Abstract
Introduction
Approximately 7.2% of children in the world suffer from attention-deficit/hyperactivity disorder (ADHD). Due to the availability of the osmotic-release oral-system methylphenidate, ADHD currently has a remission rate of up to 30.72%. Nevertheless, it has been reported that patients with ADHD tend to exhibit vitamin A and vitamin D deficiency, which may aggravate the symptoms of ADHD. This study aims to determine the effect of vitamin A and vitamin D supplementation as adjunctive therapy to methylphenidate on the symptoms of ADHD.
Methods and analysis
This is a parallel, prospective, interventional multicentric study. Patients will be enrolled from the southern, central and northern parts of China. A target of 504 patients will be followed for 8 weeks. They will be allocated into three groups (vitamin AD, vitamin D and placebo) and administered the interventions accordingly. Data on changes in the symptoms of ADHD as well as changes in the serum concentrations of vitamin A and vitamin D will be recorded. Both responders and nonresponders based on the sociodemographic and clinical data will also be described to mitigate selection bias.
Ethics and dissemination
This study is performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Children’s Hospital of Chongqing Medical University, China (approval number: (2019) IRB (STUDY) number 262). The results of the trial will be reported in peer-reviewed scientific journals and academic conferences regardless of the outcomes.
Trial registration number
Keywords: public health, neurobiology, protocols & guidelines, paediatrics
Strengths and limitations of this study.
Designed as a multicentre study across China, thereby increasing the generalisability of the study results.
First trial to examine vitamin A plus vitamin D supplementation on attention-deficit/hyperactivity disorder (ADHD).
Classification of ADHD will elucidate differential effects of vitamins A and D on ADHD subtypes and provide evidence regarding vitamin A and vitamin D supplementation in patients with ADHD.
The effects of vitamin A are unclear as the effect of vitamin A alone on ADHD was not investigated.
Methylphenidate may mask the effects of vitamin A and vitamin D owing to its strong and numerous effects.
Background
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset neurodevelopmental disorder with a worldwide prevalence of 7.2%.1 It is characterised by developmentally inappropriate levels of inattention and hyperactivity or impulsivity, which can profoundly affect children’s academic achievement, social interactions and self-esteem.2 The dopamine hypothesis is thought to be the main biological mechanism underlying ADHD. Methylphenidate and amphetamine, which are both stimulants, are the first-line pharmacological agents for the treatment of ADHD.1 A study involving 757 children with ADHD aged 6–18 years reported a 30.72% remission rate and a 16.38% recovery rate with osmotic release oral-system methylphenidate.3 This treatment method increases the activity of central dopamine and norepinephrine in the cortex and striatum, which are involved in executive and attentional function regulation.4 However, it is necessary for patients with ADHD to adjust the dose of the stimulants or be co-administered nonstimulants for therapeutic effect. In addition, it has been reported that children with ADHD suffer from deficiencies of various nutrients (vitamin D, folate etc) at a higher rate than their non-ADHD counterparts,5–7 and such nutritional deficiencies may exacerbate the symptoms of ADHD. Therefore, it is essential to find an effective adjuvant therapy with minimal side effects to maximise the effect of ADHD therapy.
Vitamin A, which is an anti-oxidant, plays an essential role in neuroplasticity via its active metabolite retinoic acid (RA).8–10 RA acts as a transcriptional regulator in the corpus striatum to regulate dopamine metabolism.11 The concentration of 5-hydroxytryptamine, which is involved in the mechanism of ADHD along with dopamine,12 is also influenced by vitamin A.13 Nevertheless, a research study noted that vitamin A deficiency (VAD) as a public health problem affects 5.16%, while marginal VAD (MVAD) affects 24.29% of Chinese children aged 12 years and below.14 There has not been any accessible epidemiologic investigations about VAD among patients with ADHD to date. However, as these individuals are particularly susceptible to VAD, more research is warranted to help this population.15 In May 2019, our group investigated 31 outpatients with ADHD aged 6–12 years, 22 of whom were diagnosed with VAD or MVAD. Despite the limited size, this study suggested that patients with ADHD tend to have lower serum concentration of retinol, which determines a patient’s vitamin A status, compared with normal children of the same age (70.97% vs 5.16% and 24.29%).
Vitamin D is an essential fat-soluble vitamin for calcium homeostasis and bone metabolism.16 It has been reported that retinoid X receptor influences the activity of vitamin D receptor.17–19 Vitamin D is also involved in prompting the development and maturation of dopaminergic neurons,20–22 which may play a potential role in the ADHD pathologies.23 Vitamin D deficiency is highly prevalent in all age groups globally.16 Serum 25-hydroxyvitamin D (25(OH)D) concentration, which measures a patient’s vitamin D status, is significantly lower in patients with ADHD than in heathy controls.24 Furthermore, a meta-analysis of five case–control studies demonstrated that lower vitamin D status is significantly related to the likelihood of ADHD (OR: 2.57; 95% CI 1.09 to 6.04; I2=84.3%).5
Aside from the exiting data, a prospective study assessing the adjuvant effects of vitamin A and vitamin D administered with methylation in the ADHD population will be performed simultaneously, under the hypothesis that vitamin A and vitamin D could enhance the effects of therapy on ADHD symptoms. Previous studies conducted by Mohammadpour et al, Dehbokri et al, Elshorbagy et al have found that ADHD symptoms were significantly relieved under vitamin D supplementation.25–27 Experiments conducted on rats revealed that high vitamin A intake during pregnancy has long-lasting programming effects on the dopamine system of the offspring.28 Basic research found that vitamin A influences vitamin D by binding to acceptors of vitamin D in vivo.29 Based on these findings, identifying the effects of vitamin A and vitamin D on the therapy of ADHD is highly essential, as patients with ADHD may be excellent candidates for vitamin A and vitamin D combination therapy.
Study objectives
General objective
To our knowledge, the combined effect of vitamin A and vitamin D on ADHD treatment has never been reported. Using the current treatment regimen involving methylphenidate, the present study aims to verify the effect of vitamin D and assess the joint effect of vitamin A and vitamin D on the symptom changes of ADHD.
Specific objectives
To verify the effect of vitamin D in addition to methylphenidate on the subtypes of ADHD with a larger sample size.
To explore whether co-administration of vitamin A with vitamin D enhances, suppresses or does not affect symptomatic relief of ADHD seen with vitamin D supplementation alone, and how this effect differs between ADHD subtypes.
Methods and analysis
Study setting
This multicentre, parallel, prospective, interventional study will be performed from February to May of the next year at the Children’s Hospital of Chongqing Medical University, Qilu Hospital of Shandong University and the First Hospital of Jilin University, which are located in the south, central and north regions of China, respectively. The Children’s Hospital of Chongqing Medical University will act as the leading organisation.
Patient and public involvement
The patients diagnosed with ADHD, who show deficiency or insufficiency in vitamin A (≤1.05 µmol/ L) and vitamin D (≤50 nmol/L) will be informed about the study and contacted for their informed consent if they are willing to be involved in the study. Enrolment of patients will last for 2 months. To collect the medical history of children with suspected ADHD, the junior developmental behaviour specialists will initially screen for the related symptoms in the children and then evaluate them using the Wechsler Intelligence Scale, Vanderbilt assessment scale and Questionnaire—Children with Difficulties (QCD). Next, parental and patient interviews will be further evaluated by developmental behaviour specialists at the associate level or above, and diagnoses will be made based on the results of a comprehensive analysis of clinical manifestations, Vanderbilt scales and QCD. The participants will be randomly assigned in a double-blind fashion, at a ratio of 1:1:1, to the vitamin AD supplementation group, vitamin D supplementation group or the placebo group. Sealed, numbered, opaque envelopes containing computer-generated random numbers, which are associated with corresponding interventions, will be used. Vitamin AD supplementation group will be administrated vitamin AD capsules (3 capsules/time, once a day for 8 weeks), which contain vitamin A (2000 IU/capsule) and vitamin D (700 IU/capsule). Vitamin D supplementation group will be administrated vitamin D capsules (400 IU/capsule, 6 capsules/time, once a day for 2 weeks, then change to 5 capsules/time, once a day for 6 weeks). The placebo capsules given to the placebo group (3 capsules/time, once a day for 8 weeks) consist of oily liquids that do not contain vitamin A and vitamin D and were produced in strict accordance with China’s drug management and packaging requirements for placebo by Shandong DYNE Marine Biopharmaceutical in China. Placebo, vitamin AD and vitamin D capsules are identical in appearance and odour to guarantee blinding. The medicine is dispensed by the staff who were not involved in the process of evaluation, diagnosis and treatment. These patients will be followed up at weeks 4 and 8 following the addition of the adjunctive therapy to methylphenidate to evaluate changes in ADHD symptoms. The serum concentration of retinol and 25(OH)D will be measured at week 8. Accordingly, the placebo group and vitamin D group will be prescribed vitamin A and vitamin D as retinol and 25 (OH)D concentration after the study. The results of the study will be disseminated to the involved patients and peer-reviewed scientific journals in the form of scientific articles. However, the personal information about the patients will not be made publicly accessible and this information will be deleted after the study.
The trial design is summarised in figure 1.
Figure 1.
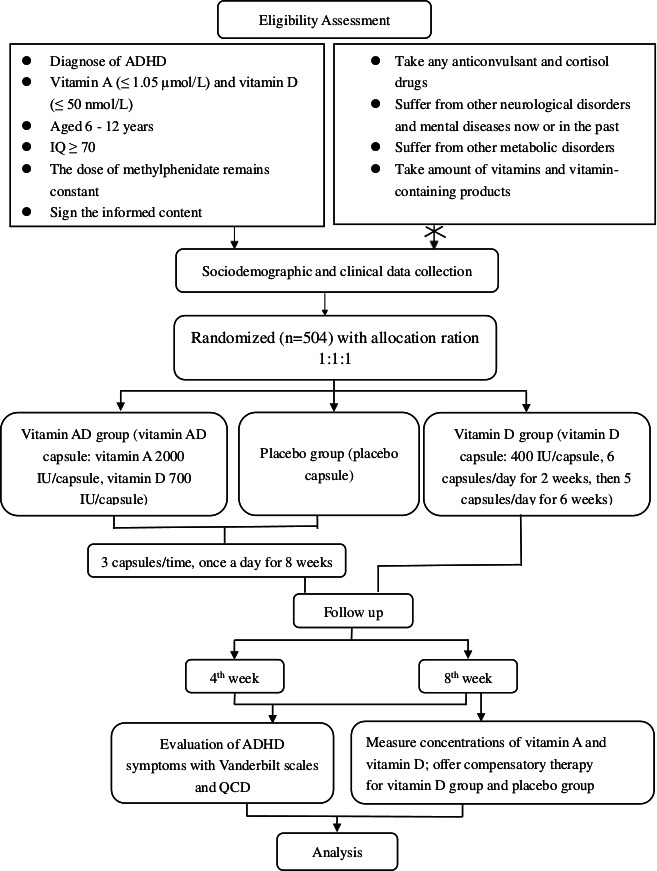
Flow diagram of the study protocol. ADHD, attention-deficit/hyperactivity disorder; QCD, Questionnaire–Children with Difficulties.
Primary outcomes
We will use the Vanderbilt parent assessment scale and Vanderbilt teacher assessment scale to estimate the symptoms of various ADHD subtypes (predominantly inattentive, predominantly hyperactivity/impulsive, and combined) at baseline. We will use the Vanderbilt parent follow-up scales and Vanderbilt teacher follow-up scales to estimate the changes in ADHD symptoms at weeks 4 and 8, respectively. Moreover, we will assess problems in the daily life of children at particular times of the day by QCD.
Secondary outcomes
The serum concentrations of vitamin A and vitamin D will be measured through high-performance liquid chromatography using peripheral blood.
Criteria
Inclusion criteria
(1) Diagnosis of ADHD based on the diagnostic criteria of Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), (2) deficiency or insufficiency in vitamin A (≤1.05 µmol/L) and vitamin D (≤50 nmol/L), (3) aged 6–12 years, (4) IQ ≥70, (5) receiving methylphenidate (trade name Concerta) 18–54 mg/day (began with 18 mg/day for a week and titrated gradually to the optimum dose not more than 54 mg/day).
Exclusion criteria
(1) Inconsistent or changing dose of methylphenidate during the participation period, (2) use of anticonvulsant drugs or hydrocortisone, (3) current or previous history of other neurological disorders or mental illnesses, such as convulsions, anxiety and depression, (4) history of metabolic disorders such as cholestasis, liver dysfunction, pancreatic insufficiency, measles, diarrhoea, respiratory illness, severe inflammation, malnutrition and so on, (5) use of vitamins and vitamin-containing products 3 months before the study, (6) IQ <70, (7) serum concentration of vitamin A >1.05 µmol/L and/or vitamin D >50 nmol/L.
Exit and termination criteria
The study will be terminated if (1) the ADHD symptoms worsen or side effects such as vitamin A poisoning, including toxicity to the liver, visual impairment, bone and muscle pain, appear during the study period and (2) the subjects drop out for other reasons.
Recruitment
Any patient meeting the inclusion criteria will be informed about the study and give informed consent at their personal discretion. Basic information including previous medical history of the patient, family history, including any neuropsychiatric disorders, such as epilepsy, depression, autism spectrum disorder and ADHD, congenital diseases and metabolic diseases in his or her family as well as results of physical examinations will be recorded. Enrolment of patients is expected to last for 2 months.
Instruments
Sociodemographic and clinical data
The sociodemographic data come from the child’s primary caregiver, detailing child’s name, gender, date of birth, height, weight, blood pressure, heart rate and supplementation of vitamin A/D products or vitamin A/D-containing products. Clinical data will be ascertained from the medical records, including information about the DSM-5 diagnosis, disease classification, current treatment and comorbid conditions. All collected data will be double checked.
Evaluation methods and tools
Chinese version of Vanderbilt Assessment Scales
The initial assessment scales are designed to measure the severity of ADHD symptoms for children aged 6–12. There are two versions available: parent assessment scale and teacher assessment scale. Each version consists of three components: symptom assessment, performance impairment and comorbid diseases. The symptom assessment screens for symptoms related to inattentive (items 1–9) and hyperactive (items 10–18) ADHD. Scores of 2 or 3 in each question of symptom assessment part (scored 0–3) reflect often-occurring or very often-occurring behaviours. The performance measures in the scale are located in items 49–56 and 36–43 of the parent and teacher assessment scales, respectively. Scores of 4 or 5 in the performance measures (scored 1–5) mean somewhat of a problem or problematic. Items 19–48 in the parent assessment scale and items 19–35 in the teacher assessment scale are used to screen for comorbid diseases—oppositional-defiant disorder, conduct disorder and anxiety/depression and scores of 2 or 3 are considered positive. To meet the DSM-5 criteria for ADHD diagnosis, one must score 2 or 3 on 6 out of either the inattentive 9 or hyperactive 9 core symptoms or both and score 4 or 5 on any of the performance questions. Higher scores indicate a worse outcome. Symptom assessment combined with performance measures are divided into three subtypes: predominantly inattentive subtype, predominantly hyperactivity/impulsive subtype and combined type. The score criteria are detailed in table 1.30 31
Table 1.
The score criteria for ADHD subtypes and comorbidities using the Vanderbilt assessment scales30
| Vanderbilt parent assessment scale | Vanderbilt teacher assessment scale | ||||
| Symptom section | Performance section | Symptom section | Performance section | ||
| Predominantly inattentive subtype | At least 6 of questions 1–9 must score a 2 or 3. | At least 1 of questions 49–56 must score a 4 or 5. | At least 6 of questions 1–9 must score a 2 or 3. | At least 1 of questions 36–43 must score a 4 or 5. | |
| Predominantly hyperactivity/impulsive subtype | At least 6 of questions 10–18 must score a 2 or 3. | At least 1 of questions 49–56 must score a 4 or 5. | At least 6 of questions 10–18 must score a 2 or 3. | At least 1 of questions 36–43 must score a 4 or 5. | |
| Combined type | At least 6 of questions 1–9 and 6 of questions 10–18 must score a 2 or 3. | At least 1 of questions 49–56 must score a 4 or 5. | At least 6 of questions 1–9 and 6 of questions 10–18 must score a 2 or 3. | At least 1 of questions 36–43 must score a 4 or 5. | |
| Comorbidities | Oppositional-defiant disorder | At least 4 of questions 19–26 must score a 2 or 3. | A score of 4 or 5 on any of questions 49–56. | At least 3 of questions 19–28 must score a 2 or 3. | A score of 4 or 5 on any of questions 36–43. |
| Conduct disorder | At least 3 of questions 27–41 must score a 2 or 3. | A score of 4 or 5 on any of questions 49–56. | At least 3 of questions 19–28 must score a 2 or 3. | A score of 4 or 5 on any of questions 36–43. | |
| Anxiety/depression | At least 3 of questions 42–48 must score a 2 or 3. | A score of 4 or 5 on any of questions 49–56. | At least 3 of questions 29–35 items must score a 2 or 3. | A score of 4 or 5 on any of questions 36–43. | |
ADHD, attention-deficit/hyperactivity disorder.
With the same assessment items on symptom (items 1–18) and performance (items 19–26) as the initial scales, the parent and teacher follow-up scales have added a side-effect reporting scale that can be applied to evaluate and monitor the occurrence of adverse effects to prescribed medications, if any, such as headache, stomach ache, change of appetite, extreme sadness or unusual crying and so on. The scoring criteria evaluated at weeks 4 and 8 are as follows: (1) calculating total symptom score for questions 1–18 and (2) calculating average performance score for questions 19–26. Higher scores indicate worse outcome.
Chinese-Wechsler Intelligence Scale for Children
Chinese-Wechsler Intelligence Scale for Children (C-WISC) is revised from the Wechsler Intelligence Scale for Children based on the Chinese cultural background and tests the individual intelligence for children aged from 6 to 16. It is composed of 11 subtests: information, sort, arithmetic, comprehension, digit span and vocabulary for verbal intelligence; coding, picture completion, block design, picture arrangement, mazes and object assembly for performance intelligence. The C-WISC raw total score obtained by the sum of verbal and performance scores is transformed to IQ, including verbal IQ, nonverbal IQ and full-scale IQ, based on an algorithm. An IQ less than 70 is considered as abnormal.32
The Chinese version of QCD
The QCD assesses problems in the daily life of children aged 6–18 years at a particular time of the day, including in the morning or evening, during or after school and overall difficulties over the entire day and night. The Chinese version of QCD has been shown to have good validity and reliability. Filled in by the parents, the scale consists of 20 questions with respect to ADHD-related difficulties. Each question is scored on a 4-point scale: 0=completely disagree, 1=somewhat (partially) agree, 2=mostly agree and 3=completely agree. A score of 30–35 is considered the cut-off range for functional impairment and a score of less than 30 indicates functional impairment (highest score possible: 57). Lower scores indicate lower life function and more difficulty in the children’s daily activities.33
Determination of vitamin A and vitamin D status
The serum concentrations of retinol and 25(OH)D are measured by high-performance liquid chromatography from 2 mL of venous blood. Vitamin A status is categorised as follows:<0.35 µmol/L is considered very deficient, 0.35–0.70 µmol/L is deficient, 0.70–1.05 µmol/L is marginal and >1.05 µmol/L is adequate. The values of serum vitamin D level are classified into four categories: <30 nmol/L is regarded as deficiency, 30–50 nmol/L is insufficiency, 50–250 nmol/L is normal and >250 nmol/L is toxic.
Sample size
Based on an alpha of 0.05, power of 80% and a dropout rate of 10%, we adopted an analysis of variance F-test by using the PASS software 2020 to evaluate the sample size. This study is a randomised double-blind controlled trial. Intervention groups are vitamin AD group and vitamin D group, while the control is the placebo group. The primary outcome index is changes in ADHD symptoms as evaluated by Vanderbilt assessment scales at weeks 4 and 8 compared with that at baseline. In the study conducted by Mohammadpour et al,25 where the score generated using Conner’s Parent Rating Scale (CPRS) was considered the main outcome, the mean±SD of ADHD index in CPRS was 55.84±10.20 for the vitamin D+methylphenidate group (n=25) and 56.79±9.60 for the placebo +methylphenidate group (n=29). The Vanderbilt assessment scale is considered as effective as the CPRS in assessing the changes of ADHD symptoms.31 Based on the hypothesis described above, vitamin A, along with vitamin D, promotes the improvement of ADHD symptoms. We cautiously presume that the mean score±SD for vitamin AD+methylphenidate group is lower than that of vitamin D+methylphenidate group, while the control group scores the highest using the Vanderbilt assessment scales, with a score of 54.00±9.88 for the vitamin AD+methylphenidate group, 55.84±10.20 for the vitamin D+methylphenidate group and 56.79±9.60 for the control group. The number of subjects to be enrolled in the study is 504.
Statistical analysis
All data will be analysed using the Statistical Package for the Social Sciences V.19. The normality of variables will be assessed by Kolmogorov Smirnov test. F test and Kruskal-Wallis test will be carried out for the comparison of parametric and nonparametric variables between groups, respectively. Paired t-test and Wilcoxon signed-rank test will be used to investigate within-group differences. Confounding factors will be adjusted by the analysis of covariance.
Bias control
To achieve masking to prevent bias, the participants and care providers are unaware of which group they are enrolled in. Furthermore, the clinicians’ roles are limited to recruiting the patients, informing the patients about the study and then randomly assigning the patients in a 1:1:1 ratio to group A, group B or group C. The intervention assignments are designated by computer-generated random numbers, which are concealed in numbered, sealed opaque envelopes. The drugs will be dispensed by the staff, who is responsible for taking notes about the patients' basic information and medication records and not involved in the process of evaluation, diagnosis and treatment. After the study, the staff will give the unblinded results to the outcomes assessor to complete statistical analysis, to the clinicians to provide compensatory therapy for the patients. In addition, we will describe both responders and nonresponders on sociodemographic and clinical data in detail to mitigate the selection bias. Furthermore, to decrease the rate of missed follow-ups, we will contact the patients’ guardians to inform them regarding adherence to the treatment regimen by Wechat (a digital communication platform), e-mail or telephone.
Discussion
To our knowledge, this is the first trial to examine vitamin A and vitamin D supplementation in ADHD. Based on the known theoretic foundation, vitamin A binding to the vitamin D receptors to influence the metabolism of vitamin D, the study is expected to provide more substantial findings regarding the potential use of vitamin A and vitamin D in addition to methylphenidate in cases of ADHD complicated by vitamin A and vitamin D deficiency and to provide supporting data to supplement and help revise the current ADHD clinical guidelines.
As the study will be carried out in the southern, central and northern parts of China, regional differences will be minimised. This study design not only verifies the effect of vitamin D on the treatment of ADHD using a larger sample size25–27 but also explores whether vitamin A along with vitamin D is effective in the treatment of ADHD. In terms of dosage, the dose of vitamin D—2100IU—is not higher than the previous study, neither the reported 3000 IU/day of vitamin D lasting for 12 weeks in the study of Dr Hatem Hamed Elshorbagy27 nor 50 000 IU/week of vitamin D lasting for 6 weeks in the study of Dr Nadia Dehbokri,26 and it is as similar as 2000 IU/day lasting for 8 weeks from Nakisa Mohammadpour.25 Additionally, according to the category criterion of WHO, China is still a country with mild VA deficiency. Data from the Chinese Dietary Reference Intakes show that the tolerable upper intake levels (UL) of vitamin A in children above 4 years old is 6600 IU/day.34 Furthermore, the Nelson textbook of paediatrics 21st edition showed that ‘chronic daily intakes of 15 000 µg and 6000 µg can be toxic in adults and children, respectively’.35 And the reported chronic toxic dose in Chinese paediatrics textbook is 50 000 IU/day to 100 000 IU/day for children, more than 6 months.36 Considering the proportion of vitamin A and D dosage forms in China, we chose 6000 IU/day of vitamin A during 3 months observation period in our study, which is lower than UL and chronic toxic dose. It is a safe dose. Apart from the fact that all the patients are diagnosed, treated alone and they all take medicine separately at home, the staff dispensing the drugs does not participate in the patient’s diagnosis and treatment process. Therefore, the results of the study are not biased even though the oral amount of vitamin D capsules is different from that of the other groups. Moreover, the classification of ADHD will be conducted to further elucidate the effects of vitamin A and vitamin D on ADHD and to lay a foundation to explore the mechanism underlying this condition. Although the sample size is calculated by referring to the CPRS scale rather than the Vanderbilt assessment scales, our study proposes a much larger sample size than previous studies in the literature, reducing selection bias as much as possible. There are still some limitations in our study. We will not be administering vitamin A alone as our intervention due to the restrictions on pharmaceutical production, which may pose limitations in determining the exact effect of vitamin A. Considering ethical conditions, we will be enrolling all patients with deficiency or insufficiency in vitamin A and vitamin D and administer methylphenidate along with these vitamins to improve patient adherence. As a result, we cannot conclude the effect of vitamin A or vitamin D on patients with ADHD with normal serum concentrations of vitamin A and vitamin D. Furthermore, methylphenidate may mask the effects of vitamin A and vitamin D owing to its strong and numerous effects. However, these restrictions are not the Achilles' heel of this study, and the topics of the study remain to be further investigated, as the mechanism of action of vitamins A and D on ADHD should be explored based on its promise shown in the current literature.
Ethics and dissemination
This study was approved by the Institutional Review Board of Children’s Hospital of Chongqing Medical University, China (approval number: (2019) IRB (STUDY) Number 262). The patients who participated in the study will sign the informed consent (obtained from the guardian and patients). The participants, care providers and investigators will be masked in the clinical trial. The drugs will be dispensed by a staff not involved in the process of diagnosis and treatment. The authorisation from parents on the patient’s health information remains valid until the study is completed. After that, investigators will delete private information from the study record. The results of the study will be disseminated in form of academic conferences or publication in peer view of journals.
Supplementary Material
Acknowledgments
We wish to thank Dr. Mark Lee Wolraich for authorisation to use of Vanderbilt assessment scales, Shandong DYNE Marine Biopharmaceutical in China for offering the vitamins and placebos for free and Beijing Harmony Health Medical Diagnostics for the technology support to measure concentrations of vitamin A and vitamin D, as well as the study participants, their families and clinicians for their contribution.
Footnotes
Contributors: PZ designed the study and wrote the paper. LC designed the study and conducted the writing. MLW conducted the writing and gave some advices and supports on the study. A-hC, TL, QC, F-YJ, BL, YL, TY, JC gave some advices and supports on the study. CL and BP gave statistical advices. LZ and XL revised the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by Chongqing Municipal Education Commission (project number: CYS19199).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Etrhics approval is obtained from the Institutional Review Board, Children's Hospital of Chongqing Medical University.
References
- 1. Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2019;144:e20192528. 10.1542/peds.2019-2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallo EF, Posner J. Moving towards causality in attention-deficit hyperactivity disorder: overview of neural and genetic mechanisms. Lancet Psychiatry 2016;3:555–67. 10.1016/S2215-0366(16)00096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tzang R-F, Wang Y-C, Yeh C-B, et al. Naturalistic exploration of the effect of osmotic release oral system-methylphenidate on remission rate and functional improvement in Taiwanese children with attention-deficit-hyperactivity disorder. Psychiatry Clin Neurosci 2012;66:53–63. 10.1111/j.1440-1819.2011.02289.x [DOI] [PubMed] [Google Scholar]
- 4. Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev 2018;87:255–70. 10.1016/j.neubiorev.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khoshbakht Y, Bidaki R, Salehi-Abargouei A. Vitamin D status and attention deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. Adv Nutr 2018;9:9–20. 10.1093/advances/nmx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotsi E, Kotsi E, Perrea DN. Vitamin D levels in children and adolescents with attention-deficit hyperactivity disorder (ADHD): a meta-analysis. Atten Defic Hyperact Disord 2019;11:221–32. 10.1007/s12402-018-0276-7 [DOI] [PubMed] [Google Scholar]
- 7. Saha T, Chatterjee M, Verma D, et al. Genetic variants of the folate metabolic system and mild hyperhomocysteinemia may affect ADHD associated behavioral problems. Prog Neuropsychopharmacol Biol Psychiatry 2018;84:1–10. 10.1016/j.pnpbp.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 8. Evans E, Piccio L, Cross AH. Use of vitamins and dietary supplements by patients with multiple sclerosis: a review. JAMA Neurol 2018;75:1013–21. 10.1001/jamaneurol.2018.0611 [DOI] [PubMed] [Google Scholar]
- 9. Fragoso YD, Stoney PN, McCaffery PJ. The evidence for a beneficial role of vitamin A in multiple sclerosis. CNS Drugs 2014;28:291–9. 10.1007/s40263-014-0148-4 [DOI] [PubMed] [Google Scholar]
- 10. Ono K, Yamada M. Vitamin A and Alzheimer's disease. Geriatr Gerontol Int 2012;12:180–8. 10.1111/j.1447-0594.2011.00786.x [DOI] [PubMed] [Google Scholar]
- 11. McCaffery P, Dräger UC. High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci U S A 1994;91:7772–6. 10.1073/pnas.91.16.7772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oades RD. Dopamine-Serotonin interactions in attention-deficit hyperactivity disorder (ADHD). Prog Brain Res 2008;172:543–65. 10.1016/S0079-6123(08)00926-6 [DOI] [PubMed] [Google Scholar]
- 13. Guo M, Zhu J, Yang T, et al. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): a pilot study. Brain Res Bull 2018;137:35–40. 10.1016/j.brainresbull.2017.11.001 [DOI] [PubMed] [Google Scholar]
- 14. Song P, Wang J, Wei W, et al. The prevalence of vitamin A deficiency in Chinese children: a systematic review and Bayesian meta-analysis. Nutrients 2017;9. 10.3390/nu9121285. [Epub ahead of print: 25 Nov 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kassebaum NJ, GBD 2013 Anemia Collaborators . The global burden of anemia. Hematol Oncol Clin North Am 2016;30:247–308. 10.1016/j.hoc.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 16. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 2014;144 Pt A:138–45. 10.1016/j.jsbmb.2013.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wagner CE, Jurutka PW, Marshall PA, et al. Retinoid X receptor selective agonists and their synthetic methods. Curr Top Med Chem 2017;17:742–67. 10.2174/1568026616666160617091559 [DOI] [PubMed] [Google Scholar]
- 18. Long MD, Sucheston-Campbell LE, Campbell MJ. Vitamin D receptor and RXR in the post-genomic era. J Cell Physiol 2015;230:758–66. 10.1002/jcp.24847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de la Fuente AG, Errea O, van Wijngaarden P, et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol 2015;211:975–85. 10.1083/jcb.201505119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moretti R, Morelli ME, Caruso P. Vitamin D in neurological diseases: a rationale for a pathogenic impact. Int J Mol Sci 2018;19. 10.3390/ijms19082245. [Epub ahead of print: 31 Jul 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pertile RAN, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience 2016;333:193–203. 10.1016/j.neuroscience.2016.07.020 [DOI] [PubMed] [Google Scholar]
- 22. Pertile RAN, Cui X, Hammond L, et al. Vitamin D regulation of GDNF/RET signaling in dopaminergic neurons. Faseb J 2018;32:819–28. 10.1096/fj.201700713R [DOI] [PubMed] [Google Scholar]
- 23. Seyedi M, Gholami F, Samadi M, et al. The effect of vitamin D3 supplementation on serum BDNF, dopamine, and serotonin in children with attention-deficit/hyperactivity disorder. CNS Neurol Disord Drug Targets 2019;18:496–501. 10.2174/1871527318666190703103709 [DOI] [PubMed] [Google Scholar]
- 24. Fasihpour B, Moayeri H, Shariat M, et al. Vitamin D deficiency in school-age Iranian children with attention-deficit/hyperactivity disorder (ADHD) symptoms: a critical comparison with healthy controls. Child Neuropsychol 2020;26:1–15. 10.1080/09297049.2019.1665638 [DOI] [PubMed] [Google Scholar]
- 25. Mohammadpour N, Jazayeri S, Tehrani-Doost M, et al. Effect of vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: a randomized, double blind, placebo-controlled trial. Nutr Neurosci 2018;21:202–9. 10.1080/1028415X.2016.1262097 [DOI] [PubMed] [Google Scholar]
- 26. Dehbokri N, Noorazar G, Ghaffari A, et al. Effect of vitamin D treatment in children with attention-deficit hyperactivity disorder. World J Pediatr 2019;15:78–84. 10.1007/s12519-018-0209-8 [DOI] [PubMed] [Google Scholar]
- 27. Elshorbagy HH, Barseem NF, Abdelghani WE, et al. Impact of vitamin D supplementation on attention-deficit hyperactivity disorder in children. Ann Pharmacother 2018;52:623–31. 10.1177/1060028018759471 [DOI] [PubMed] [Google Scholar]
- 28. Sánchez-Hernández D, Poon AN, Kubant R, et al. High vitamin A intake during pregnancy modifies dopaminergic reward system and decreases preference for sucrose in Wistar rat offspring. J Nutr Biochem 2016;27:104–11. 10.1016/j.jnutbio.2015.08.020 [DOI] [PubMed] [Google Scholar]
- 29. Riccio P, Diet RR, Microbiota G. And vitamins D + A in multiple sclerosis. Neurotherapeutics 2018;15:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Attention Deficity disorder toolkit. Available: http://www.nccpeds.com/adhd_toolkit.htm; [Accessed 8 May 2020].
- 31. Lin Z, Fei L, Li C. Application of four commonly used rating scales in diagnosis and follow-up management of children with attention deficit hyperactivity disorder. Journal of Chongqing Medical University 2020;45:32–5. [Google Scholar]
- 32. Gong Y, Cai T. Chinese-Wechsler intelligence scale for children. Chinese Journal of Clinical Psychology 1994:1–6. [Google Scholar]
- 33. Zheng Y, Du Y, Su LY, et al. Reliability and validity of the Chinese version of Questionnaire - Children with Difficulties for Chinese children or adolescents with attention-deficit/hyperactivity disorder: a cross-sectional survey. Neuropsychiatr Dis Treat 2018;14:2181–90. 10.2147/NDT.S166397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Society CN . Chinese dietary reference intakes (Chinese DRIs) (2013 edition. 322. Science Publishing House, 2014. [Google Scholar]
- 35. Robert M, Kliegman JSG. Nelson textbook of pediatrics. 21th edn. Elsevier, 2019: 1938–40. [Google Scholar]
- 36. Yonghao G, Xindong X. Pediatics.: People’s Medical Publishing House Co., LTD. 84, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


