Abstract
Purpose:
Infants born before 26 complete weeks of gestation represent fewer than 1% of live births in the US but account for roughly 40% of infant mortality and 20% of hospital-based obstetric costs. Ethicists and others have argued, but never tested, that these “periviable” births increased early in the 21st century when efforts to reduce stillbirth also increased. We use data from California, where 13% of US births occur, to address two questions implied by this argument. First, did stillbirths decline in the first decade of the 21st century? Second, if stillbirths did decline, did periviable live births increase simultaneously?
Methods:
Using data describing 37,440 singleton pregnancies in California that reached the 20 0/7 week of gestation but ended before 26 complete weeks of gestation, we constructed 240 monthly conception cohorts starting with that conceived in January 1991.We then answered our questions using Box-Jenkins time-series methods that address confounding by autocorrelation, including secular trends and seasonality.
Results:
We detected a downward shift in stillbirths in April 2007 that coincided with an upward shift in periviable live births.
Conclusions:
Our findings imply that, since 2007, fewer Californians than expected have had to contend with the sequelae of stillbirth, but more than expected have had to contend with those of periviable birth.
Keywords: Stillbirth, periviable, evidence-based medicine
Introduction
Patient advocacy (1,2) and political action (3–6) in the US and elsewhere (7–10) led to increased efforts early in the 21st century to reduce stillbirth defined in the US as fetal death after 20 0/7 weeks of gestation (7–10). These efforts included public health programming intended to reduce maternal, fetal, and environmental antecedents of stillbirth (11). They also included interventions, encouraged by increasing efficacy of neonatal intensive care, to save at-risk fetuses at parturition (12,13). Clinicians and ethicists have, however, expressed concern that these latter efforts have increased “periviable” births or those before 26 complete weeks (17,19). Concern arises, at least in part, because periviable infants, who represent fewer than 1% of births in the US, account for 40% of infant mortality (18, 20–22). Those who survive the first year of life, moreover, likely suffer considerable developmental deficiencies (18). Caring for these infants has also proved very costly (24). In California, for example, periviable deliveries account for an estimated 20% of hospital obstetric costs (23).
Despite the concerns noted above, the epidemiologic literature includes no attempts to estimate the association between still and periviable births. The claim that periviable births rose when stillbirths fell remains untested. We attempt to fill this gap by using data from California, where roughly 13% of US births occurred in the first decade of the 21st century.
Based on the literature describing the campaign to reduce stillbirths (1), we assume that physicians and prospective parents in California increasingly opted to “resuscitate” (25,26) fetuses otherwise stillborn in the periviable period. If these attempts proved effective, stillbirths would have shifted detectably below values expected from the population at risk and from history, but periviable live births would have shifted above expected values. We test both these predictions.
Methods
Study Population and Data
Data for this study come from the 1991–2010 California Linked Birth Cohort Files. These files link birth certificate data with fetal or infant death certificate data for all parturitions to women resident in California. We used the linked records for all singleton live births or stillbirths with known sex and gestational age (based on last menstrual period) of more than 20 0/7 but fewer than 43 0/7 weeks. These inclusion criteria yielded 9,880,536 parturitions from which we selected the 37,440 that occurred before 26 complete weeks of gestation. We then estimated a conception month for each parturition by subtracting the length of gestation in days from delivery date. We assigned each parturition to one of 240 monthly conception cohorts beginning with that conceived in January 1991 and ending with that conceived in December 2010.
Statistical Analyses
Statistical tests of association typically measure the degree to which two variables differ from their expected values in the same cases. Such tests assume that the values of each variable vary independently, thereby making their mean the expected value. Variables measured over time, however, often exhibit “autocorrelation” or historical patterns that violate the assumption of independence, implying that their mean is not the expected value. Consistent with convention in epidemiologic literature (27), we solved this problem by estimating the expected value of periviable or stillbirths yielded by a conception cohort as a function not only of the population at risk (i.e., the number of gestations in the cohort surviving to the 20 0/7 week of gestation) but also of autocorrelation in the series.
Testing our hypotheses required 5 analytic steps. We tested our first hypothesis, that stillbirths detectably fell below levels expected from the population at risk (i.e., the number of gestations reaching 20 0/7 weeks of gestation) and from history, in three steps. Testing our second hypothesis, that periviable births detectably rose above levels expected from history when stillbirths fell, required two additional steps.
-
1
We regressed the number of stillbirths in the periviable period yielded by 240 monthly conception cohorts (beginning January 1991 and ending with that conceived in December 2010) on the number of gestations in the cohort that survived to 20 0/7 weeks of gestation. This regression removed the effect of the size of the population at risk on variation over the conception cohorts in their yield of stillbirths.
-
2We used Box-Jenkins “transfer function” methods to detect and model autocorrelation in the residuals of the regression estimated in step 1 (28). These well-described methods appear frequently in social and natural science, as well as in clinical, research (29). They detect and model trends, cycles (including seasonality), and the tendency to regress slowly or quickly after high or low values. The methods use differencing (i.e., subtracting values at t from those at t+1), as well as moving average and autoregressive parameters to express autocorrelation. Residuals from Box-Jenkins transfer functions measure the degree to which stillbirths yielded by a given cohort in the periviable period differed from the value expected from autocorrelation and from the number of pregnancies in the cohort surviving to 20 weeks. The general Box-Jenkins transfer function for our test was as follows.
[1] St is the number of stillbirths in the periviable period yielded by the cohort conceived in month t. Δd is the differencing operator indicating that the series exhibited trends or, if d is greater than 1, detectable cycles. C is a constant. H is the population at risk or number of pregnancies, in 1000’s, conceived in month t that reached the 20th week of gestation. ω0 gauges the association between the population at risk and stillbirths. θ is a moving average parameter. B is the backshift operator or value of ε at t-q or t-p. ϕ is the autoregressive parameter. ε is the residual for the cohort conceived at month t.
Box and Jenkins (28) provided rules for detecting which combination of C, Δ, θ, and ϕ, as well as which values of d, p and b, yield best-fitting estimates of a series.
-
3
We applied the methods of Chang, Tiao, and Chen (30) to detect level shifts in the residuals of the model identified in step 1. These methods describe any outlying sequence of values as a function of a binary predictor variable added to the Box-Jenkins transfer function developed in step 2. The added variable has a value of 1 for the case at which the shift begins as well as for as many subsequent cases in which the shift persists, and 0 otherwise. The Chang, Tiao, and Chen method also adjusts, if necessary, moving average and autoregressive parameters in the transfer function to reflect the contribution of detected level shifts to autocorrelation in the series. We searched for level shifts for which the estimated coefficient of the implied binary variable would, when added to the model estimated in step 1, be detectably greater than 0 at p<.005 (2-tailed test).
We tested our second hypothesis, that periviable births detectably rose above levels expected from history when stillbirths fell, in 2 additional steps.
-
4
We used Box-Jenkins methods to detect and model autocorrelation in the number of live periviable births, adjusted for the population at risk, yielded by the monthly conception cohorts.
-
5We estimated a test equation formed by expanding the Box-Jenkins model identified for live periviable births (i.e., the model estimated in the above step) to include any binary predictor variable, discovered in step 3, that indicates the onset and persistence of a level shift in stillbirths in the periviable period. Our test equation, therefore, was as follows.
Lt is the number of live births in the periviable period yielded by the cohort conceived in month t. It is a binary variable scored 1 for cohorts in which the Chang, Tiao, and Chen method detected (in step 3) downshifted periviable-period stillbirths. The parameter ω1 gauges the shift in live births when stillbirths shifted detectably to an unexpected level. All other notation remains as in equation 1 above.[2]
The argument that efforts to reduce stillbirth increased the number of periviable live births implies that ω1 will be detectably greater than 0. We set the confidence interval for detection at p<.01 (two-tailed test) but show standard errors so that readers can set whichever confidence interval they choose.
We specified the population at risk as a predictor of still and live births rather than using rates as the dependent variable. We did so in order to allow us to express our findings in numbers of infants saved from stillbirth or put at risk of the sequelae of periviable birth. To ensure that using rates would have yielded the same results, we also repeated steps 2 and 3 above with the rate of stillbirth (i.e., per 1000 gestations reaching the 20th week of gestation) in the periviable period as the dependent variable.
Results
Gestations reaching 20 0/7 weeks (i.e., the population at risk) from the 240 monthly conception cohorts exhibited a mean of 41,169 gestations (SD = 2,785) per month with a range from 36,745 to 49,637 from 1991 through 2010. Stillbirths in the periviable period had a mean of 65.33 (SD = 10.05) per month with a range from 45 to 105 while periviable live births averaged 90.85 (SD = 11.56) with a range of 64 to 126.
Steps 1 and 2 in our test of hypothesis 1 yielded the following Box-Jenkins transfer function for the number of stillbirths in the periviable period yielded by the 240 monthly conception cohorts.
| [3] |
The standard errors for 1.593 and −0.235 (i.e., 0.019 and 0.064 respectively) implied high confidence in detection (p <.0005). The autoregressive parameter (i.e., −0.235 at t+9) implies an “echo” of high or low values 9 months after their appearance.
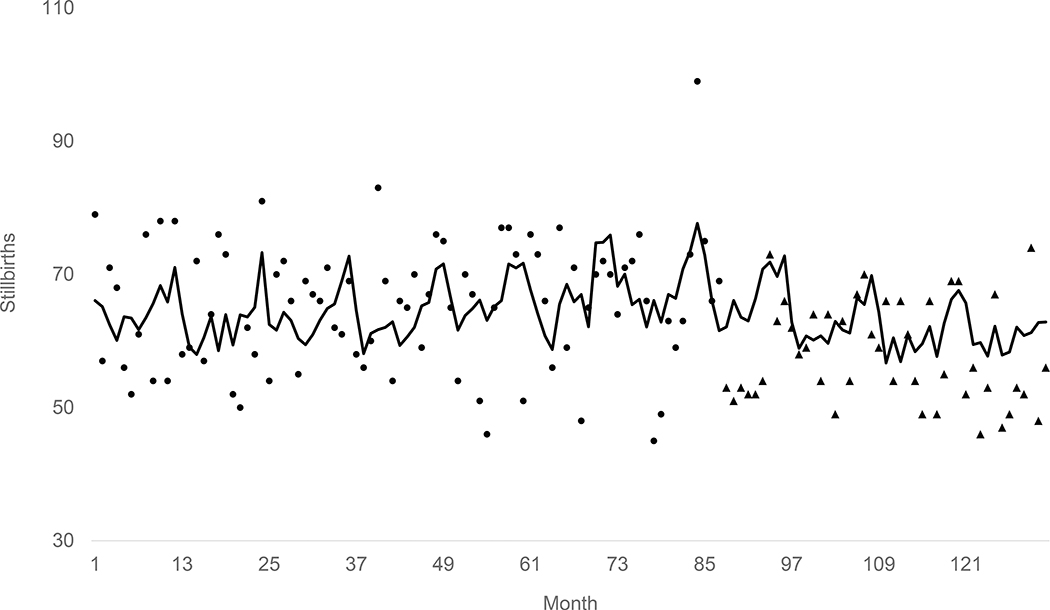
Step 3, applying the methods of Chang, Tiao, and Chen to the residuals of the model estimated in step 2 detected, consistent with hypothesis 1 and with high confidence (p< .00005), an outlying sequence of 7.3 fewer stillbirths per month than expected starting with the cohort conceived in April 2007 and continuing through the test period. Figure 1 shows this result graphically by plotting the expected (i.e. from the population at risk and autocorrelation) stillbirths as a line, the observed values before April 2007 as points, and the observed values from April 2007 through December 2010 as triangles. The Figure includes only the years 2000 through 2010 because plotting all 240 test months in a fixed space causes considerable loss of detail.
Figure 1.
Line shows expected (from population at risk and autocorrelation) stillbirths in the periviable period for cohorts conceived in California from January 2000 through December 2010. Points show observed stillbirths before April 2007. Triangles show observed stillbirths during and after April 2007.
Table 1 shows the results of testing hypothesis 2 that periviable births detectably rose above levels expected from history when stillbirths fell. Column 1 shows the results of step 4 -- using Box-Jenkins methods to detect and model autocorrelation in the number of live periviable births adjusted for the population at risk. As expected, the population at risk predicts the number of live periviable births per conception cohort. As indicated in Table 1, we added no Box-Jenkins parameters because the residuals of the regression of stillbirths on the population at risk exhibited no autocorrelation. Column 2 shows the estimates when the binary variable marking the downward shift, starting in April 2007, in stillbirths during the periviable period enters the model (i.e., step 5). The coefficient (i.e., 3.058; SE = 1.502; p<.05, two-tailed test) for the downward shift in stillbirths implies that conversion of anticipated still to live births increased periviable births an average of 3 per month for each of the remaining 45 cohorts in our test period.
Table 1.
Coefficients (standard errors in parentheses) for base and test models predicting the number of live periviable births in 240 monthly California conception cohorts beginning 1/1991 and ending 12/2010.
| Base Model | Test Model | |
|---|---|---|
| Binary variable scored 1 from April 2007 to December 2010 | 3.058* (1.502) | |
| Population at risk (in 1000’s) | 2.208** (0.144) | 2.195** (0.158) |
| Autocorrelation |
None | None |
p<.05; two-tailed test
p<.01;two-tailed test
These findings imply that reforms in the first decade of the 21st century coincided with approximately 328 fewer stillbirths (i.e., 7.3 X 45) in the periviable period than expected. Periviable births, however, increased simultaneously by 135 (i.e., 3 X 45) above expected.
As noted in the analysis section, we repeated steps 2 and 3 above with the rate of stillbirth (i.e., per 1000 gestations reaching the 20th week of gestation) in the periviable period as the dependent variable to ensure that using rates would have yielded the same results as using counts (and adding the population at risk as a predictor). We found the same downward shift beginning with the cohort conceived in April 2007.
Discussion
Research suggests that physician and patient preferences influence whether parturition in the periviable period will yield a still or live birth (19). Others have argued that patient advocacy and political action intended to reduce stillbirth influenced these preferences in the first decade of the 21st century such that the frequency of periviable births rose as stillbirths declined (3–10). Yet others have urged research to measure the association, in conception cohorts, between still and periviable births (34). We applied time-series methods to 240 monthly cohorts conceived from 1990 through 2010 in California to estimate the association between the incidence of periviable birth and stillbirth. We detected a downward level shift in periviable stillbirths beginning with the cohort conceived in April 2007 and continuing through 2010. Consistent with our argument, this downward shift in stillbirths in the periviable period coincided with an increase in periviable births.
Strengths of our analyses include examination of periviable parturition in California over a long period thereby allowing detection of “shifts” in still and live births presumably due to changes in clinical practice. Our time-series methods address confounding due to shared autocorrelation in still and live births that could arise from factors such as seasonality. In addition, use of all California births minimizes the possibility that sampling errors biased the results. Our results also demonstrate that integrating both outcomes into studies of periviable gestation helps characterize the true risk of parturition in conception cohorts (33,34). This “fetuses at risk” approach contrasts with prior studies that seldom consider how the risk of stillbirth affects morbidity and mortality associated with periviable birth.
Weaknesses of our approach include that attempts to reduce error in the measurement of gestational age could induce artifactual variation in time series. Recording of gestational age in California, for example, changed from LMP to best obstetric estimate in 2007 (35). As noted above, however, we used the LMP measure for our entire test period to avoid introducing an artifactual level shift in our data. Any measurement bias resulting from gestational age misclassification would, moreover, likely be small. Data from 2013, for example, show (36) that both methods produce similar estimates of the percentage of deliveries that occur before 28 (i.e, 0.70% using OE and 0.73% using LMP).
Our analyses cannot account for individual provider and institutional differences in the clinical management of threatened pregnancies in the periviable period and of resuscitation of infants. Further work should explore provider and health system factors that affect the likelihood of either still or live birth.
The argument that efforts to reduce stillbirth have had poorly accounted outcomes extends beyond periviable birth (19). Further research should study, for example, whether infant mortality increased when stillbirths fell. That work would also allow us to better understand the contribution of periviable births to variation in the infant mortality rate over. As noted at the outset, periviable infants now account for about 40% of infant mortality. That fraction, however, can change not only with the incidence periviable births but also with the frequency of death due to other causes.
We cannot rule out that physician and patient willingness to risk resuscitation increased due to advances in medical technology rather than the campaign to reduce stillbirth. Further research should attempt to empirically discriminate between these rival hypotheses. We, however, know of no major advances in neonatal intensive care in California that coincided with the upward level shift we detected.
We also note that conversion from an anticipated still to live birth may occur late in the periviable period while the “counterfactual” averted stillbirth could have occurred in the post periviable period. The inverse association we found between live birth and stillbirth may continue into the early preterm period during which mortality among infants would likely be lower than among periviable infants. Further research should, therefore, estimate associations between still and live births other than those in the periviable period.
Only replication can determine the external validity of our findings. The shift to fewer stillbirths may have occurred earlier or later in other regions of the US than in California. The patterns we find appear consistent with the argument that willingness among physicians and parents in California to convert imminent still to live births increased in the first decade of the 21st century. Fewer Californians than previously expected have since had to contend with the sorrow and sequelae of stillbirth. More than previously expected, however, have had to contend with the challenges of periviable birth.
Contributor Information
Holly Elser, UC Berkeley School of Public Health, Division of Epidemiology, 2121 Berkeley Way West, Berkeley, CA 94720.
Alison Gemmill, Johns Hopkins Bloomberg School of Public Health, Department of Population, Family and Reproductive Health.
Joan A. Casey, Columbia University Mailman School of Public Health, Department of Environmental Health Sciences
Deborah Karasek, University of California, San Francisco, Department of Obstetrics & Gynecology, Reproductive Sciences.
Tim Bruckner, Program in Public Health, University of California, Irvine.
Jonathan A. Mayo, Division of Neonatal and Developmental Medicine, Department of Pediatrics, March of Dimes Prematurity Research Center, Stanford University School of Medicine
Henry C. Lee, Division of Neonatal and Developmental Medicine, Department of Pediatrics, Stanford University School of Medicine
David K. Stevenson, Division of Neonatal and Developmental Medicine, Department of Pediatrics, March of Dimes Prematurity Research Center, Stanford University School of Medicine
Gary M. Shaw, Division of Neonatal and Developmental Medicine, Department of Pediatrics, March of Dimes Prematurity Research Center, Stanford University School of Medicine
Ralph Catalano, UC Berkeley School of Public Health, Division of Epidemiology.
References
- 1.Layne LL. Pregnancy and infant loss support: a new, feminist, American, patient movement? Soc Sci Med. 2006;62:602–613. [DOI] [PubMed] [Google Scholar]
- 2.Murphy S, Cacciatore J. The psychological, social, and economic impact of stillbirth on families. Semin Fetal Neonatal Med. 2017;22:129–134 [DOI] [PubMed] [Google Scholar]
- 3.Halva-Neubauer GA, Zeigler SL. Promoting fetal personhood: The rhetorical and legislative strategies of the pro-life movement after planned parenthood v. casey. Feminist Formations. 2010;10:101–123. [Google Scholar]
- 4.New York Times. When Prosecutors Jail a Mother for a Miscarriage. 2018. December 28. https://www.nytimes.com/interactive/2018/12/28/opinion/pregnancy-women-pro-life-abortion.html
- 5.Stevens A The politics of stillbirth. The American Prospect. 2007. https://prospect.org/article/politics-stillbirth/. [Google Scholar]
- 6.Wyler G Personhood movement continues to divide pro-life activists. Time Magazine. 2013. July 24. [Google Scholar]
- 7.Ateva E, Blencowe H, Castillo T, et al. Every Woman, Every Child’s ‘Progress in Partnership’ for stillbirths: a commentary by the stillbirth advocacy working group. BJOG. 2018;125:1058–1060 [DOI] [PubMed] [Google Scholar]
- 8.Costello A Renewed focus needed on stillbirth. World Health Organization. 2016. https://www.who.int/maternal_child_adolescent/news_events/news/stillbirths/en/ [Google Scholar]
- 9.Qureshi ZU, Millum J, Blencowe H, et al. Stillbirth should be given greater priority on the global health agenda. BMJ. 2015;351:h4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serour GI, Cabral SA, Lynch B. Stillbirths: the professional organisations’ perspective. Lancet. 2011;377:1471–1472. [DOI] [PubMed] [Google Scholar]
- 11.Flenady V, Wojcieszek AM, Middleton P, et al. Stillbirths: recall to action in high-income countries. Lancet. 2016;387:691–702. [DOI] [PubMed] [Google Scholar]
- 12.Fowlie PW, McGuire W. Immediate care of the preterm infant. BMJ. 2004;329:845–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page JM, Silver RM. Interventions to prevent stillbirth. Semin Fetal Neonatal Med. 2017;22:135–145.. [DOI] [PubMed] [Google Scholar]
- 14.MacDorman MF, Reddy UM, Silver RM. Trends in Stillbirth by Gestational Age in the United States, 2006–2012. Obstet Gynecol. 2015;126(6):1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohangoo AD, Buitendijk SE, Szamotulska K, et al. Gestational age patterns of fetal and neonatal mortality in Europe: results from the Euro-Peristat project. PLoS One. 2011;6:e24727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gold KJ, Sen A, Xu X. Hospital costs associated with stillbirth delivery. Matern Child Health J. 2013;17:1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levene M Is intensive care for very immature babies justified? Acta Paediatr. 2004;93(2):149–152. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JG, Baer RJ, Partridge JC, et al. Survival and Major Morbidity of Extremely Preterm Infants: A Population-Based Study. Pediatrics. 2016;138:e20154434. [DOI] [PubMed] [Google Scholar]
- 19.Cavolo A, Dierckx de Casterlé B, Naulaers G, Gastmans C. Ethics of resuscitation for extremely premature infants: a systematic review of argument-based literature. J Med Ethics. 2020;medethics-2020–106102. doi: 10.1136/medethics-2020-106102 [DOI] [PubMed] [Google Scholar]
- 20.Lau C, Ambalavanan N, Chakraborty H, Wingate MS, Carlo WA. Extremely low birth weight and infant mortality rates in the United States. Pediatrics. 2013;131:855–860. [DOI] [PubMed] [Google Scholar]
- 21.Mahgoub L, van Manen M, Byrne P, Tyebkhan JM. Policy change for infants born at the “cusp of viability”: a Canadian NICU experience. Pediatrics. 2014;134:e1405–e1410. [DOI] [PubMed] [Google Scholar]
- 22.Patel RM, Rysavy MA, Bell EF, Tyson JE. Survival of Infants Born at Periviable Gestational Ages. Clin Perinatol. 2017;44:287–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phibbs CS, Schmitt SK, Cooper M, et al. Birth Hospitalization Costs and Days of Care for Mothers and Neonates in California, 2009–2011.J Pediatr. 2019;204:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caughey AB, Burchfield DJ. Costs and cost-effectiveness of periviable care. Semin Perinatol. 2014;38:56–62. [DOI] [PubMed] [Google Scholar]
- 25.Boyle RJ, McIntosh N. Ethical considerations in neonatal resuscitation: clinical and research issues. Semin Neonatol. 2001;6:261–269. [DOI] [PubMed] [Google Scholar]
- 26.Casalaz DM, Marlow N, Speidel BD. Outcome of resuscitation following unexpected apparent stillbirth.Arch Dis Child Fetal Neonatal Ed. 1998;78:F112–F115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalano R, Serxner S. Time series designs of potential interest to epidemiologists. Am J Epidemiol. 1987;126:724–731. [DOI] [PubMed] [Google Scholar]
- 28.Box GE, Jenkins GM, Reinsel GC, Ljung GM. Time series analysis: forecasting and control. John Wiley & Sons; 2015. [Google Scholar]
- 29.De Gooijer JG, Hyndman RJ. 25 years of time series forecasting. International journal of forecasting. 2006;22:443–473. [Google Scholar]
- 30.Chang I, Tiao GC, Chen C. Estimation of time series parameters in the presence of outliers. Technometrics. 1988;30:193–204. [Google Scholar]
- 31.American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine, Ecker JL, Kaimal A, et al. Periviable birth: Interim update. Am J Obstet Gynecol. 2016;215:B2–B12.e1. [DOI] [PubMed] [Google Scholar]
- 32.Wojcieszek AM, Shepherd E, Middleton P, et al. Interventions for investigating and identifying the causes of stillbirth. Cochrane Database Syst Rev. 2018;4:CD012504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph KS, Kramer MS. The fetuses-at-risk approach: survival analysis from a fetal perspective. Acta Obstet Gynecol Scand. 2018;97(4):454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmichael SL, Blumenfeld YJ, Mayo JA, et al. Stillbirth and Live Birth at Periviable Gestational Age: A Comparison of Prevalence and Risk Factors. Am J Perinatol. 2019;36:537–544. [DOI] [PubMed] [Google Scholar]
- 35.Pearl M, Wier ML, Kharrazi M. Assessing the quality of last menstrual period date on California birth records. Paediatr Perinat Epidemiol. 2007;21 Suppl 2:50–61. [DOI] [PubMed] [Google Scholar]
- 36.Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring Gestational Age in Vital Statistics Data: Transitioning to the Obstetric Estimate. Natl Vital Stat Rep. 2015;64:1–20. [PubMed] [Google Scholar]