Abstract
Recent data indicate a resurgence of stimulant use and harms in the United States; thus, there is a need to identify risk factors to inform development of effective prevention strategies. Prior research suggests adverse childhood experiences (ACEs) are common among individuals using stimulants and may be an important target for prevention. National Epidemiological Survey on Alcohol and Related Conditions was used to estimate prevalence of ACEs among U.S. adults using amphetamine-type stimulants (ATS), cocaine, or both. Multivariable logistic regression examined associations between ACEs and stimulant use and use disorders. Among adults reporting lifetime ATS use, 22.1% had ≥4 ACEs, 24.9% had 2–3 ACEs, 22.4% had 1 ACE, 30.6% reported no ACEs. Among adults with lifetime ATS use disorder, 29.3% reported ≥4 ACEs, 28.7% reported 2–3 ACEs, 21.6% reported 1 ACE, and 20.4% reported no ACEs. Multivariable logistic regression found a significant relationship between number of ACEs and stimulant use and use disorders. In conclusion, we found a strong relationship between increasing ACE exposures and stimulant use and use disorders. Advancing comprehensive strategies to prevent ACEs and treating underlying trauma among those using stimulants holds great promise to reduce stimulant use and its health and social consequences in the United States.
Keywords: Cocaine use, Methamphetamine use, Stimulant use disorder, Adverse childhood experiences, Trauma, Trauma informed care
1. Introduction
Cocaine and amphetamine-type stimulants (ATS) such as methamphetamine are highly addictive and potent central nervous system stimulants (Ciccarone, 2011). Use of these substances, especially chronic use, is associated with a range of physical and psychological harms, including psychosis and other mental disorders, cognitive and neurologic deficits, cardiovascular and renal dysfunction, infectious disease transmission, and increased mortality (Barr et al., 2006; Butler et al., 2017; Cheng et al., 2010; Cunningham et al., 2015; Darke, et al., 2017; Darke et al., 2017; Hirsiger et al., 2019; Strathdee and Stockman, 2010; Voce et al., 2019; Wang et al., 2017). In recent years, the availability of stimulants, including cocaine and methamphetamine, throughout the U.S. has increased (Drug Enforcement Administration, 2019). Coincident with this increasing availability, indicators of use and harms have increased. In 2018, 5.5 million (2.0%) people aged 12 years or older reported past-year use of cocaine, up from 4.8 million (1.8%) in 2015; for methamphetamine, 1.9 million (0.7%) people aged 12 years or older reported past-year use in 2018, up from 1.4 million (0.5%) in 2016 (Substance Abuse and Mental Health Services Administration, 2019). Reporting use of methamphetamine at substance use treatment admission has nearly doubled, rising from 13.7% of drug-related treatment admissions in 2010 to 23.6% in 2017, with increases observed among males and females, all age groups, most racial/ethnic groups, and all U.S. census regions (Jones et al., 2020). Emergency department visits and overdose deaths involving cocaine or psychostimulants such as methamphetamine have also increased over the past decade (Hoots et al., 2020). In 2018, 14,666 overdose deaths involved cocaine, up from 4,350 deaths in 2009; 12,676 overdose deaths involved psychostimulants in 2018, up from 1,632 in 2009 (Hedegaard et al., 2020). Importantly, these increases in stimulant-related harms appear to be intertwined with the ongoing opioid overdose crisis in the U.S., posing new and complex prevention and treatment challenges (Cicero et al., 2020; Hoots et al., 2020; Jones, et al., 2020).
Given the substantial morbidity and mortality attributed to stimulant use, there is a critical need to identify risk factors that can inform the development of effective prevention and treatment strategies. Addressing adverse childhood experiences (ACEs) is a potentially powerful target for prevention of stimulant use and related harms. ACEs are preventable, potentially traumatic events that occur in childhood such as neglect, experiencing or witnessing violence, and having a family member with a suicide attempt or death by suicide. Also included are aspects of a child’s environment that can undermine their sense of safety, stability, and bonding, such as growing up in a household with substance use, mental health problems, or instability due to parental separation or incarceration of a parent, sibling, or other member of the household (Centers for Disease Control and Prevention, 2019). Decades of research have documented the impact of ACEs on health, wellbeing, and opportunity across the lifespan (Felitti et al., 1998; Hughes et al., 2017). Repeated exposure to ACEs, especially in the absence of protective factors, can lead to the development of toxic stress and chronic activation of the stress response system. This toxic stress response results in dysregulation of the limbic-hypothalamic-pituitary-adrenal axis, elevating levels of catecholamines such as cortisol, and pro-inflammatory cytokines, leading to cascading effects on the nervous, endocrine, and immune systems (De Bellis and Zisk, 2014). These changes can affect executive functioning and decision-making, attention, impulsive behaviors, brain reward systems, and emotion regulation and responses to stress throughout an individual’s life (De Bellis and Zisk, 2014; Felitti et al., 1998; Hughes et al., 2017).
ACEs have consistently been associated with increased risk for substance use, including initiating use at an early age and the development of substance use disorders (Banducci et al., 2014; Felitti et al., 1998; Hughes et al., 2017; Rhee et al., 2019; Scheidell et al., 2018; Svingen et al., 2016). Further, research has shown that individuals who have been exposed to ACEs, especially those exposed to multiple ACEs, are at increased risk for more severe substance use, initiating injection drug use at a younger age, transitioning to regular injecting, and experiencing an overdose (Banducci et al., 2014; Debeck et al., 2013; Felitti et al., 1998; Marshall et al., 2011; Scheidell et al., 2018; Stein et al., 2017; Svingen et al., 2016). Specific to stimulants, high prevalence of ACEs has been documented among people who use cocaine, methamphetamine, and other stimulants (Banducci et al., 2014; Christian et al., 2007; Marshall et al., 2011; Scheidell et al., 2018; Svingen et al., 2016; Zapolski et al., 2016). In a study of adults with methamphetamine dependence, 52% reported having experienced lifetime physical abuse and 20% reported having experienced lifetime sexual abuse (Christian et al., 2007). Among adults reporting cocaine use, 29.0% had been exposed to 4 or more ACEs, with 25.9% reporting they had experienced violence, 22.2% had experienced emotional abuse, and 20.0% had experienced sexual abuse (Scheidell et al., 2018).
Although prior research provides important insights into the association between ACEs and stimulant use, studies have generally included small convenience samples of specific populations such as people entering substance use treatment or high-risk youth in limited geographic areas, and often included only a subset of ACEs such as child abuse and neglect or sexual abuse (Banducci et al., 2014; Christian et al., 2007; Marshall et al., 2011; Scheidell et al., 2018; Svingen et al., 2016; Zapolski et al., 2016). To our knowledge no study has examined the full spectrum of ACEs and the associations of ACEs with stimulant use and stimulant use disorders using nationally representative data. To address this research gap, we used data from the National Epidemiological Survey on Alcohol and Related Conditions to estimate the prevalence of ACEs among adults using stimulants and with stimulant use disorders as well as the association of ACEs with stimulant use and use disorders in the United States.
2. Methods
2.1. Data and study sample
Data were from Wave 3 of the National Epidemiological Survey on Alcohol and Related Conditions (NESARC-III) conducted in 2012–2013 in person by the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The NESARC-III is a nationally representative sample of the non-institutionalized adult population 18 years old or older in the United States (N=36,309). The NESARC-III collected information on participants’ substance use (tobacco and alcohol use, illicit drug use, and prescription drug misuse), mental disorders, and physical health conditions. It is the only national survey that has the full complement of ACEs questions along with a wide range of substance use questions. Data were adjusted for oversampling (e.g., minority groups) and nonresponse, and then weighted to represent the noninstitutionalized U.S. civilian adult population. The overall survey response rate was 60.1% (Grant et al., 2014; Grant et al., 2015). This study utilized existing deidentified data and was deemed exempt from the Institutional Review Board review by the author’s institution. Additional details on the NESARC-III survey design and description are available elsewhere (Grant et al., 2014).
2.2. Measures
2.2.1. Adverse childhood experiences
The NESARC-III assessed respondents’ exposure to ACEs based on responses to a series of questions adopted from validated instruments (Bernstein et al., 1994; Felitti et al., 1998; Fink et al., 1995; Straus, 1979; Wyatt, 1985). The ACE score variable was created based on twenty-nine questions regarding 10 ACEs categories (see Appendix A): 1) emotional abuse, 2) physical abuse, 3) sexual abuse, 4) physical neglect, 5) emotional neglect, 6) witnessing domestic violence, 7) household substance use, 8) incarcerated household member, 9) household mental illness, and 10) parental separation or divorce. Following the same method for ACEs coding by Dong et al., questions were collapsed for each ACE category, and respondents were coded as a “1” if they were exposed to that category of ACE (Dong et al., 2004). We then summed the number of ACEs categories each respondent was exposed to (score ranged from 0 to 10). Each respondent was classified into one of the following categories: zero ACEs, one ACE, two or three ACEs, and four or more ACEs based on their exposure history.
2.2.2. Amphetamine-type stimulant (ATS) use, cocaine use, any stimulant use
The NESARC-III includes a series of questions to capture lifetime (ever before) and past-year (in the past 12 months) use of specific illicit and prescription drugs. Respondents were told “Now I’d like to ask you about your experiences with medicines and other kinds of drugs that you may have used on your own – that is, either without a doctor’s prescription; in greater amounts, more often, or longer than prescribed; or for a reason other than a doctor said you should use them. People use these medicines and drugs on their own to feel more alert, to relax or quiet their nerves, to feel better, to enjoy themselves, to get high or just to see how they work,” and then they are presented with a flashcard with specific categories of drugs to facilitate their reporting of the substances they used.
For the analysis, ATS use was defined as responding yes to using “stimulants, for example……Adderall, Concerta, Sylert, Provigil, Ritalin or Dexedrine, speed, amphetamine, methamphetamine, uppers, bennies, pep pills, crystal, crank.” Cocaine use was defined as responding yes to “cocaine or crack, for example…blow, rock, snow.” Any stimulant use was defined as reporting either ATS use, cocaine use, or both.
2.2.3. ATS use disorder, cocaine use disorder, any stimulant use disorder
The NESARC-III categorized individuals as having lifetime (ever before) and past-year (in the past 12 months) ATS use disorders or cocaine use disorders using questions (Grant et al., 2015) based on the individual diagnostic criteria contained in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). Any stimulant use disorder was defined as meeting DSM-V diagnostic criteria for either ATS use disorder, cocaine use disorder, or both.
2.2.4. Demographic measures
The NESARC-III captured demographic information of respondents, including sex, age, race/ethnicity, educational status, marital status, region, and employment status.
2.3. Statistical analyses
First, we estimated the weighted number of individuals as well as prevalence and corresponding 95% confidence intervals (CIs) for lifetime and past-year ATS use, cocaine use and any stimulant use, ATS use disorder, cocaine use disorder, and any stimulant use disorder by number of ACE exposures. Second, we calculated the percentage of individuals by each ACE category who reported lifetime and past-year ATS use and cocaine use, and lifetime and past-year ATS use disorder and cocaine use disorder. Third, we estimated the age of first ATS and cocaine use and compared the mean and 95% CIs of the ACE score by categories of the age of first-time use. Fourth, we estimated weighted prevalence and 95% CIs of the ten specific types of ACEs for each of the stimulant use and use disorder measure.
Finally, two series of individual weighted multivariable logistic regression models were performed to examine the association between ACE score and each stimulant use and use disorder measure. In the first series of models, all models were adjusted for sex, age, race/ethnicity, education, marital status, region, employment status, and the three most prevalent substances in the U.S. (including lifetime cannabis use, lifetime use of at least 100 cigarettes, and past-year binge drinking). In the second series of models, we adjusted for the same demographic variables, lifetime cannabis use, lifetime use of at least 100 cigarettes, and past-year binge drinking, and lifetime prescription opioid misuse and lifetime heroin use in order to further control for the use of opioids, the drug classes that accounted for the majority of overdose morbidity and mortality at the time of data collection. All statistical analyses were conducted using Stata SE 15 (College Station, TX) to account for the complex survey design and weights of the NESARC-III.
3. Results
Based on the weighted respondents of the 2012–2013 NESARC-III, the estimated number and prevalence of adults reporting lifetime ATS use was 19,588,798 (8.3%), 23,416,166 (10.0%) reported lifetime cocaine use, and 31,084,221 (13.2%) reported lifetime any stimulant use. Past-year use of ATS was reported by 2,890,714 (1.2%) adults, 2,397,649 (1.0%) reported past-year cocaine use, and 4,573,019 (2.0%) reported past-year any stimulant use. For stimulant use disorders, 4,034,672 (1.7%) and 754,282 (0.3%) adults reported lifetime and past-year ATS use disorders; 5,640,881 (2.4%) and 820,841 (0.3%) adults reported lifetime and past-year cocaine use disorders; and 8,164,412 (3.5%) and 1,440,806 (0.6%) reported lifetime any stimulant use disorders.
Across all stimulant use and use disorder measures, the prevalence of each measure increased as the number of ACEs increased (Table 1). For example, the prevalence of lifetime ATS use was 5.3% (95% CI=4.8%−5.8%) among respondents reporting no exposure to ACEs, 7.9% (95% CI=7.3%−8.7%) among respondents reporting 1 ACE, 11.8% (95% CI=10.8–13.0%) among respondents reporting 2–3 ACEs, and 17.6% (95% CI=16.0%−19.4%) among respondents reporting 4 or more ACEs. Similarly, for cocaine, the prevalence of lifetime cocaine use was 6.3% (95% CI=5.8%−6.8%) among respondents reporting no exposure to ACEs, 10.4% (95% CI=9.5%−11.3%) among those reporting 1 ACE, 13.8% (95% CI=12.8%−14.9%) among those reporting 2–3 ACEs, and 19.8% (95% CI=18.0%−21.7%) among those reporting 4 or more ACEs.
Table 1.
Amphetamine-type stimulant, cocaine, and any stimulant use and use disorder by adverse childhood experience score.
| Adverse Childhood Experience (ACE) Scorea | ||||||||
|---|---|---|---|---|---|---|---|---|
| Substance Variable | 0 | 1 | 2–3 | >=4 | ||||
| No.b | %c (95% CI) | No.b | %c (95% CI) | No.b | %c (95% CI) | No.b | %c (95% CI) | |
| Amphetamine-Type Stimulants | ||||||||
| Lifetimed use | ||||||||
| Yes | 777 | 5.3% (4.8%, 5.8%) | 595 | 7.9% (7.3%, 8.7%) | 688 | 11.8% (10.8%, 13.0%) | 654 | 17.6% (16.0%, 19.4%) |
| No | 16,158 | 94.7% (94.2%, 95.2%) | 8,024 | 92.1% (91.3%, 92.7%) | 6,000 | 88.2% (87.0%, 89.2%) | 3,360 | 82.4% (80.6%, 84.0%) |
| Past-year use | ||||||||
| Yes | 124 | 0.8% (0.7%, 1.0%) | 85 | 1.2% (0.9%, 1.6%) | 104 | 1.7% (1.4%, 2.1%) | 95 | 2.5% (2.0%, 3.1%) |
| No | 16,787 | 99.2% (99.0%, 99.3%) | 8,523 | 98.8% (98.4%, 99.1%) | 6,565 | 98.3% (97.9%, 98.6%) | 3,898 | 97.5% (96.9%, 98.0%) |
| Lifetimed use disordere | ||||||||
| Yes | 111 | 0.7% (0.6%, 0.9%) | 108 | 1.6% (1.3%, 1.9%) | 163 | 2.8% (2.3%, 3.4%) | 185 | 4.8% (4.0%, 5.7%) |
| No | 16,849 | 99.3% (99.1%, 99.4%) | 8,524 | 98.4% (98.1%, 98.7%) | 6,535 | 97.2% (96.6%, 97.7%) | 3,834 | 95.2% (94.3%, 96.0%) |
| Past-year use disordere | ||||||||
| Yes | 20 | 0.1% (0.1%, 0.2%) | 20 | 0.2% (0.1%, 0.4%) | 34 | 0.6% (0.4%, 0.8%) | 38 | 1.0% (0.7%, 1.5%) |
| No | 16,940 | 99.9% (99.8%, 99.9%) | 8,612 | 99.8% (99.6%, 99.9%) | 6,664 | 99.4% (99.2%, 99.6%) | 3,981 | 99.0% (98.5%, 99.3%) |
| Cocaine | ||||||||
| Lifetimed use | ||||||||
| Yes | 1,029 | 6.3% (5.8%, 6.8%) | 834 | 10.4% (9.5%, 11.3%) | 905 | 13.8% (12.8%, 14.9%) | 775 | 19.8% (18.0%, 21.7%) |
| No | 15,907 | 93.7% (93.2%, 94.2%) | 7,782 | 89.6% (88.7%, 90.5%) | 5,783 | 86.2% (85.1%, 87.2%) | 3,240 | 80.2% (78.3%, 82.0%) |
| Past-year use | ||||||||
| Yes | 113 | 0.6% (0.5%, 0.7%) | 93 | 0.9% (0.7%, 1.2%) | 117 | 1.6% (1.2%, 2.1%) | 96 | 2.3% (1.8%, 3.1%) |
| No | 16,800 | 99.4% (99.3%, 99.5%) | 8,506 | 99.1% (98.8%, 99.3%) | 6,556 | 98.4% (97.9%, 98.8%) | 3,910 | 97.7% (96.9%, 98.2%) |
| Lifetimed use disordere | ||||||||
| Yes | 197 | 1.2% (1.1%, 1.5%) | 178 | 2.0% (1.7%, 2.4%) | 244 | 3.6% (3.1%, 4.2%) | 252 | 6.5% (5.6%, 7.6%) |
| No | 16,763 | 98.8% (98.5%, 99.0%) | 8,454 | 98.0% (97.6%, 98.3%) | 6,454 | 96.4% (95.8%, 96.9%) | 3,767 | 93.5% (92.4%, 94.4%) |
| Past-year use disordere | ||||||||
| Yes | 37 | 0.2% (0.1%, 0.3%) | 28 | 0.3% (0.2%, 0.5%) | 41 | 0.4% (0.3%, 0.6%) | 46 | 1.1% (0.8%, 1.6%) |
| No | 16,923 | 99.8% (99.7%, 99,9%) | 8,604 | 99.7% (99.5%, 99.8%) | 6,657 | 99.6% (99.4%, 99.7%) | 3,973 | 98.9% (98.4%, 99.2%) |
| Any Stimulantf | ||||||||
| Lifetimed use | ||||||||
| Yes | 1,349 | 8.6% (8.0%, 9.2%) | 1,067 | 13.6% (12.6%, 14.6%) | 1,160 | 18.3% (17.1%, 19.6%) | 989 | 25.5% (23.6%, 27.6%) |
| No | 15,585 | 91.4% (90.8%, 92.0%) | 7,549 | 86.4% (85.4%, 87.4%) | 5,528 | 81.7% (80.4%, 82.9%) | 3,024 | 74.5% (72.4%, 76.4%) |
| Past-year use | ||||||||
| Yes | 208 | 1.2% (1.0%, 1.5%) | 154 | 1.8% (1.5%, 2.2%) | 191 | 2.8% (2.3%, 3.4%) | 167 | 4.1% (3.3%, 5.0%) |
| No | 16,691 | 98.8% (98.5%, 99.0%) | 8,442 | 98.2% (97.8%, 98.5%) | 6,471 | 97.2% (96.6%, 97.7%) | 3,821 | 95.9% (95.0%, 96.7%) |
| Lifetimed use disordere | ||||||||
| Yes | 271 | 1.7% (1.5%, 2.0%) | 250 | 3.1% (2.6%, 3.6%) | 354 | 5.5% (4.8%, 6.2%) | 371 | 9.2% (8.2%, 10.3%) |
| No | 16,689 | 98.3% (98.0%, 98.5%) | 8,382 | 96.9% (96.4%, 97.4%) | 6,344 | 94.5% (93.8%, 95.2%) | 3,648 | 90.8% (89.7%, 91.8%) |
| Past-year use disordere | ||||||||
| Yes | 55 | 0.3% (0.2%, 0.4%) | 44 | 0.5% (0.3%, 0.7%) | 70 | 1.0% (0.7%, 1.2%) | 76 | 1.9% (1.4%, 2.5%) |
| No | 16,905 | 99.7% (99.6%, 99.8%) | 8,588 | 99.5% (99.3%, 99.7%) | 6,628 | 99.0% (98.8%, 99.3%) | 3,943 | 98.1% (97.5%, 98.6%) |
Adverse childhood experience (ACE) score is calculated based on the number of exposures to 10 ACE categories.
Unweighted number of individuals
The percentages are weighted estimates.
Lifetime use indicates a respondent reporting ever use of the substance.
Use disorder is assessed based on self-reported questions that correspond to DSM-V diagnosis criteria.
Amphetamine-type stimulants and/or cocaine.
Source: National Epidemiological Survey on Alcohol and Related Conditions, United States, 2012–2013 (n = 36,309)
Adults reporting ACE exposures consistently accounted for the bulk of individuals reporting use of ATS or cocaine, and for reporting ATS or cocaine use disorders (Fig. 1). Among adults reporting lifetime use of ATS, an estimated 22.1% reported 4 or more ACEs, 24.9% 2–3 ACEs, and 22.4% 1 ACE; less than one-third (30.6%) reported no ACE exposure. Similar patterns are seen for lifetime cocaine use as well as past-year use of ATS and cocaine. The influence of ACEs is even more pronounced for the use disorder outcomes. Among those reporting lifetime ATS use disorder, an estimated 29.3% reported 4 or more ACEs, 28.7% 2–3 ACEs, 21.6% 1 ACE, and 20.4% no ACEs. For past-year ATS use disorder, an estimated 33.0% reported 4 or more ACEs, 31.7% 2–3 ACEs, 18.3% 1 ACE, and 17.0% no ACEs. The distribution patterns by ACE score of lifetime cocaine use disorder and past-year cocaine use disorder were similar to those of ATS use disorder.
Fig. 1.
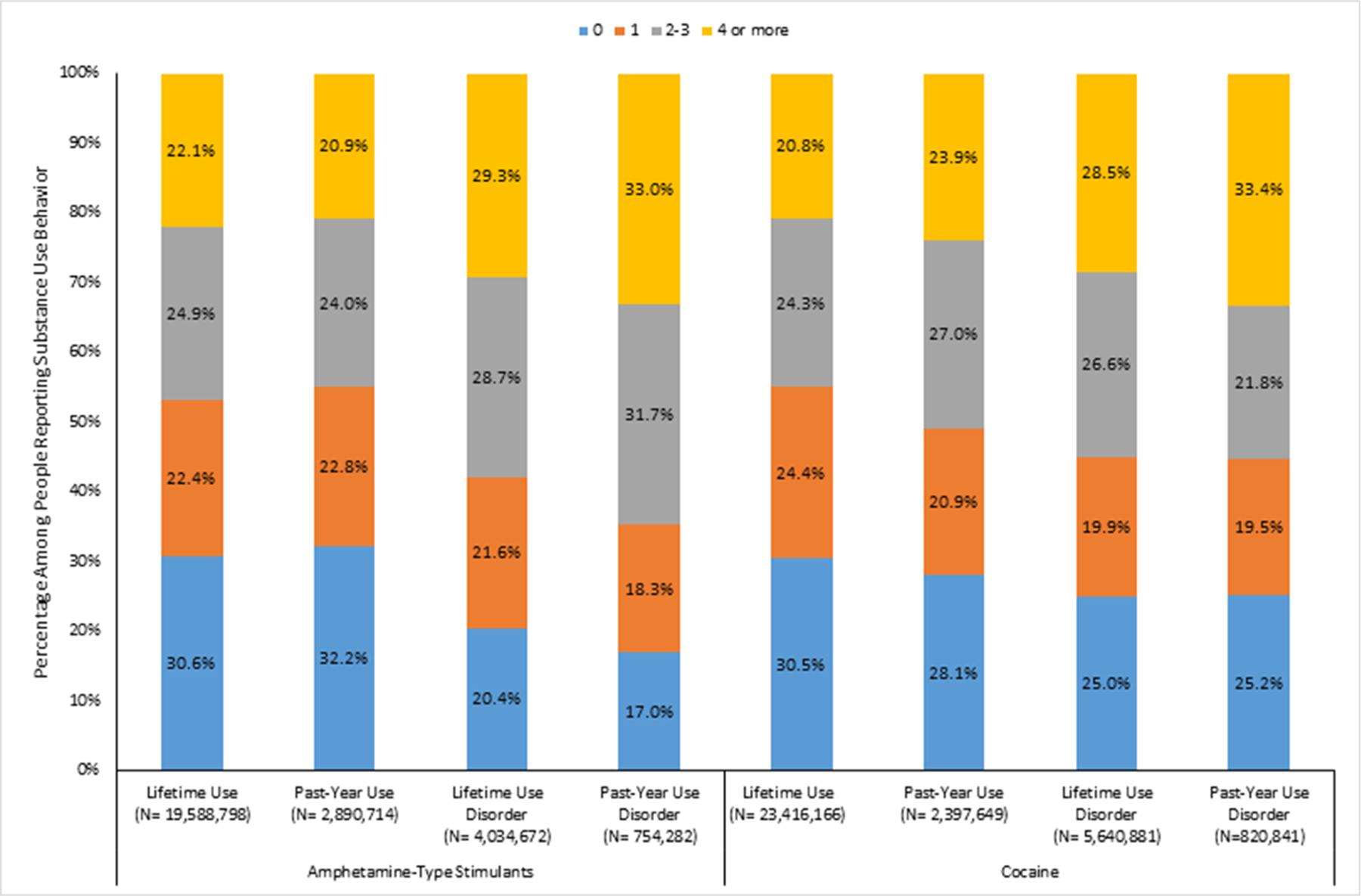
Percentage of Each Adverse Childhood Experience Score Category by Amphetamine-Type Stimulant or Cocaine Use Measuresabcd
a Adverse childhood experience (ACE) score is calculated based on the number of exposures to 10 ACE categories.
b Weighted counts are provided under each measure.
c Lifetime use indicates a respondent reporting ever use of substance.
d Use disorder is assessed based on self-reported questions that correspond to DSM-V diagnosis criteria.
Source: National Epidemiological Survey on Alcohol and Related Conditions, United States, 2012–2013 (n = 36,309).
Average ACE score by age of first ATS use and cocaine use are presented in Table 2. The results show that adults with earlier age of first use have greater numbers of ACEs as reflected by the mean ACE scores. For example, adults who reported using ATS at age 14 or younger had a mean ACE score of 2.8 (95% CI=2.4–3.2) and those who reported using cocaine at age 14 or younger had a mean ACE score of 3.4 (95% CI=2.8–4.0), compared to those who began using at age 25 or older (mean ACE score=1.9; 95% CI=1.7–2.2) for ATS and mean ACE score=1.8 (95% CI=1.6–2.0) for cocaine, as well as compared to those who reported no lifetime use (mean ACE score=1.1; 95% CI=1.1–1.2) among those reporting no lifetime use of ATS and mean ACE score=1.1 (95% CI=1.1–1.1) for those reporting no lifetime cocaine use.
Table 2.
Average adverse childhood experience score by age of first use of amphetamine-type stimulants or cocainea
| Adverse Childhood Experience Score | ||||
|---|---|---|---|---|
| Amphetamine-Type Stimulant Use | Cocaine Use | |||
| Age First Use of Substance | Meanb | (95% CI) | Meanb | (95% CI) |
| 14 or younger | 2.8 | (2.4, 3.2) | 3.4 | (2.8, 4.0) |
| 15 – 17 | 2.4 | (2.2, 2.6) | 2.5 | (2.2, 2.7) |
| 18 – 20 | 1.8 | (1.6, 1.9) | 1.9 | (1.7, 2.0) |
| 21 – 24 | 1.8 | (1.5, 2.0) | 1.7 | (1.6, 1.9) |
| 25 or older | 1.9 | (1.7, 2.2) | 1.8 | (1.6, 2.0) |
| Never misused | 1.1 | (1.1, 1.2) | 1.1 | (1.1, 1.1) |
Adverse childhood experience (ACE) score is calculated based on the number of exposures to 10 ACE categories.
The means are weighted estimates.
Source: National Epidemiological Survey on Alcohol and Related Conditions, United States, 2012–2013 (n = 36, 309).
Table 3 presents the prevalence of 10 specific types of ACEs for each stimulant use measure. Across all of these measures, prevalence of each ACE was higher among adults reporting use or a use disorder compared to those not reporting use or a use disorder. Generally, the highest prevalence across each of the stimulant measures was found among those exposed household substance use or parental divorce/separation. For example, the two most prevalent ACEs among adults reporting lifetime ATS use were household substance use (43.1%,95% CI=40.9%−45.4%) and parental divorce or separation (35.4%, 95% CI=33.0%−37.9%). The same pattern was seen among respondents reporting past-year ATS use, past-year and lifetime ATS use disorder.
Table 3.
Prevalence of specific types of adverse childhood experiences among adults by amphetamine-type stimulants or cocaine use and use disorder status.
| Adverse Childhood Experience Category | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substance Variable | Emotional abuse | Physical abuse | Sexual abuse | Emotional neglect | Physical neglect | Witnessing Domestic Violence | Household Substance Use | Incarcerated household member | Household mental illness | Parental divorce or separation |
| Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | Prevalence, %a (95% CI) | |
| Amphetamine-Type Stimulants | ||||||||||
| Lifetimeb use | ||||||||||
| Yes | 18.3% (16.8%, 20.0%) | 19.2% (17.6%, 21.0%) | 20.1% (18.2%, 22.2%) | 15.5% (13.9%, 17.2%) | 13.6% (12.2%, 15.2%) | 19.2% (17.8%, 20.6%) | 43.1% (40.9%, 45.4%) | 13.4% (11.7%, 15.2%) | 11.5% (10.3%, 12.8%) | 35.4% (33.0%, 37.9%) |
| No | 8.2% (7.8%, 8.6%) | 9.5% (9.0%, 10.0%) | 10.5% (10.0%, 11.0%) | 9.4% (8.9%, 9.9%) | 8.0% (7.6%, 8.4%) | 11.7% (11.2%, 12.3%) | 23.2% (22.4%, 23.9%) | 7.2% (6.8%, 7.6%) | 6.5% (6.2%, 6.8%) | 21.8% (21.0%, 22.6%) |
| Past-year use | ||||||||||
| Yes | 17.7% (12.7%, 24.1%) | 16.7% (13.2%, 20.9%) | 18.2% (14.2%, 23.0%) | 13.6% (10.4%, 17.6%) | 12.4% (9.0%, 16.9%) | 17.8% (13.8%, 22.7%) | 40.5% (35.1%, 46.1%) | 15.3% (11.7%, 19.8%) | 12.2% (8.8%, 16.6%) | 39.6% (33.5%, 46.1%) |
| No | 8.9% (8.4%, 9.3%) | 10.2% (9.6%, 10.7%) | 11.1% (10.6%, 11.7%) | 9.8% (9.4%, 10.3%) | 8.4% (8.0%, 8.8%) | 12.3% (11, 7%, 12.8%) | 24.6% (23.8%, 25.3%) | 7.6% (7.2%, 8.0%) | 6.8% (6.5%, 7.2%) | 22.7% (21.9%, 23.5%) |
| Lifetimeb use disorderc | ||||||||||
| Yes | 26.1% (21.6%, 31.0%) | 26.7% (22.2%, 31.8%) | 29.1% (24.9%, 33.8%) | 17.4% (13.7%, 21.8%) | 17.5% (13.8%, 22.1%) | 23.32% (19.6%, 27.5%) | 51.2% (47.5%, 56.0%) | 21.1% (17.1%, 25.8%) | 15.0% (11.8%, 18.8%) | 42.4% (37.8%, 47.1%) |
| No | 8.7% (8.3%, 9.1%) | 10.0% (9.5%, 10.6%) | 11.0% (10.5%, 11.5%) | 9.8% (9.3%, 10.3%) | 8.3% (7.9%, 8.7%) | 12.2% (11.6%, 12.7%) | 24.4% (23.6%, 25.1%) | 7.5% (7.1%, 7.8%) | 6.8% (6.4%, 7.1%) | 22.6% (21.8%, 23.3%) |
| Past-year use disorderc | ||||||||||
| Yes | 29.9% (19.5%, 43.0%) | 24.5% (16.2%, 35.3%) | 29.2% (20.4%, 39.9%) | 20.7% (13.1%, 31.1%) | 24.4% (15.6%, 36.1%) | 22.4% (15.1%, 31.9%) | 54.8% (45.0%, 64.2%) | 30.6% (20.6%, 42.9%) | 16.6% (9.6%, 27.0%) | 49.1% (35.3%, 63.0%) |
| No | 8.9% (8.5%, 9.4%) | 10.2% (9.7%, 10.8%) | 11.2% (10.7%, 11.7%) | 9.9% (9.4%, 10.4%) | 8.4% (8.0%, 8.8%) | 12.3% (11.8%, 12.9%) | 24.7% (24.0%, 25.5%) | 7.6% (7.2%, 8.0%) | 6.9% (6.6%, 7.2%) | 22.8% (22.1%, 23.6%) |
| Cocaine | ||||||||||
| Lifetimeb use | ||||||||||
| Yes | 17.5% (15.9%, 19.2%) | 18.4% (17.1%, 19.8%) | 18.1% (16.4%, 19.9%) | 15.8% (14.4%, 17.3%) | 13.3% (12.0%, 14.8%) | 18.7% (17.1%, 20.4%) | 41.6% (39.5%, 43.7%) | 13.0% (11.6%, 14.5%) | 11.6% (10.3%, 13.1%) | 34.3% (32.0%, 36.7%) |
| No | 8.1% (7.7%, 8.5%) | 9.4% (8.9%, 10.0%) | 10.5% (10.1%, 11.0%) | 9.3% (8.8%, 9.8%) | 7.9% (7.5%, 8.3%) | 11.7% (11.1%, 12.2%) | 23.0% (22.2%, 23.7%) | 7.1% (6.8%, 7.5%) | 6.4% (6.0%, 6.8%) | 21.6% (20.9%, 22.4%) |
| Past-year use | ||||||||||
| Yes | 19.8% (14.5%, 26.4%) | 18.6% (14.7%, 23.2%) | 17.4% (12.7%, 23.4%) | 14.1% (10.5%, 18.6%) | 14.2% (10.8%, 18.4%) | 21.4% (16.6%, 27.1%) | 50.3% (43.6%, 57.0%) | 18.5% (14.8%, 22.9%) | 14.9% (11.0%, 20.0%) | 39.5% (34.0%, 45.3%) |
| No | 8.9% (8.5%, 9.3%) | 10.2% (9.7%, 10.8%) | 11.2% (10.7%, 11.7%) | 9.9% (9.4%, 10.4%) | 8.4% (8.0%, 8.8%) | 12.3% (11.7%, 12.8%) | 24.5% (23.8%, 25.3%) | 7.6% (7.2%, 8.0%) | 6.8% (6.5%, 7.2%) | 22.7% (21.9%, 23.5%) |
| Lifetimeb use disorderc | ||||||||||
| Yes | 22.7% (19.2%, 26.5%) | 26.0% (22.2%, 30.2%) | 23.4% (19.9%, 27.2%) | 19.8% (16.8%, 23.1%) | 17.2% (14.2%, 20.6%) | 26.8% (23.2%, 30.8%) | 49.5% (45.8%, 53.2%) | 17.1% (14.3%, 20.3%) | 12.8% (10.1%, 16.1%) | 40.1% (35.2%, 45.3%) |
| No | 8.7% (8.3%, 9.1%) | 9.9% (9.4%, 10.5%) | 11.0% (10.5%, 11.5%) | 9.7% (9.2%, 10.2%) | 8.2% (7.8%, 8.6%) | 12.0% (11.5%, 12.5%) | 24.2% (23.5%, 25.0%) | 7.5% (7.1%, 7.9%) | 6.8% (6.4%, 7.1%) | 22.5% (21.7%, 23.3%) |
| Past-year use disorderc | ||||||||||
| Yes | 21.0% (13.7%, 30.9%) | 26.0% (19.3%, 33.9%) | 18.3% (11.6%, 27.8%) | 18.8% (11.9%, 28.4%) | 19.8% (13.4%, 28.2%) | 31.2% (21.6%, 42.6%) | 52.6% (41.2%, 63.8%) | 23.4% (16.0%, 33.0%) | 15.6% (8.9%, 25.7%) | 42.1% (30.9%, 54.1%) |
| No | 9.0% (8.6%, 9.4%) | 10.2% (9.7%, 10.8%) | 11.2% (10.8%, 11.8%) | 9.9% (9.5%, 10.4%) | 8.4% (8.0%, 8.8%) | 12.3% (11.8%, 12.9%) | 24.7% (24.0%, 25.5%) | 7.6% (7.3%, 8.0%) | 6.9% (6.5%, 7.2%) | 22.8% (22.1%, 23.6%) |
| Any Stimulant | ||||||||||
| Lifetimeb use | ||||||||||
| Yes | 17.1% (15.8%, 18.5%) | 18.1% (16.9%, 19.4%) | 18.3% (16.8%, 20.0%) | 15.2% (13.%, 16.5%) | 13.1% (11.9%, 14.4%) | 18.2% (17.0%, 19.5%) | 40.6% (38.9%, 42.3%) | 12.6% (11.3%, 14.0%) | 11.2% (10.1%, 12.3%) | 33.8% (31.9%, 35.8%) |
| No | 7.8% (7.4%, 8.2%) | 9.10% (8.6%, 9.7%) | 10.2% (9.7%, 10.7%) | 9.1% (8.6%, 9.6%) | 7.7% (7.3%, 8.2%) | 11.5% (10.9%, 12.0%) | 22.4% (21.7%, 23.2%) | 6.9% (6.6%, 7.3%) | 6.3% (5.9%, 6.6%) | 21.2% (20.5%, 22.0%) |
| Past-year use | ||||||||||
| Yes | 19.3% (14.9%, 24.5%) | 17.9% (14.9%, 21.2%) | 17.6% (14.4%, 21.5%) | 13.6% (11.0%, 16.6%) | 13.0% (10.2%, 16.4%) | 19.1% (15.7%, 23.0%) | 43.8% (39.2%, 48.4%) | 16.5% (13.8%, 19.6%) | 13.1% (10.3%, 16.6%) | 39.1% (34.5%, 43.9%) |
| No | 8.8% (8.3%, 9.2%) | 10.1% (9.6%, 10.7%) | 11.1% (10.6%, 11.6%) | 9.8% (9.3%, 10.3%) | 8.3% (7.9%, 8.7%) | 12.2% (11.7%, 12.8%) | 24.4% (23.6%, 25.1%) | 7.5% (7.1%, 7.9%) | 6.8% (6.4%, 7.1%) | 22.6% (21.8%, 23.3%) |
| Lifetimeb use disorderc | ||||||||||
| Yes | 22.6% (19.8%, 25.7%) | 25.0% (21.8%, 28.5%) | 25.2% (22.1%, 28.6%) | 18.0% (15.6%, 20.8%) | 16.6% (14.1%, 19.5%) | 25.0% (22.2%, 27.9%) | 49.2% (46.2%, 52.3%) | 18.6% (16.0%, 21.5%) | 13.5% (11.2%, 16.3%) | 41.3% (37.7%, 45.1%) |
| No | 8.5% (8.1%, 8.9%) | 9.8% (9.2%, 10.3%) | 10.8% (10.3%, 11.3%) | 9.6% (9.1%, 10.2%) | 8.2% (7.8%, 8.6%) | 11.9% (11.4%, 12.4%) | 24.0% (23.2%, 24.7%) | 7.3% (6.9%, 7.7%) | 6.7% (6.3%, 7.0%) | 22.3% (21.5%, 23.0%) |
| Past-year use disorderc | ||||||||||
| Yes | 25.8% (18.5%, 34.8%) | 25.7% (19.5%, 33.1%) | 22.5% (16.6%, 29.7%) | 19.5% (14.2%, 26.2%) | 21.8% (15.5%, 29.7%) | 26.0% (19.7%, 33.4%) | 52.4% (45.2%, 59.5%) | 24.5% (18.0%, 32.4%) | 15.0% (9.5%, 22.7%) | 45.2% (35.7%, 55.2%) |
| No | 8.9% (8.5%, 9.3%) | 10.2% (9.7%, 10.8%) | 11.2% (10.7%, 11.7%) | 9.9% (9.4%, 10.4%) | 8.4% (8.0%, 8.8%) | 12.3% (11.7%, 12.8%) | 24.7% (23.9%, 25.4%) | 7.6% (7.2%, 8.0%) | 6.9% (6.5%, 7.2%) | 22.8% (22.0%, 23.5%) |
The percentages are weighted estimates.
Lifetime use indicates a respondent reporting ever use of substance.
Use disorder is assessed based on self-reported questions that correspond to DSM-V diagnosis criteria.
Source: National Epidemiological Survey on Alcohol and Related Conditions, United States, 2012–2013 (n = 36, 309).
After controlling for sociodemographic characteristics and lifetime use of cannabis and tobacco, and past-year binge drinking, a significant relationship between the number of ACEs and stimulant use and use disorder outcomes was found, with the highest adjusted odds ratios found among adults with 4 or more ACEs compared to those with no exposure to ACEs (Table 4). Compared to adults with no exposure to ACEs, respondents with 4 or more ACEs have greater adjusted odds of: life time ATS use (adjusted odds ratio (AOR)=2.1, 95% CI=1.7–2.5), past-year ATS use (AOR=1.6, 95% CI=1.2–2.2), lifetime cocaine use (AOR=1.9, 95% CI=1.6–2.3), past-year cocaine use (AOR=1.9, 95% CI=1.2–2.9), any lifetime any stimulant use (AOR=2.0, 95% CI=1.7–2.3), and past-year any stimulant use (AOR=1.7, 95% CI:1.3–2.3). Notably, the adjusted odds ratios for DSM-V use disorders were larger when the number of ACEs increased. For instance, compared to adults with no exposure to ACEs, respondents with 4 or more ACEs have greater odds of reporting lifetime (AOR=2.7, 95% CI=2.1–3.6) and past-year (AOR=3.3, 95% CI=1.8–5.9) ATS use disorder; lifetime (AOR=2.5, 95% CI=2.0–3.2) and past-year (AOR=2.4, 95% CI = 1.2–4.9) cocaine use disorder; and lifetime (AOR=2.5, 95% CI=2.1–3.1) and past-year (AOR=2.5, 95% CI=1.5–4.0) any stimulant use disorder. Generally, the pattern of having larger adjusted odds ratios as the number of ACEs increased for the stimulant use measures was similar in the second series of multivariable logistic regression models which included additional controls for lifetime prescription opioid misuse and lifetime heroin use, with the exception of the non-significant relationships between past-year ATS use and adults with 4 or more ACEs, past-year cocaine use and adults with 4 or more ACEs, past-year cocaine use disorder and adults with 4 or more ACEs, and past-year any stimulant use and adults with 4 or more ACEs.
Table 4.
Association between adverse childhood experiences score and amphetamine-type stimulant, cocaine, or any stimulant use and use disordera
| Substance Variable | Series 1 | Series 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Adverse Childhood Experience Score | Adverse Childhood Experience Score | |||||||
| 0 | 1 | 2–3 | >=4 | 0 | 1 | 2–3 | >=4 | |
| AORb (95% CD | AORb (95% CD | AORb (95% CD | AORb (95% CD | AORc (95% CD | AORc (95% CD | AORc (95% CI) | AORc (95% CI) | |
| Amphetamine-Type Stimulant | ||||||||
| Lifetimed use | 1.00 (referent) | 1.14 (1.00, 1.31) | 1.50 (1.32, 1.71) | 2.10 (1.75, 2.53) | 1.00 (referent) | 1.08 (0.94, 1.24) | 1.32 (1.16, 1.51) | 1.70 (1.39, 2.07) |
| Past-year use | 1.00 (referent) | 1.03 (0.73, 1.45) | 1.16 (0.84, 1.59) | 1.59 (1.15, 2.18) | 1.00 (referent) | 0.97 (0.68, 1.38) | 0.99 (0.72, 1.36) | 1.25 (0.89, 1.75) |
| Lifetimed use disordere | 1.00 (referent) | 1.43 (1.07, 1.92) | 2.01 (1.50, 2.70) | 2.74 (2.07, 3.64) | 1.00 (referent) | 1.38 (1.04, 1.83) | 1.78 (1.33, 2.39) | 2.18 (1.65, 2.88) |
| Past-year use disordere | 1.00 (referent) | 1.30 (0.59, 2.87) | 2.33 (1.19, 4.56) | 3.25 (1.78, 5.95) | 1.00 (referent) | 1.23 (0.55, 2.79) | 2.06 (1.05, 4.05) | 2.52 (1.36, 4.70) |
| Cocaine | ||||||||
| Lifetimed use | 1.00 (referent) | 1.26 (1.07, 1.50) | 1.40 (1.22, 1.60) | 1.93 (1.64, 2.26) | 1.00 (referent) | 1.22 (1.03, 1.45) | 1.25 (1.08, 1.45) | 1.57 (1.34, 1.85) |
| Past-year use | 1.00 (referent) | 0.95 (0.67, 1.36) | 1.35 (0.92, 1.98) | 1.90 (1.24, 2.90) | 1.00 (referent) | 0.87 (0.59, 1.26) | 1.11 (0.73, 1.67) | 1.35 (0.89, 2.05) |
| Lifetimed use disordere | 1.00 (referent) | 1.08 (0.81, 1.44) | 1.62 (1.30, 2.01) | 2.52 (1.96, 3.24) | 1.00 (referent) | 1.02 (0.76, 1.38) | 1.40 (1.11, 1.77) | 1.98 (1.53, 2.57) |
| Past-year use disordere | 1.00 (referent) | 0.76 (0.38, 1.54) | 1.07 (0.52, 2.18) | 2.39 (1.18, 4.86) | 1.00 (referent) | 0.70 (0.34, 1.44) | 0.83 (0.39, 1.74) | 1.70 (0.85, 3.37) |
| Any Stimulantf | ||||||||
| Lifetimed use | 1.00 (referent) | 1.25 (1.09, 1.44) | 1.45 (1.29, 1.63) | 1.99 (1.70, 2.32) | 1.00 (referent) | 1.21 (1.05, 1.39) | 1.31 (1.16, 1.48) | 1.66 (1.41, 1.95) |
| Past-year use | 1.00 (referent) | 0.98 (0.75, 1.29) | 1.25 (0.96, 1.64) | 1.72 (1.30, 2.27) | 1.00 (referent) | 0.91 (0.68, 1.22) | 1.07 (0.80, 1.42) | 1.32 (0.98, 1.77) |
| Lifetimed use disordere | 1.00 (referent) | 1.20 (0.95, 1.53) | 1.79 (1.50, 2.12) | 2.55 (2.10, 3.09) | 1.00 (referent) | 1.15 (0.91, 1.47) | 1.60 (1.32, 1.91) | 2.08 (1.68, 2.56) |
| Past-year use disordere | 1.00 (referent) | 0.95 (0.55, 1.66) | 1.57 (0.98, 2.49) | 2.48 (1.55, 3.98) | 1.00 (referent) | 0.89 (0.50, 1.59) | 1.32 (0.82, 2.13) | 1.87 (1.15, 3.02) |
Note: The adjusted odds ratios (AORs) are weighted estimates; Bold text indicates statistical significance at a significance level of 5%Source: National Epidemiological Survey on Alcohol and Related Conditions, United States, 2012–2013 (n = 36, 309).
Adverse childhood experience (ACE) score is calculated based on the number of exposures to 10 ACE categories.
Models were adjusted for sex, age, race/ethnicity, education, marital status, region, employment status, lifetime cannabis use, lifetime tobacco use, and past-year binge drinking.
Models were adjusted for sex, age, race/ethnicity, education, marital status, region, employment status, lifetime cannabis use, lifetime tobacco use, past-year binge drinking, lifetime prescription opioid misuse, lifetime heroin use.
Lifetime use indicates a respondent reporting ever use of substance.
Use disorder is assessed based on self-reported questions that correspond to DSM-V diagnosis criteria.
Amphetamine-type stimulants and/or cocaine.
4. Discussion
Adverse childhood experiences were common among adults who reported use of stimulants or had stimulant use disorders in our study. In particular, exposure to parental substance use, parental divorce or separation, sexual abuse, and witnessing domestic violence were the most commonly reported ACEs. Further, adults exposed to ACEs accounted for the majority of individuals who reported lifetime or past-year stimulant use or use disorders, with approximately 1 in 3 adults with past-year ATS use disorder or cocaine use disorder reporting exposure to 4 or more ACEs. Importantly, we found that the relationship between an increased number of ACEs and elevated risk of stimulant use outcomes remained even after accounting for other substance use, including prescription opioid misuse and heroin use. In the context of rising stimulant availability and harms, these findings provide important new insights into potential underlying contributors to stimulant use and a scientific roadmap to inform stimulant prevention and treatment efforts through expansion of comprehensive prevention of both ACEs and substance use.
The finding of high prevalence of ACEs among adults using ATS and cocaine along with the finding that early age of initiation of ATS or cocaine use was associated with higher mean ACEs scores, highlighting the potential impact of ACEs prevention as a key strategy to address rising stimulant use and harms in the U.S. Fundamental to ACEs prevention is the creation of safe, stable, nurturing relationships and environments for all children and families. CDC recently developed an ACEs prevention resource, Preventing Adverse Childhood Experiences (ACEs): Leveraging the Best Available Evidence (Centers for Disease Control and Prevention, 2019), to assist states and communities in developing a comprehensive approach to preventing ACEs. The document provides six strategies that reflect the best available evidence and includes discussion of specific policies and programmatic initiatives that can be implemented to prevent ACEs, including: strengthening economic supports for families (e.g., earned income tax credits, family-friendly work policies); promoting social norms that protect against violence and adversity (e.g., public education campaigns to support parents and positive parenting, bystander approaches to support healthy relationship behaviors); ensuring a strong start for children (e.g., early childhood home visitation, high quality child care, preschool enrichment programs); enhancing skills to help parents and youths handle stress, manage emotions, and tackle everyday challenges (e.g., social emotional learning programs, safe dating and healthy relationship skill programs, parenting skill and family relationship approaches); connecting youths to caring adults and activities (e.g., mentoring and after school programs); and intervening to lessen immediate and long-term harms (e.g., enhanced primary care to screen, refer, and provide support, victim-centered services, and trauma-informed care).
Of particular importance to stimulant use prevention, several of the strategies identified by CDC have demonstrated lasting protective effects for substance use, including prevention of cocaine and methamphetamine use, and are therefore particularly important in light of the findings in this study. For example, social-emotional learning programs have been associated with both decreased violence as well as decreased youth substance use. One study found that first and second graders who received the Good Behavior Game curriculum were less likely at ages 19–21 to report substance use compared to students in other cohorts (Kellam et al., 2008). The Promoting School-community-university Partnerships to Enhance Resilience (PROSPER) program is an example of a delivery system for communities to implement evidence-based programs for preventing youth substance use and other health risk behaviors. Studies of PROSPER’s impact have shown significant and lasting community-wide reductions in illicit drug use initiation, including reductions in methamphetamine and cocaine use, with the strongest effects evident for the higher-risk youth (Spoth et al., 2007; Spoth et al., 2017; Svingen et al., 2016).
Another important finding from this study was the high prevalence of parental substance use among those reporting stimulant use and use disorders, a finding consistent with prior research (Houtepen et al., 2020; Madras et al., 2019; Svingen et al., 2016). For example, Houtepen et al., reported that parental substance use, in addition to other ACEs, was associated with illicit drug use (Houtepen et al., 2020). Svingen et al., found that age of substance use initiation occurred earlier when participants exposed to parental substance use were also physically abused (Svingen et al., 2016). Taken together, these findings underscore the need for prevention that focuses not only on substance use, but on the dynamics in the home contributing to ACEs and substance use risk. Strategies to disrupt this generational cycle include screening families for substance use and intervening early with home visitation programs or other positive parenting programs that can mitigate the impact of current ACEs and prevent future ACEs (Centers for Disease Control and Prevention, 2019). Home visitation programs such as the Nurse Family Partnership, which has been shown to reduce multiple ACEs, including child maltreatment, intimate partner violence, and maternal substance use, are a particularly impactful strategy (Olds et al., 1997). One study projected up to 42,000 child maltreatment incidents, 41,000 person-years of youth substance use, 36,000 intimate partner violence incidents, and 594,000 property and public order crimes would be prevented via home visitation programs in place between 1996–2014 (Miller, 2015). Other strategies that improve economic supports to families such as earned income tax credits and childcare subsidies have been shown to be associated with reduced parental stress and also may have a positive impact on ACEs and substance use (Centers for Disease Control and Prevention, 2019; Gordon et al., 2011; Klevens et al., 2017; Milligan and Stabile, 2011).
In addition to informing stimulant use prevention efforts, this study has important implications for stimulant use disorder treatment and recovery. In our analysis, we found that the vast majority of adults with lifetime or past-year ATS use disorder or cocaine use disorder had experienced ACEs, with approximately 65% of adults with past-year ATS use disorder and nearly 60% of adults with past-year cocaine use disorder reporting 2 or more ACEs. Further, the likelihood of stimulant use disorders was substantially elevated among those with more ACEs, even after accounting for demographic and other substance use characteristics. This finding points to the importance of integrating the impact of ACEs and trauma informed care into ongoing treatment and recovery support services. Trauma-informed care is a framework that involves recognizing and understanding the prevalence of trauma and adversity, responding by ensuring that care is rendered in accordance with trauma-informed principles, avoiding retraumatizing a client or patient, and ensuring that all policies and practices of an organization reflect a core understanding of trauma (The National Child Traumatic Stress Network 2020). In addition, specific therapeutic strategies such as family-centered treatment for substance use disorders, trauma-focused cognitive behavioral therapy, and multisystemic therapy can be provided in conjunction with substance use treatment (Cary and McMillen, 2012; Centers for Disease Control and Prevention, 2019; van der Stouwe et al., 2014). Finally, ensuring connection to recovery support services such as recovery coaches, vocational and educational training, transportation, and social services is an important component to sustaining substance use recovery (Center for Substance Abuse Treatment, 2009).
This study is subject to limitations. First, NESARC-III data are self-reported and subject to recall and social desirability biases. Second, because the survey is cross-sectional, inferring causality from the observed associations between ACE exposures and stimulant use measures is not possible. Third, NESARC-III does not include certain populations (e.g., institutionalized or homeless persons); thus, substance use and ACEs estimates in this study might not be generalizable to the total U.S. population. Fourth, NESARC-III provides estimates of persons meeting diagnostic criteria for substance use disorders based on self-reported responses to the individual questions that make up the DSM-V diagnostic criteria for specific substance use disorders, not estimates of the number of persons receiving a diagnosis from a health care provider. Finally, NESARC-III was conducted in 2012–2013, and thus our findings may not fully capture the most recent changes in ATS and cocaine use in the U.S.
5. Conclusion
The United States is experiencing a resurgence of stimulant-related use and harms that is intertwined with the ongoing opioid crisis posing unique prevention and treatment challenges (Hoots et al., 2020). Our analysis found a strong relationship between exposure to adverse childhood experiences and using stimulants and having stimulant use disorders. Advancing comprehensive strategies to prevent adverse childhood experiences and treating underlying trauma among those using stimulants holds great promise to reduce stimulant use and its health and social consequences in the United States.
Supplementary Material
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of Competing Interest
The authors report no conflicts of interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.psychres.2021.113870.
References
- Banducci AN, Hoffman E, Lejuez CW, Koenen KC, 2014. The relationship between child abuse and negative outcomes among substance users: psychopathology, health, and comorbidities. Addict Behav. 39 (10), 1522–1527. 10.1016/j.addbeh.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T, 2006. The need for speed: an update on methamphetamine addiction. J. Psychiatry Neurosci 31 (5), 301–313. [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151 (8), 1132–1136. 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Butler AJ, Rehm J, Fischer B, 2017. Health outcomes associated with crack-cocaine use: systematic review and meta-analyses. Drug Alcohol Depend 180, 401–416. 10.1016/j.drugalcdep.2017.08.036. [DOI] [PubMed] [Google Scholar]
- Cary CE, McMillen JC, 2012. The data behind the dissemination: a systematic review of trauma-focused cognitive behavioral therapy for use with children and youth. Child Youth Serv. Rev 34 (4), 748–757. [Google Scholar]
- Center for Substance Abuse Treatment, 2009. What Are Peer Recovery Support Services? HHS Publication No. (SMA), 09–4454, Issue. https://store.samhsa.gov/sites/default/files/d7/priv/sma09-4454.pdf.
- Centers for Disease Control and Prevention. (2019). Preventing adverse childhood experiences: leveraging the best available evidence [Internet]. https://www.cdc.gov/violenceprevention/pdf/preventingACES.pdf.
- Cheng WS, Garfein RS, Semple SJ, Strathdee SA, Zians JK, Patterson TL, 2010. Increased drug use and STI risk with injection drug use among HIV-seronegative heterosexual methamphetamine users. J. Psychoact. Drugs 42 (1), 11–18. 10.1080/02791072.2010.10399781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DR, Huber A, Brecht ML, McCann MJ, Marinelli-Casey P, Lord RH, Reiber C, Lu TH, Galloway GP, 2007. Methamphetamine users entering treatment: characteristics of the methamphetamine treatment project sample. Subst. Use Misuse 42 (14), 2207–2222. 10.1080/10826080701209341. [DOI] [PubMed] [Google Scholar]
- Ciccarone D (2011). Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Prim Care., 38 (1), 41–58. 10.1016/j.pop.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Kasper ZA, 2020. Polysubstance use: a broader understanding of substance use during the opioid crisis. Am. J. Public Health 110 (2), 244–250. 10.2105/ajph.2019.305412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham EB, Jacka B, DeBeck K, Applegate TL, Harrigan PR, Krajden M, Marshall BD, Montaner J, Lima VD, Olmstead AD, Milloy MJ, Wood E, Grebely J, 2015. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug Alcohol Depend. 152, 272–276. 10.1016/j.drugalcdep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Duflou J, Kaye S, 2017. Prevalence and nature of cardiovascular disease in methamphetamine-related death: a national study. Drug Alcohol Depend 179, 174–179. 10.1016/j.drugalcdep.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, Duflou J, 2017. Rates, characteristics and circumstances of methamphetamine-related death in Australia: a national 7-year study. Addiction 112 (12), 2191–2201. 10.1111/add.13897. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Zisk A, 2014. The biological effects of childhood trauma. Child Adolesc. Psychiatr. Clin. N Am 23 (2), 185–222. 10.1016/j.chc.2014.01.002 vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeck K, Kerr T, Marshall BD, Simo A, Montaner J, Wood E, 2013. Risk factors for progression to regular injection drug use among street-involved youth in a Canadian setting. Drug Alcohol Depend 133 (2), 468–472. 10.1016/j.drugalcdep.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, Giles WH, 2004. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 28 (7), 771–784. 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration. (2019). 2019 national drug threat assessment U.S. department of justice. Retrieved June 1 from https://www.dea.gov/sites/default/files/2020-01/2019-NDTA-final-01-14-2020_Low_Web-DIR-007-20_2019.pdf.
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am. J. Prev. Med 14 (4), 245–258. 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M, 1995. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am. J. Psychiatry 152 (9), 1329–1335. 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- Gordon RA, Usdansky ML, Wang X, Gluzman A, 2011. Child care and mothers’ mental health: is high-quality care associated with fewer depressive symptoms? Fam. Relat 60 (4), 446–460. [Google Scholar]
- Grant BF, Chu A, Sigman R, Ambary M, Kali J, Sugawara Y, Jiao R, Ren W, & Goldstein R (2014). Source and accuracy statement: national epidemiologic survey on alcohol and related conditions-III (NESARC-III).
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, 2015. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry 72 (8), 757–766. 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Smith SM, Jung J, Zhang H, Chou SP, Pickering RP, Ruan WJ, Huang B, Saha TD, Aivadyan C, Greenstein E, Hasin DS, 2015. The alcohol use disorder and associated disabilities interview schedule-5 (AUDADIS-5): reliability of substance use and psychiatric disorder modules in a general population sample. Drug Alcohol Depend 148, 27–33. 10.1016/j.drugalcdep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, Warner M, 2020. Drug Overdose Deaths in the United States, 1999–2018. National Center for Health Statistics. Retrieved June 1 from. https://www.cdc.gov/nchs/products/databriefs/db356.htm. [Google Scholar]
- Hirsiger S, Hänggi J, Germann J, Vonmoos M, Preller KH, Engeli EJE, Kirschner M, Reinhard C, Hulka LM, Baumgartner MR, Chakravarty MM, Seifritz E, Herdener M, Quednow BB, 2019. Longitudinal changes in cocaine intake and cognition are linked to cortical thickness adaptations in cocaine users. Neuroimage Clin. 21, 101652 10.1016/j.nicl.2019.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoots B, Vivolo-Kantor A, Seth P, 2020. The rise in non-fatal and fatal overdoses involving stimulants with and without opioids in the United States. Addiction 115 (5), 946–958. 10.1111/add.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtepen LC, Heron J, Suderman MJ, Fraser A, Chittleborough CR, Howe LD, 2020. Associations of adverse childhood experiences with educational attainment and adolescent health and the role of family and socioeconomic factors: a prospective cohort study in the UK. PLoS Med. 17 (3), e1003031 10.1371/journal.pmed.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP, 2017. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2 (8), e356–e366. 10.1016/s2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- Jones CM, Olsen EO, O’Donnell J, Mustaquim D, 2020. Resurgent methamphetamine use at treatment admission in the United States, 2008–2017. Am. J. Public Health 110 (4), 509–516. 10.2105/ajph.2019.305527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Underwood N, Compton WM, 2020. Increases in methamphetamine use among heroin treatment admissions in the United States, 2008–17. Addiction 115 (2), 347–353. 10.1111/add.14812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellam SG, Brown CH, Poduska JM, Ialongo NS, Wang W, Toyinbo P, Petras H, Ford C, Windham A, Wilcox HC, 2008. Effects of a universal classroom behavior management program in first and second grades on young adult behavioral, psychiatric, and social outcomes. Drug Alcohol Depend 95 (Suppl 1). 10.1016/j.drugalcdep.2008.01.004 (Suppl 1), S5–s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens J, Schmidt B, Luo F, Xu L, Ports KA, Lee RD, 2017. Effect of the earned income tax credit on hospital admissions for pediatric abusive head trauma, 1995–2013. Public Health Rep. 132 (4), 505–511. 10.1177/0033354917710905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madras BK, Han B, Compton WM, Jones CM, Lopez EI, McCance-Katz EF, 2019. Associations of parental marijuana use with offspring marijuana, tobacco, and alcohol use and opioid misuse. JAMA Netw. Open 2 (11), e1916015. 10.1001/jamanetworkopen.2019.16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall BD, Wood E, Shoveller JA, Buxton JA, Montaner JS, Kerr T, 2011. Individual, social, and environmental factors associated with initiating methamphetamine injection: implications for drug use and HIV prevention strategies. Prev. Sci 12 (2), 173–180. 10.1007/s11121-010-0197-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TR, 2015. Projected outcomes of nurse-family partnership home visitation during 1996–2013, USA. Prev. Sci 16 (6), 765–777. 10.1007/s11121-015-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan K, Stabile M, 2011. Do child tax benefits affect the well-being of children? Evidence from Canadian child benefit expansions. Am. Econ. J. Econ. Policy 3 (3), 175–205. [Google Scholar]
- Olds DL, Eckenrode J, Henderson CR Jr., Kitzman H, Powers J, Cole R, Sidora K, Morris P, Pettitt LM, Luckey D, 1997. Long-term effects of home visitation on maternal life course and child abuse and neglect. Fifteen-year follow-up of a randomized trial. JAMA 278 (8), 637–643. [PubMed] [Google Scholar]
- Rhee TG, Barry LC, Kuchel GA, Steffens DC, Wilkinson ST, 2019. Associations of adverse childhood experiences with past-year DSM-5 psychiatric and substance use disorders in older adults. J. Am. Geriatr. Soc 67 (10), 2085–2093. 10.1111/jgs.16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidell JD, Quinn K, McGorray SP, Frueh BC, Beharie NN, Cottler LB, Khan MR, 2018. Childhood traumatic experiences and the association with marijuana and cocaine use in adolescence through adulthood. Addiction 113 (1), 44–56. 10.1111/add.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Shin C, Greenberg M, Clair S, Feinberg M, 2007. Substance-use outcomes at 18 months past baseline: the PROSPER community-university partnership trial. Am. J. Prev. Med 32 (5), 395–402. 10.1016/j.amepre.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth R, Redmond C, Shin C, Greenberg MT, Feinberg ME, Trudeau L, 2017. PROSPER delivery of universal preventive interventions with young adolescents: long-term effects on emerging adult substance misuse and associated risk behaviors. Psychol. Med 47 (13), 2246–2259. 10.1017/s0033291717000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Conti MT, Kenney S, Anderson BJ, Flori JN, Risi MM, Bailey GL, 2017. Adverse childhood experience effects on opioid use initiation, injection drug use, and overdose among persons with opioid use disorder. Drug Alcohol Depend 179, 325–329. 10.1016/j.drugalcdep.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Stockman JK, 2010. Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Curr. HIV/AIDS Rep 7 (2), 99–106. 10.1007/s11904-010-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA, 1979. Measuring intrafamily conflict and violence: the conflict tactics (CT) scales. J. Marriage Fam 41 (1), 75–88. 10.2307/351733. [DOI] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2019). 2018 national survey on drug use and health. Detailed tables. Retrieved June 1 from https://www.samhsa.gov/data/report/2018-nsduh-detailed-tables. [PubMed]
- Svingen L, Dykstra RE, Simpson JL, Jaffe AE, Bevins RA, Carlo G, DiLillo D, Grant KM, 2016. Associations between family history of substance use, childhood trauma, and age of first drug use in persons with methamphetamine dependence. J. Addict Med 10 (4), 269–273. 10.1097/adm.0000000000000233. [DOI] [PubMed] [Google Scholar]
- The National Child Traumatic Stress Network. 2020. NCTSN resources. Retrieved June 1 from https://www.nctsn.org/treatments-and-practices/screening-and-assessment/nctsn-resources.
- van der Stouwe T, Asscher JJ, Stams GJ, Dekovíc M, van der Laan PH, 2014. The effectiveness of multisystemic therapy (MST): a meta-analysis. Clin. Psychol. Rev 34 (6), 468–481. 10.1016/j.cpr.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Voce A, Calabria B, Burns R, Castle D, McKetin R, 2019. A systematic review of the symptom profile and course of methamphetamine-associated psychosis(substance use and misuse). Subst. Use Misuse 54 (4), 549–559. 10.1080/10826084.2018.1521430. [DOI] [PubMed] [Google Scholar]
- Wang TY, Fan TT, Bao YP, Li XD, Liang CM, Wang RJ, Ma J, Han Y, Meng SQ, Wu P, Shi J, Lu L, 2017. Pattern and related factors of cognitive impairment among chronic methamphetamine users. Am. J. Addict 26 (2), 145–151. 10.1111/ajad.12505. [DOI] [PubMed] [Google Scholar]
- Wyatt GE, 1985. The sexual abuse of Afro-American and white-American women in childhood. Child Abuse Negl. 9 (4), 507–519. 10.1016/0145-2134(85)90060-2. [DOI] [PubMed] [Google Scholar]
- Zapolski TC, Baldwin P, Lejuez CW, 2016. Examining risk for frequent cocaine use: focus on an African American treatment population. Subst. Use Misuse 51 (7), 882–891. 10.3109/10826084.2016.1155618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


