Abstract
Background:
Subtle gait deficits can be seen in people with idiopathic rapid eye movement (REM) sleep behavior disorder (RBD), a prodromal stage of Parkinson’s disease (PD) and related alpha-synucleinopathies. It is unknown if the presence and level of REM sleep without atonia (RSWA, the electromyographic hallmark of RBD) is related to the severity of gait disturbances in people with PD.
Objective:
We hypothesized that gait disturbances in people with mild-to-moderate PD would be greater in participants with RSWA compared to those without RSWA and matched controls, and that gait impairment would correlate with measures of RSWA.
Methods:
Spatiotemporal characteristics of gait were obtained from 41 people with PD and 21 age-matched controls. Overnight sleep studies were used to quantify muscle activity during REM sleep and group participants with PD into those with RSWA (PD-RSWA+, n = 22) and normal REM sleep muscle tone (PD-RSWA−, n = 19). Gait characteristics were compared between groups and correlated to RSWA.
Results:
The PD-RSWA+ group demonstrated significantly reduced gait speed and step lengths and increased stance and double support times compared to controls, and decreased speed and cadence and increased stride velocity variability compared to PD-RSWA– group. Larger RSWA scores were correlated with worse gait impairment in the PD group.
Conclusion:
The presence and level of muscle tone during REM sleep is associated with the severity of gait disturbances in PD. Pathophysiological processes contributing to disordered gait may occur earlier and/or progress more rapidly in people with PD and RBD.
Keywords: Parkinson’s disease, REM sleep without atonia, gait
INTRODUCTION
An estimated seventy percent of people with Parkinson’s disease (PD) develop debilitating balance and gait deficits, including freezing of gait (FOG) [1]. People with PD and rapid eye movement (REM) sleep behavior disorder (RBD) are more likely to have a phenotype with postural instability, gait disturbances, FOG, and falls [2]. RBD is a parasomnia characterized by abnormally elevated muscle tone during REM sleep (REM sleep without atonia, RSWA) and dream enactment [2–4]. Isolated/idiopathic RBD (iRBD) is recognized as a prodromal stage of synucleinopathy, with up to 74% of RBD developing PD, dementia with Lewy bodies, or multiple system atrophy over 13 years [5]. The prevalence of RBD in the general population is low (<1%), but is common in people with PD (16–47%) [6–8]. The increased severity and prevalence of postural and gait disturbances in people with PD and RBD suggests that pathophysiological processes affecting the regulation of muscle tone during REM sleep may also impact structures and pathways contributing to the control of posture and locomotion.
Gait and postural deficits can be seen in people with iRBD three to five years prior to a diagnosis of PD [9]. Quantitative measures of gait and gait initiation show subtle parkinsonian-like impairments in people with iRBD [10, 11]. RBD [12] and the pathogenesis of postural and gait impairment (via influences on the function of pontomedullary reticular formation and central pattern generating circuits in the spinal cord and spinal motoneurons). Accordingly, the presence and level of abnormal muscle activity during REM sleep may serve as a proxy for neurodegenerative changes in brainstem nuclei that control gait.
Previous studies have shown that spatiotemporal measures of gait in people with early stage PD are significantly different from matched controls [16–19]. We hypothesized that, after separating a cohort with mild-to-moderate severity of PD into those with and without RSWA (the electrophysiological hallmark of RBD), the PD group with RSWA would show significantly more impairment in gait compared to PD subjects without RSWA and controls. We further hypothesized that measures of muscle activity during REM sleep in the PD group would correlate with gait deficits.
METHODS
Study participants
Forty-two individuals diagnosed with idiopathic PD [20] by a movement disorders neurologist and 21 age- and sex-matched controls were recruited and tested; one participant was later excluded due to a change in diagnosis. Individuals were recruited who were considered by the referring neurologist to have mild-to-moderate motor severity (based on Movement Disorders Society Unified Parkinson’s Disease Rating Scale part III score [21]). Referring neurologists were unaware of the study hypotheses. Inclusion criteria were age 21–79 and the ability to ambulate 50 m without an assistive device (so that participants could complete the gait task). Participants were excluded if they had another neurological disorder, a Montreal Cognitive Assessment (MoCA) score less than 22/30 (a cognitive screen for individuals with reduced capacity to consent [22]), musculoskeletal disorders significantly affecting movement, implanted deep brain stimulators or other surgeries to treat PD, pregnant women, or untreated sleep apnea. The University of Minnesota Institutional Review Board approved procedures and participants gave written informed consent in accordance with the Declaration of Helsinki.
Group classification via RSWA
Overnight video-based PSGs [23] were performed ON the participant’s usual Parkinson’s medications. When relevant, melatonin was withdrawn for 7 days prior to the sleep study. Electroencephalography (EEG) data were collected from 10 scalp surface electrodes using a 10–20 montage. Electromyographic (EMG) data were collected from the chin, and bilateral flexor digitorum superficialis and tibialis anterior muscles. The arm EMG data set was incomplete and therefore is not reported. Electrooculography (EOG), EEG, and EMG data were sampled at 500 Hz. PSGs were scored by a single rater (AV), blinded to disease state, using standard AASM criteria [23]. Study personnel were blinded to the PSG results until the initial data analysis was completed.
The level of tonic or phasic RSWA was used to separate the PD participants into normal (PD-RSWA−) or RSWA positive (PD-RSWA+) groups (for more detail see Linn-Evans et al. [24]). Subjects were included in the PD+RSWA+ group if: tonic chin EMG >7.5%, phasic chin EMG >4%, phasic arm EMG >11%, or phasic leg EMG >4% of REM sleep. Thresholds were defined based on the distribution and level of RSWA scores across all participants. An RBD diagnosis (n = 1) or evidence of dream enactment during the PSG also resulted in designation to the PD-RSWA+ group. Fifteen of 22 participants categorized as PD-RSWA+ met more than one threshold/criteria. While some control subjects had low levels of RSWA, as expected [25], all control subjects had scores below the threshold values. Average RSWA scores for each group are presented in Table 1.
Table 1.
Group demographics and sleep scores with statistical results. Means ± single standard deviations are presented for normally distributed data, while medians (and interquartile ranges) are presented for the nonparametric statistical tests. Number of participants are given for survey results
| Control | PD-RSWA− | PD-RSWA+ | Probability Level and Post-hoc comparisons | |
|---|---|---|---|---|
| Demographics | ||||
| N (# of females) | 21 (12) | 19 (6) | 22 (9) | NS |
| Age (y) | 60.4 ± 7.3 | 62.6 ± 8.6 | 65.1 ± 6.4 | NS |
| Time since diagnosis (years) | N/A | 1.3 (1.0–3.3) |
1.6 (1.2–4.8) |
NS |
| MDS-UPDRS III | N/A | 35 (27–43) | 38 (27–50) | NS |
| Hoehn &Yahr | N/A | 2 (2-3) | 2 (2-3) | NS |
| Levodopa Eq. (mg)a | N/A | 200 (100 – 317) | 373 (300 – 605) | **PD-RSWA+ > PD-RSWA− |
| MoCA | 27 (25–28) | 28 (24–30) | 28 (26–29) | NS |
| Survey Results | ||||
| n with FOG | N/A | 0 | 6 | |
| n with postural instabilityb | 2 | 4 | 9 | |
| n with RBD diagnosis | 0 | 0 | 1 | |
| n with self-reported dream enactment | 0 | 3c | 15 | |
| n on regular melatonin | 0 | 1 | 1 | |
| n on clonazepam | 0 | 1 | 1d | |
| n on lorazepam | 0 | 0 | 1 | |
| n on SSRIs | 1 | 0 | 4d | |
| PSG scores | ||||
| Tonic chin (%) | 0 (0 – 3.7) | 0 (0 – 1.8) | 7.1 (0 – 18.8) | ***e |
| Phasic chin (%) | 0.9 (0.2 – 1.6) | 0.6 (0.1 – 1.1) | 4.6 (2.1 – 7.1) | ***e |
| Phasic leg (%) | 0.7 (0.1 – 1.4) | 2.2 (0.3 – 4.3) | 7.2 (1.9 – 12.6) | **e |
| Phasic arm (%) | 0.4 (0 – 1.1) | 0.9 (0 – 2.8) | 6.9 (2.3 – 11.5) | **e |
Results are from the Fisher Exact Chi-Square Test (sex), One-Way ANOVA (Age, with Tukey’s HSD for post-hoc comparisons), Mann-Whitney U test (Time since diagnosis, MDS-UPDRS III, Hoehn & Yahr, and Levodopa Eq.), or Kruskal-Wallis test (MoCA score, PSG scores, post-hoc comparisons were performed using the Mann-Whitney U test with the Bonferroni correction for multiple tests).
p < 0.05,
p < 0.01,
p < 0.001, NS, not statistically significantly different. N/A, not applicable. The control group was excluded from analysis when measures were not applicable.
Levodopa equivalents were calculated using the Tomlinson method [28].
Postural instability was determined by scores > 0 on MDS-UPDRS 3.12 postural stability.
Although three participants in the PD-RSWA− group answered “yes” to the single RBD question, the descriptions, when available, did not indicate clear dream enactment.
The PD-RSWA+ participant taking clonazepam and an SSRI was also taking an anticonvulsant (lamotrigine).
All follow-ups on the PSG scores showed: PD-RSWA + > Controls, PD-RSWA+ > PD-RSWA−.
Clinical and gait assessments
Motor testing and exams were performed the morning after overnight (12 + h) withdrawal from PD medications. Motor assessment and overnight polysomnography (PSG) tests were conducted an average of 28 ± 24 days apart (range 1–97 days; in some subjects, motor testing was conducted in the morning and the PSG in the following evening). The MDS-UPDRS part III motor exam, new freezing of gait questionnaire (NFOG-Q) [26], MoCA, and a standardized steady-state gait task were performed. Participants walked clock-wise continuously in an oval-shaped course [27] containing a section with a 10 m pressure-sensitive gait mat (GAITRite, Franklin, NJ, USA) and 2 m of straight-line walking on either side of the mat. Thirty-five or more steps were collected on the mat to ensure reliability [27]. Trials took approximately 5 min to complete. Breaks were provided if requested. Spatial and temporal gait characteristics were exported (Table 2), with primary outcome gait measures of speed, step length, width, and time and their variability, and normalized double support. Coefficients of variability (CV) were calculated as the standard deviation divided by the mean and multiplied by 100.
Table 2.
One-way ANCOVA and Two-way repeated measures ANCOVA results with means and one standard deviation for each group and variable
| Controls (C) | PD-RSWA− | PD-RSWA+ | Group effect (F) | Side effect (F) | Group x side (F) | Significant Covariates | Post-hoc comparisons | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| One-Way ANCOVA Results | |||||||||||
| Speed (cm/s) | 132.5±12.8 | 130.1±14.6 | 117.8±19.2 | 5.0* | MoCA | PD-RSWA+ < Controls PD-RSWA + < PD-RSWA− |
|||||
| Normalized speed (%LL/s) | 151±17 | 145±20 | 133±22 | 4.1* | PD-RSWA+ < Controls PD-RSWA + < PD-RSWA− |
||||||
| Cadence (steps/min)b | 111.1±7.7 | 111.2±6.7 | 106.8±8.7 | 1.8* | Sex | PD-RSWA + < PD-RSWA− | |||||
| Mean Step Length CV (%)b | 2.7±0.8 | 3.1±1.1 | 3.8±1.9 | 1.7 | |||||||
| Mean Step Width CV (%) | 23.8±11.1 | 23.7±9.5 | 25.6±8.0 | 0.5 | |||||||
| Mean Step Time CV (%) | 3.0±1.3 | 2.7±0.8 | 3.4±2.0 | 1.9 | |||||||
| Mean Stance Time CV (%) | 3.0±0.9 | 2.9±2.0 | 3.3±2.0 | 2.1 | |||||||
| Mean Stride Velocity CV (%) | 3.6±1.5 | 2.9±1.1 | 4.4±2.0 | 3.6* | PD-RSWA + > PD-RSWA− | ||||||
| Mean Double support time CV (%) | 27.1±2.4 | 28.8±3.1 | 30.1±4.4 | 0.2 | |||||||
| Two-way Repeated Measures ANCOVA | |||||||||||
| MA | LA | MA | LA | MA | LA | ||||||
| MDS-UPDRS laterality/ asymmetry | N/A | N/A | 17±5 | 10±4 | 18±5 | 12±5 | 0.4 | 1.07 | 1.74 | ||
| Normalized step length (%LL) | 81±6 | 81±6 | 78±9 | 78±8 | 74±10 | 74±8 | 4.2* | 0.03 | 0.01 | PD-RSWA+ < Controls | |
| Step width (cm) | 9.0±2.3 | 9.0±2.3 | 9.2±2.5 | 9.1±2.6 | 8.9±2.0 | 8.8±2.0 | 0.01 | 0.31 | 0.31 | ||
| Step time (s) b | 0.54±0.04 | 0.54±0.04 | 0.55±0.03 | 0.53±0.03 | 0.57±0.05 | 0.56±0.06 | 1.8 | 0.01 | 9.2*** | PD-RSWA−: MA > LA | |
| Stance time (s) b | 0.69±0.05 | 0.69±0.05 | 0.69±0.05 | 0.70±0.05 | 0.73±0.10 | 0.74±0.10 | 2.4 | 0.23 | 3.5* | MoCA | PD-RSWA−: LA > MA |
| Stance percent of gait cycle (%)b | 63.5±1.1 | 63.5±1.4 | 63.8±1.7 | 64.9±1.7 | 64.7±2.5 | 65.4±2.1 | 4.4* | 0.21 | 4.0* | MoCA | PD-RSWA+ > Controls LA side: PD > Controls PD: LA > MA |
| Double support percent of gait cycle (%)b | 27.1±2.3 | 27.1±2.4 | 28.7±3.1 | 28.8±3.2 | 30.1±4.3 | 30.2±4.4 | 4.3* | 0.002 | 0.52 | MoCA | PD-RSWA+ > Controls |
PD-RSWA+, Parkinson’s disease with abnormally elevated REM sleep without atonia; PD-RSWA−, Parkinson’s disease with abnormal REM sleep without atonia; MA, More affected side; LA, Less affected side; %LL, percent leg length (normalized to leg length); CV, coefficient of variability.
PSG scores calculated per the American Academy of Sleep Medicine Scoring Manual (AASM Manual for the Scoring of Sleep and Associated Events, Version 2.2, 2015). For the one-way ANCOVA, post-hoc analyses used Tukey’s HSD test. For the repeated measures two-way ANCOVA, post-hoc tests used the Sidak’s correction with significance set at p < 0.05 (
p < 0.05,
p < 0.01,
p < 0.001). Covariates included age, sex, and MoCA score.
denotes variables that did not pass the Shapiro-Wilk test of normality. These measures were assessed using the Kruskal-Wallis test and post-hoc analyses were conducted using the Mann-Whitney U test with the Bonferroni correction for multiple comparisons.
Statistics
Statistical analyses were performed in SPSS (version 25, IBM, New York). Group demographics were tested with a Fisher Exact Chi-Square Test (sex), One-Way ANOVA (age, Tukey’s HSD forpost-hoc comparisons), Mann-Whitney U test (time since diagnosis, MDS-UPDRS III, Hoehn & Yahr, and Levodopa Eq. [28]) or a Kruskal-Wallis test (MoCA score and PSG scores, post-hoc comparisons with the Mann-Whitney U test and Bonferroni corrections). For general gait characteristics (e.g., cadence), an ANCOVA was performed to test for group differences (significance threshold set to p < 0.05). Covariates tested included age, sex, and MoCA score.
A rank analysis of covariance was used for measures that were not normally distributed [29]. Post-hoc comparisons used Tukey’s Honestly Significant Difference (HSD) test.
For bilateral measures (e.g., step length), two-way repeated measures ANCOVAs were performed to test for effects of group and side (more or less affected side) and group x side interactions (significance set to p < 0.05). The more (MA) and less (LA) affected sides were determined by summing the right and left scores in the MDS-UPDRS III (items 3.3-3.8 and 3.15–3.17), with the higher score determining the more affected side. MA and LA were randomly assigned in control participants such that the ratio of right sides assigned to more affected (and vice versa) was similar to the PD group. Post-hoc comparisons were conducted using Sidak’s adjustments.
The relationships between RSWA scores (tonic chin, phasic chin, or phasic leg) and gait characteristics within the PD group were investigated using Spearman’s correlations, to account for non-normal distributions. Control subjects were not included as their RSWA scores were at or near zero. To minimize the potential for a Type I error, correlations were constrained to primary outcome gait measures. Phasic arm RSWA scores were omitted to limit the correlations, deal with incomplete arm data, and because tonic and phasic chin scores may be better at distinguishing synucleinopathy without the presence of dream enactment [25].
RESULTS
Demographics
The final three groups were composed of 22 participants with PD with RSWA, 19 with PD without RSWA, and 21 controls (Table 1). Between the three groups, there were no significant differences in age or MoCA score. Between the PD groups, there were no significant differences in time since diagnosis or MDS-UDRS III motor score. The PD-RSWA+ group had significantly higher average levodopa equivalent dose [28] and EMG activity during REM sleep (p < 0.01.) All six PD participants with freezing of gait symptoms and 9 out of 15 PD participants with abnormal postural stability (item 3.12 on the MDS-UPDRS) were in the PD-RSWA+ group.
Group differences in gait
Gait metrics are summarized in Table 2 and Fig. 1. There was a main effect of group for gait speed (m/s) and normalized gait speed (leg length/s) (all p < 0.05, unless otherwise noted). Gait speed in the PD-RSWA+ group was significantly slower than the control and PD-RSWA− groups and was predominantly driven by significantly reduced step lengths compared to controls and reduced cadence compared to the PD-RSWA− group. A main effect of group was also observed for mean stride velocity variability. Stride velocity was significantly more variable in the PD-RSWA+ compared with the PD-RSWA− group. MoCA was a significant covariate for (non-normalized) gait speed, while sex was significant for cadence.
Fig. 1.
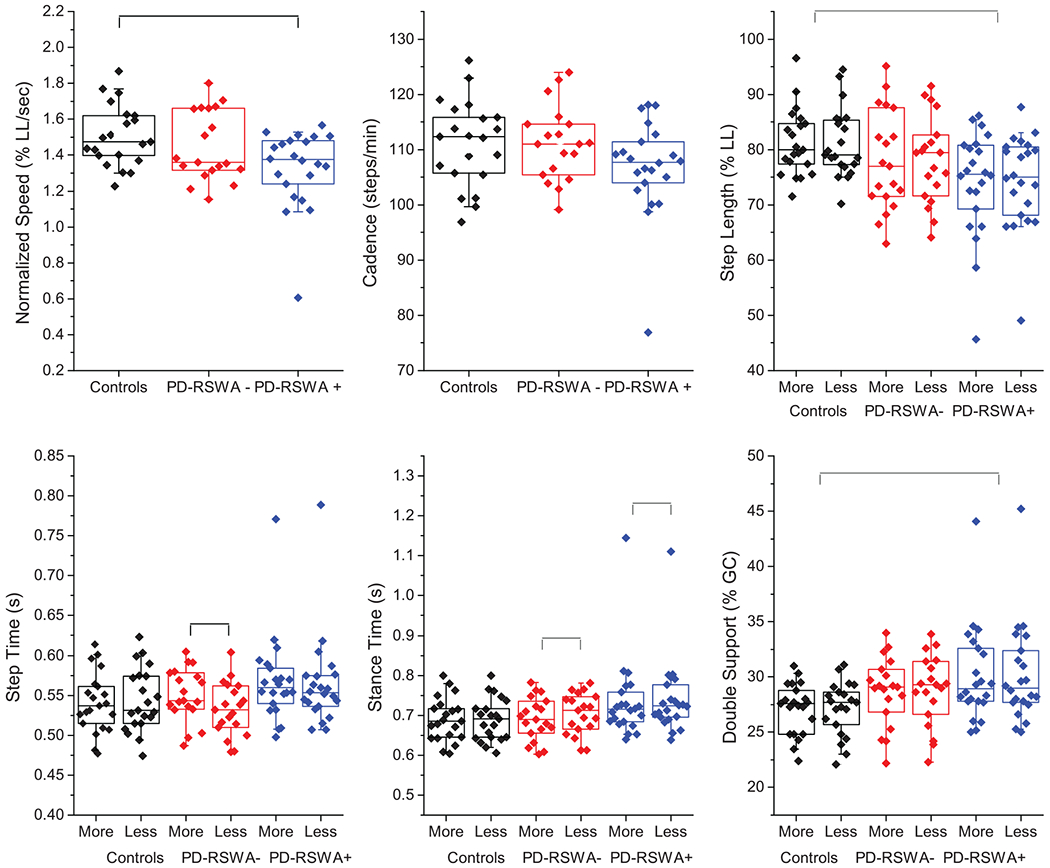
Box and whiskers plots demonstrating group differences in key gait measures including speed (normalized to percentage of leg length), cadence, step lengths (normalized to percentage of leg length), step time, stance time, and double support time (normalized to percentage of the gait cycle, %GC). Measures that involved separate legs were sorted according to more and less affected limb as determined by the MDS-UPDRS III motor exam. Those who were symmetrical were randomly assigned. The means of each individual subject’s data are overlaid on the box plots. Colors assigned to each group are: controls (black), PD-RSWA− (red), PD-RSWA+ (blue).
Main effects of group (all p < 0.05, unless noted) were seen in the percentage of the gait cycle spent in stance and double support, with PD-RSWA+ having increased durations compared to controls. Stance percentage also had an interaction effect, with post-hoc tests showing the PD groups had spent a higher percentage of the gait cycle on the less affected side compared to the more affected side and higher percentages compared to controls for the less affected side only. Step and stance times had significant interaction effects (p < 0.001 and p < 0.05, respectively) such that only the PD-RSWA− group had longer step times on the more affected leg (p < 0.001) and a longer time spent in stance on the less affected leg (p < 0.001), however, the differences between sides were small (mean of 0.04 s) and thus unlikely to be clinically relevant. MoCA score was a significant covariate for stance time and stance and double support percent of gait cycle (p < 0.01). The timing variables (step, stance, and double support), along with cadence and mean step length CV, did not pass the Shapiro-Wilk test of normality, due to a single PD-RSWA+ participant with very slow gait. Significance was re-tested without that subject, which did not appreciably change the mean values, and so this data was included in the tables. However, removal of this participant resulted in the loss of the significant group effect for cadence, but added sex as a significance covariate to the analysis of step time and stance time, and the percentage of the gait cycle spent in stance and double support.
Gait measures correlated to RSWA scores
Correlations between primary outcome gait variables and RSWA measures are summarized in Fig. 2 and Table 3. Phasic chin EMG activity was significantly (all p < 0.05) inversely correlated with gait speed, normalized gait speed, and cadence, and positively correlated with step times and mean coefficients of variability (averaged across the two legs) for step time and step length. Tonic chin EMG activity was significantly inversely correlated to cadence and positively correlated to step time CV. Phasic leg EMG activity was significantly positively correlated to normalized gait speed and mean step time variability, and inversely correlated to normalized step lengths on the more and less affected sides. RSWA scores were not significantly correlated with step widths or mean step width CV.
Fig. 2.
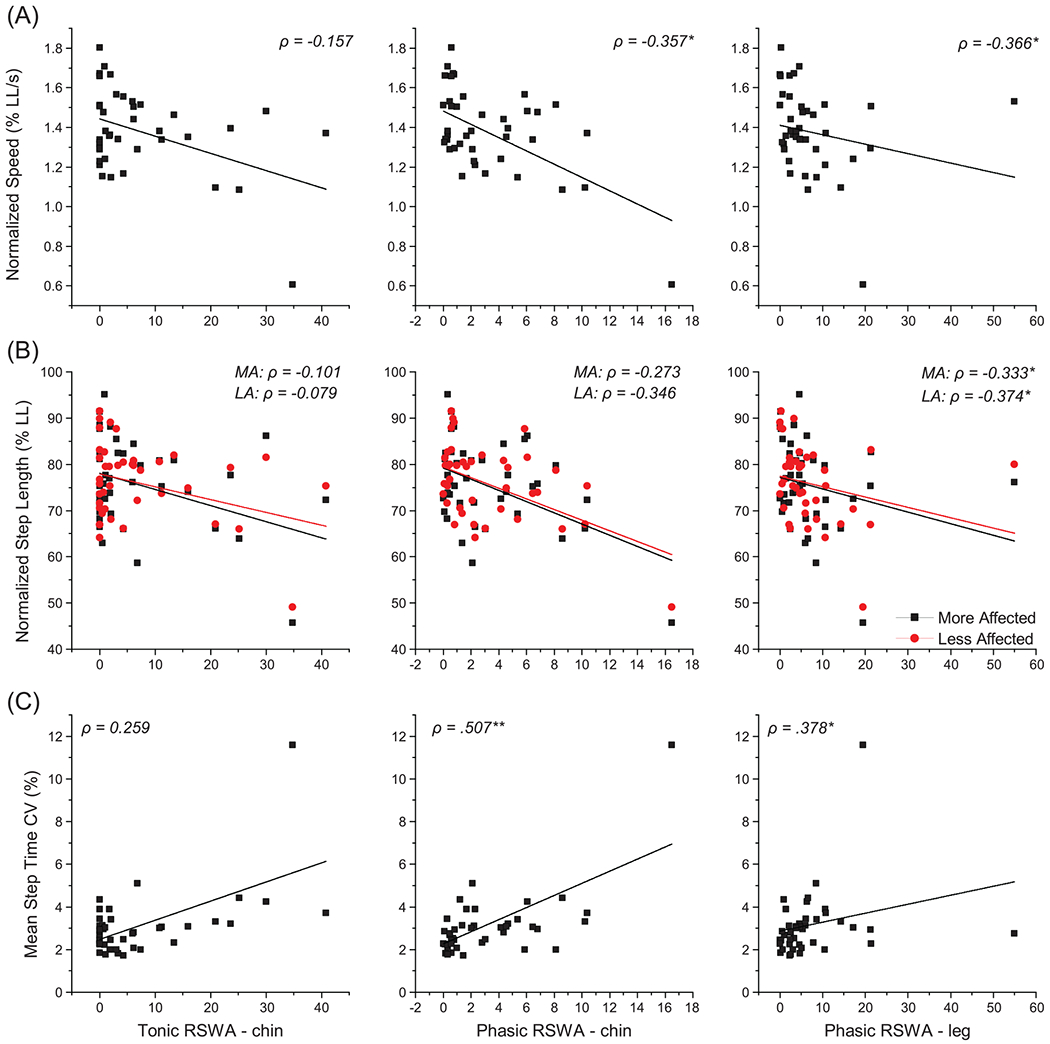
Correlations of gait measures to PSG RSWA scores within the participants with Parkinson’s disease (n = 41). The relationship of gait measures including (A) speed and (B) step length, both normalized to leg length, and (C) average step time coefficient of variation are plotted relating to tonic and phasic chin EMG activity and phasic leg EMG activity during REM sleep. Black squares represent the mean value for each participant on figures (A) and (C). On figure (B), black squares represent the mean of each participant’s more affected leg, while red circles represent the mean of each participant’s less affected leg. Lines are the lines of best fit for the data of matching color. *p < 0.05, **p < 0.01, ***p < 0.001.
Table 3.
Relationships between tonic and phasic chin muscle RSWA scores to gait measures within the PD participants (n = 41). Spearman’s coefficients (ρ) are reported
| Tonic Chin EMG | Phasic Chin EMG | Phasic Leg EMG | |
|---|---|---|---|
| Speed | −0.257 | −0.505** | −0.299 |
| Normalized speed (%LL/s) | −0.157 | −0.357* | 0.366* |
| Cadence | −0.358* | −0.377* | −0.170 |
| Normalized step length MA (%LL) | −0.101 | −0.273 | −0.333* |
| Normalized step length LA | −0.079 | −0.346 | −0.374* |
| Step width MA | −0.020 | −0.104 | 0.003 |
| Step width LA | −0.064 | −0.042 | −0.011 |
| Step time MA | 0.270 | 0.317* | 0.157 |
| Step time LA | 0.407** | 0.409** | 0.197 |
| Mean double support (%GC) | 0.215 | 0.157 | 0.159 |
| Mean step length CV (%) | 0.124 | 0.320* | 0.262 |
| Mean step width CV (%) | 0.079 | 0.109 | 0.033 |
| Mean step time CV (%) | 0.259 | 0.507** | 0.378* |
p < 0.05,
p < 0.01,
p < 0.001. %LL, percent leg length; %GC, percent of the gait cycle; CV, coefficient of variability; MA, more affected side; LA, less affected side.
DISCUSSION
We found, by separating a cohort of individuals with mild-to-moderately severe PD into groups based on RSWA, the presence of elevated muscle activity during REM sleep (PD-RSWA+) was associated with significantly greater impairment in gait than controls and people with PD without elevated muscle activity (PD-RSWA−). In contrast, few significant differences were observed between controls and the PD-RSWA− group. Gait impairment in the PD-RSWA+ group was characterized by significantly reduced gait speed, step lengths and double support as a percentage of gait cycle, compared to controls, and decreased speed and cadence and increased stride velocity variability and step and stance times, compared to the PD-RSWA− group. Moreover, the level of muscle activity during REM sleep correlated with measures of gait such that increased tonic or phasic EMG was associated with worsening of gait metrics. These findings demonstrate that the presence and level of disordered muscle tone during REM sleep is associated with the severity of gait disturbances.
RSWA and gait impairment in PD
Gait impairment is a common symptom of PD and becomes a major contributor to morbidity and decreased quality of life [19]. Gait deficits in PD are characterized by reduced speed, predominantly due to reductions in step/stride length [30–32], and increased step-to-step variability [16–19]. Even in the earliest stages of PD, statistically significant deficits in gait pace, rhythm, variability and asymmetry can be seen when compared with age- and sex-matched healthy adults [16–19]. Our findings in mild-to-moderate PD are consistent with these studies except that the differences from controls were predominantly found in the PD group with RSWA. Gait speed and step lengths were reduced by an average of 11% (12.3 cm/s) and 9% (14.7 cm/s) respectively in the PD-RSWA+ group compared to controls, but only 2% and 4% respectively in the PD-RSWA− group. The decrease in gait speed in the PD-RSWA+ group reflects a clinically meaningful impairment in gait [33]. The percentage decrease in gait speed between the PD-RSWA+ group and controls was comparable to the decrease of 11% reported by Galna et al. [17] between a large cohort with early stage PD (n = 121) and controls (n = 184). In general, the PD cohort in the Galna et al. study walked slower (mean =1.12 cm/s) than in the present study. Differences in findings between studies might be explained by the gait course, task duration and instructions, medication status, and participant demographics. Nonetheless, it can be assumed that the PD cohort in the Galna et al. study and other previous studies of gait in early PD [16–19] was comprised of both RSWA phenotypes (RSWA− and RSWA+). It would be interesting to test if subgroups with more and less gait impairment could be discerned when these cohorts were separated based on the presence/absence of RSWA and/or RBD.
RSWA, postural instability and freezing of gait in PD
Gait abnormalities in PD are often linked to additional deficits in postural stability and freezing of gait. Increases in stance time, asymmetry, step width, and variability, may reflect disturbances in dynamic postural control during gait and be related to falls [16, 17, 34–36]. People with PD who are fallers tend to have slower gait speed, and increased stance time variability and swing time asymmetry [37]. Slower gait speed and increased stance time are predictive of first falls in people with PD [37]. In early stage PD, step width and double support time are typically not different from controls [37], but increase with advancing disease [18]. In the current study, the PD with RSWA group showed significant changes in gait speed, step length, stance and double support time, which could be a harbinger of postural instability. However, studies using factor analyses have shown that gait and balance (e.g., postural sway) measures are largely independent [38], and impairments reflect dysfunction in different neural control domains [39].
The co-expression of PD and RBD (which includes both RSWA and dream enactment) is associated with a predominantly akinetic-rigid phenotype, with increased severity of postural instability and gait impairments, fall frequency, and FOG incidence [2, 4]. The presence of FOG is also associated with significantly higher RSWA compared to those with PD without FOG [40]. Our findings further demonstrate that the presence and level of RSWA expression is correlated with gait deficits during wakefulness. In addition, all participants in the PD cohort with FOG (n = 6) and 63% of the participants with an abnormal postural stability response (item 3.12 of the MDS-UPDRS III) were in the PD-RSWA+ group. Thus, the presence of RSWA in PD was associated with increased dysfunction of systems controlling gait and possibly balance.
Gait, cognition, and RBD
Cognitive decline may also parallel early alterations in specific domains of gait, such as declines in speed, rhythm, and pace variables, and increases in variability [41–44]. In PD, gait factors of pace, variability and postural control predict a decline in attention and memory, even when baseline neuropsychological measures do not [18]. The co-expression of PD with RBD may be a marker of a more complex and aggressive subtype of synucleinopathy that includes motor decline with mild cognitive impairment or dementia [45]. In our study, the PD-RSWA+ group had a more variable stride velocity, a factor associated with cognitive decline [46], but MoCA score was not a significant covariate for this measure. While there were no significant group differences in MoCA scores, MoCA was a significant covariate for speed and timing measures. Thus, changes in gait speed and stance and double support timing may reflect, or be harbingers of, cognitive decline in the group with PD elevated RSWA. Future studies employing neuropsychological assessments may add to this line of investigation.
Do RSWA and gait share a common region of degeneration?
The gait measures that correlated with the phasic chin or leg RSWA scores included variables that are related to pace (speed, cadence, and step length), rhythm (step time) and variability (step time variability). Elevated submentalis (chin) RSWA has recently been shown to be a potential biomarker that distinguishes synucleinopathy (probable PD or multiple system atrophy) from tauopathy (probable progressive supranuclear palsy or corticobasal degeneration) in people with parkinsonism with high sensitivity and specificity [47]. Our findings suggest that synuclein-related neurodegeneration that affects systems controlling muscle tone during REM sleep may parallel degenerative changes leading to gait deficits in people with PD.
Degeneration of the brainstem neurons that contribute to both the regulation of muscle tone during REM sleep and the control of locomotion may explain the correlations between sleep and gait measures. In particular, cholinergic neurons of the pedunculopontine nucleus (PPN) have been implicated in the control of both REM atonia [12] and the generation and regulation of locomotor patterns [48]. Stimulation in caudal PPN, an area with a relatively higher proportion of cholinergic neurons, is associated with suppression of muscle tone via projections to the ventromedial medullary reticular formation [49]. Conversely, stimulation in rostral regions of the PPN evokes a pattern of flexor-extensor muscle activity in the legs via inputs to reticulospinal neurons in the medullary reticular formation that control posture and locomotion [50]. Cholinergic neurons of the PPN degenerate in both RBD and PD [12, 51]. Loss of ascending and descending cholinergic projections from the PPN has been implicated in the pathogenesis of gait deficits in PD. Thalamic cholinergic denervation resulting from degeneration of the PPN is associated with decreased gait speed and an increased risk of falls in people with PD [52, 53]. Together, this evidence links abnormal RSWA to potential gait disorders. In keeping with this idea, several studies have shown that people with RBD (but without a PD diagnosis) demonstrate significant changes in gait and gait initiation compared to matched controls and these deficits resemble the impairments seen in PD [10, 11, 54].
Limitations
While the majority of the participants with PD could be considered as having relative early stage disease (overall median time from diagnosis = 1.5 years, interquartile range of 1–3.7 years; mean MDS-UPDRS = 37, interquartile range of 27–50) there was considerable skew in the distribution of disease durations and severity. As such, the PD cohort can be generally described as having mild-to-moderate disease severity [21]. The PD-RSWA+ group did not have significantly higher MDS-UDRS III scores or disease durations than the RSWA− group, but the levodopa daily equivalent dosage was significantly higher in the PD-RSWA+ group, which is consistent with other studies comparing PD with and without RBD [2]. Participants who were exercising regularly and/or receiving physical therapy may have performed better on the gait task, but these data were not collected. The correlational analyses included many variables without correcting for Type I errors, and should be interpreted cautiously.
All PD with FOG were classified as PD-RSWA+, which is not surprising given that FOG is more common in people with PD and RBD [40]. Since PD and FOG have greater gait disturbances than those without FOG [1], this may have contributed to differences in gait between the PD groups. As discussed above, the increased prevalence of FOG in the PD-RSWA+ group could be attributed to earlier and more extensive pathology in brainstem circuits that contribute to the control of both REM sleep muscle tone and locomotion, which have been implicated in the pathogenesis of FOG [55, 56]. Indeed, RSWA was significantly higher in people with PD and FOG compared to those without FOG [40]. Frontal executive function deficits are also more common in people with PD and RBD [57] and may contribute to gait disturbances and FOG [58]. Our cognitive assessment was limited to the MoCA and future studies should include more a more comprehensive neuropsychological battery in conjunction with measures of dual-task gait [59–61].
CONCLUSION
The study findings support our hypothesis that the presence and extent of changes in REM sleep motor tone correlate to daytime changes in steady-state gait measures among people with PD. Specifically, the expression of gait impairment manifests differently between those with and without RSWA. Importantly, clinically relevant [33] deficits in gait were seen in the PD-RSWA+ group compared to controls and PD-RSWA− participants. We suggest that the co-expression of abnormal REM sleep muscle tone and changes in daytime gait measures may reflect progressive involvement of related brainstem structures. Our current findings are critical for the development of therapies for PD, and support utilizing clinical PSGs to identify people with PD and RSWA who could benefit from more intense and customized physical therapy focused on gait and postural stability. Prospective studies that include longitudinal tracking of populations will also be critical for developing predictive models of disease progression. In these future models, measures of REM sleep and daytime gait may be used as biophysiological markers of disease progression and employed to evaluate symptomatic and possibly disease modifying therapies [62].
ACKNOWLEDGMENTS
We thank the volunteers for participation in this research, Devin O’Connell, BS, for assisting with data collection and analysis, Joshua De Kam, BA, CCRP for research coordination, and Chiahao Lu, PhD for statistical assistance. We are grateful for our additional referring movement disorders specialists including Dr. Martha Nance, MD, Dr. Daniel Kuyper, MD, and Dr. Sotirios Parashos, MD at Struthers Parkinson’s Center and Dr. Julia Johnson, MD at HealthPartners Parkinson’s Center. This work was supported by grants NIH RO1 NS070264 & NS088679 (CDM), NIH Clinical and Translational Science Award at the University of Minnesota (8UL1TR000114-02, Research support), and the National Center for Advancing Translational Sciences (NCATS) of the NIH (Grant Number UL1TR000114, Research support). Additional support for individuals came from: NIH R21 NS108022 (AV), NIH training grant T32GM008471 (MLE), NSF NRT Fellowship DGE-1734815 (MLE), the Wallin Neuroscience Foundation (JWC), the MnDRIVE Fellowship (SLAH, JWC, MNP), the Parkinson Study Group (MNP), the Parkinson’s Disease Foundation’s Advancing Parkinson’s Treatments Innovations Grant (MNP), and the Udall Center for Excellence in Parkinson’s Disease (NIH P50 NS09857) (SLAH, CDM).
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
- [1].Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A (2011) Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol 10, 734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J (2008) REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry 79, 1117–1121. [DOI] [PubMed] [Google Scholar]
- [3].Schenck CH, Hogl B, Videnovic A, eds. (2019) Rapid-Eye-Movement Sleep Behavior Disorder, Springer, Cham, Switzerland. [Google Scholar]
- [4].Romenets SR, Gagnon JF, Latreille V, Panniset M, Chouinard S, Montplaisir J, Postuma RB (2012) Rapid eye movement sleep behavior disorder and subtypes of Parkinson’s disease. Mov Disord 27, 996–1003. [DOI] [PubMed] [Google Scholar]
- [5].Postuma RB, Iranzo A, Hu M, Högl B, Boeve BF, Manni R, Oertel WH, Arnulf I, Ferini-Strambi L, Puligheddu M, Antelmi E, Cochen De Cock V, Arnaldi D, Mollenhauer B, Videnovic A, Sonka K, Jung KY, Kunz D, Dauvilliers Y, Provini F, Lewis SJ, Buskova J, Pavlova M, Heidbreder A, Montplaisir JY, Santamaria J, Barber TR, Stefani A, Louis SEK, Terzaghi M, Janzen A, Leu-Semenescu S, Plazzi G, Nobili F, Sixel-Doering F, Dusek P, Bes F, Cortelli P, Ehgoetz Martens K, Gagnon JF, Gaig C, Zucconi M, Trenkwalder C, Gan-Or Z, Lo C, Rolinski M, Mahlknecht P, Holzknecht E, Boeve AR, Teigen LN, Toscano G, Mayer G, Morbelli S, Dawson B, Pelletier A (2019) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 142, 744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sixel-Döring F, Trenkwalder C (2019) REM sleep behavior disorder associated with Parkinson’s disease and multiple system atrophy. In Rapid-Eye-Movement Sleep Behavior Disorder, Schenck CH, Högl B, Videnovic A, eds. Springer International Publishing, Cham, pp. 53–65. [Google Scholar]
- [7].Liu Y, Zhu XY, Zhang XJ, Kuo SH, Ondo WG, Wu YC (2017) Clinical features of Parkinson’s disease with and without rapid eye movement sleep behavior disorder. Transl Neurodegener 6, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Högl B, Stefani A, Videnovic A (2018) Idiopathic REM sleep behaviour. Nat Rev Neurol 14, 40–55. [DOI] [PubMed] [Google Scholar]
- [9].Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB (2019) Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 142, 2051–2067. [DOI] [PubMed] [Google Scholar]
- [10].McDade EM, Boot BP, Christianson TJH, Pankratz VS, Boeve BF, Ferman TJ, Bieniek K, Hollman JH, Roberts RO, Mielke MM, Knopman DS, Petersen RC (2013) Subtle gait changes in patients with REM sleep behavior disorder. Mov Disord 28, 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alibiglou L, Videnovic A, Planetta PJ, Vaillancourt DE, MacKinnon CD (2016) Subliminal gait initiation deficits in rapid eye movement sleep behavior disorder: A harbinger of freezing of gait? Mov Disord 31, 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, Benarroch EE, Ahlskog JE, Smith GE, Caselli RC, Tippman-Peikert M, Olson EJ, Lin SC, Young T, Wszolek Z, Schenck CH, Mahowald MW, Castillo PR, Del Tredici K, Braak H (2007) Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 130, 2770–2788. [DOI] [PubMed] [Google Scholar]
- [13].MacKinnon CD (2018) Sensorimotor anatomy of gait, balance, and falls. Handb Clin Neurol 159, 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].MacKinnon CD, Alibiglou L, Videnovic A (2019) Gait and postural disorders in REM sleep behavior disorder. In Rapid-Eye-Movement Sleep Behavior Disorder, Schenck CH, Högl B, Videnovic A, eds. Springer International Publishing, Cham, pp. 547–556. [Google Scholar]
- [15].Braak H, Bohl JR, Müller CM, Rüb U, de Vos RAI, Del Tredici K (2006) Stanley Fahn lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21, 2042–2051. [DOI] [PubMed] [Google Scholar]
- [16].Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L (2013) Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J Gerontol Ser A Biol Sci Med Sci 68, 820–827. [DOI] [PubMed] [Google Scholar]
- [17].Galna B, Lord S, Burn DJ, Rochester L (2015) Progression of gait dysfunction in incident Parkinson’s disease: Impact of medication and phenotype. Mov Disord 30, 359–367. [DOI] [PubMed] [Google Scholar]
- [18].Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, Khoo TK, Yarnall AJ, Burn DJ, Rochester L (2017) Gait rather than cognition predicts decline in specific cognitive domains in early Parkinson’s disease. J Gerontol Ser A Biol Sci Med Sci 72, 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, Hass CJ, Hausdorff JM, Pelosin E, Almeida QJ (2019) Gait impairments in Parkinson’s disease. Lancet Neurol 4422, 1–12. [DOI] [PubMed] [Google Scholar]
- [20].Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30, 1591–1601. [DOI] [PubMed] [Google Scholar]
- [21].MartÍnez-MartÍn P, RodrÍguez-Blázquez C, Alvarez M, Arakaki T, Arillo VC, Chaná P, Fernández W, Garretto N, MartÍnez-Castrillo JC, RodrÍguez-Violante M, Serrano-Dueñas M, Ballesteros D, Rojo-Abuin JM, Chaudhuri KR, Merello M (2015) Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat Disord 21, 50–54. [DOI] [PubMed] [Google Scholar]
- [22].Karlawish J, Cary M, Moelter ST, Siderowf A, Sullo E, Xie S, Weintraub D (2013) Cognitive impairment and PD patients’ capacity to consent to research. Neurology 81, 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Berry Richard B., MD; Brooks Rita, MEd, RST, RPSGT; Charlene E. Gamaldo M, Harding Susan M., MD; Lloyd Robin M. MCLM (2015) American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications, Version 2.2. Am Acad Sleep 28, 391–397. [Google Scholar]
- [24].Linn-Evans ME, Petrucci MN, Amundsen-Huffmaster SL, Chung JW, Tuite PJ, Howell MJ, Videnovic A, MacKinnon CD (2020) REM sleep without atonia is associated with increased rigidity in patients with mild to moderate Parkinson’s disease. Clin Neurophysiol 131, 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McCarter SJ, Tabatabai GM, Jong H-Y, Sandness DJ, Timm PC, Johnson KL, McCarter AR, Savica R, Vemuri P, Machulda MM, Kantarci K, Mielke MM, Boeve BF, Silber MH, St Louis EK (2020) REM sleep atonia loss distinguishes synucleinopathy in older adults with cognitive impairment. Neurology 94, e15–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N (2009) Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait Posture 30, 459–463. [DOI] [PubMed] [Google Scholar]
- [27].Galna B, Lord S, Rochester L (2013) Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol. Gait Posture 37, 580–585. [DOI] [PubMed] [Google Scholar]
- [28].Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25, 2649–2653. [DOI] [PubMed] [Google Scholar]
- [29].Quade D (1967) Rank analysis of covariance. J Am Stat Assoc 62, 1187–1200. [Google Scholar]
- [30].Morris ME, Iansek R, Matyas TA, Summers JJ (1994) Ability to modulate walking cadence remains intact in Parkinson’s disease. J Neurol Neurosurg Psychiatry 57, 1532–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Morris ME, Iansek R, Matyas TA, Summers JJ (1996) Stride length regulation in Parkinson’s disease. Brain 119, 551–568. [DOI] [PubMed] [Google Scholar]
- [32].Morris M, Iansek R, Matyas T, Summers J (1998) Abnormalities in the stride length-cadence relation in parkinsonian gait. Mov Disord 13, 61–69. [DOI] [PubMed] [Google Scholar]
- [33].Hass CJ, Bishop M, Moscovich M, Stegemöller EL, Skinner J, Malaty IA, Shukla AW, McFarland N, Okun MS (2014) Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J Neurol Phys Ther 38, 233–238. [DOI] [PubMed] [Google Scholar]
- [34].Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA (2005) Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J NeuroEngineering Rehabil 2, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Maki BE (1997) Gait changes in older adults: Predictors of falls or indicators of fear? J Am Geriatr Soc 45, 313–320. [DOI] [PubMed] [Google Scholar]
- [36].Taylor ME, Delbaere K, Mikolaizak AS, Lord SR, Close JCT (2013) Gait parameter risk factors for falls under simple and dual task conditions in cognitively impaired older people. Gait Posture 37, 126–130. [DOI] [PubMed] [Google Scholar]
- [37].Lord S, Galna B, Yarnall AJ, Coleman S, Burn D, Rochester L (2016) Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naïve cohort. Mov Disord 31, 1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Horak FB, Mancini M, Carlson-Kuhta P, Nutt JG, Salarian A (2016) Balance and gait represent independent domains of mobility in Parkinson disease. Phys Ther 96, 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mancini M, Nutt J, Horak F (2020) Balance Dysfunction in Parkinson’s Disease: Basic Mechanisms to Clinical Management, Academic Press, London. [Google Scholar]
- [40].Videnovic A, Marlin C, Alibiglou L, Planetta PJ, Vaillancourt DE, MacKinnon CD (2013) Increased REM sleep without atonia in Parkinson disease with freezing of gait. Neurology 81, 1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J (2010) The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 67, 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA (2005) Gait slowing as a predictor of incident dementia: 6-Year longitudinal data from the Sydney Older Persons Study. J Neurol Sci 229-230, 89–93. [DOI] [PubMed] [Google Scholar]
- [43].Jayakody O, Breslin M, Srikanth VK, Callisaya ML (2019) Gait characteristics and cognitive decline: A longitudinal population-based study. J Alzheimers Dis 71, S5–S14. [DOI] [PubMed] [Google Scholar]
- [44].Verghese J, Wang C, Lipton RB, Holtzer R, Xue X (2007) Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 78, 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios Romenets S, Panisset M, Gagnon JF (2012) Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: Aprospective study. Mov Disord 27, 720–726. [DOI] [PubMed] [Google Scholar]
- [46].Morris R, Lord S, Lawson RA, Coleman S, Galna B, Duncan GW, Khoo TK, Yarnall AJ, Burn DJ, Rochester L (2017) Gait rather than cognition predicts decline in specific cognitive domains in early Parkinson’s disease. J Gerontol A Biol Sci Med Sci 72, 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].McCarter SJ, Feemster JC, Tabatabai GM, Sandness DJ, Timm PC, McCarter AR, Talley HN, Junna MR, Savica R, Singer W, Coon EA, Benarroch EE, Josephs KA, Boeve BF, Silber MH, St. Louis EK (2019) Submentalis rapid eye movement sleep muscle activity: A potential biomarker for synucleinopathy. Ann Neurol 86, 969–974. [DOI] [PubMed] [Google Scholar]
- [48].Roseberry TK, Lee AM, Lalive AL, Wilbrecht L, Bonci A, Kreitzer AC (2016) Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 164, 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Takakusaki K (2017) Functional neuroanatomy for posture and gait control. J Mov Disord 10, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG (2008) Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57, 183–191. [DOI] [PubMed] [Google Scholar]
- [51].Tubert C, Galtieri D, Surmeier DJ (2019)The pedunclopontine nucleus and Parkinson’s disease. Neurobiol Dis 128, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bohnen NI, Frey KA, Studenski S, Koeppe RA, Scott PJH, Albin RL, Müller MLTM (2013) Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology 81, 1611–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bohnen NI, Müller MLTM, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, Albin RL (2009) History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology 73, 1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ehgoetz Martens KA, Matar E, Hall JM, Phillips J, Szeto JYY, Gouelle A, Grunstein RR, Halliday GM, Lewis SJG (2019) Subtle gait and balance impairments occur in idiopathic rapid eye movement sleep behavior disorder. Mov Disord 34, 1374–1380. [DOI] [PubMed] [Google Scholar]
- [55].Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB (2013) Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136(Pt 8), 2405–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I (2011) Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain 134(Pt 1), 59–72. [DOI] [PubMed] [Google Scholar]
- [57].Jozwiak N, Postuma RB, Montplaisir J, Latreille V, Panisset M, Chouinard S, Bourgouin P-A, Gagnon J-F (2017) REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep 40, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ehgoetz Martens KA, Peterson DS, Almeida QJ, Lewis SJG, Hausdorff JM, Nieuwboer A (2020) Behavioural manifestations and associated non-motor features of freezing of gait: A narrative review and theoretical framework. Neurosci Biobehav Rev 116, 350–364. [DOI] [PubMed] [Google Scholar]
- [59].Hausdorff JM, Schaafsma JD, Balash Y (2003) Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res 149, 187–194. [DOI] [PubMed] [Google Scholar]
- [60].Kelly VE, Eusterbrock AJ, Shumway-Cook A (2012) A review of dual-task walking deficits in people with Parkinson’s disease: Motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis 2012, 918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].de Souza Fortaleza AC, Mancini M, Carlson-Kuhta P, King LA, Nutt JG, Chagas EF, Freitas IF, Horak FB (2017) Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait Posture 56, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schenck CH, Montplaisir JY, Frauscher B, Hogl B, Gagnon JF, Postuma R, Sonka K, Jennum P, Partinen M, Arnulf I, Cochen de Cock V, Dauvilliers Y, Luppi PH, Heidbreder A, Mayer G, Sixel-Döring F, Trenkwalder C, Unger M, Young P, Wing YK, Ferini-Strambi L, Ferri R, Plazzi G, Zucconi M, Inoue Y, Iranzo A, Santamaria J, Bassetti C, Möller JC, Boeve BF, Lai YY, Pavlova M, Saper C, Schmidt P, Siegel JM, Singer C, St Louis E, Videnovic A, Oertel W (2013) Rapid eye movement sleep behavior disorder: Devising controlled active treatment studies for symptomatic and neuroprotective therapy-a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep Med 14, 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]


