Abstract
A robust spore wall was a key requirement of terrestrialization by early plants. Sporopollenin in spore and pollen grain walls is thought to be polymerized and cross-linked to other macromolecular components partly through oxidative processes involving H2O2. Therefore, we investigated effects of scavengers of reactive oxygen species (ROS) on formation of spore walls in the moss, Physcomitrella patens. Exposure of sporophytes, containing spores in the process of forming walls, to ascorbate, dimethylthiourea or 4-hydroxy-TEMPO prevented normal wall development in a dose, chemical and stage-dependent manner. Mature spores, exposed while developing to a ROS scavenger, burst when mounted in water on a flat slide under a coverslip (a phenomenon we named “augmented osmolysis” since they did not burst in phosphate-buffered saline or in water on a depression slide). Additionally, walls of exposed spores were more susceptible to alkaline hydrolysis than those of control spores and some were characterized by discontinuities in the exine, anomalies in perine spine structure, abnormal intine and aperture and occasionally wall shedding. Our data support involvement of oxidative cross-linking in spore wall development, including sporopollenin polymerization or deposition, as well as a role for ROS in intine/aperture development.
Keywords: augmented osmolysis, exine, perine, intine, oxidative cross-linking, sporopollenin polymerization, microscopy
Introduction
The evolution of land plants ~500 million years ago (Morris et al. 2018) required innovations to counter challenges posed by dehydration, UV radiation and gravity. One such innovation was a durable spore wall, which provided protection from dehydration and UV radiation. The bryophyte spore wall is composed of a minimum of two layers, an inner intine and an outer exine. An additional outermost perine layer is found in moss and fern spores (Brown and Lemmon 1990; Wallace et al. 2011). Sporopollenin is the main polymeric constituent of the walls of spores (exine and perine) and pollen grains (exine). It is chemically resistant and composed of polyhydroxylated fatty acid derivatives and oxygenated aromatic compounds linked together by extensive ester and ether bonds (Dominguez et al. 1999). A recent study provides chemical and spectroscopic evidence that acetal linkages are also prevalent in pine sporopollenin (Li et al. 2019).
Several enzymes involved in the biosynthesis of sporopollenin monomers and transcription factors regulating their expression are known (Ariizumi and Toriyama 2011; Quilichini et al. 2015). In Arabidopsis, a fatty acyl reductase (MS2), two cytochrome P450-dependent fatty acid hydroxylases (CYP703A2, CYP704B1), an acyl-CoA synthetase (ACOS5), two type III polyketide synthases (PKSA, PKSB), and two tetraketide α-pyrone reductases (TKPR1, TKPR2) participate in biosynthesis of sporopollenin monomers (Morant et al. 2007; de Azevedo Souza et al. 2009; Dobritsa et al. 2009; Grienenberger et al. 2010; Kim et al. 2010; Chen et al. 2011). Also in Arabidopsis, two transcription factors, ABORTED MICROSPORES and MALE STERILE 188, form a regulatory feed-forward loop in which the former induces the latter, and they co-operatively induce MS2, CYP703A2, PKSA and PKSB expression (Wang et al. 2018). Data from phylogenetic and gene expression studies of Physcomitrella orthologs imply that the sporopollenin biosynthetic pathway is widely conserved in land plants (Colpitts et al. 2011). This conclusion is strengthened by the finding that Physcomitrella strains possessing deletion mutations of MS2 and PKSA/B orthologs produce spores with defective walls (Wallace et al. 2015; Daku et al. 2016). However, the enzymes that catalyze sporopollenin polymerization and deposition are yet to be discovered.
Chemical stability of lignin, suberin, and the polymeric matrices of the plant primary cell wall is due largely to C–C and C–O (ether) bonds formed by oxidative coupling reactions (Bernards et al. 1999; Burr and Fry 2009; Shigeto and Tsutsumi 2016). Class III peroxidases catalyze the oxidative coupling reactions of lignin polymerization using H2O2 as oxidant. It is reasonable to expect similar processes to occur in sporopollenin polymerization and its deposition in the walls of spores and pollen grains. Matveyeva et al. (2012) reported evidence, using a fluorescent dye, that the superoxide anion radical (O2–•) and probably other reactive oxygen species (ROS) are present in the exine of tobacco microspores at the tetrad stage when sporopollenin polymerization and deposition are occurring.
In culture, Physcomitrella sporophytic development, including sporogenesis, occurs when sporophytes are submerged in water, thus enabling both chemical rescue and inhibitor studies. To obtain evidence of the involvement of ROS in sporopollenin polymerization and spore wall formation, we investigated the effects of ROS scavengers on the formation of spore walls by exposing sporophytes containing developing spores to ascorbate (AsA), N,Nʹ-dimethylthiourea (DMTU) and 4-hydroxy-Tempo (Tempol). AsA is a natural antioxidant, and DMTU and Tempol are representative thiourea and nitroxide antioxidants, respectively. These scavengers are highly soluble in water, less toxic and had already been studied in planta within the context of plant developmental processes (Soule et al. 2007; Akram et al. 2017; Mei et al. 2017).
Materials and methods
Plant material, culture conditions and chemicals
The pabB4 strain of Physcomitrella patens (Hedw.) Bruch, Schimp & W. Gümbel (Ashton and Cove 1977) was used in this study. PabB4 was obtained originally by NTG mutagenesis and shown by conventional genetic analysis through sexual crossing to possess a single pab biochemical mutation (Ashton and Cove 1977; Courtice et al. 1978). When grown on medium containing adequate p-aminobenzoic acid (paba) (1.8 and 18 µM for gametophytes and sporophytes respectively), the pabB4 control strain is phenotypically (i.e. morphologically and developmentally) indistinguishable from the original Gransden wild type strain from which it was derived. Like the original Gransden wild type, obtained from a single spore isolated from nature by H.L.K. Whitehouse in 1962, it produces abundant sporophytes containing numerous viable spores with normal spore coat ornamentation and typical stratification of the spore wall into outer perine, middle exine and inner intine layers (Ashton and Cove 1977; Courtice et al. 1978; Singer et al. 2007; Singer and Ashton 2007; Daku et al. 2016). Gametophytic plants were grown axenically in 30 mL glass tubes containing ABC medium (Knight et al. 1988) solidified with 1.5% agar and supplemented with 1.8 µM paba. Cultures were maintained under continuous white light (25‒40 µmol cm‒2 s‒1) at 20‒22 °C and 30‒50% relative humidity. To produce sporophytes, 2–3 month-old gametophytes were subjected to cold stress at 16 °C for 3 weeks and then irrigated with 5 mM HEPES buffer (pH 8.0) containing 18 µM paba to facilitate fertilization and support growth and development of the paba-requiring sporophytes.
AsA, DMTU and Tempol were purchased from Sigma-Aldrich (Oakville, ON, Canada).
Effects of ROS scavengers on the development of sporophytes and spores
At 0, 10 or 20 days post-irrigation (dpi), ROS scavengers were added at 0.1, 1 and 10 mM to the irrigation solution in which plants had been submerged. By 35 dpi, sporophytes and the spores within them had reached maturity (late orange stage) (Daku et al. 2016). For each treatment, three culture tubes were used. Under the standard conditions, ~80 sporophytes were produced in each tube. Two or more sporophytes from each of three culture tubes were collected and spores were gently released into 200 µL of water or PBS (phosphate buffered saline, pH 7.4). Spores were immediately mixed by gentle pipetting and 10 µL aliquots, each containing ~1000 spores, were mounted on slides. For each observation, minimally three slides were prepared and representative images reported. All experiments involving 10 mM scavengers added at 20 dpi were duplicated.
Swelling and bursting of spores
Aliquots (10 µL) of spore suspension in water or in PBS were mounted on a flat glass slide or on a depression slide. The slide was overlaid with a coverslip and its edges were sealed with nail polish to prevent evaporation. Images of spores were taken as soon as possible after mounting (within 5 min), and the spores were monitored for up to 2 h to detect swelling and bursting. Times taken for bursting to occur were recorded. To quantify swelling in water, spore diameters were measured from spore images taken within 5 min of mounting and 30 min after mounting. Spore diameter was taken as the mean of the largest diameter and that perpendicular to it.
For Sudan IV-staining of oil droplets, spores from 10 mM AsA-treated sporophytes in 20 µL water were placed between a slide and a cover slip. After bursting, aliquots (5 µL) were withdrawn and mixed with an equal volume of Sudan IV solution (0.1% in ethylene glycol) for staining. Images were obtained with a Nikon Eclipse 80i compound light microscope equipped with a DSRi1 digital camera.
Transmission electron microscopy
Capsules of mature sporophytes were punctured with a razor blade or fine needle to facilitate infiltration of solutions and resin. Specimens were fixed in 2% glutaraldehyde in 50 mM sodium phosphate buffer (pH 7.2) for 1 h at room temperature, then overnight at 20 °C. Following three rinses in buffer for 30 min each, sporophytes were post-fixed for 1 h in buffered 2.5% OsO4 followed by three 10-min rinses in distilled water. Plants were dehydrated in a graded ethanol series, rinsed 3 times in 100% ethanol and slowly infiltrated over 4–7 d by increasing the percentage of Spurr’s resin to ethanol. Plants were placed in molds with fresh resin and cured for 16 h at 65 °C. Thick sections (500–1000 nm) were mounted on glass slides and stained with 1.5% toluidine blue in distilled water to monitor spore stage and integrity using light microscopy. Thin sections (60–90 nm) were collected on nickel grids and observed unstained and post-stained with methanolic uranyl acetate and basic lead citrate. Specimens were observed on a Hitachi H7650 transmission electron microscope at 60 kV and images were captured digitally. Three to ten capsules of each treatment were used to observe ultrastructural features of mature spores.
Scanning electron microscopy
Three to six capsules were harvested from each treatment, air-dried and opened on stubs to disperse spores. Specimens were sputter-coated with 50 nm of Au/Pd using a Denton Desk II Vacuum Sputter Coater and imaged using a scanning electron microscope, QUANTA FEG 450 (FEI) with 5 kV acceleration voltage.
Alkaline hydrolysis
For alkaline hydrolysis, spores from sporophytes, which had been treated with 10 mM ROS scavengers at 20 dpi, were heated at 70 °C in 2 M KOH for various periods of time. After treatment, the spores were washed with water (3×) by centrifugation, and their images recorded. For each treatment, six sporophytes from three culture tubes were used, and two slides were prepared for each observation.
Statistical analysis
The significance of a difference between a given pair of means was assessed with the Student’s t-test.
Results
Effects of ROS scavengers on spore development
Although in culture it is impossible to achieve complete synchronization of sporophytic development since fertilization may occur prior to and at different times following irrigation, most Physcomitrella sporophytes are in the late green stage at 20 dpi. During this stage, the formation of sporopollenin-containing walls is initiated around developing spores (Daku et al. 2016). During the subsequent yellow stage, which lasts ~5 days, spines of the perine begin to appear and both exine and perine become pigmented. Spore maturation and wall development continue for approximately another 10 days through the orange stage, when the perine spines develop further and pigmentation intensifies. The effects on developing spores of exposure to ROS scavengers in comparison to untreated spores (Fig. 1a) are stage-dependent: the earlier and longer the exposure, the greater the severity. Thus, for example, when 10 mM AsA was present from 0 dpi, some spores became malformed and oblong-shaped (5 of 25 spores), and all lacked prominent perine spines (Fig. 1b). When the same scavenger was added at 10 dpi, spherical spores without prominent spines were formed. When mounted on a slide for observation, it was apparent immediately that there were fissures in the spore wall and the spores were already flattened, having lost most of their contents (Fig. 1c). By contrast, when 10 mM AsA was added at 20 dpi, the spores appeared to be normal and possessed prominent perine spines and dark pigmentation by 35 dpi (Fig. 1d). However, when mounted in water on a flat slide with a coverslip and left for >15 min, the spores swelled and burst, releasing Sudan IV-positive oil droplets. Similar stage-dependent effects on spore morphologies were also obtained with DMTU and Tempol (data not shown). We concluded that swelling and bursting, which did not occur unless developing spores had been exposed to a ROS scavenger, are indicative of a partially compromised spore wall. Additional experiments were performed to elucidate the nature of this phenomenon.
Fig. 1.
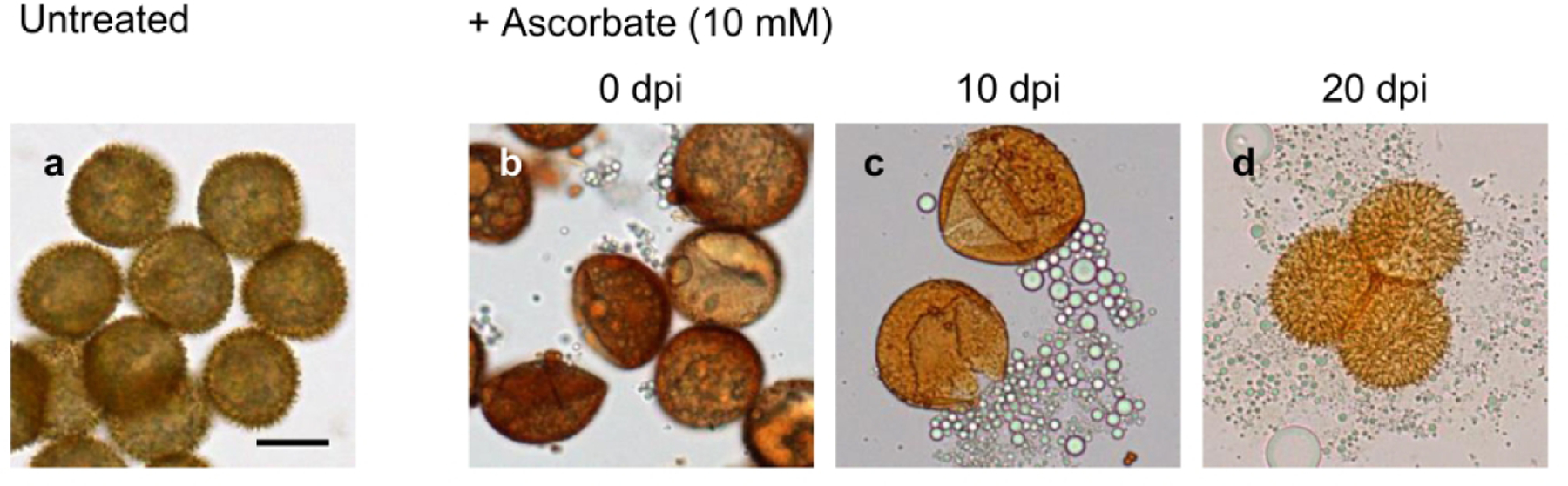
Bright-field images of Physcomitrella spores that developed in the absence (a) or presence (b–d) of 10 mM ascorbate from 0, 10, or 20 days post-irrigation (dpi) until 35 dpi. All images are to the same scale. Scale bar in (a) = 20 µm.
Effects of ROS scavengers on spore wall integrity: swelling and bursting
When mounted in water on a flat slide with a coverslip, spores that had developed in the absence of scavenger (untreated) remained unchanged during the observation period (90 min) (Fig. 2a, b). However, spores exposed to a ROS scavenger during the maturation period from 20 to 35 dpi swelled and eventually burst releasing oil droplets and other spore contents (Fig. 2c–t; Supplementary video). The rapid outflow of spore contents through an opening typically lasted 20–30 sec. Most bursting spores released their contents through a single opening but simultaneous release through two openings was occasionally observed. The extent of swelling and the time it took for spores to burst were scavenger and dose-dependent. Swelling without bursting was observed when spores had been exposed during development to a ROS scavenger at a low concentration (0.1 mM). Oil droplets were visible through the translucent walls of the swollen spores as seen in Fig. 2d and 2p. Previous exposure to higher concentrations (1 and 10 mM) of ROS scavengers resulted in the mature spores swelling and ultimately bursting. AsA was the most effective in causing bursting and all spores exposed while developing burst within 50 min at 1 mM AsA (Fig. 2f) and 15 min at 10 mM AsA (Fig. 2h). Tempol was the least effective requiring 80 min (Fig. 2r) and 60 min (Fig. 2t) at 1 and 10 mM, respectively. Bursting was observed in all the treated (10 mM) spores that were examined. When mounted in PBS (instead of water) on a flat slide under a coverslip, spores previously treated with DMTU at 10 mM swelled slightly (0.69 ± 0.06 µm, n = 8) but did not burst during the observation period (60–90 min) (Fig. 3c, d), similar to spores from untreated sporophytes (Fig. 3a, b). Also, bursting depended on the type of slide employed. Thus, spores exposed to 10 mM DMTU while developing burst within 40 min when mounted in water on a flat slide under a coverslip (Fig. 2n, 3g). However, despite becoming swollen, they did not burst during the observation period (120 min) when mounted in water on a depression slide with a coverslip (Fig. 3h). The mean diameter of the DMTU-exposed spores increased after 30 min in water from 22.88 to 24.80 µm when mounted on a flat slide under a cover slip, while that of control spores changed from 22.72 to 22.82 µm measured under the same conditions. Thus, the magnitude of swelling, expressed as increase in spore diameter, of DMTU-exposed spores in water (1.92 ± 0.10 µm) was significantly larger than that of control spores (0.10 ± 0.01 µm) (P < 0.01) (Fig. 3i). Pre-soaking in water had little effect on the time taken to burst. When spores, previously treated with 10 mM DMTU, were soaked in water for 2 h before being mounted on a flat slide under a coverslip, they took approximately the same time (40–45 min) to burst as in the absence of pre-soaking. Only when the spores were pre-soaked in water for 24 h did a small percentage (5%) of them burst within 10 min, while the rest (95%) still took 40–45 min to burst. By contrast, spores, which had not been exposed to a ROS scavenger while they were developing, failed to burst under any of the experimental circumstances described above even after 2h (Fig. 3e, f).
Fig. 2.
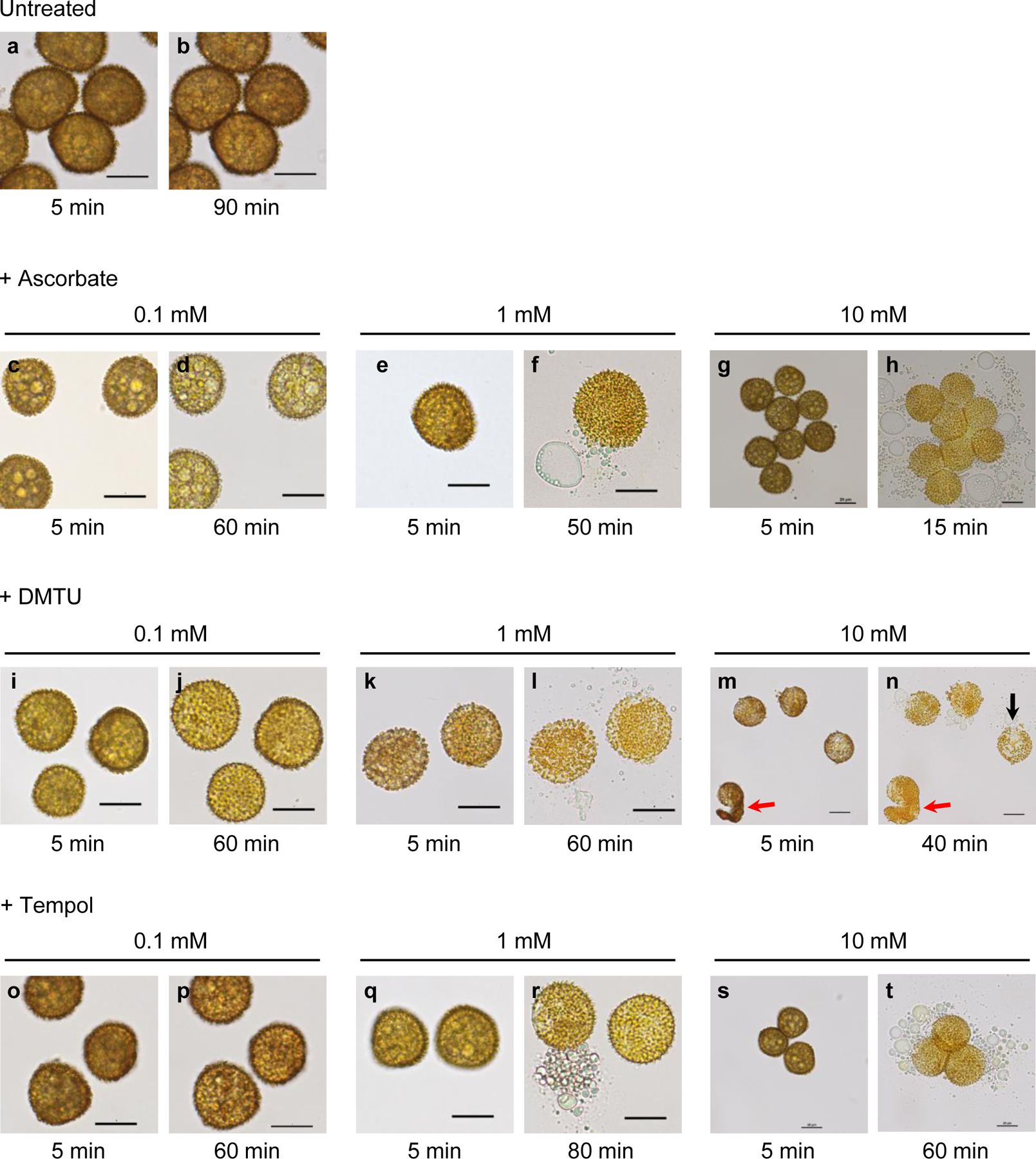
Bright-field images of spores, which developed in the absence (a, b) or presence (c–t) of a ROS scavenger during the period from 20 to 35 days post-irrigation (dpi). At 35 dpi, spores were harvested in water and immediately mounted on a flat slide with a coverslip. Spores were monitored for a minimum of 60 min to detect swelling and bursting. Shown are representative images of spores, which had been exposed to a ROS scavenger at 0.1, 1 or 10 mM, taken at the beginning of the observation period (5 min post-harvest) and at the time when most spores had burst (e.g. 50 min for 1 mM ascorbate, f). While unexposed spores neither swelled nor burst within 90 min (a, b), scavenger-treated spores swelled and burst dose-dependently. Spores that had been exposed to a ROS scavenger at 0.1 mM swelled but did not burst within 60 min (d, j, p). Spores exposed to a scavenger at 1 or 10 mM burst after 15–80 min depending on the scavenger and dose applied (f, h, l, n, r, t). Some spores exposed to 10 mM DMTU exhibited wall shedding (red arrows, m, n) or under-developed spines (black arrow, n). Scale bars 20 µm.
Fig. 3.
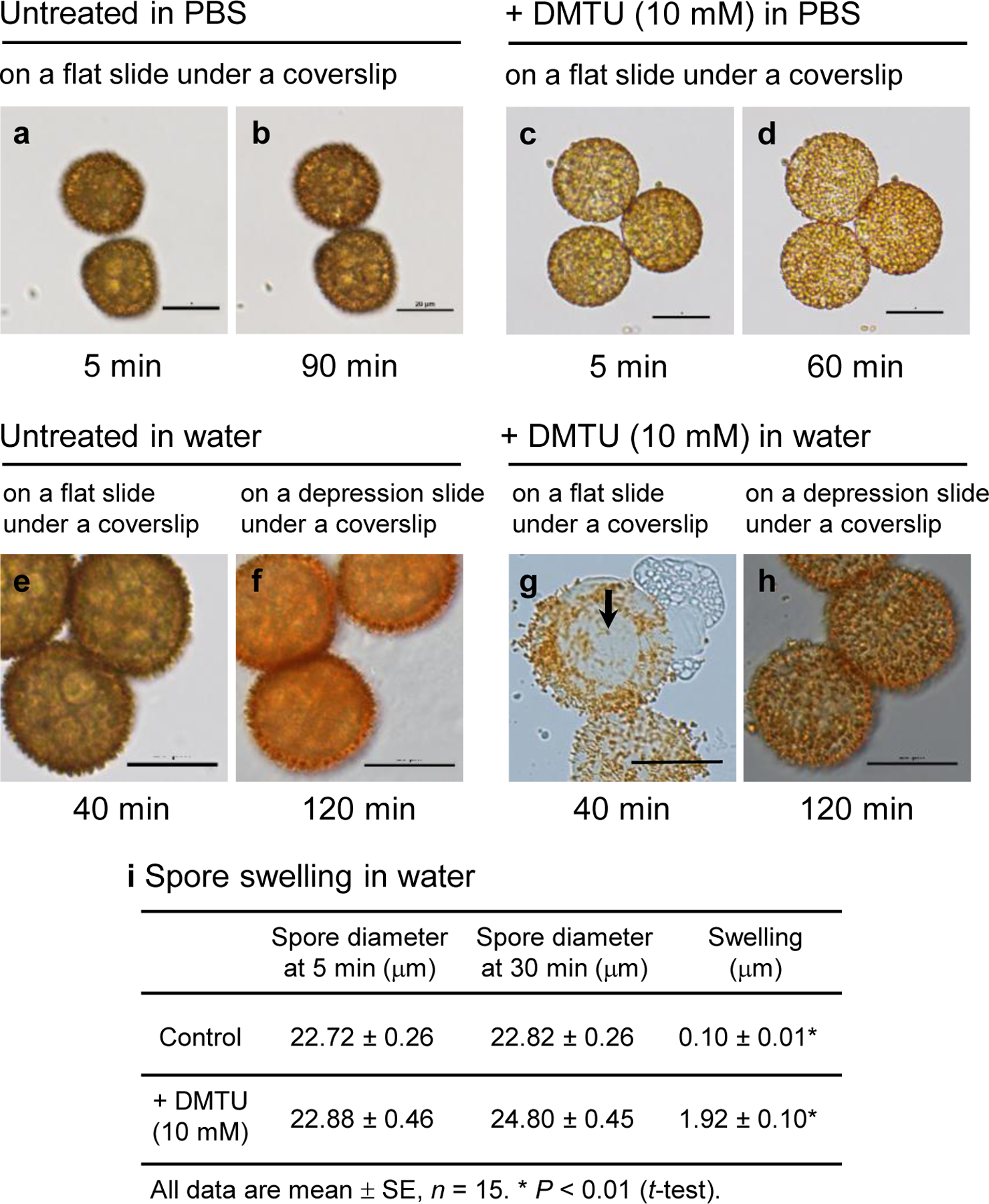
Bright-field images of spores that developed in the absence (a, b, e, f) or presence (c, d, g, h) of 10 mM DMTU from 20 to 35 days post-irrigation (dpi). At 35 dpi, spores were harvested in water or PBS and immediately mounted on a flat slide with a coverslip or on a depression slide with a coverslip. The spores from untreated sporophytes exhibited little swelling and did not burst after 90 min in PBS (a, b), while the spores from DMTU-treated sporophytes did not burst after 60 min in PBS (c, d). In water on a flat slide under a coverslip, the spores from DMTU-treated sporophytes swelled and burst within 40 min (g) whereas in water on a depression slide (where there was no direct contact between the coverslip and the spores) they swelled but had not burst after 120 min (h). The spores from untreated sporophytes also did not burst in water on a flat (e) or a depression slide (f). An area with under-developed spines, also seen in Fig. 2n, is clearly recognizable in g (black arrow). Scale bars 20 µm. (i) Swelling of spores from untreated and DMTU-treated sporophytes in water. Spore diameter was taken as the mean of the largest diameter and that perpendicular to it.
Except for swelling and bursting in water, most spores exposed from 20 dpi to AsA or Tempol appeared in the light microscope to be similar morphologically to control spores. By contrast, some spores previously exposed to 10 mM DMTU exhibited surface regions with poorly developed perine spines that became more noticeable after spore swelling (black arrows, Fig. 2n, 3g); occasionally, wall (presumably perine) shedding was also observed (red arrow, Fig. 2m, n). Spores exposed to 10 mM DMTU were also examined by SEM and compared with untreated spores (Fig. 4). Following air-drying and no chemical processing, untreated spores maintained their rounded shape with slight compression along the proximal spore face (Fig. 4a, c). In contrast, most DMTU-exposed spores completely collapsed along the proximal surface at the aperture during drying (Fig. 4b, d). Disrupted perine deposition in many DMTU-treated spores resulted in abnormal aggregations of sporopollenin globules terminating perine spines (Fig. 4f), differing markedly from the layered spines with gradually tapering ends on untreated spores (Fig. 4e). Small rosettes of aggregated sporopollenin were very abundant on tapetal cell walls, especially along radial walls, in DMTU-treated (Fig. 4h) and less so in untreated plants (Fig. 4g). Rosettes are comprised of globules of sporopollenin and small spines with layers similar to those of spore wall spines (Fig. 4i).
Fig. 4.
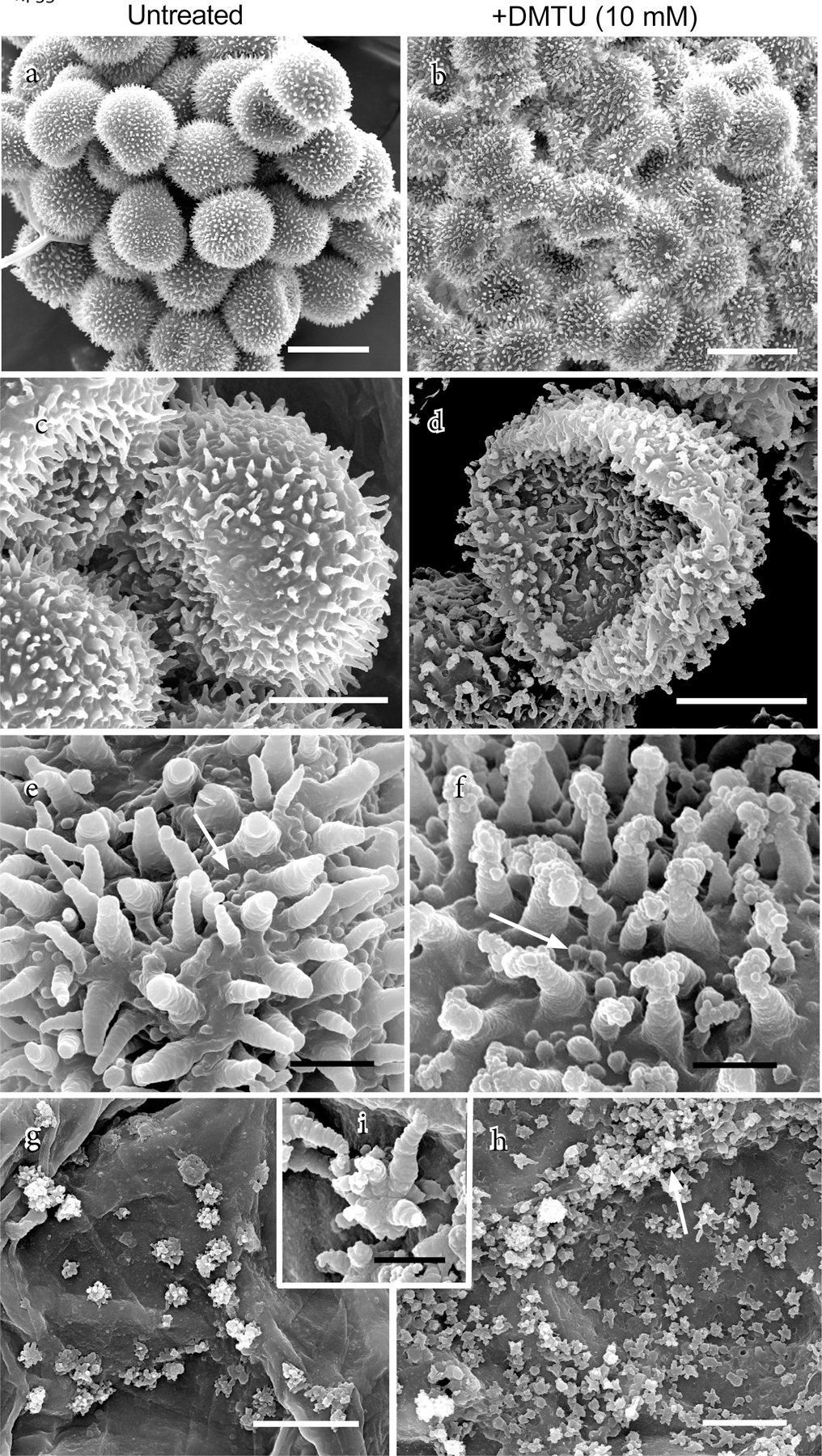
Scanning electron micrographs of spores and tapetum from air-dried capsules that developed in the absence (left column) or presence of 10 mM DMTU (right column). (a) Cluster of untreated spores that are compressed on the proximal surface and rounded distally. (b) DMTU-treated spores are irregularly shrunken with collapsed proximal faces. (c) A tetrad of untreated spores showing distal perine spines and slightly compressed proximal surface. (d) Single DMTU-treated spore showing abnormal perine spines and collapsed proximal surface that marks the site of the aperture. (e) Higher magnification reveals details of the distal spore surface perine with striated spines and surface papillae (arrow) on an untreated spore. (f) DMTU-treated spore surface with papillae (arrow) and aberrantly constructed spines that are striated at their bases and terminated by abnormal aggregates of perine globules. (g) Scattered rosettes of sporopollenin globules on inner walls of tapetal cells in untreated capsules. (h) Abundant rosettes of sporopollenin globules on inner walls of tapetal cells, especially along radial walls (arrow), in DMTU-treated capsules. (i) Higher magnification of a rosette in (g) showing striated nature of sporopollenin globules similar to that in spore spines. Scale bars a, b = 25 µm; c, d, g, h = 10 µm; e, f, = 2 µm; i = 1 µm.
The distal spore wall of untreated plants consists of an electron-lucent intine, thin continuous exine and spiny perine (Fig. 5a). The aperture extends across the proximal surface and includes a swollen intine and thin exine subtended by a fibrillar layer (Fig. 5b). Spore wall anomalies are evident in the TEM in all three treatments. Ultrastructural details of DMTU-treated spores corroborate SEM observations relating to the spore wall ornamentation. Sporopollenin globules aggregated around perine spines (Fig. 5c) are the same size (~100 nm in diameter) and abundance as those in Fig. 4f. Not visible in the SEM but visible in the TEM are aperture abnormalities found in some DMTU-treated spores (Fig. 5d). Whereas in untreated spores, the exine is uniform in thickness, the exine in DMTU-treated spores thins and is discontinuous in places along the aperture (Fig. 5d). Moreover, the subtending fibrillar wall is disrupted in DMTU-treated spores and the thickened intine contains electron-dense fibers, probably representing the disruption of the fibrillar layer beneath the exine of untreated spores. The distal spore walls of AsA-treated spores (Fig. 5e) are similar ultrastructurally to untreated spores, while the aperture in some spores has thin exine areas and contains aggregates of small electron-dense droplets (Fig. 5f). While many Tempol-treated spores do not display abnormal ultrastructure, other spores show an anomalous fibrillar network in the intine (Fig. 5g) and dense droplets in the aperture region resembling those in AsA treatments (Fig. 5h).
Fig. 5.
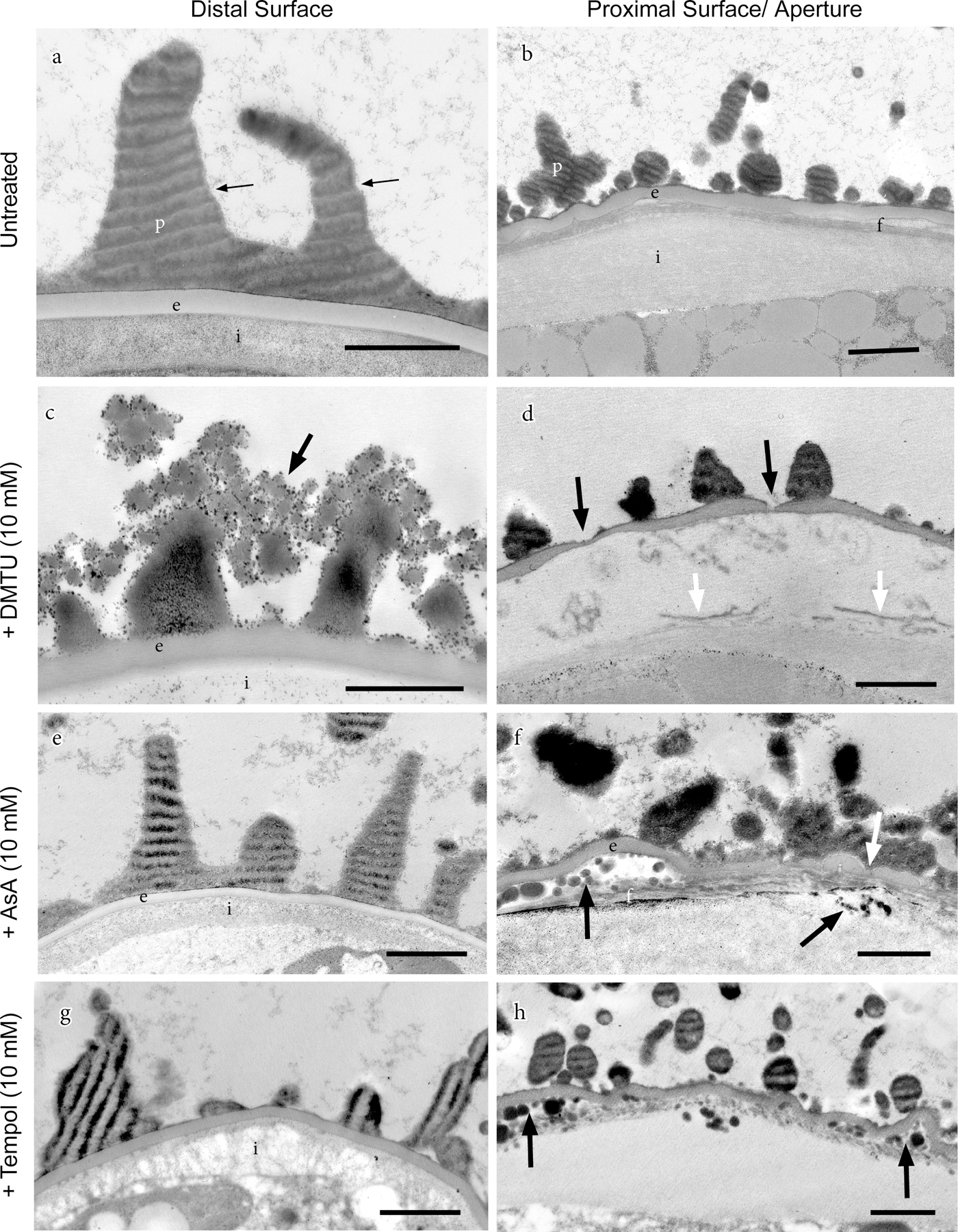
Transmission electron micrographs showing the effects of treatments with ROS scavengers, introduced at 20 days post-irrigation, on distal and proximal spore wall construction. Light-dark stripes in perine are artifacts that result from the resistance created by dense sporopollenin during the sectioning process. (a) Untreated spore with perine (p), including spines, over a homogeneous layer of exine (e) and electron-lucent intine (i). (b) Untreated spore showing proximal aperture with irregular perine spines (p) and wavy exine (e) subtended by fibrillar layer (f) and thickened intine (i). (c) DMTU-treated spore showing defective perine spines terminated by aggregations of sporopollenin globules (arrow). (d) Aperture of DMTU-treated spore has exine with thin to broken areas (black arrows) and disrupted fibrillar layer (white arrows). (e) Perine spines (p), exine (e) and intine (i) of ascorbate (AsA)-treated spores resemble those of untreated spores. (f) Aperture of AsA-treated spore exhibits abnormalities in the exine thickness (white arrow) and accumulation of lipid droplets below the exine and below the fibrillar (f) layer (black arrows). (g) The disrupted intine (i) in Tempol-treated spores has an abnormal fibrillar substructure. (h) Aggregations of lipid droplets (arrows) in Tempol-treated spores resemble those in AsA treatments (f). Scale bars 1 µm.
Alkaline hydrolysis
Spore wall integrity was tested by alkaline hydrolysis, which cleaves ester bonds in wall polymers, including sporopollenin, as well as amide bonds in proteins and glycosidic bonds in polysaccharides. Spore wall pigmentation and perine were very sensitive to alkaline hydrolysis. Control spores and spores exposed to any of the ROS scavengers became colorless and perine-less within 30 min in 2 M KOH at 70 °C. However, all spores exposed to a ROS scavenger at 10 mM while developing, were more susceptible to alkaline hydrolysis than untreated spores. Only untreated spores remained intact after 4 h in KOH (Fig. 6a–d). Spores treated with 10 mM Tempol were the most sensitive, bursting and fragmenting after 2 h and 4 h in KOH, respectively (Fig. 6r, s). Among the ROS scavengers, 10 mM DMTU appeared to have the least effect on sensitivity to alkaline hydrolysis with spores retaining most of their protoplasm after 4 h in KOH (Fig. 6n). After 6 h in KOH, the integrity of spore walls had been breached in all cases and the spores flattened under the weight of the coverslips.
Fig. 6.
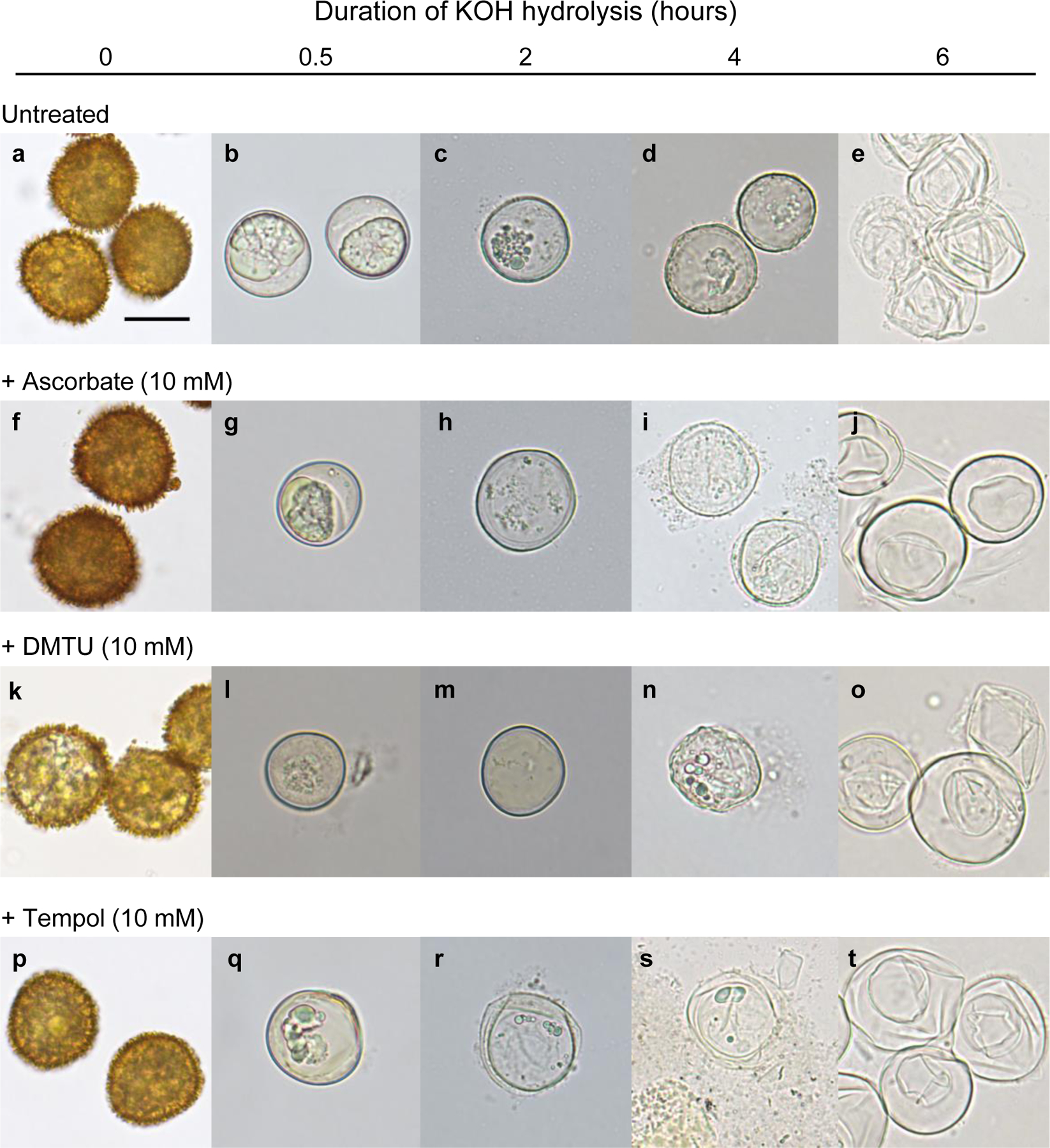
Bright-field images of spores subjected to alkaline hydrolysis with 2 M KOH at 70 °C. The spores had developed in the absence (A) or presence (B–D) of a ROS scavenger at 10 mM during the period from 20 to 35 days post-irrigation (dpi). At regular time intervals from 0 to 6 h, images of the KOH-treated spores were recorded. All spores exposed to a ROS scavenger during their maturation were more susceptible to alkaline hydrolysis than control spores from untreated sporophytes. All images are to the same scale. Scale bar in (a) = 20 µm.
Discussion
ROS are required for the formation of spore and pollen grain walls
Although the enzymes in sporopollenin polymerization and deposition have yet to be identified, it has been reported that a ROS, the superoxide anion radical, is involved in sporopollenin polymerization in the exine of developing tobacco pollen grains (Matveyeva et al. 2012) and that the class III peroxidases, PRX9 and PRX40, may participate in Arabidopsis pollen grain development (Jacobowitz et al. 2019), perhaps indirectly via cross-linking extensins in tapetal cell walls.
Our findings are the first to demonstrate that ROS scavengers compromise the formation and structural integrity of the moss spore coat and imply the involvement of ROS and peroxidases in normal spore wall development, including sporopollenin polymerization and/or deposition and intine/ aperture development. Physcomitrella mature spores, which have been exposed to an ROS scavenger during their development, swell and burst in a scavenger-, dose- and developmental stage-dependent manner when placed in water under a coverslip. Their walls are more susceptible to alkaline hydrolysis than those of untreated spores while some of them are characterized by discontinuities in the exine, anomalies in perine spine structure, abnormal intine and aperture and occasionally, in DMTU-exposed spores, wall shedding. Some variability in morphology/ultrastructure among spores exposed to a scavenger can be attributed to variability in the stage of sporophytic development at the time of treatment (since the development of sporophytes, and thus also of spores within them, is imperfectly synchronized), and to the plane of section of spores.
The three ROS scavengers employed in this investigation elicited different responses from maturing spores. For example, Tempol-treated developing spores are the most sensitive to alkaline hydrolysis, while those exposed to DMTU exhibited abnormal perine spines and on rare occasions wall shedding. Most striking are the aggregates of electron-dense globules, presumed to be sporopollenin, on the tips of perine spines and the abundance of rosettes composed of sporopollenin aggregates that cover the inner spore sac (tapetal cells) in DMTU-treated capsules. This aberrant deposition of sporopollenin on the perine spines implies inhibition of sporopollenin polymerization and/or deposition by the ROS scavenger. These differences probably reflect dissimilarities in the bioavailability, chemical stability and reactivity towards ROS of the scavengers. AsA has a strong affinity for H2O2, while DMTU is a potent hydroxyl radical scavenger that also reacts with H2O2 and the superoxide radical (Curtis et al. 1988; Kelner et al. 1990). Tempol reacts with H2O2 and scavenges the superoxide radical (Soule et al. 2007). Further investigation will be needed to elucidate in detail the biochemical mechanisms responsible for some of the effects of ROS scavengers, especially those of DMTU. For example, DMTU-induced wall shedding (presumably of the perine) implies attachment of perine to the exine is partially compromised. Similar wall shedding has not been reported previously in the literature and little is known about how the exine and perine are chemically connected. Our results suggest that oxidative coupling of components (proteins, carbohydrates, or sporopollenin) of these layers may be involved in a manner analogous to, for example, strengthening of the plant primary cell wall matrix by cross-linking of polymer-esterified hydrocinnamic acids and wall polysaccharides or tyrosine-containing glycoproteins (e.g. extensins). In this case, the cross-linking reactions are mediated by peroxidases and H2O2 (Lindsay and Fry 2007). Further biochemical studies will be required to confirm this supposition.
Defects in the intine of AsA and Tempol-treated spores are not surprising given that the enzymes involved in cross-linking cell wall constituents require ROS (Ros Barceló and Gómez Ros 2009). The intine is the inner spore wall layer that lacks sporopollenin and has a carbohydrate composition similar to that of the primary cell wall, namely cellulose, pectins, hemicelluloses and protein (Owen and Makaroff 1995; Huang et al. 2009). These cell wall polymers are cross-linked to form a three-dimensional and insoluble network, a scaffolding that can withstand internal turgor pressure. The intine is the immediate barrier between the spore protoplasm and sporopollenin layers. Disruption of intine construction would have consequences related to porosity, strength and flexibility of the spore coat as the spore is subjected to dehydration prior to dispersal and imbibition at the time of germination. Less is known about the molecular composition of the spore aperture. With a thickened intine, this region probably contains the major primary cell wall constituents (Renzaglia et al. 2020). As the site of germination, the aperture is modified to take up water and allow for cell wall expansion that ruptures the spore wall and initiates the development of the filamentous protonema. During normal spore maturation, the aperture is the site of wall shrinking that presumably is critical to successful dehydration and the maintenance of cell wall-plasmalemma integrity as the spore protoplasm decreases in volume. Anomalous development of the spore aperture could have an impact on a range of spore functions, including dehydration, water uptake and emergence of the protonema from the spore case. Extracellular ROS have been detected in aperture zones during pollen tube growth, and it is proposed that •OH causes local loosening of the intine in aperture zones (Smirnova et al. 2014). ROS are also implicated in tobacco pollen grain activation that precedes the formation of the pollen tube (Smirnova et al. 2009).
It is reasonable to conclude that the collective effects of ROS scavengers on spore wall formation in Physcomitrella result mainly from inhibition of oxidative polymerization and/or cross-linking of sporopollenin and other cell wall components. Although indirect effects of scavengers on spore wall formation through the synthesis and secretion of sporopollenin monomers appear to be minor as indicated by the normal morphologies of sporopollenin globules in DMTU-treated capsules (Fig. 4h), the possibility of indirect effects from the inhibition of other functions required for normal spore wall formation, for example, the production or secretion of wall precursors, cannot be excluded.
Composition of the exine is different from that of the perine of the Physcomitrella spore
Another notable observation made in this study is that the exine and perine of Physcomitrella spores differ with respect to their electron opacity and alkaline resistance. In TEMs, the perine is more electron-dense than the exine and, unlike the exine, perine spines dissolve in hot alkaline solution. Different reactivity to alkaline hydrolysis implies different contributions of hydrolyzable (ester) and non-hydolyzable (C–C and C–O) linkages. Thus, our findings suggest there are compositional and/or linkage differences between exine and perine sporopollenin and associated wall polymers.
Augmented osmolysis, wall shedding, chemical inhibition: Physcomitrella is a convenient model for studying spore wall formation
Bursting of pollen grains by osmotic shock has been reported in the literature. About 40% of tobacco pollen grains burst when left for 15 min in a hypotonic medium (Smirnova et al. 2014). When inaperturate pine pollen grains are subjected to osmotic down-shock (e.g. transfer from a saturated salt solution to water), their exines rupture and protoplasts are released (Bohne et al. 2005). In a study of the walls of various pollen grains, Matamoro-Vidal et al. (2016) observed exine rupture and cytoplasmic leakage ‘through a tiny breakage of the exine,’ when pollen grains lacking apertures and with thin exines are placed in a hypotonic medium. By contrast, Physcomitrella spores do not normally burst in water, indicating physical robustness of their walls.
We have concluded that the observed swelling in water of mature spores, which were exposed to a ROS scavenger during their development, is osmotically-driven and depends upon their possession of a spore wall whose structural integrity and tensile strength have been compromised, as exemplified by a discontinuous exine (Fig. 5d) and disrupted fibrillar layer (Fig. 5d, f). Subsequent bursting when mounted in water on a flat slide under a coverslip is a consequence of additional pressure from the weight of the coverslip being applied directly to the already swollen spores. We have named this phenomenon “augmented osmolysis”. Our interpretation is reinforced by the finding that spores, previously exposed to a scavenger, exhibit little or no swelling and do not burst when mounted in PBS under a coverslip. Also, when placed in water on a depression slide, spores are not subjected to the additional pressure resulting from direct contact with the coverslip. In this latter case, the spores swell but do not burst.
In conclusion, the structural integrity of the walls of spores in the process of developing and maturing within Physcomitrella sporophytes submerged in aqueous solutions containing ROS scavengers is compromised. The spores with defective walls are susceptible to augmented osmolysis and sometimes display wall (perine) shedding. Spores from sporophytes exposed to DMTU accumulate sporopollenin globules around their perine spines, while those subjected to other treatments show structural defects in the intine and/or aperture. These findings provide support for the proposed oxidative cross-linking during polymerization or deposition of sporopollenin and other wall constituents within maturing spore walls. Augmented osmolysis of Physcomitrella spores with defective walls provides a direct and simple test for studying the effects of chemicals on spore wall formation.
Supplementary Material
Supplementary video. Bursting of mature spores that had developed in the presence of 10 mM DMTU from 20 to 35 days post-irrigation (dpi). At 35 dpi, spores were released from sporophytic capsules, placed in water and mounted for observation on a flat slide with a coverslip. Time elapsed from the beginning of observation is shown on the screen.
Acknowledgements
This work was funded by a Natural Sciences and Engineering Research Council of Canada Discovery grant (RGPIN-2018–04286) to D-YS and by grants from the National Science Foundation (NSF 1758497) and the National Institutes of Health (NIH 107760) to KSR. FR was supported in part by University of Regina Graduate Scholarships. FR was a Saskatchewan Innovation Opportunity Graduate Scholarship recipient. We acknowledge technical assistance from William Browning and Ryan Welch, undergraduates at Southern Illinois University Carbondale.
Contributor Information
Fazle Rabbi, Department of Chemistry and Biochemistry, University of Regina, Regina, SK S4S 0A2, Canada.
Karen S. Renzaglia, Department of Plant Biology, Southern Illinois University, Carbondale, IL 62901, USA
Neil W. Ashton, Department of Chemistry and Biochemistry, University of Regina, Regina, SK S4S 0A2, Canada
Dae-Yeon Suh, Department of Chemistry and Biochemistry, University of Regina, Regina, SK S4S 0A2, Canada.
References
- Akram NA, Shafiq F, and Ashraf M 2017. Ascorbic acid–a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci 8: 613. 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi T, and Toriyama K 2011. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol 62: 437–460. 10.1146/annurev-arplant-042809-112312. [DOI] [PubMed] [Google Scholar]
- Ashton NW, and Cove DJ 1977. The isolation and preliminary characterization of auxotrophic and analogue resistant mutants in the moss Physcomitrella patens. Mol. Genet. Genomics, 154(1): 87–95. [Google Scholar]
- Bernards MA, Fleming WD, Llewellyn DB, Priefer R, Yang X, Sabatino A, and Plourde GL 1999. Biochemical characterization of the suberization-associated anionic peroxidase of potato. Plant Physiol 121: 135–145. 10.1104/pp.121.1.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne G, Woehlecke H, and Ehwald R 2005. Water relations of the pine exine. Ann. Bot 96(2): 201–208. 10.1093/aob/mci169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, and Lemmon BE 1990. Sporogenesis in bryophytes. In Microspores Evolution and Ontogeny. Edited by Blackmore S, and Knox RB. Academic Press, London, UK. pp, 55–94. [Google Scholar]
- Burr SJ, and Fry SC 2009. Feruloylated arabinoxylans are oxidatively cross-linked by extracellular maize peroxidase but not by horseradish peroxidase. Mol. Plant, 2(5): 883–892. 10.1093/mp/ssp044. [DOI] [PubMed] [Google Scholar]
- Chen W, Yu X-H, Zhang K, Shi J, De Oliveira S, Schreiber L, et al. 2011. Male Sterile2 encodes a plastid-localized fatty acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol 157(2): 842–853. 10.1104/pp.111.181693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpitts CC, Kim SS, Posehn SE, Jepson C, Kim SY, Wiedemann G, et al. 2011. PpASCL, a moss ortholog of anther-specific chalcone synthase-like enzymes, is a hydroxyalkylpyrone synthase involved in an evolutionarily conserved sporopollenin biosynthesis pathway. New Phytol 192(4): 855–868. 10.1111/j.1469-8137.2011.03858.x. [DOI] [PubMed] [Google Scholar]
- Courtice GRM, Ashton NW, and Cove DJ 1978. Evidence for the restricted passage of metabolites into the sporophyte of the moss, Physcomitrella patens. J. Bryol 10(2): 191–198. 10.1179/jbr.1978.10.2.191. [DOI] [Google Scholar]
- Curtis WE, Muldrow ME, Parker NB, Barkley R, Linas SL, and Repine JE 1988. N,Nʹ-Dimethylthiourea dioxide formation from N,N’-dimethylthiourea reflects hydrogen peroxide concentrations in simple biological systems. Proc. Natl. Acad. Sci. U. S. A 85(10): 3422–3425. 10.1073/pnas.85.10.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daku RM, Rabbi F, Buttigieg J, Coulson IM, Horne D, Martens G, et al. 2016. PpASCL, the Physcomitrella patens anther-specific chalcone synthase-like enzyme implicated in sporopollenin biosynthesis, is needed for integrity of the moss spore wall and spore viability. PLoS ONE, 11: e0146817. 10.1371/journal.pone.0146817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo Souza C, Kim SS, Koch S, Kienow L, Schneider K, McKim SM, et al. 2009. A novel fatty acyl-CoA synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell, 21(2): 507–525. 10.1105/tpc.108.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobritsa AA, Shrestha J, Morant M, Pinot F, Matsuno M, Swanson R, et al. 2009. CYP704B1 is a long-chain fatty acid ω-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol 151(2): 574–589. 10.1104/pp.109.144469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez E, Mercado JA, Quesada MA, and Heredia A 1999. Pollen sporopollenin: degradation and structural elucidation. Sex. Plant Reprod 12: 171–178. [Google Scholar]
- Grienenberger E, Kim SS, Lallemand B, Geoffroy P, Heintz D, de Azevedo Souza C, et al. 2010. Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell, 22(12): 4067–4083. 10.1105/tpc.110.080036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Ye Y, Zhang Y, Zhang A, Liu T, and Cao J 2009. BcMF9, a novel polygalacturonase gene, is required for both Brassica campestris intine and exine formation. Ann. Bot 104(7): 1339–1351. 10.1093/aob/mcp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobowitz JR, Doyle WC, and Weng J-K 2019. PRX9 and PRX40 are extensin peroxidases essential for maintaining tapetum and microspore cell wall integrity during Arabidopsis anther development. Plant Cell, 31(4): 848–861. 10.1105/tpc.18.00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner MJ, Bagnell R, and Welch KJ 1990. Thioureas react with superoxide radicals to yield a sulfhydryl compound. Explanation for protective effect against paraquat. J. Biol. Chem 265(3): 1306–1311. [PubMed] [Google Scholar]
- Kim SS, Grienenberger E, Lallemand B, Colpitts CC, Kim SY, de Azevedo Souza C, et al. 2010. LAP6/POLYKETIDE SYNTHASE A and LAP5/POLYKETIDE SYNTHASE B encode hydroxyalkyl α-pyrone synthases required for pollen development and sporopollenin biosynthesis in Arabidopsis thaliana. Plant Cell, 22(12): 4045–4066. 10.1105/tpc.110.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CD, Cove DJ, Boyd PJ, and Ashton NW 1988. The isolation of biochemical and developmental mutants in Physcomitrella patens. In Methods in bryology. Edited by Glime JM Hattori Botanical Laboratory. Nichinan, Japan. pp. 47–58. [Google Scholar]
- Li F-S, Phyo P, Jacobowitz J, and Weng J-K 2019. The molecular structure of plant sporopollenin. Nat. Plants, 5(1): 41–46. 10.1038/s41477-018-0330-7. [DOI] [PubMed] [Google Scholar]
- Lindsay SE, and Fry SC 2007. Redox and wall-restructuring. In The expanding cell Edited by Verbelen J-P, and Vissenberg K. Springer, Berlin, Germany. pp. 159–190. [Google Scholar]
- Matamoro-Vidal A, Raquin C, Brisset F, Colas H, Izac B, Albert B, and Gouyon P-H 2016. Links between morphology and function of the pollen wall: an experimental approach. Bot. J. Linn. Soc 180(4): 478–490. 10.1111/boj.12378. [DOI] [Google Scholar]
- Matveyeva NP, Polevova SV, Smirnova AV, and Yermakov IP 2012. Sporopollenin accumulation in Nicotiana tabacum L. microspore wall during its development. Cell Tissue Biol 6(3): 293–301. 10.1134/S1990519X12030078. [DOI] [PubMed] [Google Scholar]
- Mei Y, Chen H, Shen W, Shen W, and Huang L 2017. Hydrogen peroxide is involved in hydrogen sulfide-induced lateral root formation in tomato seedlings. BMC Plant Biol 17: 162. 10.1186/s12870-017-1110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morant M, Jørgensen K, Schaller H, Pinot F, Møller BL, Werck-Reichhart D, and Bak S 2007. CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell, 19(5): 1473–1487. 10.1105/tpc.106.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, et al. 2018. The timescale of early land plant evolution. Proc. Natl. Acad. Sci. U. S. A 115(10): E2274–E2283. 10.1073/pnas.1719588115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen HA, and Makaroff CA 1995. Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae). Protoplasma, 185: 7–21. [Google Scholar]
- Renzaglia KS, Lopez RA, Welsh RD, Owen HA, and Merced A 2020. Callose in sporogenesis: novel composition of the inner spore wall in hornworts. Plant Syst. Evol 306: 16. 10.1007/s00606-020-01631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros Barceló A, and Gómez Ros LV 2009. Reactive oxygen species in plant cell walls. In Reactive oxygen species in plant signaling. Edited by del Río LA, and Puppo A. Springer, Heidelberg, Germany. pp. 73–93. [Google Scholar]
- Quilichini TD, Grienenberger E, and Douglas CJ 2015. The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack. Phytochemistry, 113: 170–182. 10.1016/j.phytochem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Shigeto J, and Tsutsumi Y 2016. Diverse functions and reactions of class III peroxidases. New Phytol 209(4): 1395–1402. 10.1111/nph.13738. [DOI] [PubMed] [Google Scholar]
- Singer SD, and Ashton NW 2007. Revelation of ancestral roles of KNOX genes by a functional analysis of Physcomitrella homologues. Plant Cell Rep 26(12): 2039–2054. 10.1007/s00299-007-0409-5. [DOI] [PubMed] [Google Scholar]
- Singer SD, Krogan NT, and Ashton NW 2007. Clues about the ancestral roles of plant MADSbox genes from a functional analysis of moss homologues. Plant Cell Rep 26(8): 1155–1169. 10.1007/s00299-007-0312-0. [DOI] [PubMed] [Google Scholar]
- Smirnova AV, Matveyeva NP, Polesskaya OG, and Yermakov IP 2009. Generation of reactive oxygen species during pollen grain germination. Russ. J. Dev. Biol 40, 345–353. 10.1134/S1062360409060034. [DOI] [PubMed] [Google Scholar]
- Smirnova AV, Matveyeva NP, and Yermakov IP 2014. Reactive oxygen species are involved in regulation of pollen wall cytomechanics. Plant Biol. 16(1): 252–257. 10.1111/plb.12004. [DOI] [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, and Mitchell JB 2007. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med 42(11): 1632–1650. 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace S, Chater CC, Kamisugi Y, Cuming AC, Wellman CH, Beerling DJ, and Fleming AJ 2015. Conservation of Male Sterility 2 function during spore and pollen wall development supports an evolutionarily early recruitment of a core component in the sporopollenin biosynthetic pathway. New Phytol 205(1): 390–401. 10.1111/nph.13012. [DOI] [PubMed] [Google Scholar]
- Wallace S, Fleming A, Wellman CH, and Beerling DJ 2011. Evolutionary development of the plant and spore wall. AoB Plants, 2011: plr027. 10.1093/aobpla/plr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Guo Z-L, Zhou W-T, Zhang C, Zhang Z-Y, Lou Y, et al. 2018. The regulation of sporopollenin biosynthesis genes for rapid pollen wall formation. Plant Physiol 178(1): 283–294. 10.1104/pp.18.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video. Bursting of mature spores that had developed in the presence of 10 mM DMTU from 20 to 35 days post-irrigation (dpi). At 35 dpi, spores were released from sporophytic capsules, placed in water and mounted for observation on a flat slide with a coverslip. Time elapsed from the beginning of observation is shown on the screen.


