Abstract
Nearly all fatalities arising from breast tumors are attributable to distant metastases. Breast cancer liver metastasis (BCLM) is associated with poor prognoses, with the median survival time being 2 to 3 years. Tumor intrinsic subtype directs preferential metastasis to specific organs, with HER2-enriched tumors demonstrating the highest rates of metastasis to the liver, though all subtypes can grow in the liver. There is no singular established standard-of-care for BCLM; therapeutic selection is driven by histologic and molecular hallmarks of the primary tumor or biopsied metastasis samples. Given the poor prognosis of patients with hepatic spread, pre-clinical studies are necessary to identify and evaluate promising new treatment strategies. It is critical that these laboratory studies accurately recapitulate the BCLM disease process, standard progression, and histological attributes. In this review, we summarize the histologic and molecular characteristics of BCLM, evaluate the efficacy of existing surgical and medical treatment strategies, and discuss future approaches to preclinical study of BCLM.
Keywords: Breast cancer, Liver metastasis, Surgery, Targeted therapies, In vivo models
Introduction
Breast cancer is the most common cancer diagnosis in women, and the second-leading cause of cancer-related death of women in the United States. In 2021, 284,200 novel cases of invasive disease are estimated, and 44,130 women and 530 men are estimated to succumb to the disease [1]. Breast cancer mortality is attributable to complications arising from the formation and growth of distant metastases, most commonly that of the liver, lungs, and bone. Between 5 and 8% of breast cancer patients present with distant metastasis at the time of diagnosis; only 24–39% of patients with metastases survive for 5 years [2, 3]. Approximately 20% of breast cancer patients will experience relapse, and 50–70% of metastatic breast cancer cases involve the liver [4]. Prognosis is poor following metastasis to the liver, with the median survival rate being only 2–3 years [5]. Complications that arise from liver metastasis include sudden hepatic failure, refractory ascites, portal vein thrombosis, and nutritional compromise [6].
Breast cancer is a heterogenous disease, in terms of histology and molecular profile. While the majority of breast cancers are invasive ductal carcinoma, additional classifications include invasive lobular carcinoma, medullary carcinoma, metaplastic breast cancer, and inflammatory breast cancer. Historically, breast cancers were stratified by hormone receptor expression, namely that of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Starting in the early 1990s, hormone receptor status, histologic grade (I, II, and III), and node status were the primary factors utilized for prognostic indication. Advancements in transcriptomics, however, has led to the development of the intrinsic subtyping system, which is based on gene expression patterns [7]. These molecular subtypes are luminal A, luminal B, HER2-enriched, basal-like, and claudin-low. The intrinsic subtypes have unique patterns of both primary tumor growth and tissue distribution of distant metastasis.
Despite the prevalence and relative commonality of breast cancer liver metastasis (BCLM), specialized therapeutic options for this patient population are lacking. This may be partially attributable to a lack of standard-of-care currently ascribed to BCLM. As is presented in this review, models of BCLM demonstrate clear, unique molecular patterns intrinsic to this disease, which may ultimately lend themselves to the emergence of novel therapeutic targets for future studies.
Tissue distribution of metastases by intrinsic subtype
The “seed and soil” hypothesis proposes that favorable interactions between the metastatic tumor cell (the “seed”) and the organ-specific microenvironment (the “soil”) are required for successful expansion of cancer cells in different vital organs [8, 9]. Better understanding the mechanism(s) of this interaction could greatly facilitate the uncoupling of this relationship, thereby effectively preventing and/or treating metastatic disease. Recent studies have examined the relationship between primary tumor characteristics and metastatic dissemination patterns, in order to understand this predilection. A 2011 study examining the maintenance of gene expression signatures between matched primary and metastatic breast cancer samples uncovered that > 90% of genetic signatures found in the primary tumor are maintained in distant metastases [10]. Interestingly, the gene signatures with the highest rate of discordance between primary and metastatic samples were associated with extracellular matrix (ECM) proteins, which could be a result of a direct influence of the unique ECM itself on gene expression, or simply differing levels of fibroblasts found in primary tumors versus metastases. Overall, these results confirmed that primary breast tumor gene profiles are strong predictors of metastatic behavior.
Early studies that stratified tumors into different breast cancer subtypes, both histologic and molecular, noted clear organ-specific patterns of metastatic colonization that were unique to each subtype. For example, basal-like breast cancers frequently metastasize to the lungs and brain, luminal breast cancers tend to metastasize to the bone, and HER2+ breast cancers aggressively colonize the liver [10–12]. Relative to the other frequent sites of metastasis, liver is one of the most common sites of metastatic relapse, with a 40–50% clinical incidence rate, and 50–62% autopsy incidence [4, 5, 13]. Furthermore, BCLM is highly associated with poor prognosis, and the median survival time of patients with liver metastasis is 4–8 months without intervention [14]. Understanding the underlying mechanisms that promote liver metastasis is crucial towards making a significant impact on disease outcome.
One repository that has provided significant insight into the distribution patterns and mechanisms of liver metastasis is the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database [15]. SEER consists of a network of tumor registries that encompasses almost 30% of the US population, harboring data from 1975 to 2017 [15]. Using these data, one 2020 study determined that, of the 2.4 million cancer patients described within this database, 5.14% presented with synchronous liver metastases [16]. Within this broader analysis, the most common site of primary lesion for women (age 20–50) was breast, wherein 1.4% of breast cancer patients assessed harbored liver metastasis at the time of primary diagnosis, and that the presence of liver metastasis significantly reduced patient overall survival (OS) compared to patients without liver metastasis (HR 1.94 [1.86, 2.02]) [16]. These results were corroborated by univariate logistic regression analyses, confirming the significant impact of liver metastasis on OS in patients with stage IV disease [17].
Analyses performed using SEER data have also corroborated that patients with HER2+ breast cancer, namely that which is ER−/PR−/HER2+, have the highest incidence rate of liver metastasis (4.6% of all patients, 32.7–46.5% among those with metastatic disease) [5, 18–20]. Patients with ER+/PR+/HER2+ also have a high degree of liver metastasis, with an average of 20.3% of patients with stage IV disease relapsing to this organ [18]. Patients with luminal A, ER+/PR+/HER2− disease are the least likely to experience relapse to the liver, with an incidence rate 4-times lower than that of HER2-enriched malignancies [10]. However, patients with liver metastatic triple negative breast cancer (TNBC) have worse OS than patients with HER2+ metastatic disease, and comorbidity with extrahepatic metastases significantly worsens 5-year prognosis [5]. With treatment, however, patients with liver metastasis exhibit an average OS of 31.0 months [18].
This association between HER2-enriched breast cancers and liver metastasis has been observed in other population-based studies as well, beyond those which utilized SEER data [11, 21]. For example, one moderately-sized study examined metastatic distribution as a function of hormone receptor status, specifically in women of Kenyan descent [22]. Similar to what was observed with the studies utilizing SEER data, patients with HER2-enriched breast cancer were significantly more likely to develop metastases to the liver than those of the other subtypes [22]. A separate, highly-powered population-based study, examined HER2-enriched patient data reposited within the National Cancer Database (n = 31,946) [23]. This study uncovered that breast cancers which are ER+/PR+/HER2+ are significantly more likely to metastasize to the bone when compared to ER−/PR−/HER2+ disease, and that patients with ER−/PR−/HER2+ disease have a worse overall prognosis than those with hormone receptor-positive (HR+), HER2+ malignancy [23].
Given the intrinsic subtype-specific manner by which breast cancers metastasize to different organs, investigators have examined the underlying molecular signatures of primary tumors, metastatic samples, and the tumor microenvironment, in order to understand this predilection. RNA-sequencing (RNAseq) studies utilizing patient-derived xenograft (PDX) samples, for example, demonstrated significant upregulation in S100a9 (neutrophil marker), Acta2 (smooth muscle actin), and Lcn2 (lipocalin) transcripts in the liver microenvironment during liver metastatic growth, demonstrating neutrophil involvement in this process [24]. Analyses of colonized livers discovered a recurrent 27-gene signature upregulated in the liver microenvironment (termed, “the liver microenvironment-induced gene signature”), regardless of PDX sample examined [24]. Ingenuity pathway analysis (IPA) of this signature discovered that the top function of these 27 genes lies in the inflammatory response, mirroring the upregulation of genes associated with neutrophil involvement along the tumor periphery. Considering this, a separate study analyzed the presence of CD68 positivity, as a marker for macrophage influx, in the liver microenvironment and found a significant association between CD68 positivity and metastatic expansion [25]. This same trend was observed in comparing metastatic size and neutrophil abundance, as measured by S100A9 IHC staining [25]. These data are consistent with more recent findings that indicate neutrophil activity promotes metastatic outgrowth within the liver microenvironment [26].
Regarding molecular attributes that are hypothesized to be critical driving forces of metastatic dissemination: one recent study demonstrated TGFβ and MAPK are critically involved in the extravasation step of BCLM, and their over-expression was shown to be significantly associated with poor 5-year prognosis [27]. Ren et al. demonstrated that both of these pathways serve antagonistic roles in the actions of bone morphogenic proteins (BMPs), the latter of which has a well-documented negative effect on epithelial-to-mesenchymal transition (EMT) and metastasis. Treatment of the claudin-low cell line MDA-MB-231 with MAPK (MEK) inhibitors was sufficient to inhibit both liver and bone colonization in this model, highlighting the specificity of MAPK activity in liver metastasis [27]. A number of transcriptomic-based studies have also provided insight in this manner. For instance, microarray technology has been critical in parsing unique genomic signatures which are predictive of organ-specific relapse. One study uncovered that the most informative gene signature for liver metastasis is comprised of genes typically observed in HER2-enriched breast cancer [10]. More recent analyses, also utilizing microarray data, have further corroborated BCLM is associated with a unique set of gene expression patterns. For example, Wang et al. collected microarray data from a number of publicly-available repositories and found that the KEGG pathways “cytokine-cytokine receptor interaction” and “calcium signaling” are significantly enriched in BCLM [28]. TGFβ signaling was also uncovered as an alternatively active pathway, corroborating the significant involvement of this pathway in extravasation described by Ren and colleagues [27, 28]. A separate microarray-based analysis uncovered FGFR4 signaling is significantly enriched in Her2-enriched patients, as determined by analysis utilizing TCGA data, and the signature of which could potentially be used as a biomarker for breast cancer metastasis to the liver, lung, and brain [29]. Lastly, looking from a genetic mutation perspective, one moderately large (n = 290) Chinese cohort study found an enrichment in PIK3CA, PTEN, ARID1A, and RB1 mutations in patients with liver metastasis [30]. This study, in particular, noted ethnicity-specific differences in metastatic breast cancer, in comparing the mutational frequency of their patient cohort (exclusively of Chinese descent) with that of the TCGA-BRCA cohort (predominantly European-Americans) [30].
While the majority of breast cancers present as invasive ductal carcinoma (IDC), approximately 10% are invasive lobular carcinoma (ILC). ILC has been demonstrated to preferentially metastasize to the gastrointestinal tract and gynecologic sites, while IDC predominantly metastasizes to the lungs, bone, and liver [31]. To this effect, ILC has been demonstrated to metastasize significantly less-readily to the liver than IDC [31]. Some evidence indicates that this organotropism may be a result of the loss of E-cadherin, which is typically found in ILC and is believed to be critical for this particular histologic subtype [31, 32]. Proteomic studies have also uncovered an enrichment in PI3K/AKT and p90-RSK pathways in ILC, associating with a upregulation in angiogenesis and genes involved in the extracellular matrix, which could provide insight into the metastatic dissemination pattern of ILCs [33].
Histological growth patterns
During metastatic colonization and growth in the liver, at least three different histologic growth patterns have been observed, namely replacement, desmoplastic, and pushing (Fig. 1) [34]. Two additional yet rare histologic growth patterns are sinusoidal and portal [34]. Each of these growth patterns are distinguishable from one another by the respective characteristics of the cancer/normal liver interface, as are discussed here. Furthermore, while these growth patterns were originally observed using human patient samples, these patterns have also been subsequently observed in mouse models of liver metastasis [25].
Fig. 1.
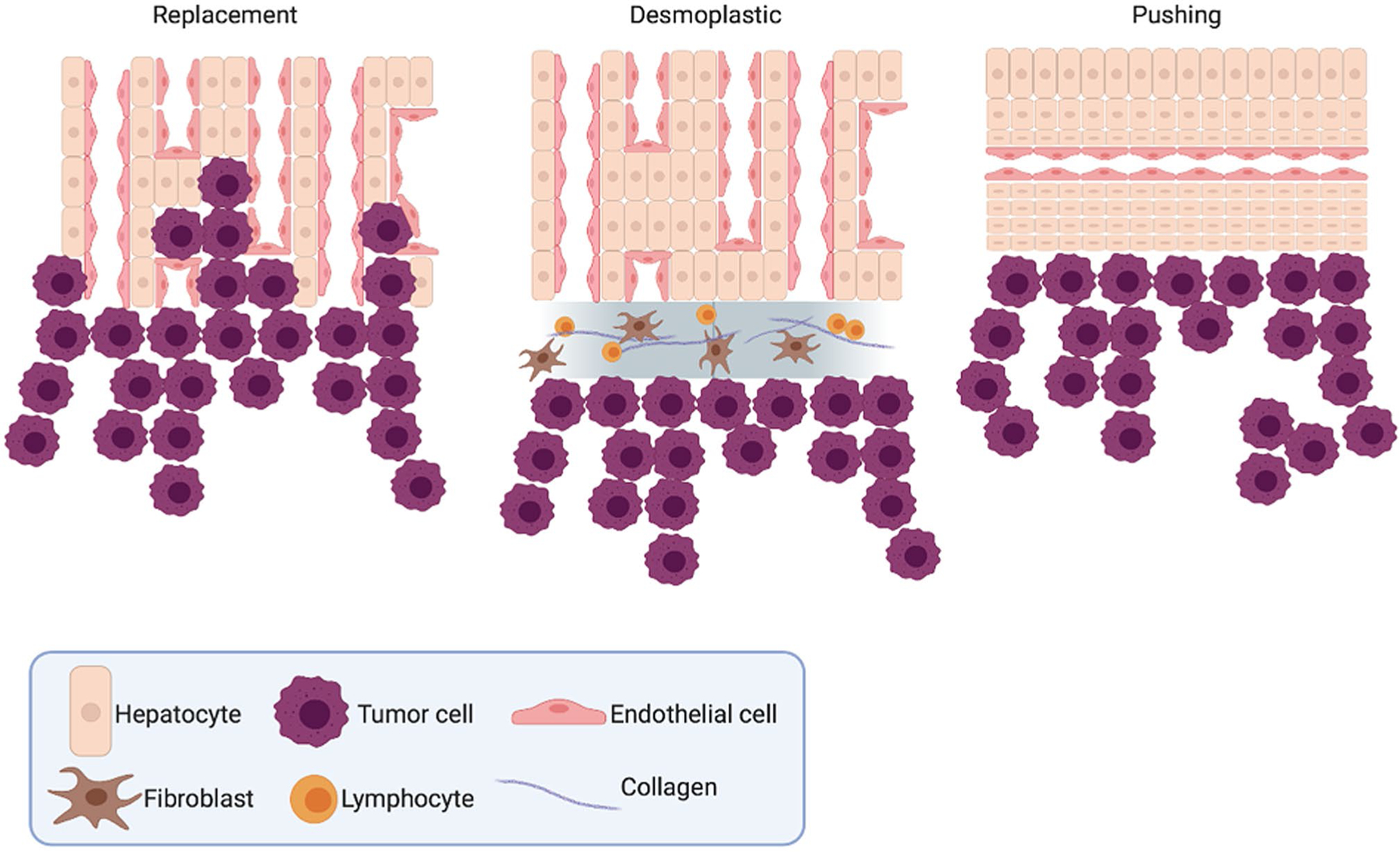
The three most common histological growth patterns of liver metastasis
The replacement growth pattern consists of tumor cells replacing the host hepatocytes to form muralia (i.e. a style of cellular arrangement comprised of a one cell-thick wall typically with a sinusoid on either side) and is characterized by a lack of inflammation and desmoplastic stroma with nominal levels of proliferating endothelial cells. Liver metastases demonstrating a replacement growth pattern tend to co-opt the sinusoidal liver vasculature rather than relying on angiogenesis, which has been observed to contribute to anti-angiogenic therapy resistance [35, 36]. This observation, in particular, is significantly associated with worse patient prognosis [35]. Breast cancer liver metastases with the replacement phenotype are additionally significantly less likely to have central hypoxia than metastases with a non-replacement growth phenotype [37]. The replacement growth pattern is the most common histologic phenotype of breast cancer metastatic growth [34, 35, 37].
The desmoplastic histologic growth pattern, conversely, is defined by extensive stromal remodeling at the tumor-hepatocyte juncture, alongside significant sprouting angiogenesis [34, 35]. This remodeling separates the normal liver parenchyma from the invading tumor cells by a sheath of fibrotic stromal tissue. During this histologic growth pattern, there is no metastatic mimicry of the normal liver architecture, and the metastatic growth does not directly come into contact with the normal hepatocytes. In colon cancer liver metastasis, desmoplastic growth patterns associate with upregulated type I and type IV collagens as well as augmentation of the uPA-uPAR-PAI-1 proteolytic system, which facilitates the interaction between the growing metastasis and its new tissue environment [38, 39]. Patients with desmoplastic colorectal liver metastasis are consistently observed as having a significantly more favorable outcome than those with the other histologic growth subtypes [40].
The pushing histologic growth pattern, on the other hand, is characterized by the flattening and compressing of hepatocytes by the metastasis, and there is no intervening fibrosis [34]. Similar to the desmoplastic histologic growth pattern, the metastatic growth observed here does not mimic normal liver cellular architecture. Pushing histologic growth patterns are more highly proliferative than either desmoplastic or replacement patterns, in examining both the endothelial and tumor cell fractions, and this augmentation in proliferative potential positively correlates with gross metastasis size [36]. The pushing group is also the most angiogenic of the histologic growth patterns, and this angiogenesis appears to be partially hypoxia-driven [36].
The two additional histologic growth patterns, sinusoidal and portal, are rarely observed in BCLM. During sinusoidal growth, metastases exist as emboli within the liver sinusoidal blood vessels and/or in the peri-sinusoid [34]. In this manner, the sinusoidal blood vessels are utilized for means of metastatic vascularization, and has been found in patients with more rapidly-progressing disease [41]. During the portal histologic growth pattern, BCLM grows exclusively in the portal tracts, liver capsule, and septa [34, 42].
While these histologic growth patterns are entirely unique from one another, it is critical to note that these growth patterns display a significant degree of plasticity. For example, antiangiogenic systemic treatment of colorectal cancer liver metastases (CRLM) has been shown to result in a phenotypic switch, from angiogenic desmoplastic growth to non-angiogenic replacement growth [35, 43]. This type of switching event has also been observed pre-clinically, for lung and brain metastasis [44, 45]. Furthermore, while a clear enrichment for BCLM is observed for patients with HER2+ disease, subtype-specific patterns of metastatic histologic growth are not as clearly-defined. Frentzas et al. demonstrated histologic growth patterns in patient tumors are not statistically different between breast cancer intrinsic subtypes, with the majority phenotype being replacement growth [35]. Indeed, only one of the seventeen samples assayed (luminal B, HER2−) deviated from this majority, depicting a desmoplastic phenotype [35]. A separate study utilizing 14 different PDX models demonstrated a similar pattern, however studies of larger sample sizes and an even distribution of intrinsic subtype are needed to fully understand the putative relationship between intrinsic subtype and histologic growth pattern [25].
Local therapies
Targeted treatment of BCLM is limited to local, interventional procedures. Such procedures are standard of care in the management of CRLM; however, such is not the case for BCLM. The most common local treatment for liver metastases, both CRLM and BCLM, is liver resection (LR; Fig. 2). This invasive procedure involves surgically removing tumor growth in the liver as well as surrounding tissue. Tasleem et al. performed a systematic review of 25 articles describing clinical benefit rates arising from liver resection in BCLM patients [46]. They included data from 1080 BCLM patients. They found that LR produced favorable clinical outcomes, both meeting and exceeding outcomes reported in the literature following systemic therapy. They found a median overall survival (MOS) of 29.5 months to 116 months amongst patients who underwent LR for isolated liver metastases, and a MOS of 32 to 58 months was described amongst patients with oligometastatic disease who underwent the same procedure. While LR is standard of care for CRLM management, it is infrequently considered in the management of BCLM. Howlader et al. found that BCLM LR 5-year survival outcomes were similar to those of CRLM LR [47]. These findings suggest that LR be more frequently considered in the management of BCLM.
Fig. 2.
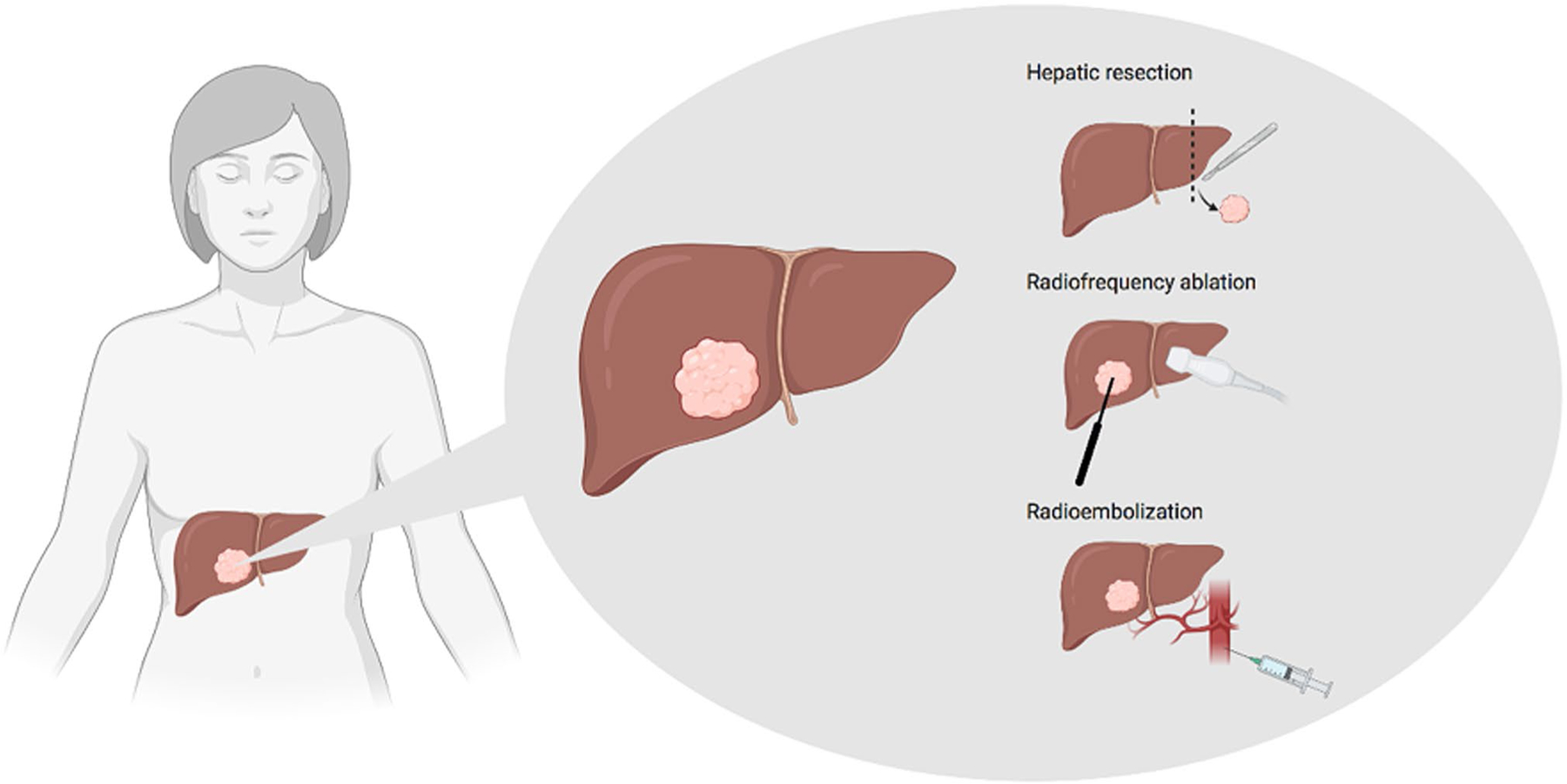
Local treatment options for patients with BCLM
However, not all BCLM patients are strong candidates for LR. There are multiple well-described clinical characteristics of BCLM that predict a positive response to liver resection, as summarized by Golse and Adam [48]. These characteristics include: small liver metastases (< 4–5 cm), positive hormone receptor status, radical resection, stable disease, and greater than 1–2 years between primary and secondary lesions. They also reported that patient age and lymph node status have no significant bearing on LR outcome and should not be considered prognostically [48]. In their review, Bale et al. report similar prognostic criteria.
Recently, minimally-invasive local procedures to treat liver metastases have emerged. Well-described local procedures include radiofrequency ablation (RFA) and radioembolization (Fig. 2). These procedures are used less frequently than LR in the treatment of liver metastases, but they provide promising alternative treatment strategies. RFA uses electrical current and heat to kill cancer cells. A radiologist inserts a thin needle through the skin and guides it to the cancerous tissue using ultrasound computer tomography (CT) or magnetic resonance imaging (MRI) [49]. Preliminary studies have demonstrated clinical benefit from RFA in the treatment of BCLM, reporting MOS times of 29.9 months and 60 months following first RFA [50, 51]. A follow-up meta-analysis by Xiao et al., however, demonstrated that LR produced better clinical outcomes than radiofrequency ablation [52]. LR was associated with significantly higher 5-year OS and 5-year disease-free survival (DFS) than RFA. However, their analysis also found that RFA was associated with fewer complications and shorter hospital stays than LR. Given these findings, Xiao et al. suggest that RFA be reserved for patients who are poor candidates for LR [52].
In radioembolization, a catheter is inserted into the hepatic artery to allow for targeted delivery of radioactive particles at the site of the cancerous tissue (Fig. 2). The radioactive particles consist of small resin or glass spherical particles containing yttrium-90 [53]. Chemoembolization uses a similar technique to allow for targeted drug delivery to the cancerous tissue. A retrospective review performed by Chang et al. examined the outcomes of patients who received transarterial chemoembolization (TACE) or transarterial radioembolization (TARE) for liver-only or liver-dominant breast cancer metastases [54]. Their findings suggested improved patient outcomes following TARE versus TACE (MOS 12.9 months and 4.6 months, respectively), but these findings were not statistically significant. They also found significantly more all-grade adverse events including nausea/vomiting and pain in the TACE group. Their findings suggest that TARE should be prioritized over TACE in the management of BCLM.
The role of surgery and less-invasive local procedures in the management of BCLM is poorly defined and requires more comparative studies. Bale et al. performed an all-inclusive systematic review of local treatment of BCLM [55]. Bale et al. provide a table describing clinical benefit rates of liver resection, RFA, kryoablation, radioembolization, transarterial chemoembolization, brachytherapy, and stereotactic body radiation therapy. Their findings can be used to prioritize future study of local treatment of BCLM. However, they note that the available studies of local treatment of BCLM are limited by small sample sizes and a variety of confounding variables including primary tumor characteristics, multiorgan metastases, and varying systemic therapies.
Systemic therapies
Endocrine therapies
Currently, BCLM patients receive systemic therapies as dictated by primary breast tumor characteristics, as molecular features of the primary tumor are retained during metastatic dissemination more often than not [10]. Endocrine therapies are the first line of treatment for patients with hormone receptor positive (HR+) metastatic breast cancer (MBC). In HR+ MBC, estrogen binds to estrogen receptors on cancerous breast cells and activates genes responsible for cell division and the inhibition of apoptosis. Selective estrogen receptor modulator (SERM) tamoxifen and selective estrogen receptor degrader (SERD) fulvestrant demonstrated similar efficacy and tolerability in postmenopausal women with advanced or metastatic breast cancer [56]. Aromatase inhibitors (AIs) block the activity of the enzyme aromatase, an enzyme that converts androgens to estrogen. Multiple clinical trials report that AIs were superior to tamoxifen as first-line therapies for advanced/metastatic breast cancer, both in terms of tolerability and disease management [57–59]. However, several studies report longer median PFS for patients treated with a SERD (i.e. fulvestrant) compared to patients treated with AIs (i.e. anastrazole) [60, 61]. It is unclear whether therapy with a SERM or SERD in combination with an AI is superior to treatment with either single agent. One study observed increased MOS in untreated metastatic breast cancer patients who received combinatorial anastrazole and fulvestrant as opposed to anastrazole as a single agent (47.7 months vs 41.3 months) [62]. However, both Bergh et al. and Johnston et al. found that combination treatment produced no increase in MOS or PFS in women with locally advanced or metastatic breast cancer [63, 64].
CDK4/6 inhibitors have become standard of care in the treatment of advanced HR+BC after improving patient outcomes in phase III clinical trials [65–67]. Approved CDK4/6 inhibitors include abemaciclib, palbociclib, and ribociclib. These drugs have been revolutionary in the treatment of metastatic HR+BC. A meta-analysis comprised of eight clinical trials studying the efficacy of combination therapy with CDK4/6 inhibitors and endocrine therapy reported that combination therapy can significantly increase PFS and improve overall response rate (ORR) [68]. Despite these advances, some tumors are either inherently resistant to CDK4/6 therapy or acquire resistance after prolonged treatment. Preclinical studies have identified phosphoinositide 3-kinase (PI3K) as a promising molecular target to treat CDK 4/6 resistant HR+ advanced BC. For example, PI3K inhibition by alpelisib was shown to result in tumor regression in both mutant and wild-type PIK3CA CDK4/6 resistant HR+ xenografts [69]. A number of recent clinical trials have examined the efficacy of combination therapies in patients with HR+ metastatic breast cancer. One such study found that combination treatment of fulvestrant and alpelisib significantly increased PFS time in patients with PIK3CA-mutated, ER+/PR+/HER2−advanced breast cancer [70]. A Phase Ib study examined the efficacy of tamoxifen plus buparlisib, another PI3K inhibitor, or alpelisib in premenopausal women with HR+/HER2− advanced BC [71]. In this preliminary study, alpelisib displayed greater clinical benefit rates than buparlisib. In the alpelisib group, 50% of patients had the best overall response of partial response compared to 23.1% of patients in the buparlisib group. Targeted therapies are promising opportunities in the treatment of advanced metastatic disease.
HER2-targeted therapies
Trastuzumab, lapatinib, pertuzumab, and ado-trastuzumab emtansine have been approved for use in HER2+ MBC. Standard of care for patients with HER2-enriched cancers is HER2-targeted agents in combination with endocrine therapy and/or chemotherapy. Trastuzumab can be combined with chemotherapeutics such as vinorelbine, docetaxel, and/or capecitabine [72, 73]. Combination HER2 blockade and treatment with an AI has also demonstrated high rates of clinical benefit. Kaufman et al. performed a Phase III clinical trial in 207 post-menopausal women with HR+/HER2+ MBC [74]. PFS was significantly higher in the group receiving combination therapy. There was no significant difference in OS between the treatment groups, but 70% of patients in the control (anastrazole only) group crossed over to receive combination therapy with trastuzumab following disease progression on single-agent anastrazole treatment. Dual HER2 blockade has also demonstrated significant clinical benefit in clinical trials. For example, Baselga et al. found a MOS of 18.5 months in MBC patients receiving treatment with trastuzumab plus docetaxel plus pertuzumab compared to a MOS of 12.4 months in patients only receiving trastuzumab plus docetaxel [75]. For this reason, dual HER2 blockade with trastuzumab and pertuzumab plus chemotherapy is considered first-line standard of care for patients with HER2-enriched tumors [76].
The second-line standard of care is TDM-1 [76]. However, other second-line therapeutic approaches are being evaluated for efficacy. For example, lapatinib, a tyrosine kinase inhibitor, has been evaluated for efficacy in combination with capecitabine. Lapatinib and capecitabine combination therapy demonstrated greater clinical benefit than capecitabine alone [77]. However, lapatinib and capecitabine combination therapy offered less clinical benefit than TDM-1 as a second-line therapy as determined by a retrospective study [78]. Acquired anti-HER2 resistance is often observed in patients with advanced HER2+ disease. There is a lack of established molecular targets for patients demonstrating this clinical phenomenon. Given the strong association between HER2 expression and LM, further study of novel therapeutic strategies for the treatment of metastatic HER2-enriched BC is critical to developing stronger standards of care for BCLM.
Chemotherapy
Triple-negative breast cancers (TNBC) are devoid of the clinical biomarkers used to indicate endocrine therapy or HER2-directed therapy. TNBC also has a high propensity to metastasize to the liver, and TNBC patients with liver metastasis have worse prognosis than patients with HER2-enriched disease [5]. Due to the lack of clinical biomarkers and actionable targets, chemotherapy is the current standard of care for metastatic TNBC. Common chemotherapeutic agents include anthracyclines, taxanes, platinum-based compounds, anti-metabolites, and vinca alkaloids. Combinatorial chemotherapeutic regimens have demonstrated the highest levels of efficacy in treating metastatic TNBC, over that of single-agents [79]. Natori et al. performed a meta-analysis of eight studies evaluating the efficacy of standard chemotherapy with or without capecitabine. They found that inclusion of capecitabine in TNBC treatment regimens significantly increased DFS and OS. Chalakur-Ramireddy and Pakala published a review summarizing ongoing TNBC clinical trials and reported that nearly 80% of clinical trials are evaluating the efficacy of combinatorial therapies in TNBC [80]. The future of TNBC treatment lies in identifying the most efficacious combinatorial therapies. Combination treatment with chemotherapeutics and targeted drugs is a promising line of study in the treatment of metastatic TNBC. Multiple clinical trials have also evaluated the efficacy of PARP inhibitors in combination with standard chemotherapeutic options. Several studies have reported no significant increase in clinical benefit rate for TNBC MBC patients treated with both chemotherapy and PARP inhibitors [81, 82]. However, in the more recent OlympiAD trial, combination treatment with olaparib and physician’s choice of chemotherapy (TPC) demonstrated a meaningful increase in OS over TPC alone in patients with HER2-negative MBC with germline BRCA mutations [83]. PARP inhibition warrants further study in the TNBC MBC setting. MTOR inhibitors have also been evaluated for safety and efficacy in treating metastatic TNBC. A phase I trial found that combination therapy with everolimus and eribulin was safe [84]. Everolimus was effective in combination with carboplatin in treating metastatic TNBC [85]. However, the addition of everolimus to a cisplatin/paclitaxel regimen produced more adverse side effects without an improvement in pathologic complete response or clinical response [86]. A summary of the clinical trials discussed here can be found in Table 1.
Table 1.
Select MBC Phase III clinical trials
| Study | Disease | Treatments | N= | ORR (%) | CBR (%) | TTP (months) | PFS (months) | MOS (months) |
|---|---|---|---|---|---|---|---|---|
| Howell [56] | HR+ | Fulvestrant, tamoxifen | 313, 274 | 31.6, 33.9 | 54.3, 62 | 6.8, 8.3 | N/A | 36.9, 38.7 |
| Nabholtz [57] | HR+ | Anastrazole, tamoxifen | 171, 182 | 21, 17 | 59, 46 | 11.1, 5.6 | N/A | N/A |
| Mouridsen [58] | HR+ | Letrozole, tamoxifen | 458, 458 | 32, 21 | 50, 38 | 9.4, 6.0 | N/A | 34, 30 |
| Gelmon [65] | HR+/HER2− | Palbociclib or placebo + letrozole | 168, 99 | 57, 52 | 80, 67 | N/A | 25.4, 13.7 | |
| Sledge [66] | HR+/HER2− | Abemaciclib or placebo + fulvestrant | 446, 223 | 48.1, 21.3 | N/A | 23.1, 20.6 | 16.4, 9.3 | 46.7, 37.3 |
| Yardley [67] | HR+/HER2− | Ribociclib or placebo + letrozole | 100, 113 | 39, 26.5 | 76, 67.3 | N/A | 27.6, 15.0 | N/A |
| Geyer [77] | HER2+ | Capecitabine (+, − lapatinib) | 164, 152 | 22, 14 | 27, 18 | 8.4, 4.4 | 8.4, 4.1 | 10.4, 8 |
| Dieras [73] | HER2+ | TDM-1, lapatinib + capecitabine | 495, 496 | N/A | N/A | N/A | 9.6, 6.4 | 29.9, 25.9 |
| O’Shaughnessy [82] | HR−/HER2− | Gemcitabine + carboplatin (+, −iniparib) | 261, 258 | 33.7, 30.2 | N/A | N/A | 5.1, 4.1 | 12.2, 11.1 |
| Robson [83] | HER2− | TPC (+, −olaparib) | 205, 97 | 57.6, 22.2 | N/A | N/A | N/A | 19.3, 17.1 |
ORR overall response rate, CBR clinical benefit rate, TTP time to progression, PFS median progression free survival, MOS median overall survival, OS overall survival, TPC physician’s choice of chemotherapy
Future therapeutic approaches
Despite advances in the treatment of MBC, there is still a lack of established regimens for patients with organ-specific metastases. There are no specific standard-of-care therapeutic strategies indicated for patients with BCLM. Systemic therapies have shown limited efficacy in managing liver metastases, and patient prognoses are still relatively poor. Few clinical trials regarding MBC provide organ-specific metastasis response following treatment. Moving forward, it is necessary to identify systemic therapies that specifically demonstrate efficacy in treating BCLM. Given the liver’s unique role in detoxification and drug metabolism, many traditional therapeutic agents may be quickly rendered ineffective in the liver. Future directions should consider examining if the molecular hallmarks of BCLM are potentially relevant targets of systemic BCLM therapy. Future directions may also include developing optimized drug administration to the liver, perhaps by modifying drugs’ chemical structure or route of administration, such as in hepatic arterial therapy.
The most promising strategy in BCLM management incorporates local intervention with systemic chemotherapy. It is unclear whether systemic pharmacologic or surgical management of BCLM produces greater clinical benefit rates. A 2013 case-matched control study at the Instutit Curie found a 3.04-fold higher mortality rate when BCLM patients did not receive surgical resection [87]. However, a subsequent 2016 case–control study suggested no significant difference in outcomes for patients who received surgery and/or ablation to those who received standard of care systemic therapies [88]. The latter study also found that the 5-year OS for the surgical cohort was 38% compared to a 5-year OS of 39% for the drug-treated cohort [88]. In consideration of the combinatorial approach, Adam et al. reported favorable outcomes in a retrospective study following BCLM patients who received adjuvant surgery alongside systemic chemotherapy [14]. This study found that BCLM patients who received adjuvant hepatic resection had a 37% 5-year survival rate, which compared favorably to previously reported survival rates of BCLM patients. However, Adam et al. report that their study only included BCLM patients with favorable prognostic characteristics such as limited extrahepatic metastases and well-controlled disease. Thus, the high survival rate may be attributable to these favorable prognostic characteristics. A more recent case matched comparison study by Ruiz et al. compared long term outcomes of patients with BCLM who received systemic chemotherapy alone versus those who received systemic chemotherapy in conjunction with LR [89]. BCLM patients who received both systemic chemotherapy and LR had significantly better outcomes. The systemic group had a median survival of 31 months compared to the resection group’s median survival of 82 months (P < 0.001). Citing the success of LR in the treatment of liver metastases, they suggest greater incorporation of LR into BCLM management.
Experimental models for studying liver metastasis
Rodent syngeneic models
Syngeneic models of BCLM are derived from spontaneous mammary gland tumors in mice which are then transplanted into an immunocompetent host of the same genetic background. One such murine cell line, 410.4, was established from a mammary tumor in a BALB/cfCH3H mouse. 410.4 is a spontaneously arising, metastatic adenocarcinoma cell line. 4T1, a 6-thioguanine resistant cell line, was derived from the 410.4 cell line. Both 410.4 and 4T1 have been reported to spontaneously metastasize to the liver following orthotropic introduction into an immune-competent mouse [90–92]. Another set of spontaneously metastatic sublines, D2A1-m1 and D2A1-m2, have been derived from the BALB/c derived D2A1 cell line [93]. Both sublines spontaneously metastasized to the liver. The primary advantage of the rodent syngeneic model is the ability to use immunocompetent mice. This allows for the study of interactions between the immune system and tumor cells, as well as the study of immunotherapies.
Genetically engineered mouse models (GEMMs) are also used in the study of BCLM, though less frequently than other experimental models. One GEMM is the H19-IGF2 single-transgenic mouse, which has been reported to spontaneously develop metastases to the liver [94, 95]. The composite-transgenic mouse p53fp/fp MMTV-Cre Wap-Cre mouse has also been reported to develop spontaneous metastases to the liver [95, 96]. While likely the most representative of the complete metastatic process, there are limitations to the use of GEMMs. In these models, liver metastasis formation is neither consistent nor reliable. Furthermore, spontaneous metastases to other organs render liver-organotropic studies challenging [95].
Human xenotransplantation models
Xenotransplantation models involve the introduction of human-derived tumor cells into immunocompetent mice (Fig. 3). Tumor cells can be obtained from established cell lines or patient-derived xenografts (PDXs). The MDA-MB-231 human breast cancer cell line has been reported to spontaneously metastasize to the liver following orthotopic injection [97]; however, the cells also metastasize to other organs in tandem. The presence of multi-organ metastases hinders the study of liver metastases and targeted treatments. Furthermore, liver metastases arising from primary mammary gland tumors are far less common than metastases to other sites, such as the lungs and bones [98]. Thus, many researchers utilize hematogenous introduction of tumor cells into the mouse. In hematogenous introduction, tumor cells are directly implanted into the bloodstream. This experimental model is not without its inherent limitations. As summarized by Fidler, the formation of metastases is a complex multi-step process involving the primary tumor, proliferation/angiogenesis, detachment/invasion, embolism/circulation, transport, arrest in organs, adherence to vessel wall, and extravasation [8]. Hematogenous establishment of metastases fails to depict the initial steps of malignant dissemination, from the formation of a primary tumor to detachment/invasion.
Fig. 3.
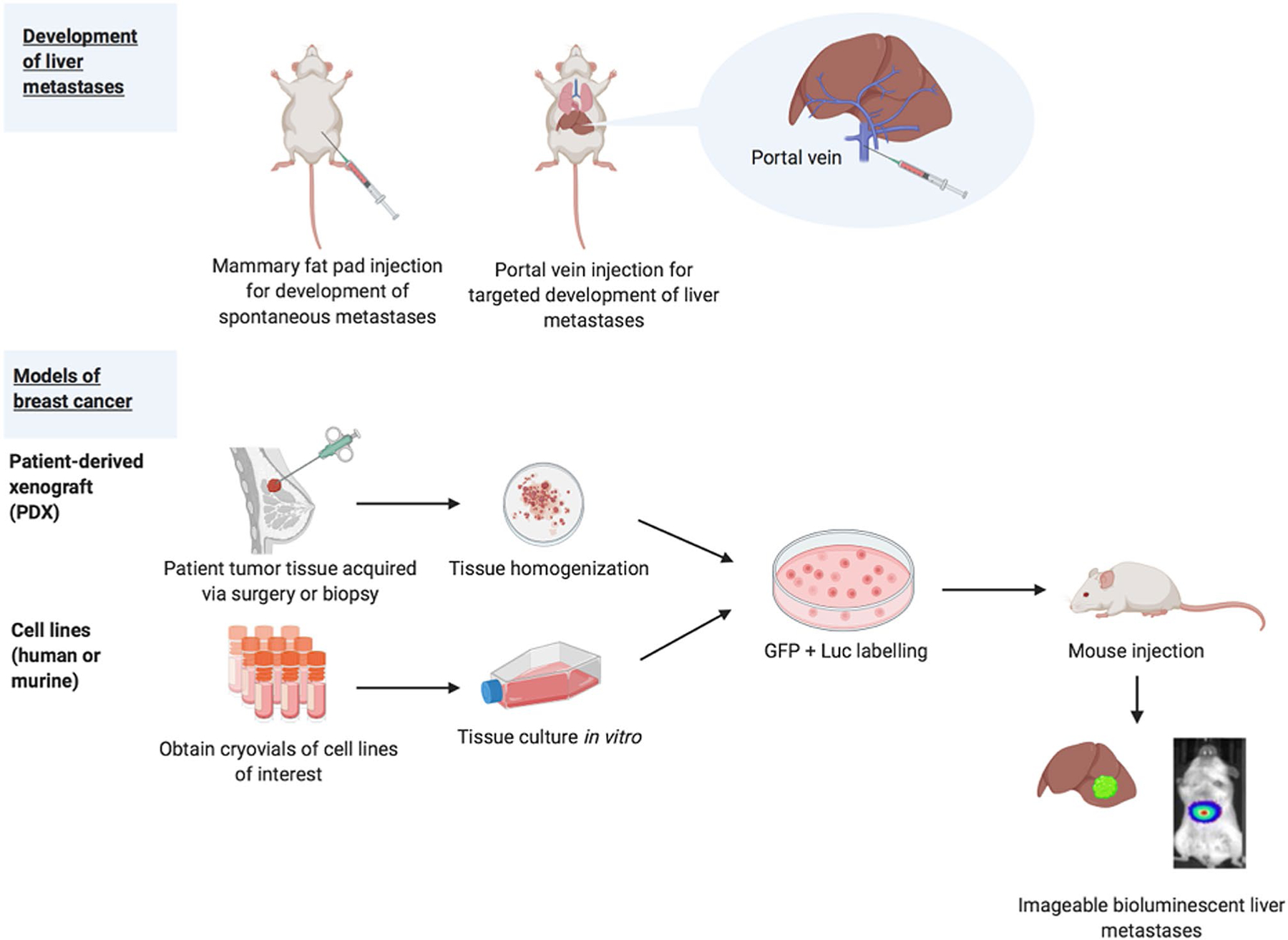
Current in vivo models of BCLM
There are multiple models of hematogenous liver metastasis establishment described in the literature, which include intracardiac, intrasplenic, and portal vein. During intracardiac injection, cells are injected into the left ventricle and disseminated by the aorta. Intracardiac injection frequently yields multi-system metastases, thereby hindering the ability to study BCLM [98]. Tumor cells can also be introduced via direct implantation in the spleen or injection into the splenic vein. Splenectomy must be performed following intrasplenic injection to avoid the formation of splenic tumors [98]. However, the removal of the spleen impacts immune function and impedes comprehensive study of immune–tumor interactions [98]. During portal vein injection, tumor cells are introduced directly into the murine liver, limiting the formation of extrahepatic metastases. Of these experimental models, the portal vein injection has become the most commonplace in the study of BCLM. In the portal vein technique, a single cell suspension of tumor cells is injected into the portal vein of the mouse. This technique has been used to successfully produce BCLM. Our lab recently demonstrated the utility of this technique with 14 genetically distinct BC PDX cells injected into the portal veins of mice [25]. This study found that growth in the liver occurred following injection of 12 of the 14 PDXs, which included several intrinsic subtypes.
While cultured cell lines are useful for preliminary assessment of drug response, they are not sufficient for complete preclinical analysis of treatment efficacy. Gillet et al. found that passaged cell lines underwent clonal selection and a loss of heterogeneity [99]. In fact, this study found that the passaged cell lines bore greater genetic resemblance to each other than to the clinical samples from which they were originally derived. Inasmuch, cell lines are not always reliable, stable models of disease, as high levels of genetic variation have been found among eight MCF7 sublines [100]. It is therefore necessary to incorporate models that more faith-fully recapitulate the heterogeneity of MBC. This feature is important since intratumoral heterogeneity contributes to the formation of metastases, with specific subpopulations of cells being most fit to intravasate into the circulation and extravasate into vital organs [101, 102].
PDXs have been shown to largely retain clinical biomarker status, subtype, and DNA copy number variations [103] and maintain transcriptomic, proteomic, and genomic stability [104] over time. PDX models also display promising concordance with clinical samples, indicating stronger translation from bench to clinic. Marangoni et al. reported that out of seven analyzable cases, five of the breast cancer PDXs displayed a preclinical response consistent with the patient’s response [105]. Johnson et al. found a correlation between in vivo drug activity on xenograft models and activity in phase II clinical trials [106]. Given their ability to capture the heterogeneity of patient samples and predict clinical response, PDXs should be prioritized in the study of BLCM. However, one limitation to utilizing PDXs is that orthotopic transplantation does not always recapitulate the metastatic pattern of the original patient, and other modalities of tumor introduction (e.g. tail vein, portal vein, and/or intracardiac injection) are sometimes necessary to achieve the desired metastatic dissemination pattern [107].
Ongoing studies in vivo
Ongoing studies are utilizing in vivo models of BCLM to identify superior systemic and local therapies. The majority of studies focus on liver metastases arising from colorectal cancer. However, these methodologies can be translated to BCLM to some extent. In one study, PDXs were established from sixteen patients with colorectal liver metastases, and mice harboring these PDXs were treated with a variety of chemotherapeutic agents [108]. Of the 16 total PDXs established, 6 (37.5%) were effective in mimicking the derivative patient’s response to treatment. Of these six PDXs, four demonstrated comparable chemo sensitivity [108]. Another study explored if mechanism of drug delivery could improve patient response. Guo et al. developed a nanoprecipitate from the active forms of folinic acid and oxaliplatin [109]. They found that this nanoprecipitate significantly decreased liver metastases and showed enhanced activity in combination with the anti-PD-L1 antibody. These studies support the use of personalized precision medicine in the treatment of BCLM. Given the liver’s unique vasculature and metabolic capacity, it is necessary to identify targeted drugs and optimize the drug delivery vehicle to maximize its activity.
Murine models of colorectal cancer liver metastasis have been used to evaluate the efficacy of novel surgical techniques. Incomplete hepatic resection of metastases is associated with poorer prognosis, so Schneider et al. explored the use of a fluorescent probe-based confocal laser endomicroscopy to identify cancerous tissue [110]. Several other studies explore the pro-metastatic effects of incomplete RFA through mouse models of RFA. Shi et al. report that incomplete RFA of liver metastases arising from colorectal cancer is associated with metastases, poor survival, and decreased efficacy of anti-PD-1 therapy [111]. Kumar et al. report that STAT3 is upregulated following RFA [112]. STAT3 has oncogenic effects, and Kumar et al. suggest adjuvant STAT3 inhibitor administration as a means of combating these oncogenic effects. Zhang et al. report that incomplete RFA promotes the growth of colorectal cancer via heat shock response [113]. Such studies can be used to optimize local procedures in the preclinical setting.
Discussion
While the mechanism of malignant liver colonization is not fully understood, it is generally hypothesized that migrating tumor cells in circulation come to adhere to sinusoidal endothelium through which they invade, proliferate, and ultimately colonize the liver [114]. This metastatic growth is associated with a significant number of critical health complications, including sudden hepatic failure and nutritional compromise, which contribute to the high mortality rate of BCLM [6]. An understanding of the histologic and molecular characteristics of BCLM is critical to the development of more efficacious treatment strategies. HER2-enriched tumors demonstrate the highest rates of liver metastasis, which associate with their own unique gene-expression patterns that may ultimately provide promising drug targets. The literature evaluating existing BCLM treatment strategies supports a multi-faceted approach for the treatment of BCLM, and surgical resection should be actively considered alongside systemic therapy. Similarly, combinatorial therapies with targeted drugs should be explored for clinical efficacy in BCLM.
Despite recent advances, continued efforts are needed to establish efficacious treatment options for patients with BCLM. Robust studies are needed to identify and evaluate treatment options in the pre-clinical setting. Indeed, both transgenic approaches and PDX models should be prioritized over established cell line studies alone, due to their faithful recapitulation of clinical tumor biology. Ongoing in vivo studies are evaluating different modalities of drug delivery and surgical techniques. Considering the unique vasculature of the liver and its heightened capacity for metabolic activity, further studies of optimized drug delivery and surgical technique are essential.
Acknowledgement
All figures were created using BioRender.com.
Funding
This work was supported by Grants to JCH from the NIH/NCI (1R01CA246182-01A1), the Susan G. Komen Foundation (CCR19608826), and the Jeffress Trust.
Footnotes
Conflict of interest The authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics. CA Cancer J Clin 71(1):7–33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A (2016) Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin 66(1):31–42. 10.3322/caac.21320 [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE et al. (2019) Breast cancer statistics, 2019. CA Cancer J Clin 69(6):438–451. 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 4.Cummings MC et al. (2014) Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol 232(1):23–31. 10.1002/path.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao HY, Gong Y, Ye FG, Ling H, Hu X (2018) Incidence and prognostic factors of patients with synchronous liver metastases upon initial diagnosis of breast cancer: a population-based study. Cancer Manag Res 10:5937–5950. 10.2147/CMAR.S178395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond JR, Finlayson CA, Borges VF (2009) Hepatic complications of breast cancer. Lancet Oncol 10(6):615–621. 10.1016/S1470-2045(09)70029-4 [DOI] [PubMed] [Google Scholar]
- 7.Perou CM et al. (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. 10.1038/35021093 [DOI] [PubMed] [Google Scholar]
- 8.Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited—the role of tumor–stroma interactions in metastasis to different organs. 10.1002/ijc.26031 [DOI] [PMC free article] [PubMed]
- 9.Paget S (1989) The distribution of secondary growths in cancer of the breast 1889. Cancer Metastasis Rev 8(2):98–101 [PubMed] [Google Scholar]
- 10.Harrell JC et al. (2012) Genomic analysis identifies unique signatures predictive of brain, lung, and liver relapse. Breast Cancer Res Treat 132(2):523–535. 10.1007/s10549-011-1619-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smid M et al. (2008) Subtypes of breast cancer show preferential site of relapse. Cancer Res 68(9):3108–3114. 10.1158/0008-5472.CAN-07-5644 [DOI] [PubMed] [Google Scholar]
- 12.Kennecke H et al. (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277. 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- 13.Chan S et al. (1999) Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J Clin Oncol 17(8):2341–2354. 10.1200/jco.1999.17.8.2341 [DOI] [PubMed] [Google Scholar]
- 14.Adam R et al. (2006) Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 244(6):897–907. 10.1097/01.sla.0000246847.02058.1b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surveillance, Epidemiology, and End Results (SEER) Program. seer.cancer.gov
- 16.Horn SR et al. (2020) Epidemiology of liver metastases. Cancer Epidemiol 67:101760. 10.1016/j.canep.2020.101760 [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y et al. (2020) Early death incidence and prediction in Stage IV breast cancer. Med Sci Monit 26:e924858. 10.12659/msm.924858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Y, Liu YR, Ji P, Hu X, Shao ZM (2017) Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep 7(1):1–10. 10.1038/srep45411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Feng Y, Swinnen J, Oyen R, Li Y, Ni Y (2020) Incidence and prognosis of liver metastasis at diagnosis: a pan-cancer population-based study. Am J Cancer Res 10(5):1477–1517 [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D et al. (2020) Breast subtypes and prognosis of breast cancer patients with initial bone metastasis: a population-based study. Front Oncol 10:1. 10.3389/fonc.2020.580112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan MA et al. (2016) Biological subtypes and distant relapse pattern in breast cancer patients after curative surgery (study of Anatolian Society of Medical Oncology). Breast Care 11(4):248–252. 10.1159/000448186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekpe E, Shaikh AJ, Shah J, Jacobson JS, Sayed S (2019) Metastatic breast cancer in Kenya: presentation, pathologic characteristics, and patterns—findings from a tertiary cancer center. J Glob Oncol 5:1–11. 10.1200/JGO.19.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arciero CA et al. (2019) ER+/HER2+ breast cancer has different metastatic patterns and better survival than ER−/HER2+ breast cancer. Clin Breast Cancer 19(4):236–245. 10.1016/j.clbc.2019.02.001 [DOI] [PubMed] [Google Scholar]
- 24.Alzubi MA et al. (2019) Separation of breast cancer and organ microenvironment transcriptomes in metastases. Breast Cancer Res 21(1):36. 10.1186/s13058-019-1123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alzubi MA et al. (2019) Quantitative assessment of breast cancer liver metastasis expansion with patient-derived xenografts. Clin Exp Metastasis 36(3):257–269. 10.1007/s10585-019-09968-z [DOI] [PubMed] [Google Scholar]
- 26.Coffelt SB, Wellenstein MD, De Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16(7):431–446. 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 27.Ren J, Wang Y, Ware T, Iaria J, Ten Dijke P, Zhu H-J (2020) Reactivation of BMP signaling by suboptimal concentrations of MEK inhibitor and FK506 reduces organ-specific breast cancer metastasis. Cancer Lett 493:41–54. 10.1016/j.canlet.2020.07.042 [DOI] [PubMed] [Google Scholar]
- 28.Wang L et al. (2019) Identification of alternatively-activated pathways between primary breast cancer and liver metastatic cancer using microarray data. Genes (Basel) 10(100):753. 10.3390/genes10100753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Recio S et al. (2020) FGFR4 regulates tumor subtype differentiation in luminal breast cancer and metastatic disease. J Clin Investig 130(9):4871–4887. 10.1172/jci130323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao Z et al. (2020) Characterizations of cancer gene mutations in Chinese metastatic breast cancer patients. Front Oncol 10:1023. 10.3389/fonc.2020.01023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St. Romain P, Madan R, Tawfik OW, Damjanov I, Fan F (2012) Organotropism and prognostic marker discordance in distant metastases of breast carcinoma: fact or fiction? A clinic-pathologic analysis. Hum Pathol 43(3):398–404. 10.1016/j.humpath.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 32.Masciari S et al. (2007) Germline E-cadherin mutations in familial lobular breast cancer. J Med Genet 44(11):726–731. 10.1136/jmg.2007.051268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasdemir N et al. (2020) Proteomic and transcriptomic profiling identifies mediators of anchorage-independent growth and roles of inhibitor of differentiation proteins in invasive lobular carcinoma. Sci Rep 10(1):11487. 10.1038/s41598-020-68141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dam PJ et al. (2017) International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer 117(10):1427–1441. 10.1038/bjc.2017.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frentzas S et al. (2016) Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med 22(11):1294–1302. 10.1038/nm.4197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Den Eynden GG, Bird NC, Majeed AW, Van Laere S, Dirix LY, Vermeulen PB (2012) The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin Exp Metastasis 29(6):541–549. 10.1007/s10585-012-9469-1 [DOI] [PubMed] [Google Scholar]
- 37.Stessels F et al. (2004) Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 90(7):1429–1436. 10.1038/sj.bjc.6601727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Illemann M et al. (2009) Two distinct expression patterns of urokinase, urokinase receptor and plasminogen activator inhibitor-1 in colon cancer liver metastases. Int J Cancer 124(8):1860–1870. 10.1002/ijc.24166 [DOI] [PubMed] [Google Scholar]
- 39.Nyström H, Naredi P, Berglund A, Palmqvist R, Tavelin B, Sund M (2012) Liver-metastatic potential of colorectal cancer is related to the stromal composition of the tumour. Anticancer Res 32(12):5185–5191 [PubMed] [Google Scholar]
- 40.Fernández Moro C, Bozóky B, Gerling M (2018) Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastroenterol 5(1):e000217. 10.1136/bmjgast-2018-000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simone C, Murphy M, Shifrin R, Zuluaga Toro T, Reisman D (2012) Rapid liver enlargement and hepatic failure secondary to radiographic occult tumor invasion: two case reports and review of the literature. J Med Case Rep 6:402. 10.1186/1752-1947-6-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossniklaus HE (2013) Progression of ocular melanoma metastasis to the liver: the 2012 Zimmerman lecture. JAMA Ophthalmol 131(4):462–469. 10.1001/jamaophthalmol.2013.2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mentha G et al. (2009) Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg 96(1):95–103. 10.1002/bjs.6436 [DOI] [PubMed] [Google Scholar]
- 44.Bridgeman VL et al. (2017) Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J Pathol 241(3):362–374. 10.1002/path.4845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leenders WPJ et al. (2004) Antiangiogenic therapy of cerebral melanoma metastases results in sustained tumor progression via vessel co-option. Clin Cancer Res 10(18 I):6222–6230. 10.1158/1078-0432.CCR-04-0823 [DOI] [PubMed] [Google Scholar]
- 46.Tasleem S et al. (2018) The role of liver resection in patients with metastatic breast cancer: a systematic review examining the survival impact. Ir J Med Sci 187(4):1009–1020. 10.1007/s11845-018-1746-9 [DOI] [PubMed] [Google Scholar]
- 47.Howlader M, Heaton N, Rela M (2011) Resection of liver metastases from breast cancer: towards a management guideline. Int J Surg 9(4):285–291. 10.1016/j.ijsu.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 48.Golse N, Adam R (2017) Liver metastases from breast cancer: what role for surgery? Indications and results. Clin Breast Cancer 17(4):256–265. 10.1016/j.clbc.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 49.McDermott S, Gervais DA (2013) Radiofrequency ablation of liver tumors. Semin Interv Radiol 30(1):49–55. 10.1055/s-0033-1333653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sofocleous CT et al. (2007) Radiofrequency ablation in the management of liver metastases from breast cancer. Am J Roentgenol 189(4):883–889. 10.2214/AJR.07.2198 [DOI] [PubMed] [Google Scholar]
- 51.Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT (2009) Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation—intermediate and long-term survival rates. Radiology 253(3):861–869. 10.1148/radiol.2533081968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bin Xiao Y, Zhang B, Lian Wu Y (2018) Radiofrequency ablation versus hepatic resection for breast cancer liver metastasis: a systematic review and meta-analysis. J Zhejiang Univ Sci B 19(11):829–843. 10.1631/jzus.B1700516 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Tong AKT, Kao YH, Too C, Chin KFW, Ng DCE, Chow PKH (2016) Yttrium-90 hepatic radioembolization: clinical review and current techniques in interventional radiology and personalized dosimetry. Br J Radiol 89(1062):20150943. 10.1259/bjr.20150943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang J et al. (2018) Liver-dominant breast cancer metastasis: a comparative outcomes study of chemoembolization versus radioembolization. Anticancer Res 38(5):3063–3068. 10.21873/anticanres.12563 [DOI] [PubMed] [Google Scholar]
- 55.Bale R, Putzer D, Schullian P (2019) Local treatment of breast cancer liver metastasis. Cancers 11(9):1341. 10.3390/cancers11091341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howell A et al. (2004) Comparison of fulvestrant versus tamoxifen for the treatment of advanced breast cancer in postmenopausal women previously untreated with endocrine therapy: a multinational, double-blind, randomized trial. J Clin Oncol 22(9):1605–1613. 10.1200/JCO.2004.02.112 [DOI] [PubMed] [Google Scholar]
- 57.Nabholtz JM et al. (2000) Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol 18(22):3758–3767. 10.1200/JCO.2000.18.22.3758 [DOI] [PubMed] [Google Scholar]
- 58.Mouridsen H et al. (2003) Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol 21(11):2101–2109. 10.1200/JCO.2003.04.194 [DOI] [PubMed] [Google Scholar]
- 59.Paridaens RJ et al. (2008) Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol 26(30):4883–4890. 10.1200/JCO.2007.14.4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robertson JFR et al. (2012) Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: follow-up analysis from the randomized ‘FIRST’ study. Breast Cancer Res Treat 136(2):503–511. 10.1007/s10549-012-2192-4 [DOI] [PubMed] [Google Scholar]
- 61.Robertson JFR et al. (2016) Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388(10063):2997–3005. 10.1016/S0140-6736(16)32389-3 [DOI] [PubMed] [Google Scholar]
- 62.Mehta RS et al. (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367(5):435–444. 10.1056/NEJMoa1201622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergh J et al. (2012) FACT: an open-label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30(16):1919–1925. 10.1200/JCO.2011.38.1095 [DOI] [PubMed] [Google Scholar]
- 64.Johnston SRD et al. (2013) Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 14(10):989–998. 10.1016/S1470-2045(13)70322-X [DOI] [PubMed] [Google Scholar]
- 65.Gelmon et al. (2020) Efficacy and safety of palbociclib plus endocrine therapy in North American women with hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. Breast J 26(3):368–375. 10.1111/tbj.13516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sledge et al. (2019) The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 6(1):116–124. https://doi.org/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yardley et al. (2019) Efficacy and safety of Ribociclib with Letrozole in US patients enrolled in the MONALEESA-2 study. Clinical Breast Cancer 19(4):268–277.e1. 10.1016/j.clbc.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 68.Xie N, Qin T, Ren W, Yao H, Yu Y, Hong H (2020) Efficacy and safety of cyclin-dependent kinases 4 and 6 inhibitors in HR+/HER2− advanced breast cancer. Cancer Manag Res 12:4241–4250. 10.2147/CMAR.S254365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Brien NA et al. (2020) Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res 22(1):89. 10.1186/s13058-020-01320-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.André F et al. (2019) Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med 380(20):1929–1940. 10.1056/NEJMoa1813904 [DOI] [PubMed] [Google Scholar]
- 71.Lu Y-S et al. (2020) A Phase Ib study of alpelisib or buparlisib combined with tamoxifen plus goserelin in premenopausal women with HR-positive HER2-negative advanced breast cancer. Clin Cancer Res 27(2):408–417. 10.1158/1078-0432.ccr-20-1008 [DOI] [PubMed] [Google Scholar]
- 72.Figueroa-Magalhães MC, Jelovac D, Connolly RM, Wolff AC (2014) Treatment of HER2-positive breast cancer. Breast 23(2):128–136. 10.1016/j.breast.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diéras V et al. (2017) Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 18(6):732–742. 10.1016/S1470-2045(17)30312-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaufman B et al. (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27(33):5529–5537. 10.1200/JCO.2008.20.6847 [DOI] [PubMed] [Google Scholar]
- 75.Baselga J et al. (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119. 10.1056/NEJMoa1113216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dormann C (2020) Metastatic human epidermal growth factor receptor 2-positive breast cancer: current treatment standards and future perspectives. Breast Care 15(6):570–578. 10.1159/000512328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geyer CE, Forster J, Lindquist D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. Adv Breast Cancer 5(2):45. 10.1056/nejmoa064320 [DOI] [PubMed] [Google Scholar]
- 78.Ramagopalan SV, Pisoni R, Zenin A, Rathore LS, Ray J, Sammon C (2020) Comparative effectiveness of trastuzumab emtansine versus lapatinib plus capecitabine for HER2+ metastatic breast cancer. J Comp Eff Res. 10.2217/cer-2020-0201 [DOI] [PubMed] [Google Scholar]
- 79.Natori A, Ethier JL, Amir E, Cescon DW (2017) Capecitabine in early breast cancer: a meta-analysis of randomised controlled trials. Eur J Cancer 77:40–47. 10.1016/j.ejca.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 80.Chalakur-Ramireddy NKR, Pakala SB (2018) Combined drug therapeutic strategies for the effective treatment of Triple Negative Breast Cancer. Biosci Rep 38(1):BSR20171357. 10.1042/BSR20171357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Llombart-Cussac A et al. (2015) SOLTI NeoPARP: a phase II randomized study of two schedules of iniparib plus paclitaxel versus paclitaxel alone as neoadjuvant therapy in patients with triple-negative breast cancer. Breast Cancer Res Treat 154(2):351–357. 10.1007/s10549-015-3616-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Shaughnessy J et al. (2014) Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol 32(34):3840–3847. 10.1200/JCO.2014.55.2984 [DOI] [PubMed] [Google Scholar]
- 83.Robson ME et al. (2019) OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol 30(4):558–566. 10.1093/annonc/mdz012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JS et al. (2019) Phase I clinical trial of the combination of eribulin and everolimus in patients with metastatic triple-negative breast cancer. Breast Cancer Res 21(1):119. 10.1186/s13058-019-1202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh JC et al. (2014) Phase 2 trial of everolimus and carboplatin combination in patients with triple negative metastatic breast cancer. Breast Cancer Res 16(2):R32. 10.1186/bcr3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jovanovic B et al. (2017) A randomized phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III triple-negative breast cancer (TNBC): responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC subtype, AR status, and Ki67. Clin Cancer Res 23(15):4035–4045. 10.1158/1078-0432.CCR-16-3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mariani P et al. (2013) Liver metastases from breast cancer: surgical resection or not? A case-matched control study in highly selected patients. Eur J Surg Oncol 39(12):1377–1383. 10.1016/j.ejso.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 88.Sadot E et al. (2016) Hepatic resection or ablation for isolated breast cancer liver metastasis. Ann Surg 264(1):147–154. 10.1097/SLA.0000000000001371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruiz A et al. (2018) Surgical resection versus systemic therapy for breast cancer liver metastases: results of a European case matched comparison. Eur J Cancer 95:1–10. 10.1016/j.ejca.2018.02.024 [DOI] [PubMed] [Google Scholar]
- 90.Nizam E, Köksoy S, Erin N (2020) NK1R antagonist decreases inflammation and metastasis of breast carcinoma cells metastasized to liver but not to brain; phenotype-dependent therapeutic and toxic consequences. Cancer Immunol Immunother 69(8):1639–1650. 10.1007/s00262-020-02574-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wei W, Aitken D, Rogers C, McInerney D, Miller F (1986) Use of drug resistance markers to recover clonogenic tumor cells from occult metastases in host tissues. Invasion Metastasis 6(4):197–208. [PubMed] [Google Scholar]
- 92.Lelekakis M et al. (1999) A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis 17(2):163–170. 10.1023/A:1006689719505 [DOI] [PubMed] [Google Scholar]
- 93.Jungwirth U et al. (2018) Generation and characterisation of two D2A1 mammary cancer sublines to model spontaneous and experimental metastasis in a syngeneic BALB/c host. Dis Model Mech 11(1):dmm031740. 10.1242/dmm.031740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pravtcheva DD, Wise TL (1998) Metastasizing mammary car-cinomas in H19 enhancers-Igf2 transgenic mice. J Exp Zool 281(1):43–57. [DOI] [PubMed] [Google Scholar]
- 95.Fantozzi A, Christofori G (2006) Mouse models of breast cancer metastasis. Breast Cancer Res 8(4):212. 10.1186/bcr1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin SCJ et al. (2004) Somatic mutation of p53 leads to estrogen receptor α-positive and -negative mouse mammary tumors with high frequency of metastasis. Cancer Res 64(10):3525–3532. 10.1158/0008-5472.CAN-03-3524 [DOI] [PubMed] [Google Scholar]
- 97.Rikhi R et al. (2016) Murine model of hepatic breast cancer. Biochem Biophys Rep 8:1–5. 10.1016/j.bbrep.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goddard ET, Fischer J, Schedin P (2016) A portal vein injection model to study liver metastasis of breast cancer. J Vis Exp 2016(118):1–10. 10.3791/54903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gillet JP et al. (2011) Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc Natl Acad Sci USA 108(46):18708–18713. 10.1073/pnas.1111840108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nugoli M et al. (2003) Genetic variability in MCF-7 sublines: evidence of rapid genomic and RNA expression profile modifications. BMC Cancer 3:13. 10.1186/1471-2407-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wagenblast E et al. (2015) A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature 520(7547):358–362. 10.1038/nature14403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis RT et al. (2020) Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat Cell Biol 22(3):310–320. 10.1038/s41556-020-0477-0 [DOI] [PubMed] [Google Scholar]
- 103.DeRose YS et al. (2013) Patient-derived models of human breast cancer: protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol 14:14.23. 10.1002/0471141755.ph1423s60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X et al. (2013) A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res 73(15):4885–4897. 10.1158/0008-5472.CAN-12-4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marangoni E et al. (2007) A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 13(13):3989–3998. 10.1158/1078-0432.CCR-07-0078 [DOI] [PubMed] [Google Scholar]
- 106.Johnson JI et al. (2001) Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer 84(10):1424–1431. 10.1054/bjoc.2001.1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whittle JR, Lewis MT, Lindeman GJ, Visvader JE (2015) Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 10.1186/s13058-015-0523-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brown KM et al. (2018) Using patient-derived xenograft models of colorectal liver metastases to predict chemosensitivity. J Surg Res 227:158–167. 10.1016/j.jss.2018.02.018 [DOI] [PubMed] [Google Scholar]
- 109.Guo J, Yu Z, Das M, Huang L (2020) Nano codelivery of oxaliplatin and folinic acid achieves synergistic chemo-immunotherapy with 5-fluorouracil for colorectal cancer and liver metastasis. ACS Nano 14(4):5075–5089. 10.1021/acsnano.0c01676 [DOI] [PubMed] [Google Scholar]
- 110.Schneider C et al. (2017) Identification of liver metastases with probe-based confocal laser endomicroscopy at two excitation wavelengths. Lasers Surg Med 49(3):280–292. 10.1002/lsm.22617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi L et al. (2019) Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat Commun. 10.1038/s41467-019-13204-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar G et al. (2018) Targeting STAT3 to suppress systemic pro-oncogenic effects from hepatic radiofrequency ablation. Radiology 286(2):524–536. 10.1148/radiol.2017162943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Z et al. (2019) Incomplete radiofrequency ablation pro-vokes colorectal cancer liver metastases through heat shock response by PKCα/Fra-1 pathway. Cancer Biol Med 16(3):542–555. 10.20892/j.issn.2095-3941.2018.0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ma R et al. (2015) Mechanisms involved in breast cancer liver metastasis. J Transl Med 13:64. 10.1186/s12967-015-0425-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


