Abstract
The effects of heterogeneity in group composition remain a major hurdle to our understanding of collective behavior across disciplines. In social insects, division of labor (DOL) is an emergent, colony-level trait thought to depend on colony composition. Theoretically, behavioral response threshold models have most commonly been employed to investigate the impact of heterogeneity on DOL. However, empirical studies that systematically test their predictions are lacking because they require control over colony composition and the ability to monitor individual behavior in groups, both of which are challenging. Here, we employ automated behavioral tracking in 120 colonies of the clonal raider ant with unparalleled control over genetic, morphological, and demographic composition. We find that each of these sources of variation in colony composition generates a distinct pattern of behavioral organization, ranging from the amplification to the dampening of inherent behavioral differences in heterogeneous colonies. Furthermore, larvae modulate interactions between adults, exacerbating the apparent complexity. Models based on threshold variation alone only partially recapitulate these empirical patterns. However, by incorporating the potential for variability in task efficiency among adults and task demand among larvae, we account for all the observed phenomena. Our findings highlight the significance of previously overlooked parameters pertaining to both larvae and workers, allow the formulation of theoretical predictions for increasing colony complexity, and suggest new avenues of empirical study.
This study uses automated tracking of clonal raider ants and mathematical modeling to reveal how previously overlooked traits of larvae and workers might shape social organization in heterogeneous ant colonies. By incorporating the potential for variability in task efficiency among adults and task demand among larvae, the authors were able to account for all empirically observed phenomena.
Introduction
The study of collective behavior and self-organization is an active area of research across fields, from animal movement [1] to robotics [2], from tissue engineering [3] to public health [4], and from voting [5] to conservation [6]. Despite considerable theoretical and empirical advances, however, our understanding remains limited by a poor grasp on the impacts of heterogeneity in group composition on collective organization. This limitation stems from the difficulty in precisely controlling the sources of heterogeneity and rigorously and comprehensively measuring their impacts experimentally. This empirical challenge, in turn, has hindered the systematic testing and refining of the conceptual and theoretical frameworks employed to investigate the mechanisms underlying the collective dynamics.
The colonies of social insects are striking examples of highly integrated, complex biological systems that can self-regulate without centralized control [7]. Consequently, social insects have emerged as powerful systems to study collective behavior and social dynamics, both experimentally and theoretically [8–12]. An emergent, colony-level trait that has long been thought to depend on colony composition (e.g., in age, genotype, or morphology) is division of labor (DOL), the nonrandom interindividual variation in task performance among members of a social group that is consistent over time [13,14]. However, few experimental studies have comprehensively measured this dependence because the inherent complexity of social insect colonies usually renders their composition intractable: A typical social insect colony consists of 1 or more queens, dozens to thousands of workers of different (and often unknown) age, genotype, and morphology, and various brood development stages. This difficulty in controlling and replicating colony composition has hampered attempts to systematically test and refine the theoretical framework for collective organization in insect societies. Consequently, we have a limited understanding of how colony composition affects individual behavior and the emergent DOL, which, in turn, limits our understanding of the evolution of collective organization [15].
While several proximate mechanisms have been proposed to explain DOL in social insects (see [14] for a review), the “vast majority of studies on the impact of variability on colony behaviour have so far focused on the distribution of individual response thresholds and how this distribution affects the collective response behaviour” (see [16], p. 679). In this framework, colony members are assumed to differ in their response thresholds, i.e., in their propensity to respond to task-specific stimuli indicating the group-level demand for a given task [17–23]. Individuals with lower thresholds perform the corresponding task more readily than individuals with higher thresholds. Stimulus intensity, in turn, decreases with the number, efficiency, and time investment of individuals performing the task. With this negative feedback loop, response thresholds offer a simple mechanism for both robust and flexible allocation of individuals to tasks [14]. While refinements of response threshold models have included a self-reinforcement mechanism, whereby thresholds are modulated through experience such that individuals become more likely to perform a task that they have already performed [14,24], DOL can emerge in the absence of threshold reinforcement so long as individuals differ in their response thresholds. Indeed, the simplest version of the model, which only assumes intrinsic (i.e., fixed) variation in individual thresholds, has been successful in recapitulating certain empirically observed patterns of DOL [11,25–31].
Empirically, worker behavior in social insect colonies often correlates with individual traits [16]. For example, within a colony, workers of different age [32–35], experience [36], genotype [37] (e.g., patrilines [38,39] or matrilines [40]), or morphology (e.g., size [38,41–44]) can vary in their propensity to engage in tasks such as foraging, nursing, or nest construction. Such behavioral variation is often attributed to the developmental or genetic modulation of response thresholds. However, empirical evidence suggests that response thresholds are only one of several axes of possible individual variation. For example, workers can also vary in the efficiency with which they perform tasks [45–47] or in the average time spent performing a given task [48]. These empirical findings suggest that previously underexplored parameters may vary depending on developmental or genetic factors and may play a role in colony organization. This possibility has led to recent calls for a diversity of parameters to be considered when investigating the relationship between colony composition and DOL [16,49,50].
Here, we combine theoretical modeling with behavioral tracking experiments in the clonal raider ant, Ooceraea biroi, to both assess the explanatory power of existing behavioral response threshold models and explore other axes of individual variation. The unique biology of this species affords unparalleled control over the main axes of colony composition that are thought to affect individual- and group-level behavior in social insects: genotype, age, and morphology. Specifically, colonies of clonal raider ants are naturally queenless and exclusively composed of workers that all reproduce asexually and synchronously, so that all adults within a colony are genetically almost identical and emerge in discrete age cohorts. Furthermore, individuals show variation in ovariole number that is associated with body size and other morphological features [51], making it possible to approximately sort individuals into “regular workers” (2 to 3 ovarioles) and “intercastes” (4 to 6 ovarioles) based on their size [51]. Intercastes typically represent a small fraction (3.7% to 6.3% [52]) of individuals in unmanipulated colonies, but colonies with higher fractions of intercastes (50% or more) do occur occasionally and are functional [51]. Conveniently, workers of different clonal genotypes, age cohorts, and morphologies can be mixed to create functional chimeric experimental colonies [51]. Additionally, colony behavior is controlled by larvae [27,53,54], which solicit food and care from the workers and induce them to forage. This means that colony-level task demand can be standardized or manipulated across colonies by controlling larvae number or, potentially, genotype. Finally, while colonies collected in the field contain between approximately a dozen and several hundred workers [55–57], smaller colonies of approximately 10 workers have high fitness and show complex collective behavior (e.g., group raiding [58], stable DOL, and phasic reproduction [27]) in the laboratory. Taking advantage of these features, we quantify individual and collective behavior of O. biroi in response to precise, independent manipulations of colony genetic, morphological, and demographic composition, as is uniquely possible in this system.
Results and discussion
Theoretical model
We adopt the simplest and most commonly employed formulation of the response threshold model, which assumes that individual thresholds do not change over time [18]. We consider a colony of n individuals, NX of which are of type X and NY = n - Nx are of type Y. Types X and Y represent any pair of the experimentally manipulated subcolony compositions (i.e., genotypes A and B, Young and Old, or Regular Workers and Intercastes). The colony must perform m tasks; for consistency with the experimental approach (see below), we assume that there are 2 tasks (m = 2). At a given time step, an individual can be either performing one of the m tasks (active) or not performing any (inactive). The task state of individual i at time t is given by the binary variable xij,t: If individual i is active and performing task j at time t, then xij,t = 1 and xij’,t = 0 for all j’ ≠ j; if individual i is inactive and resting, then xij,t = 0 for all j.
Each task j has an associated stimulus sj,t, signaling the group-level demand for that task. The stimulus for a task changes depending on the rate at which the demand increases (e.g., the demand for foraging increases due to increased hunger in the colony), the efficiency with which workers perform the task (e.g., more efficient foragers decrease hunger faster), and the number of individuals performing the task. Mathematically, the stimulus sj,t is governed by Eq (1):
| (1) |
where δj is the task-specific demand rate, taken to be constant over time; αjX (respectively, αjY) is the task-specific performance efficiency (i.e., the rate with which an individual decreases stimulus intensity by performing the corresponding task) of type X (respectively, type Y) individuals; and and are the numbers of type X and Y individuals performing task j at time t, respectively. We assume that individuals i = 1, …, NX are of type X and individuals i = NX + 1, …, n are of type Y.
Each individual i is assumed to have an internal threshold for each task j, θij, drawn at time t = 0 from a normal distribution with mean μj and normalized standard deviation σj (i.e., expressed as a fraction of the corresponding mean μj). Thus, an individual can, and typically does, have different thresholds for different tasks. Although thresholds may change over the individuals’ lifetime [59], they are assumed to be fixed over the timescale of the experiments and, consequently, over the simulation runs. We refer to μj as the mean task threshold (or mean threshold) and to σj as the threshold variance for task j; each can be type and/or task specific (i.e., μjX, μjY, σjX, σjY).
At each time step, inactive individuals assess the m task stimuli in a random sequence until they either begin performing a task or have encountered all stimuli without landing on a task. For each encountered stimulus, individual i evaluates whether to perform the task by comparing the stimulus level to its internal threshold. Specifically, given stimulus sj,t and internal threshold θij, individual i commits to performing task j with probability
| (2) |
where parameter η governs the steepness of this response threshold function. The larger the value of η, the more deterministic the behavior; in the limit η → ∞, the response function becomes a step function. Active individuals spontaneously quit their task with a constant quit probability τ. Active individuals can neither evaluate stimuli nor switch tasks without first quitting their current task.
Each agent-based simulation began with both stimuli set to 0 (i.e., sj,t = 0 for j = 1, 2) and lasted T = 10,000 time steps (see S1 Table for parameter settings).
Baseline model predictions
To establish baseline predictions for ant colonies with different compositions, we use the simplest implementation of this model, which assumes that ant types differ only in mean response threshold [18]. We simulated colonies that were either homogeneous (pure), with a single type of ant, or heterogeneous (mixed), with two types in equal proportions. The individual thresholds for each type were drawn from a normal distribution with the type-specific mean (μjX = μX or μjY = μY). All other model parameters—task performance efficiency, demand rate, and threshold variance—were constant across types. Thus, the only source of heterogeneity in pure colonies was the distribution of individual response thresholds, while in mixed colonies that heterogeneity was compounded by differences in the means of the type-specific distributions.
To quantify individual behavior, we computed each individual’s task performance frequency for each task, defined as the fraction of time that an individual spent performing a given task. For example, if an ant spent 2,000 time steps performing task 1 (e.g., foraging), 4,000 performing task 2 (e.g., nursing), and the remaining 4,000 being inactive in a simulation of 10,000 time steps, then it had a task 1 performance frequency of 0.2 and a task 2 performance frequency of 0.4. To quantify the mean behavior of ants in a given colony for a given task, we then averaged the individual task performance frequencies for that task across all individuals in that colony. In a mixed colony, we also quantified the type-specific mean behavior for a given task by taking the average across all individuals of a given type in the colony instead. To quantify DOL, we measured two colony-level properties: behavioral variation, defined as the standard deviation of task performance frequency across all individuals in a colony; and specialization, defined as the mean correlation in individual task performance frequencies across time, measured as the Spearman rank correlation on consecutive windows of 200 time steps. Thus, specialization measures how consistent ants in a colony are in their task performance relative to each other.
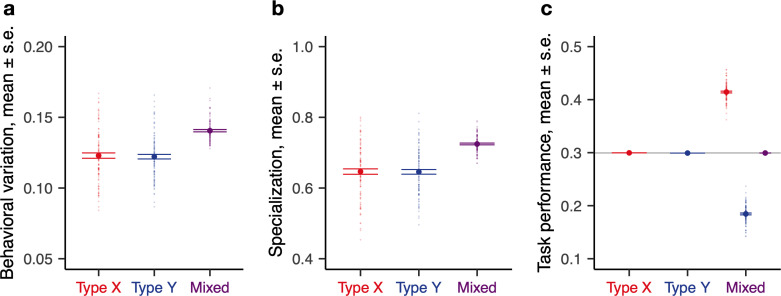
In pure colonies, there is a single normal distribution of individual thresholds for a given task. In contrast, mixed colonies have a bimodal distribution of thresholds for each task, with the thresholds of the two types clustered around the different modes. This wider distribution of thresholds resulted in both greater behavioral variation (because individuals from the lower end of the distribution for a task are more sensitive to the stimulus for that task, they tended to perform that task more often than those from the higher end) and greater colony-level specialization (those performing a task in a given time step are likely to be from the lower end of the distribution and therefore also likely to be performing that task in a future time step) relative to pure colonies, resulting in more pronounced DOL (Fig 1A and 1B). However, all colonies, irrespective of their composition, had the same mean behavior (Fig 1C). This is because while colonies may differ in how they allocate workers to tasks—within mixed colonies, the two ant types differed in their mean task performance because the type with the lower average threshold for a given task took up that task more often than the other type—they must perform the same amount of work overall to satisfy a given demand. Thus, on average, colony members spent the same fraction of time performing each task across pure and mixed colonies.
Fig 1. Baseline theoretical predictions.
Division of labor (DOL, measured by colony-level behavioral variation (a), colony-level specialization (b)) and task performance frequency for a single task (c) shown as a function of colony composition. Opaque circles represent individual replicate colonies (n = 100 replicates for each composition); solid circles represent the average value across replicates; horizontal lines represent s.e.; and the horizontal gray line in (c) represents the average of the pure colonies (first 2 columns). Types X and Y differ in mean threshold: μX = 10, μY = 20; all other parameters are identical across types (see S1 Table). Simulation code and data are available at https://github.com/marikawakatsu/mixing-model. DOL, division of labor.
In summary, the simple model predicted that (P1) mixed colonies would exhibit higher overall DOL but that (P2) all colonies would have the same mean behavior (Fig 1C), although (P3) the two types would diverge behaviorally in mixed colonies (Fig 1C). The same predictions held if, instead of differences in the means of the response thresholds, we assumed differences in the variances (S1 Fig).
Empirical tests of the theoretical predictions
We then tested these theoretical predictions in experimental colonies that were either pure or 1:1 mixes of clonal raider ants that differed in one of 3 factors thought to influence DOL: genotype (A versus B [27,51]), age (around 3-month-old “old” ants versus 1-month-old “young” ants; the life span of workers in this species is around 1 year), and size (large intercastes versus smaller regular workers [51]) (S2 Table). Colonies contained larvae of the same genotype as the workers; in the case of genotype effects, the experiment was performed twice, once with larvae of each genotype (see Materials and methods). We analyzed individual behavior in 120 experimental colonies using automated tracking [27].
Because work in insect societies is spatially organized (e.g., foraging and waste disposal occur away from the nest, whereas nursing only occurs at the nest), individual spatial distribution can be used as a proxy for individual behavioral roles [60–63]. Here, the spatial distribution of each ant was measured as the two-dimensional root–mean–square deviation (r.m.s.d.) of its spatial coordinates:
where xi and yi are the coordinates of the focal ant in frame i, and are the coordinates of the center of mass of the focal ant’s overall spatial distribution, and F is the number of frames in which the focal ant was detected. As previously shown [27], the r.m.s.d. of an ant captures its tendency to leave the nest: workers that spend a lot of time at the nest with the brood (e.g., nursing the larvae) and little time performing extranidal tasks (e.g., foraging or waste disposal) have low r.m.s.d. values, whereas workers that spend more time away from the brood have higher r.m.s.d. values (Fig 2A). Consequently, the mean r.m.s.d. of a colony reflects its collective foraging activity, as shown by the fact that r.m.s.d. increases in response to experimentally inflated nutritional demand [27]. We therefore use the r.m.s.d. as a proxy for the propensity to perform tasks away from the nest (e.g., foraging) rather than at the nest (e.g., nursing) [27]. Analogously to the simulations, we quantified the mean behavior of a given ant type as the average r.m.s.d. of all ants of that type in a colony; similarly, to quantify colony-level DOL, we computed behavioral variation as the standard deviation across r.m.s.d. values of all ants in a colony and specialization as the mean correlation in individual r.m.s.d. across time, measured as the Spearman rank correlation on consecutive days in the experiment [27] (see Materials and methods).
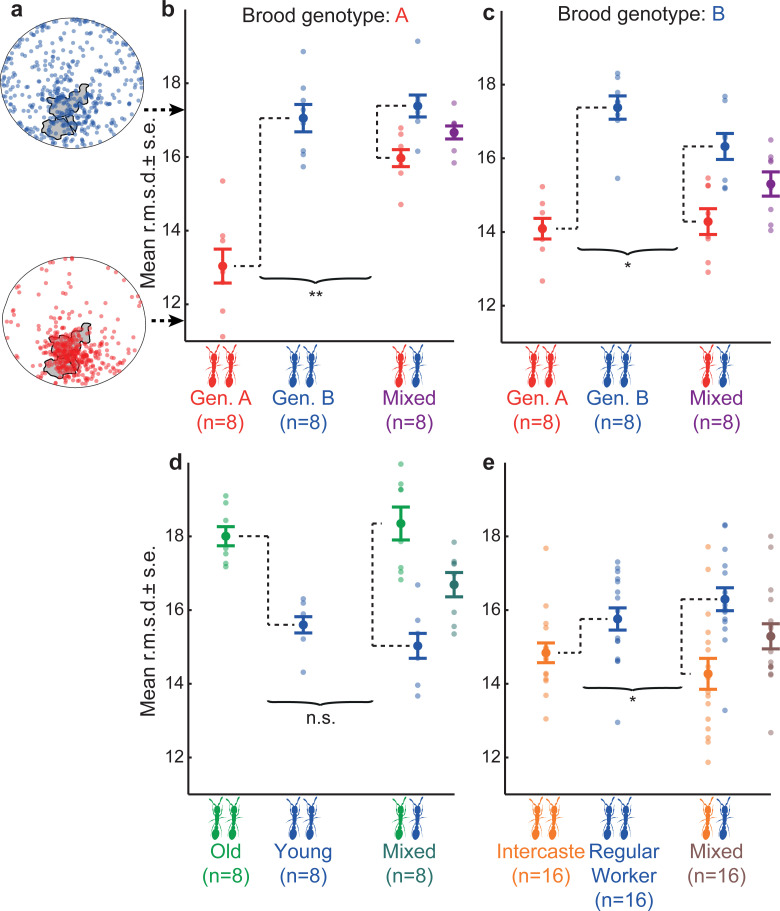
Fig 2. Behavior as a function of colony composition.
(a) Spatial distributions of 2 ants with high (blue; genotype B) and low (red; genotype A) activity outside the nest. Arrows point to corresponding r.m.s.d. values. Gray areas represent the position of the larvae. (b–e) Mean behavior (mean r.m.s.d.) as a function of colony composition. Opaque circles represent mean behavior across individuals in replicate colonies or subcolonies. Solid circles represent average behavior across replicate colonies or subcolonies. For mixed colonies, data are shown both as type-specific and colony-level mean behavior (in “average” color). Sample sizes indicate the number of replicate colonies. Black curly brackets represent the effect of mixing on behavioral differences between types. (b) Behavioral convergence in genetically mixed colonies with A brood. Bpure − Apure vs. Bmixed − Amixed: t test: t = 3.86, p = 0.002. (c) Behavioral convergence in genetically mixed colonies with B brood. Bpure − Apure vs. Bmixed − Amixed: t = 2.63, p = 0.025. (d) No effect of mixing in demographically mixed colonies. Oldpure − Youngpure vs. Oldmixed − Youngmixed, t = −1.50, p = 0.157. (e) Behavioral divergence in morphologically mixed colonies. Regular Workerpure − Intercastepure vs. Regular Workersmixed − Intercastemixed t = −2.44, p = 0.022. n.s., nonsignificant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Raw data are available at doi.org/10.5061/dryad.hx3ffbgdd. r.m.s.d., root–mean–square deviation.
Colonies with different compositions often differed in mean behavior (Fig 2B–2D), inconsistent with prediction (P2). For instance, pure colonies of genotype A on average spent more time at the nest than pure colonies of genotype B (Fig 2B: Bpure versus Apure, LME post hoc tests: z = 7.75, p = 3.64*10−14; Fig 2C: Bpure versus Apure: z = 7.45, p = 2.80*10−13). Similarly, colonies of young workers spent more time at the nest than colonies of old workers (Fig 2D: Oldpure versus Youngpure: z = −6.05, p = 4.39*10−09). That ants of different genotype [38–40] and age [33,34,64] differ in their task performance is consistent with observations in other social insects. However, such behavioral differences are theoretically only predicted to emerge (and have empirically mostly been documented) within mixed colonies (as also observed here: Fig 2B: Bmixed versus Amixed: z = 4.61, p = 8.06*10−06, Fig 2C: Bmixed versus Amixed: z = 7.68, p = 6.57*10−14, Fig 2D: Oldmixed versus Youngmixed: z = −13.31, p < 2*10−16), and not across pure colonies (Fig 1C). Moreover, while the simple model predicted behavioral divergence between types in mixed colonies relative to pure colonies (P3), experiments produced all possible outcomes. Most surprisingly, mixing different genotypes resulted in behavioral convergence (see definitions in Materials and methods), whereby genotypes behaved more similarly in mixed colonies than in separation (i.e., across pure colonies) (Fig 2B and 2C). In contrast, mixing different age cohorts had no detectable effect on mean behavior (henceforth no effect) (Fig 2D). Only mixing regular workers and intercastes produced behavioral divergence as predicted by the simple model: Intercastes spent more time at the nest than regular workers in mixed colonies (Fig 2E: Regular Workermixed versus Intercastemixed: z = 8.95, p < 2*10−16) but not in pure ones (Fig 2E: Regular Workerpure versus Intercastepure: z = 2.14, p = 0.098). Because intercastes have more ovarioles than regular workers, this behavioral difference is consistent with observations that reproductive potential often negatively correlates with the propensity to forage [52,65,66].
While in most cases mixing had symmetric effects on behavior—i.e., the behavior of both types was equally affected (Fig 2D: |Youngmixed − Youngpure| versus |Oldmixed − Oldpure|: t = 0.94, p = 0.365; Fig 2E: |Regular Workermixed − Regular Workerpure| versus |Intercastemixed − Intercastepure|: t = −0.68, p = 0.501; see Materials and methods)—we found that asymmetric effects are also possible: In genetically mixed colonies with A larvae, mixing affected the behavior of A workers more than that of B workers, manifesting in asymmetric behavioral convergence (Fig 2B: |Amixed − Apure| versus |Bmixed − Bpure| t test: t = −3.86, p = 0.002). Such an asymmetry was not apparent in the presence of B larvae, however (Fig 2C: |Amixed − Apure| versus |Bmixed − Bpure|: t = 0.53, p = 0.607).
Consistent with these behavioral patterns, mixed colonies had overall higher DOL than pure colonies in the age and morphology experiments, in line with the baseline model prediction (P1) (S2 and S3 Figs). However, this trend was weakened (i.e., half of the pairwise comparisons were not significant) in the genotype experiments by the emergent behavioral convergence (S2 and S3 Figs), so that mixed colonies did not systematically have higher DOL than pure colonies in all experiments, violating (P1).
Taken together, our experimental results revealed a greater diversity of behavioral patterns than predicted by the simple model: Colonies differed in mean behavior, thus violating (P2) (Fig 2); the direction and magnitude of behavioral changes in mixed colonies depended on the specific source of workforce heterogeneity, thus violating (P3) (Fig 2); and consequently, DOL was not necessarily higher in mixed than in pure colonies, thus violating (P1) (S2 and S3 Figs). Thus, heterogeneity in response thresholds alone was insufficient to explain our observations. This discrepancy prompted us to consider other biologically realistic sources of heterogeneity in the model.
An expanded model of DOL
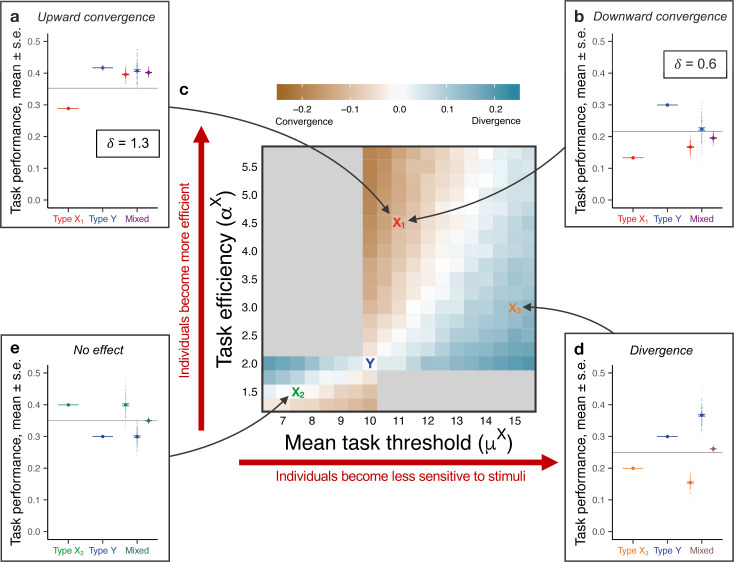
Previous work revealed that the developmental trajectory of O. biroi larvae—i.e., the size of the resulting adults—depends on nonlinear interactions between the larval genotype and the genotype of the caregiving adults [51]. This finding suggests (a) that larvae of different genotypes signal different levels of demand, e.g., for food or care; and (b) that workers of different genotypes differ in their response to a given level of larval demand, possibly via differences in their response thresholds or in the efficiency with which they perform the corresponding task. Indeed, when we added differences in task performance efficiency and in larval-induced task demand to the simple model (with between-type differences in response thresholds, i.e., consistent variation in threshold across types), we were able to qualitatively recapitulate the phenomena observed in genotype-mixing experiments (Fig 3A and 3B). Differences in task performance efficiency were, in fact, sufficient to robustly produce both colonies with different mean behaviors and behavioral convergence in mixed colonies, where the more efficient ants compensated for the less efficient ones by spending more time performing the task than they did in pure colonies. By affecting how much more the efficient ants needed to work in the mixed colonies, the differences in larval-induced task demand determined the asymmetry of the convergence. In particular, when task demand was so high that the less efficient type could not keep up with the demand on their own, we recovered the experimental pattern in Fig 2B (see Fig 3A; see also stimulus dynamics in S4 Fig and S1 Text). While the simulations assumed, for simplicity, that the two tasks had the same level of demand, the analytical calculations suggest that varying demand across tasks would produce patterns qualitatively identical to either Fig 3A or Fig 3B, depending on the demand levels (see S1 Text).
Fig 3. Theoretical predictions of the expanded model.
(a, b, d, and e) Task performance frequency for a single task as a function of colony composition. Opaque circles represent replicate colonies (n = 100 replicates per composition); solid circles represent the average across replicates; horizontal bars represent s.e.; and horizontal gray lines represent the average of the pure colonies (first two columns). Identical colors indicate ants of the same type; in particular, type Y ants are the same across all panels (αY = 2, μY = 10). (a and b) Differences in both task efficiency and mean threshold (αX₁ = 4.5, μX ₁ = 11) capture asymmetric behavioral convergence, with directionality determined by the demand rate: (a) upward (δ = 1.3) and (b) downward (δ = 0.6). (d and e) Differences in both task efficiency and mean threshold capture both (d) behavioral divergence (αX₃ = 3, μX₃ = 15) and (e) a lack of effects from mixing (αX₂ = 1.5, μX₂ = 7.5). (c) Change in relative task performance between mixed and pure colonies (measured as (Ym-Xm)-(Yp-Xp)) as a function of type X’s efficiency and mean threshold (n = 50 replicates per parameter combination). Types X1, X2, X3, and Y correspond to those in a, b, d, and e. Blue gradient indicates behavioral divergence (Ym-Xm>Yp-Xp); brown gradient indicates convergence (Ym-Xm<Yp-Xp); and light gray indicates regions with behavioral patterns falling outside our definitions (see Materials and methods). See S1 Table for other parameter values. Simulation code and data are available at https://github.com/marikawakatsu/mixing-model.
Exploring the efficiency–threshold parameter space broadly recapitulated not only the behavioral convergence observed in the genotype experiments, but also the divergence and no effect patterns observed in the morphology and age experiments, respectively (Fig 3C). The emergent pattern in mixed colonies depended on the interplay between differences in efficiency, which increased behavioral similarity, and differences in threshold, which decreased similarity. Manipulating genotypic composition corresponded to regions of the parameter space with relatively strong effects of differences in efficiency and relatively weak effects of differences in threshold (Figs 2A and 2B and 3A and 3B). Manipulating morphological composition corresponded to regions where differences in threshold had a relatively stronger effect (Figs 2E and 3D). Finally, manipulating age composition corresponded to an intermediate scenario in which the 2 effects balanced each other out (Figs 2D and 3E). Consistent with the experiments, DOL was higher in mixed colonies than in pure colonies when threshold effects were at least as strong as efficiency effects—i.e., in areas of behavioral divergence or no effect—but not when threshold effects were weaker—i.e., in areas of behavioral convergence (S5 and S6 Figs).
Conclusions
In most social insect colonies, all factors studied here (worker genotype, age, morphology, and larval genotype) influence behavior simultaneously and in largely intractable ways. However, the unique biology of O. biroi allows us to break this complexity down experimentally and study each effect independently, thereby providing insight into the basic organizing principles of behavior in social groups. Our finding that the magnitude and direction of effects on DOL depend on the specific factor being manipulated underscores the importance of considering and controlling the various sources of heterogeneity that naturally act in social groups in order to study the different (and possibly opposing) effects that they have on collective organization. Moreover, our work also underscores the importance of considering factors beyond the usual suspects (e.g., age and morphology): While larval cues [67] are known to affect worker physiology [68,69] and behavior [54,70], our results highlight larvae as important players in the actual regulation of DOL between workers, something that has rarely been considered. And, on longer timescales, our findings suggest the need to consider a broader array of factors when investigating the evolution of DOL [15].
The integrated empirical and theoretical analysis reveals that models based on threshold variation alone fail to recapitulate the diverse outcomes observed in heterogeneous colonies. However, consistent with recent calls to expand theoretical investigations to other sources of heterogeneity [16,49,50], incorporating differences in larval-induced demand and in worker task performance efficiency, two parameters that, like response thresholds [13,25,71,72], are known to vary in nature [45–47,73,74], allowed us to recapitulate all empirically observed behavioral patterns. Importantly, the expanded threshold model could recapitulate these patterns using only simple individual behavioral rules and without invoking social interactions. For example, behavioral convergence—a phenomenon that intuitively appears to rely on direct social interactions—could emerge without invoking complex social processes, such as social learning [75,76] or direct information transfer between group members [77,78]. Although the theoretical treatment can only suggest candidate mechanisms, it is reassuring that the observed behaviors are robust and generic, i.e., the parameter values chosen to illustrate the versatility of the model are representative of large regions of parameter space. Nevertheless, rigorous empirical quantifications of thresholds, efficiency, and demand for realistic task–stimulus pairs—which have only rarely been attempted [48,71,79] and remain very challenging—are a critical next step toward bridging the gap between theory and empirical observations.
While we focused on the simplest model that could recapitulate our empirical results, we recognize that DOL can be influenced by an even broader set of parameters, whose roles deserve further empirical and theoretical work. For example, experience and social interactions [28,80–82] might dynamically change individual thresholds [36] and/or task efficiency [34,83] over time, potentially modulating the effects observed here. It will be important to consider such effects in future theoretical extensions. At the same time, this simple model can nevertheless be used to make rich testable predictions for colonies with increasingly complex composition. A first attempt using different ratios of ant types led to a striking range of patterns even among the four parameter combinations in Fig 3A–3D: The model predicts that behavior can change linearly or nonlinearly as a function of colony composition depending on the between-type differences in mean threshold (S7 Fig, S1 Text). In other words, despite one type of ant being more efficient than the other in all cases considered, replacing an individual of the former with one of the latter led to proportional, greater-than-proportional, or less-than-proportional changes in task performance. Testing these predictions empirically will accelerate the productive crosstalk between theory and experiments.
Our findings add to the growing literature on the role of individual heterogeneity in the collective behavior of complex biological (e.g., schools of fish, neurons in a brain, pathogen strains sharing a host, etc.) and artificial (e.g., robot swarms, synthetic microbial communities, etc.) systems. Much like colonies of the clonal raider ant, these systems exhibit patterns that can be interpreted as behavioral convergence [76,78,84–86], divergence [87], and nonlinear effects of mixing on group-level phenotypes [88–90]. In turn, these patterns affect important processes such as collective decision-making [5], the transmission and evolution of disease [91,92], and the evolution of cooperative behavior [93,94]. While different variants of threshold-based models have been employed to study several of these systems [95–98], we still lack a unified theoretical framework to understand the consequences of individual differences on collective dynamics [99]. Thus, a comparative approach to the study of the basic organizing principles of heterogeneous systems across scales constitutes an important next step toward understanding the behavior of complex biological systems.
Materials and methods
Experimental design
Four experiments were performed to investigate the effect of genetic composition (2 experiments differing in the brood genotype used), age composition (1 experiment), and morphological composition (1 experiment). Each experiment comprised three treatments (2 with pure colonies, 1 with mixed colonies; S2 Table). All colonies within one experiment were monitored in parallel, but the different experiments were performed separately.
Experimental colonies were composed of workers of controlled age, genotype, and morphology (S2 Table), as well as larvae of controlled genotype and age. Colonies were housed in airtight Petri dishes 5 cm in diameter (corresponding to about 25 ant body lengths) with a plaster of Paris floor, in which the workers formed a nest by freely choosing a location where they piled their larvae. To control individual genotype, clonally related workers were sourced from the same stock colony. We used two commonly used genotypes, A and B [27,51,100,101]. To control individual age, workers were sourced from a single age cohort from the same stock colony. Owing to the synchronized reproduction of O. biroi, all age-matched workers collected this way had eclosed within a day of each other [57]. Young ants were 1 cycle old (approximately 1 month old), and old ants were 3 cycles old (approximately 3 months old). The estimated life span of workers of this species under laboratory conditions is approximately 1 year. To control individual morphology, age-matched regular workers and intercastes from the same stock colony were screened based on body size (small or large) and the absence or presence of vestigial eyes, respectively. From the time they were collected (1 to 3 days after eclosion) until the start of experiments, workers of a given type were kept as a group. All workers were tagged with color marks on the thorax and gaster using oil paint markers. Experimental colonies contained 16 (genetic composition and age composition experiments) or 8 (morphological composition experiment) workers and a matching number of age-matched larvae (4 to 5 days old). This 1:1 larvae-to-workers ratio corresponds to the estimated ratio found in a typical laboratory stock colony. We used 8 (genetic composition and age composition experiments) or 16 (morphological composition experiment) replicate colonies for each group composition, for a total of 120 colonies.
Colony number and size varied across experiments due to constraints on the number of available slots in the tracking system at the time each experiment was performed. However, all colony sizes employed here were previously shown to have high fitness and stable DOL [27], and all experiments were analyzed separately so that variation in colony size should not impact the results.
The experiments took place in a climate room at 25°C and 75% relative humidity under constant light (O. biroi is blind, and its behavior is not affected by light). Every three days, we cleaned and watered the plaster and added one prey item (live pupae of fire ant minor workers) per live O. biroi larva at a random location within the Petri dish.
Behavioral data acquisition and analysis
Image acquisition and analysis were performed as in Ulrich and colleagues [27]. We used an automated scan sampling approach, in which a picture of each colony was acquired every approximately 400 seconds throughout the experiment by a custom setup comprising 28 webcams (B910 or C910; Logitech, Lausanne, Switzerland) and controlled LED lighting. Each webcam acquired images (5 megapixels, RGB) of 4 colonies, and the position of colonies within the setup was randomized. Custom software (available at https://doi.org/10.5281/zenodo.1211644) was used to detect individual ants in images. For all behavioral analyses, ants were excluded from the dataset if they were detected in less than 30% of the frames acquired within the considered time frame (brood care phase or day); for ants that died during the brood care phase, the considered time frame was the portion of the brood care phase preceding death.
O. biroi colonies switch between reproductive phases (of approximately 18 days), in which all workers stay in the nest and lay eggs, and brood care phases (of approximately 16 days), in which workers nurse the larvae in the nest but also leave the nest to scout, forage, or dispose of waste. For each colony, behavioral analyses were restricted to the brood care phase, which started at the beginning of the experiment and ended when all larvae had either reached the nonfeeding prepupal stage or died.
For each colony or subcolony, mean behavior was computed as the average of individual r.m.s.d. values, and behavioral variation was computed as the standard deviation of individual r.m.s.d. values. Both metrics were then compared across treatments.
To quantify specialization, we use a metric appropriate for use on continuous behavioral data (here, r.m.s.d.). For each colony, specialization was defined as the Spearman correlation coefficient between individual r.m.s.d. ranks on consecutive days of the brood care phase, averaged over days. Mean rank-correlation coefficients were then compared across treatments.
Statistical analyses
Statistical analyses were performed in R [102] separately for each of the four experiments. As the experiments were performed at different times using different cohorts of ants, we cannot rule out “batch” effects and therefore avoid any statistical analyses comparing treatments across experiments.
Effects of individual traits on behavior
To evaluate whether type-specific behavior depended on colony composition, we tested for a statistical interaction between the effects of individual attributes (genotypes A versus B, Young versus Old, or Regular worker versus Intercaste) and of colony composition (pure versus mixed) on individual behavior (individual r.m.s.d.) using linear mixed effects (LMEs, function lmer of package lme4 [103]) models with colony as a random factor. If a significant interaction between colony composition and individual attributes was detected, we then used a second LME model with a four-level independent fixed variable combining colony composition and individual attributes (Xp, Yp, Xm, and Ym, where Xk and Yk are the mean behavior of ant types X and Y, respectively, in pure (k = p) or mixed colonies (k = m)), followed by a Tukey post hoc test with Bonferroni–Holm correction (function glht of package multcomp [104]) for the following planned pairwise comparisons: Xp versus Xm, Yp versus Ym, Xp versus Yp, and Xm versus Ym. The two models are functionally equivalent but were used to test different hypotheses regarding interaction between terms (first model) and pairwise differences between groups (second model). When needed, the response variable was transformed (r.m.s.d.2 in the genotype experiment with brood of genotype A and the age experiment, r.m.s.d3/5 in the genotype experiment with brood of genotype B; no transformation for the morphology experiment) to satisfy model assumptions. We evaluated the significance of terms by comparing pairs of nested models using χ2 log-likelihood ratio tests following deletion of the term of interest (the interaction in the first model and the four-level variable combining colony composition and individual attributes in the second model) using the function drop1 in R.
Effects of genetic, demographic, and morphological mixing on DOL
The effects of the treatment (a three-level variable: pure X, pure Y, and mixed XY) on colony-level DOL (behavioral variation and specialization) were investigated using generalized linear models (GLMs). The significance of treatment was evaluated as above. Pairwise comparisons between treatments were evaluated using Tukey post hoc tests with Bonferroni–Holm correction. Behavioral variation was square root–transformed in the genotype experiment with B larvae to satisfy model assumptions.
Effects of genetic, demographic, and morphological mixing on behavior
To assess how type-specific behavior was affected by mixing, and more specifically, whether the difference in behavior between types of ants was affected by mixing, we compared the difference in mean behavior (type-specific mean r.m.s.d. in each colony) between types across pure colonies to the difference in mean behavior between the same types within mixed colonies (i.e., Yp − Xp versus Ym − Xm, where Yp > Xp and Ym > Xm; see below for definitions of behavioral patterns), using unpaired t tests, after verifying assumptions of normality. In mixed colonies, the difference in mean behavior was calculated between types of ants within a colony (e.g., old and young workers from the same colony); in pure colonies, the difference in mean behavior was calculated between arbitrary pairs of pure colonies (e.g., old workers from the pure colony #1 and young ants from pure colony #1, where 1 is a replicate number assigned randomly at the beginning of the experiment). We further tested whether the amplitude of the effect differed across types by comparing the magnitude of change in type-specific behavior between pure and mixed colonies across the 2 ant types (i.e., |Xm − Xp| ≠ |Ym − Yp|) with unpaired t tests, after verifying assumptions of normality.
Definitions of behavioral patterns
We used the following definitions to characterize the qualitative outcomes of mixing individuals with different behavioral tendencies on individual behavior. We assume that Yp > Xp and Ym > Xm, to reflect our observation that the type with higher r.m.s.d. in pure colonies always also had higher r.m.s.d. in mixed colonies. Given this assumption, mixing could, in principle, result in one of the following patterns:
No effect of mixing on individual behavior: The mean behavioral difference between types across pure colonies is the same as the mean behavioral difference between types within mixed colonies, so that Yp − Xp = Ym − Xm;
Behavioral convergence: Individuals of different types are behaviorally more similar on average to each other when mixed, so that Yp − Xp > Ym − Xm; and
Behavioral divergence: Individuals of different types are behaviorally more different on average from each other when mixed, so that Yp − Xp < Ym − Xm.
Supporting information
(PDF)
Task performance frequency for a single task as a function of colony composition. Opaque circles represent individual replicate colonies (N = 16; n = 100 replicates per composition), and solid circles represent average value (± s.e.) across replicates. Horizontal gray lines represent the average of the pure colonies (first two columns). Types X and Y differ in threshold variance: σX = 0.1, σY = 0.5; all other parameters are identical (see S1 Table). Simulation code and data are available at https://github.com/marikawakatsu/mixing-model.
(PDF)
Small opaque circles represent individual colonies, and large solid circles represent the average values across n replicate colonies. Identical colors across panels indicate ants of the same genotype, age, and morphological types. (a) Behavioral variation as a function of colony genetic composition in colonies with A brood (N = 16; Bpure vs. Mixed: z = −2.85, p = 0.013; Apure vs. Mixed: z = 0.81, p = 0.421). (b) Behavioral variation as a function of colony genetic composition in colonies with B brood (N = 16; Bpure vs. Mixed: z = −2.76, p = 0.012; Apure vs. Mixed: z = −0.81, p = 0.419). (c) Behavioral variation as a function of colony age composition (N = 16; Youngpure vs. Mixed: z = 2.01, p = 0.090; Oldpure vs. Mixed: z = 3.89, p = 3.07*10−04). (d) Behavioral variation as a function of colony morphological composition (N = 8; Regular Workerpure vs. Mixed: z = −2.85, p = 0.013, Intercastepure vs. Mixed: z = 1.53, p = 0.254). n.s., nonsignificant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Raw data are available at doi.org/10.5061/dryad.hx3ffbgdd. r.m.s.d., root–mean–square deviation.
(PDF)
Small opaque circles represent individual colonies, and large solid circles represent the average values across n replicate colonies. Identical colors across panels indicate ants of the same genotype, age, and morphological types. (a) Specialization as a function of colony genetic composition in colonies with A brood (N = 16; GLM post hoc tests; Bpure vs. Mixed: z = −2.78, p = 0.017; Apure vs. Mixed: z = 1.25, p = 0.256). (b) Specialization as a function of colony genetic composition in colonies with B brood (N = 16; Bpure vs. Mixed: z = −2.41, p = 0.048; Apure vs. Mixed: z = 0.88, p = 0.378). (c) Specialization as a function of colony age composition (N = 16; Youngpure vs. Mixed: z = 3.01, p = 0.005; Oldpure vs. Mixed: z = 5.01, p = 1.63*10−06). (d) Specialization as a function of colony morphological composition (N = 8; Regular Workerpure vs. Mixed: z = −4.35, p = 4.07*10−05, Intercastepure vs. Mixed: z = 2.73, p = 0.013). n.s., nonsignificant; *, p < 0.05; **, p < 0.01; ***, p < 0.001. Raw data are available at doi.org/10.5061/dryad.hx3ffbgdd. r.m.s.d., root–mean–square deviation.
(PDF)
Each point shows the simulated stimulus level for the 2 tasks (task 1 on the horizontal axes and task 2 on the vertical axes) in the generation indicated by its color. Each of panels a, b, d, and e shows a pure colony of the type indicated; each of panels c and f shows a mixed colony of Types X and Y. Panels a–c (δ = 1.3) correspond to Fig 3A and d–f (δ = 0.6) to Fig 3B. (a–c)When the demand is higher (δ = 1.3), the more efficient type (Type X) can keep up with the demand on its own (a) but the less efficient type (Type Y) cannot, as demonstrated by the continual growth of the stimuli (b); however, mixed colonies can keep up with the higher level of demand (c). (d–f) When the demand is lower (δ = 0.6), the stimulus levels grow quickly at first but then stabilizes to an oscillatory pattern around a point, demonstrating that both pure and mixed colonies can keep up with the demand. Each simulation ran for 1,000 time steps; all other parameters are identical to those in the corresponding panels in Fig 3. Simulation code and data are available at https://github.com/marikawakatsu/mixing-model.
(PNG)
Behavioral variation was quantified as the standard deviation of task performance frequency across individuals in a colony. Opaque circles represent individual replicate colonies (N = 16; n = 100 replicates per composition), and solid circles represent the average value (mean ± s.e.) across replicates. Types X1, X2, X3, and Y and their corresponding parameters are identical to those in Fig 3. See S1 Table for other parameters. Simulation code and data are available at https://github.com/marikawakatsu/mixing-model.
(PDF)
Colony-level specialization was quantified using Spearman rank correlation on consecutive windows of 200 time steps. Opaque circles represent individual replicate colonies (N = 16; n = 100 replicates per composition), and solid circles represent the average value (mean ± s.e.) across replicates. Types X1, X2, X3, and Y and their corresponding parameters are identical to those in Fig 3. See S1 Table for other parameters. Simulation code and data are available at https://github.com/marikawakatsu/mixing-model.
(PDF)
Colonies with varying ratios of X and Y individuals were simulated under different conditions of threshold values, task performance efficiency, and task demand (n = 100 replicates per colony composition). Each large circle represents the mean task performance (task 1) for that mix of X and Y individuals; the neighboring smaller circles represent the means of X and Y individuals, respectively, within that mix. Dashed lines indicate the null hypothesis of linear behavioral effects of mixing types. The boxes highlight the behavioral patterns characterizing the 1:1-mixes, and their labels indicate correspondence with Fig 3 (a: Fig 3E; b: Fig 3B; c: Fig 3A; and d: Fig 3D). Types X1, X2, X3, and Y and their corresponding parameters as in Fig 3. See S1 Table for other parameters. Simulation code and data are available at https://github.com/marikawakatsu/mixing-model.
(PDF)
(PDF)
Text in bold denotes the variable of interest for each experiment. All mixed colonies contained a 1:1 ratio of each ant type.
(PDF)
Acknowledgments
We thank A. Gal for advice on analyses and O. Feinerman and M. Liu for contributions to the tracking algorithms. This is Clonal Raider Ant Project paper #13.
Abbreviations
- DOL
division of labor
- GLM
generalized linear model
- LME
linear mixed effect
- r.m.s.d.
root–mean–square deviation
Data Availability
All simulation code is available at https://github.com/marikawakatsu/mixing-model. Additionally, software for image analysis is available at https://doi.org/10.5281/zenodo.1211644. Behavioral tracking data (raw position data and summary statistics for each individual, colony, and subcolony), as well as R scripts for statistical analyses are available in the Dryad repository https://doi.org/10.5061/dryad.hx3ffbgdd.
Funding Statement
Research reported in this publication was supported by grants from the Faculty Scholars Program of the Howard Hughes Medical Institute (www.hhmi.org/programs/biomedical-research/faculty-scholars), the Pew Biomedical Scholars Program (www.pewtrusts.org/en/projects/pew-biomedical-scholars), and the National Institute of General Medical Sciences of the National Institutes of Health (www.nih.gov/) under Award Number R35GM127007 (D.J.C.K.); Swiss National Science Foundation (www.snf.ch/en/) Advanced Postdoc Mobility (P300P3-147900) and Ambizione (PZ00P3_168066) fellowships, and a Rockefeller University Women & Science fellowship (www.rockefeller.edu/support-our-science/women-and-science/) (Y.U.); Army Research Office (www.arl.army.mil) Grant W911NF-18-1-0325 (M.K.); National Science Foundation Graduate Research Fellowship (www.nsfgrfp.org) no. DGE1656466 (C.K.T.); and a Henry and Marie-Josée Kravis Postdoctoral Fellowship (J.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Howard Hughes Medical Institute, or the Pew Biomedical Scholars Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sumpter DJT. The principles of collective animal behaviour. Philos Trans R Soc B Biol Sci. 2006;361:5–22. doi: 10.1098/rstb.2005.1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Werfel J, Petersen K, Nagpal R. Designing collective behavior in a termite-inspired robot construction team. Science. 2014;343:754–8. doi: 10.1126/science.1245842 [DOI] [PubMed] [Google Scholar]
- 3.Cohen DJ, James Nelson W, Maharbiz MM. Galvanotactic control of collective cell migration in epithelial monolayers. Nat Mater. 2014;13:409–17. doi: 10.1038/nmat3891 [DOI] [PubMed] [Google Scholar]
- 4.Nadell CD, Bucci V, Drescher K, Levin SA, Bassler BL, Xavier JB. Cutting through the complexity of cell collectives. Proc R Soc B Biol Sci. 2013;280:20122770. doi: 10.1098/rspb.2012.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart AJ, Mosleh M, Diakonova M, Arechar AA, Rand DG, Plotkin JB. Information gerrymandering and undemocratic decisions. Nature. 2019;573:117–21. doi: 10.1038/s41586-019-1507-6 [DOI] [PubMed] [Google Scholar]
- 6.Westley PAH, Berdahl AM, Torney CJ, Biro D. Collective movement in ecology: from emerging technologies to conservation and management. Philos Trans R Soc B Biol Sci. 2018;373:20170004. doi: 10.1098/rstb.2017.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon DM. The organization of work in social insect colonies. Nature. 1996;380:121–4. doi: 10.1038/380121a0 [DOI] [Google Scholar]
- 8.Seeley TD, Visscher PK, Schlegel T, Hogan PM, Franks NR, Marshall JAR. Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science. 2012;335:108–11. doi: 10.1126/science.1210361 [DOI] [PubMed] [Google Scholar]
- 9.Khuong A, Gautrais J, Perna A, Sbaï C, Combe M, Kuntz P, et al. Stigmergic construction and topochemical information shape ant nest architecture. Proc Natl Acad Sci U S A. 2016;113:1303–8. doi: 10.1073/pnas.1509829113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z-Y, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol. 1996;39:147–58. doi: 10.1007/s002650050276 [DOI] [Google Scholar]
- 11.Brahma A, Mandal S, Gadagkar R. Emergence of cooperation and division of labor in the primitively eusocial wasp Ropalidia marginata. Proc Natl Acad Sci U S A. 2018;115:756–61. doi: 10.1073/pnas.1714006115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald EE, Baltiansky L, Feinerman O. Individual crop loads provide local control for collective food intake in ant colonies. Elife. 2018;7:e31730. doi: 10.7554/eLife.31730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson G. Regulation of division of labor in insect societies. Annu Rev Entomol. 1992;37:637–65. doi: 10.1146/annurev.en.37.010192.003225 [DOI] [PubMed] [Google Scholar]
- 14.Beshers SN, Fewell JH. Models of division of labor in social insects. Annu Rev Entomol. 2001;46:413–40. doi: 10.1146/annurev.ento.46.1.413 [DOI] [PubMed] [Google Scholar]
- 15.Duarte A, Weissing FJ, Pen I, Keller L. An evolutionary perspective on self-organized division of labor in social insects. Annu Rev Ecol Evol Syst. 2011;42:91–110. doi: 10.1146/annurev-ecolsys-102710-145017 [DOI] [Google Scholar]
- 16.Jeanson R, Weidenmüller A. Interindividual variability in social insects—proximate causes and ultimate consequences. Biol Rev. 2014;89:671–87. doi: 10.1111/brv.12074 [DOI] [PubMed] [Google Scholar]
- 17.Huber F. Nouvelles observations sur les abeilles. 2nd ed. chez J.J. Paschoud; 1814. doi: 10.3931/e-rara-22653 [DOI] [Google Scholar]
- 18.Bonabeau E, Theraulaz G, Deneubourg JL. Quantitative study of the fixed threshold model for the regulation of division of labour in insect societies. Proc R Soc B Biol Sci. 1996;263:1565–9. doi: 10.1098/rspb.1996.0229 [DOI] [Google Scholar]
- 19.Gautrais J, Theraulaz G, Deneubourg JL, Anderson C. Emergent polyethism as a consequence of increased colony size in insect societies. J Theor Biol. 2002;215:363–73. doi: 10.1006/jtbi.2001.2506 [DOI] [PubMed] [Google Scholar]
- 20.Myerscough MR, Oldroyd BP. Simulation models of the role of genetic variability in social insect task allocation. Insectes Soc. 2004;51:146–52. doi: 10.1007/s00040-003-0713-1 [DOI] [Google Scholar]
- 21.Page RE, Mitchell SD. Self-organization and the evolution of division of labor. Apidologie. 1998;29:171–90. doi: 10.1051/apido:19980110 [DOI] [Google Scholar]
- 22.Bonabeau E, Theraulaz G, J-LL D. Fixed response thresholds and the regulation of division of labor in insect societies. Bull Math Biol. 1998;60:753–807. doi: 10.1006/bulm.1998.0041 [DOI] [Google Scholar]
- 23.Waibel M, Floreano D, Magnenat S, Keller L. Division of labour and colony efficiency in social insects: effects of interactions between genetic architecture, colony kin structure and rate of perturbations. Proc R Soc B Biol Sci. 2006;273:1815–23. doi: 10.1098/rspb.2006.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theraulaz G, Bonabeau E, Deneubourg JL. Response threshold reinforcement and division of labour in insect societies. Proc R Soc B Biol Sci. 1998;265:327–32. doi: 10.1098/rspb.1998.0299 [DOI] [Google Scholar]
- 25.Pankiw T, Page RE. Response thresholds to sucrose predict foraging division of labor in honeybees. Behav Ecol Sociobiol. 2000;47:265–7. doi: 10.1007/s002650050664 [DOI] [Google Scholar]
- 26.Holbrook CT, Kukuk PF, Fewell JH. Increased group size promotes task specialization in a normally solitary halictine bee. Behaviour. 2013;150:1449–66. doi: 10.1163/1568539X-00003104 [DOI] [Google Scholar]
- 27.Ulrich Y, Saragosti J, Tokita CK, Tarnita CE, Kronauer DJC. Fitness benefits and emergent division of labor at the onset of group-living. Nature. 2018;560:635–8. doi: 10.1038/s41586-018-0422-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeanson R, Fewell JH, Gorelick R, Bertram SM. Emergence of increased division of labor as a function of group size. Behav Ecol Sociobiol. 2007;62:289–98. doi: 10.1007/s00265-007-0464-5 [DOI] [Google Scholar]
- 29.Gordon DM. Dynamics of task switching in harvester ants. Anim Behav. 1989;38:194–204. doi: 10.1016/S0003-3472(89)80082-X [DOI] [Google Scholar]
- 30.Fewell JH, Page RE. The emergence of division of labour in forced associations of normally solitary ant queens. Evol Ecol Res. 1999;1:537–48. [Google Scholar]
- 31.Jeanson R, Fewell JH. Influence of the social context on division of labor in ant foundress associations. Behav Ecol. 2008;19:567–74. doi: 10.1093/beheco/arn018 [DOI] [Google Scholar]
- 32.Naug D, Gadagkar R. The role of age in temporal polyethism in a primitively eusocial wasp. Behav Ecol Sociobiol. 1998;42:37–47. doi: 10.1007/s002650050409 [DOI] [Google Scholar]
- 33.Seeley TD. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol. 1982;11:287–93. doi: 10.1007/BF00299306 [DOI] [Google Scholar]
- 34.Tripet F, Nonacs P. Foraging for work and age-based polyethism: the roles of age and previous experience on task choice in ants. Ethology. 2004;110:863–77. doi: 10.1111/j.1439-0310.2004.01023.x [DOI] [Google Scholar]
- 35.Hinze B, Leuthold RH. Age related polyethism and activity rhythms in the nest of the termite Macrotermes bellicosus (Isoptera, Termitidae). Insectes Soc. 1999;46:392–7. doi: 10.1007/s000400050162 [DOI] [Google Scholar]
- 36.Ravary F, Lecoutey E, Kaminski G, Châline N, Jaisson P. Individual experience alone can generate lasting division of labor in ants. Curr Biol. 2007;17:1308–12. doi: 10.1016/j.cub.2007.06.047 [DOI] [PubMed] [Google Scholar]
- 37.Fewell JH, Page RE. Genotypic variation in foraging responses to environmental stimuli by honey bees, Apis mellifera. Experientia. 1993;49:1106–12. doi: 10.1007/BF01929923 [DOI] [Google Scholar]
- 38.Eyer P-A, Freyer J, Aron S. Genetic polyethism in the polyandrous desert ant Cataglyphis cursor. Behav Ecol. 2012;24:144–51. doi: 10.1093/beheco/ars146 [DOI] [Google Scholar]
- 39.Frumhoff PC. Baker J. A genetic component to division of labour within honey bee colonies. Nature. 1988;333:358–61. doi: 10.1038/333358a0 [DOI] [Google Scholar]
- 40.Blatrix R, Durand J-L, Jaisson P. Task allocation depends on matriline in the ponerine ant Gnamptogenys striatula Mayr. J Insect Behav. 2000;13:553–62. doi: 10.1023/A:1007815703049 [DOI] [Google Scholar]
- 41.Kwapich CL, Valentini G, Hölldobler B. The non-additive effects of body size on nest architecture in a polymorphic ant. Philos Trans R Soc B Biol Sci. 2018;373:20170235. doi: 10.1098/rstb.2017.0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wetterer J. The ecology and evolution of worker size-distribution in leaf-cutting ants (Hymenoptera: Formicidae). Sociobiology. 1999;34:119–44. [Google Scholar]
- 43.Blanchard GB, Orledge GM, Reynolds SE, Franks NR. Division of labour and seasonality in the ant Leptothorax albipennis: worker corpulence and its influence on behaviour. Anim Behav. 2000;59:723–38. doi: 10.1006/anbe.1999.1374 [DOI] [PubMed] [Google Scholar]
- 44.Spaethe J, Weidenmüller A. Size variation and foraging rate in bumblebees (Bombus terrestris). Insectes Soc. 2002;49:142–6. doi: 10.1007/s00040-002-8293-z [DOI] [Google Scholar]
- 45.Wilson EO. Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). Behav Ecol Sociobiol. 1980;7:157–65. doi: 10.1007/BF00299521 [DOI] [Google Scholar]
- 46.Kay A, Rissing SW. Division of foraging labor in ants can mediate demands for food and safety. Behav Ecol Sociobiol. 2005;58:165–74. doi: 10.1007/s00265-005-0914-x [DOI] [Google Scholar]
- 47.Mertl AL, Traniello JFA. Behavioral evolution in the major worker subcaste of twig-nesting Pheidole (Hymenoptera: Formicidae): does morphological specialization influence task plasticity? Behav Ecol Sociobiol. 2009;63:1411–26. doi: 10.1007/s00265-009-0797-3 [DOI] [Google Scholar]
- 48.Weidenmüller A. The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav Ecol. 2004;15:120–8. doi: 10.1093/beheco/arg101 [DOI] [Google Scholar]
- 49.Jeanson R. Within-individual behavioural variability and division of labour in social insects. J Exp Biol. 2019;222: jeb190868. doi: 10.1242/jeb.190868 [DOI] [PubMed] [Google Scholar]
- 50.Weidenmüller A, Chen R, Meyer B. Reconsidering response threshold models—short-term response patterns in thermoregulating bumblebees. Behav Ecol Sociobiol. 2019;73:1–13. doi: 10.1007/s00265-019-2709-5 [DOI] [Google Scholar]
- 51.Teseo S, Châline N, Jaisson P, Kronauer DJC. Epistasis between adults and larvae underlies caste fate and fitness in a clonal ant. Nat Commun. 2014;5:3363. doi: 10.1038/ncomms4363 [DOI] [PubMed] [Google Scholar]
- 52.Ravary F, Jaisson P. Absence of individual sterility in thelytokous colonies of the ant Cerapachys biroi Forel (Formicidae, Cerapachyinae). Insectes Soc. 2004;51:67–73. doi: 10.1007/s00040-003-0724-y [DOI] [Google Scholar]
- 53.Ravary F, Jahyny B, Jaisson P. Brood stimulation controls the phasic reproductive cycle of the parthenogenetic ant Cerapachys biroi. Insectes Soc. 2006;53:20–6. doi: 10.1007/s00040-005-0828-7 [DOI] [Google Scholar]
- 54.Ulrich Y, Burns D, Libbrecht R, Kronauer DJC. Ant larvae regulate worker foraging behavior and ovarian activity in a dose-dependent manner. Behav Ecol Sociobiol. 2016;70:1011–8. doi: 10.1007/s00265-015-2046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuji K, Yamauchi K. Production of females by parthenogenesis in the ant, Cerapachys biroi. Insectes Soc. 1995;42:333–6. doi: 10.1007/BF01240430 [DOI] [Google Scholar]
- 56.Trible W, McKenzie SK, Kronauer DJC. Globally invasive populations of the clonal raider ant are derived from Bangladesh. Biol Lett. 2020;16:20200105. doi: 10.1098/rsbl.2020.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravary F, Jaisson P. The reproductive cycle of thelytokous colonies of Cerapachys biroi Forel (Formicidae, Cerapachyinae). Insectes Soc. 2002;49:114–9. doi: 10.1007/s00040-002-8288-9 [DOI] [Google Scholar]
- 58.Chandra V, Gal A, Kronauer DJC. Colony expansions underlie the evolution of army ant mass raiding. Proc Natl Acad Sci U S A. 2021;118(22):e2026534118. doi: 10.1073/pnas.2026534118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson GE. Regulation of honey bee age polyethism by juvenile hormone. Behav Ecol Sociobiol. 1987;20:329–38. doi: 10.1007/BF00300679 [DOI] [Google Scholar]
- 60.Sendova-Franks AB, Franks NR. Spatial relationships within nests of the ant Leptothorax unifasciatus (Latr.) and their implications for the division of labour. Anim Behav. 1995;50:121–36. doi: 10.1006/anbe.1995.0226 [DOI] [Google Scholar]
- 61.Pamminger T, Foitzik S, Kaufmann KC, Schützler N, Menzel F. Worker personality and its association with spatially structured division of labor. PLoS ONE. 2014;9:e79616. doi: 10.1371/journal.pone.0079616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mersch DP, Crespi A, Keller L. Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science. 2013;340:1090–3. doi: 10.1126/science.1234316 [DOI] [PubMed] [Google Scholar]
- 63.Crall JD, Gravish N, Mountcastle AM, Kocher SD, Oppenheimer RL, Pierce NE, et al. Spatial fidelity of workers predicts collective response to disturbance in a social insect. Nat Commun. 2018;9:1–13. doi: 10.1038/s41467-018-03561-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biedermann PHW, Taborsky M. Larval helpers and age polyethism in ambrosia beetles. Proc Natl Acad Sci U S A. 2011;108:17064–9. doi: 10.1073/pnas.1107758108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito F. Higashi S. A linear dominance hierarchy regulating reproduction and polyethism of the queenless ant Pachycondyla sublaevis. Naturwissenschaften. 1991;78:80–2. doi: 10.1007/BF01206263 [DOI] [Google Scholar]
- 66.Bernadou A, Schrader L, Pable J, Hoffacker E, Meusemann K, Heinze J. Stress and early experience underlie dominance status and division of labour in a clonal insect. Proc R Soc B Biol Sci 2018;285:20181468. doi: 10.1098/rspb.2018.1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mas F, Kölliker M. Maternal care and offspring begging in social insects: chemical signalling hormonal regulation and evolution. Anim Behav. 2008;76:1121–31. doi: 10.1016/j.anbehav.2008.06.011 [DOI] [Google Scholar]
- 68.Maisonnasse A, Lenoir J-C, Costagliola G, Beslay D, Choteau F, Crauser D, et al. A scientific note on E-β-ocimene, a new volatile primer pheromone that inhibits worker ovary development in honey bees. Apidologie. 2009;40:562–4. doi: 10.1051/apido/2009024 [DOI] [Google Scholar]
- 69.Oldroyd BP, Wossler T, Ratnieks F. Regulation of ovary activation in worker honey-bees (Apis mellifera): larval signal production and adult response thresholds differ between anarchistic and wild-type bees. Behav Ecol Sociobiol. 2001;50:366–70. doi: 10.1007/s002650100369 [DOI] [Google Scholar]
- 70.Pankiw T, Page RE Jr, Kim Fondrk M. Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera). Behav Ecol Sociobiol. 1998;44:193–8. doi: 10.1007/s002650050531 [DOI] [Google Scholar]
- 71.Detrain C, Pasteels JM. Caste differences in behavioral thresholds as a basis for polyethism during food recruitment in the ant, Pheidole pallidula (Nyl.) (Hymenoptera: Myrmicinae. J Insect Behav. 1991;4:157–76. doi: 10.1007/BF01054609 [DOI] [Google Scholar]
- 72.Pankiw T, Page RE Jr. The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A. 1999;185:207–13. doi: 10.1007/s003590050379 [DOI] [PubMed] [Google Scholar]
- 73.Dornhaus A. Specialization does not predict individual efficiency in an ant. PLoS Biol. 2008;6:2368–75. doi: 10.1371/journal.pbio.0060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaptein N, Billen J, Gobin B. Larval begging for food enhances reproductive options in the ponerine ant Gnamptogenys striatula. Anim Behav. 2005;69:293–9. 10.1016/j.anbehav.2004.04.012 [DOI] [Google Scholar]
- 75.Alem S, Perry CJ, Zhu X, Loukola OJ, Ingraham T, Søvik E, et al. Associative mechanisms allow for social learning and cultural transmission of string pulling in an insect. PLoS Biol. 2016;14:e1002564. doi: 10.1371/journal.pbio.1002564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate’s foraging decisions. Science. 2013;340:483–5. doi: 10.1126/science.1232769 [DOI] [PubMed] [Google Scholar]
- 77.Rosenthal SB, Twomey CR, Hartnett AT, Wu HS, Couzin ID. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc Natl Acad Sci U S A. 2015;112:4690–5. doi: 10.1073/pnas.1420068112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berdahl A, Torney CJ, Ioannou CC, Faria JJ, Couzin ID. Emergent sensing of complex environments by mobile animal groups. Science. 2013;339:574–6. doi: 10.1126/science.1225883 [DOI] [PubMed] [Google Scholar]
- 79.Merling M, Eisenmann S, Bloch G. Body size but not age influences phototaxis in bumble bee (Bombus terrestris, L.) workers. Apidologie. 2020;51:763–76. doi: 10.1007/s13592-020-00759-0 [DOI] [Google Scholar]
- 80.Pacala S, Gordon D, Godfray H. Effects of social group size on information transfer and task allocation. Evol Ecol. 1996;10:127–65. doi: 10.1007/BF01241782 [DOI] [Google Scholar]
- 81.Tokita CK, Tarnita CE. Social influence and interaction bias can drive emergent behavioural specialization and modular social networks across systems. J R Soc Interface. 2020;17:20190564. doi: 10.1098/rsif.2019.0564 [DOI] [PMC free article] [PubMed]
- 82.Fewell JH, Bertram SM. Division of labor in a dynamic environment: Response by honeybees (Apis mellifera) to graded changes in colony pollen stores. Behav Ecol Sociobiol. 1999;46:171–9. doi: 10.1007/s002650050607 [DOI] [Google Scholar]
- 83.O’Donnell S, Jeanne RL. Forager success increases with experience in Polybia occidentalis (Hymenoptera: Vespidae). Insectes Soc. 1992;39:451–4. doi: 10.1007/BF01240628 [DOI] [Google Scholar]
- 84.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–9. doi: 10.1056/NEJMsa066082 [DOI] [PubMed] [Google Scholar]
- 85.Broly P, Deneubourg J-L. Behavioural contagion explains group cohesion in a social crustacean. PLoS Comput Biol. 2015;11:1–18. doi: 10.1371/journal.pcbi.1004290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Centola D. How Behavior Spreads: The Science of Complex Contagions. Princeton University Press; 2018. doi: 10.2307/j.ctvc7758p [DOI] [Google Scholar]
- 87.Bettenworth V, Steinfeld B, Duin H, Petersen K, Streit WR, Bischofs I, et al. Phenotypic heterogeneity in bacterial quorum sensing systems. J Mol Biol. 2019;431:4530–46. doi: 10.1016/j.jmb.2019.04.036 [DOI] [PubMed] [Google Scholar]
- 88.Kaushik S, Katoch B, Nanjundiah V. Social behaviour in genetically heterogeneous groups of Dictyostelium giganteum. Behav Ecol Sociobiol. 2006;59:521–30. doi: 10.1007/s00265-005-0077-9 [DOI] [Google Scholar]
- 89.Buttery NJ, Thompson CRL, Wolf JB. Complex genotype interactions influence social fitness during the developmental phase of the social amoeba Dictyostelium discoideum. J Evol Biol. 2010;23:1664–71. doi: 10.1111/j.1420-9101.2010.02032.x [DOI] [PubMed] [Google Scholar]
- 90.Pande S, Velicer GJ. Chimeric synergy in natural social groups of a cooperative microbe. Curr Biol. 2018;28:262–7. doi: 10.1016/j.cub.2017.11.043 [DOI] [PubMed] [Google Scholar]
- 91.Bell AS. De. Roode JC, Sim D, Read AF. Within-host competition in genetically diverse malaria infections: parasite virulence and competitive success. Evolution. 2006;60:1358–71. doi: 10.1111/j.0014-3820.2006.tb01215.x [DOI] [PubMed] [Google Scholar]
- 92.Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–102. doi: 10.1126/science.1059410 [DOI] [PubMed] [Google Scholar]
- 93.Strassmann JE, Zhu Y, Queller DC. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature. 2000;408:965–7. doi: 10.1038/35050087 [DOI] [PubMed] [Google Scholar]
- 94.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–4. doi: 10.1038/nature06279 [DOI] [PubMed] [Google Scholar]
- 95.Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci U S A. 1982;79:2554–8. doi: 10.1073/pnas.79.8.2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dodds PS, Watts DJ. A generalized model of social and biological contagion. J Theor Biol. 2005;232:587–604. doi: 10.1016/j.jtbi.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 97.Ward AJW, Sumpter DJT, Couzin ID, Hart PJB, Krause J. Quorum decision-making facilitates information transfer in fish shoals. Proc Natl Acad Sci U S A. 2008;105:6948–53. doi: 10.1073/pnas.0710344105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melke P, Sahlin P, Levchenko A. Jönsson H. A cell-based model for quorum sensing in heterogeneous bacterial colonies. PLoS Comput Biol. 2010;6:1–12. doi: 10.1371/journal.pcbi.1000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jolles JW, King AJ, Killen SS. The role of individual heterogeneity in collective animal behaviour. Trends Ecol Evol. 2020:278–91. doi: 10.1016/j.tree.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 100.Kronauer DJC, Pierce NE, Keller L. Asexual reproduction in introduced and native populations of the ant Cerapachys biroi. Mol Ecol. 2012;21:5221–35. doi: 10.1111/mec.12041 [DOI] [PubMed] [Google Scholar]
- 101.Oxley PR, Ji L, Fetter-Pruneda I, McKenzie SK, Li C, Hu H, et al. The genome of the clonal raider ant Cerapachys biroi. Curr Biol. 2014;24:451–8. doi: 10.1016/j.cub.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 103.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 104.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–63. doi: 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]