsive to 1st fluid resuscitation. Discuss with CAR-T team regarding the use of tocilizumab. |
| 8. Consider invasive hemodynamic monitoring for patients with shock who have reduced LV systolic function and/or refractory to low dose vasopressor. |
A single center retrospective study demonstrated that an elevation in serum troponin (checked at the discretion of treating team) was noted in more than half of the patients undergoing CAR T-cell therapy. (34) Cardiac troponin elevation was seen more commonly in patients with pre-existing cardiac structural abnormalities and was associated with subsequent cardiovascular events.(30) Future studies are needed to define the role of routine cardiac biomarker assessment in patients with different CRS severity.
Tocilizumab is a monoclonal antibody that competitively inhibits the binding of IL-6 to its receptor (IL-6R) and in turn prevents IL-6 signal transduction to inflammatory mediators. Tocilizumab blocks both classic and trans-IL-6 signaling via direct binding to membrane-bound IL-6R or the soluble IL-6R. It is widely used as a first-line treatment for CRS associated toxicity and has been FDA approved for CAR T-cell therapy related CRS.(20,21) Some concerns exist that tocilizumab may lessen the efficacy of the CAR T-cells as it is unclear to what extent the cytokine mediated immune response is required for the anti-tumor response.(21,23) Thus, there has been some hesitation towards its utilization in early CRS. There is no universal consensus regarding the time of tocilizumab initiation (with respect to the severity of CRS) and practices are likely to vary among different institutions. Some believe that the goal of tocilizumab administration is often not to abolish all the manifestations of CRS, but rather to prevent life-threatening toxicities. For example, tocilizumab is often reserved for patients that exhibit hypotension requiring BP support for longer than 24 hours, or those with unstable arrhythmia, evidence of myocardial damage (elevated troponin) or new cardiomyopathy with LVEF <40%. On the other hand, there is growing evidence that early administration of tocilizumab, even at the CRS onset, might be safe and this approach has been adopted in clinical practice at some institutions. (4)
Corticosteroids are also effective in the treatment of CRS but generally considered as second-line therapy reserved for CRS symptoms refractory to tocilizumab, given concerns that steroids may adversely affect the anti-tumor activity of the engineered T-cells. (31) Despite concerns of potentially decreased CAR T-cell efficacy with tocilizumab or steroids, the results from initial clinical trials did not show an association between their use and worse oncology outcomes.(32)
Siltuximab is another monoclonal antibody that blocks IL-6 signaling by binding to IL-6 itself and preventing it from activating immune effector cells. Although it has not been formally studied for the management of CRS and hence not FDA approved, siltuximab has a higher affinity for IL-6 than tociluzumab and can be considered in patients not responding to tocilizumab and corticosteroids.(19) An additional theoretical advantage of siltuximab compared to tocilizumab is that by binding IL-6 directly it may decrease CNS levels of IL-6, while the blockage of the IL-6R by tocilizumab results in increased systemic levels and possibly CNS levels, which could precipitate or worsen neurotoxcitiy.(32) The ASTCT convened a group of experts in July 2019 and is currently developing consensus guidelines for the management of CAR T cell toxicities, including CRS and neurotoxicty, that will address both the use and timing of anticytokine therapy and steroids (MA Perales, personal communication.).
Role of the Cardiovascular Specialist in the Multidisciplinary CAR-T Team
Management of CAR T-cell related toxicities requires the involvement of multidisciplinary team members from hematology/oncology, critical care, neurology, pharmacy, nursing and subspecialty medicine. Cardiovascular specialist may be asked to participate in patient management before, during and after CAR T-cell therapy. Monitoring and treatment of patients with pre-existing LV dysfunction, for example, is likely to involve predicting risk and hemodynamic support in the setting CRS or other CAR T-cell therapy complications such as sepsis. In patients with pre-existing CAD or vascular complications, monitoring for development of cytopenia, coagulopathy and bleeding events is needed to guide adjustment of antiplatelet and anticoagulation therapy. For patients receiving antihypertensive medications prior to CAR T-cell therapy, dose reduction or discontinuation altogether may be considered given the risk of hypotension with CRS, sepsis or tumor lysis syndrome. Multidisciplinary cooperation in patient selection, pre-therapy CV optimization, management of any early cardiotoxicity and then long-term surveillance are the key to building a successful CAR T-cell program.
Gaps In Knowledge of CAR T-Cell Therapy Related Acute and Late Cardiovascular Risks
To date, the major risk to the CV system during CAR T-cell therapy appears to be hemodynamic stress due to CRS that, for most patients, is reversible. Transient reductions in LVEF in the setting of distributive shock have been observed. There has not been convincing evidence to suggest that LVEF decline is sustained, or that there is a substantial risk of ACC/AHA stage C or D heart failure in the acute setting. However, given the lack of systematic, CV surveillance in the clinical trials, the true incidence of cardiac injury and LVEF decline is not known.
Our understanding about genetically engineered T-cell related direct cardiotoxicity is limited but rapidly evolving. Two patients developed fever and progressive cardiogenic shock with death after receiving T-cells targeting MAGE-A3 which was attributed to autoimmunity with off-target cross-reactivity of T-cells against titin, a striated muscle protein in the heart.(33,34) T-cell receptors genetically modified with enhanced affinity against a tumor-specific antigen have considerable effector functions in vivo but may cause safety concerns due to potential cross-reactivity with unrelated peptides expressed by normal tissue.(33) Preclinical studies using peptide scanning and complex cell cultures including myocardial cells will be needed to identify the effects on cardiomyocytes and identify strategies to mitigate the risk of off-target toxicity.(34)
Another significant knowledge gap relates to defining the potential late or long-term CV effects of altering the immune system. It remains unknown whether there could be a latent period with an altered immune system and continued circulation of CAR T-cells that can lead to the accelerated development of metabolic syndrome, hypertension, vascular disease and cardiomyopathy. The answer to these important questions will only come from longitudinal studies that characterize the CV profile of the survivors. To date, the majority of patients have been treated in clinical trials with all of the selection biases associated therein. Little is known of the CV risk in a real-world population that may have more pre-treatment CV risk factors and extant CV disease. Since only a limited number of centers have access to this therapy for a relatively limited number of patients, multi-institutional collaboration will be required to fully understand the benefits, and risks of this breakthrough therapy.
Conclusion and Future Directions
CAR T-cell therapy has demonstrated considerable promise against refractory hematologic malignancies and will likely expand to other treatment indications. However, toxicity is currenty a major barrier to more effective CAR T-cell therapies, and ongoing efforts seek to better understand toxicities and design safer CAR T-cells. As CAR T-cell therapy evolves to include more patients, risk stratification and prompt recognition and treatment of cardiotoxicity will become increasingly more important. Best strategies for prevention and management of cardiotoxicity to minimize major adverse cardiac events remain undefined. A best practice approach that includes systematic but selective assessment of cardiovascular clinical symptoms, cardiac biomarkers and imaging-based indices of cardiac function should be implemented to enhance our understanding of cardiotoxicity during and after CAR T-cell therapy. Multi-institutional collaboration with creation of geographically broad-based registries will be needed to inform evidence-based practice guidelines that optimize CAR T-cell therapy and patient outcomes.
Highlights:
Chimeric antigen receptor (CAR) T-cell therapy is associated with potentially life-threatening adverse effects due to cytokine release syndrome.
Clinical cardiovascular manifestations include tachycardia, hypotension, troponin elevation, reduced LV ejection fraction, pulmonary edema and cardiogenic shock.
As the use of CAR-T cell therapy expands to include wider patient population, careful patient selection, pre-therapy cardiac work-up as well as optimization of CV status should be part of the CAR T-cell treatment protocol.
Defining a CV surveillance strategy during treatment is important to mitigate life-threatening CRS.
Acknowledgement:
We gratefully thank Dr Nikola Kolundzic (www.kold.design) for preparing the illustration (Figure 1).
Funding:
MA Perales: Research support for clinicial trials from Incyte, Kite/Gilead and Milenyi Biotec V Zaha: Research support from the Cancer Prevention Research Institute of Texas
Abbreviations:
- ALL
acute lymphoblastic leukemia
- CAD
coronary artery disease
- CAR
chimeric antigen receptor
- CRS
cytokine release syndrome
- CV
cardiovascular
- DLBCL
diffuse large B-cell lymphoma
- FDA
Food and Drug Administration
- HF
heart failure
- IL-6
interleukin-6
- LVEF
left ventricular ejection fraction
Footnotes
Disclosures:
J Park: consulting/advisory board for Kite Pharma, Novartis, Amgen, Allogene, Autolus, GlaxoSmithKline, AstraZeneca
MA Perales: Honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Merck, Novartis, Nektar Therapeutics, and Takeda; serves on DSMBs for Servier and Medigene, and the scientific advisory boards of MolMed and NexImmune.
A Barac: Honoria from Bristol-Myers Squibb, DSMB for CTI BioPharma
The remaining authors have nothing to disclose.
References:
- 1.Rosenbaum L, Tragedy, Perseverance, and Chance - The Story of CAR-T Therapy. N Engl J Med 2017;377:1313–1315. [DOI] [PubMed] [Google Scholar]
- 2.June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med 2018;379:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84–91. [DOI] [PubMed] [Google Scholar]
- 4.Neelapu SS, Tummala S, Kebriaei P et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Zeng G. Cancer and innate immune system interactions: translational potentials for cancer immunotherapy. J Immunother 2012;35:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn OJ. Cancer immunology. N Engl J Med 2008;358:2704–15. [DOI] [PubMed] [Google Scholar]
- 7.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raje N, Berdeja J, Lin Y et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2019;380:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry TJ, Shah NN, Orentas RJ et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maude SL, Laetsch TW, Buechner J et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neelapu SS, Locke FL, Bartlett NL et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma.N Engl J Med 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Riviere I, Gonen M et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med 2018;378:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maldini CR, Ellis GI, Riley JL. CAR T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol 2018;18:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teachey DT, Lacey SF, Shaw PA et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov 2016;6:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obstfeld AE, Frey NV, Mansfield K et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood 2017;130:2569–2572. [DOI] [PubMed] [Google Scholar]
- 17.Shimabukuro-Vornhagen A, Gödel P, Subklewe M et al. Cytokine release syndrome. Journal for ImmunoTherapy of Cancer 2018;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riegler LL, Jones GP, Lee DW. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag 2019;15:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maude SL, Frey N, Shaw PA et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davila ML, Riviere I, Wang X et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pathan N, Hemingway CA, Alizadeh AA et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet 2004;363:203–9. [DOI] [PubMed] [Google Scholar]
- 23.Titov A, Petukhov A, Staliarova A et al. The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis 2018;9:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DW, Santomasso BD, Locke FL et al. ASBMT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac Profile of Chimeric Antigen Receptor T Cell Therapy in Children: A Single-Institution Experience. Biol Blood Marrow Transplant 2018;24:1590–1595. [DOI] [PubMed] [Google Scholar]
- 26.Kochenderfer JN, Somerville RPT, Lu T et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J Clin Oncol 2017;35:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armenian SH, Lacchetti C, Barac A et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017;35:893–911. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganatra S, Parikh R, Neilan TG. Cardiotoxicity of Immune Therapy. Cardiol Clin 2019;37:4. [DOI] [PubMed] [Google Scholar]
- 30.Alvi RM, Mahmood S, Hassan MZO et al. THE CARDIOVASCULAR EFFECTS OF CHIMERIC ANTIGEN RECEPTOR T-CELLS. Journal of the American College of Cardiology 2019;73:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brentjens RJ, Davila ML, Riviere I et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locke FL, Neelapu SS, Bartlett NL et al. Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axi-cel; KTE-C19) Treatment for Patients with Refractory,Aggressive Non-Hodgkin Lymphoma (NHL). Blood 2017;130:1547–1547. [Google Scholar]
- 33.Linette GP, Stadtmauer EA, Maus MV et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122:863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cameron BJ, Gerry AB, Dukes J et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 2013;5:197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feher A, Kampaktsis PN, Parameswaran R, Stein EM, Steingart R, Gupta D. Aspirin Is Associated with Improved Survival in Severely Thrombocytopenic Cancer Patients with Acute Myocardial Infarction. Oncologist 2017;22:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]


