Abstract
Since the initial use of vaccination in the eighteenth century, our understanding of human and animal immunology has greatly advanced and a wide range of vaccine technologies and delivery systems have been developed. The COVID-19 pandemic response leveraged these innovations to enable rapid development of candidate vaccines within weeks of the viral genetic sequence being made available. The development of vaccines to tackle emerging infectious diseases is a priority for the World Health Organization and other global entities. More than 70% of emerging infectious diseases are acquired from animals, with some causing illness and death in both humans and the respective animal host. Yet the study of critical host–pathogen interactions and the underlying immune mechanisms to inform the development of vaccines for their control is traditionally done in medical and veterinary immunology ‘silos’. In this Perspective, we highlight a ‘One Health vaccinology’ approach and discuss some key areas of synergy in human and veterinary vaccinology that could be exploited to accelerate the development of effective vaccines against these shared health threats.
Subject terms: Translational research, Vaccines, Vaccines
Emerging diseases that affect humans often arise due to the crossover of infectious agents from animal reservoirs. In this Perspective, George Warimwe and colleagues discuss the concept of ‘One Health vaccinology’, an approach that aims to use key lessons from human and veterinary immunology to develop more effective vaccination strategies for emerging infectious diseases.
Introduction
Vaccination to control infectious diseases has had a major direct impact on human health and welfare and has also secured food supply by improving animal health and controlling zoonotic diseases. For instance, childhood vaccination prevents more than 2 million deaths every year, with vaccination coverage being a strong indicator of the incidence of vaccine-preventable diseases in humans (for example, measles, yellow fever and polio)1. Similarly, veterinary vaccination against endemic diseases increases survival and productivity in food-producing animals such as cattle and poultry, with net gains in disposable household income and access to protein-rich animal source foods improving human nutrition2–5. However, despite these and many other examples of vaccine impact, very little interaction occurs between human and animal vaccine developers and policymakers.
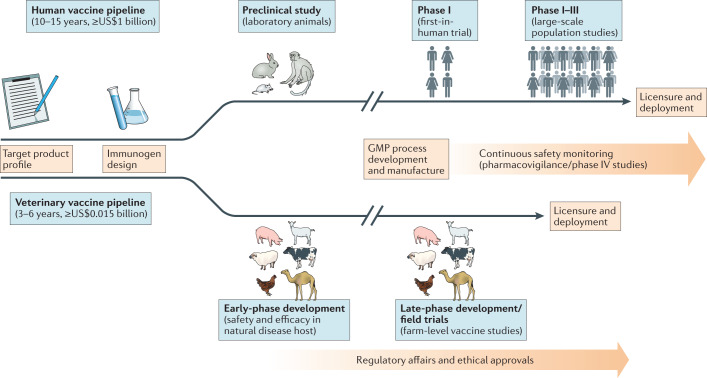
The development pipelines for human and animal vaccines are similar processes, including biological and scientific parallels in vaccine design and evaluation, as well as common bottlenecks that influence the success of vaccine development programmes6 (Fig. 1). However, there are differences in the complexity of the vaccine pipelines, largely due to the differing types of clinical data and regulatory requirements for licensure and the associated bottlenecks that are unique to the animal or human vaccine pipeline6. One example is the need for vaccine safety and efficacy data as assessed by experimental infection of vaccinated and unvaccinated target animal species in the veterinary field; in humans, phase II and phase III randomized controlled studies for estimation of vaccine efficacy against natural exposure are used, although human infection studies are now used for some vaccine programmes7. Despite these differences, the solutions to address bottlenecks in the animal and human vaccine development pipelines tend to be similar6. For instance, optimizing the immunogenicity of vaccines, whether in animals or in humans, involves iterative study of vaccination regimens or adjuvant combinations to inform ‘go’ or ‘no-go’ decisions with regard to subsequent development of promising vaccine candidates (Fig. 1).
Fig. 1. Vaccine development pipeline.
The typical vaccine development pipeline is shown, starting from target product profiling to licensure and deployment. The respective stages and approximate costs for veterinary and human vaccines are shown. Although presented as a linear chronological process, some of the different stages of the pipeline for a ‘multispecies’ vaccine can occur in parallel. For instance, the candidate ChAdOx1 RVF vaccine against Rift Valley fever12 will soon undergo evaluation in human clinical trials in parallel with veterinary development, having been made with the same manufacturing starting material. GMP, good manufacturing practice.
Most human infectious diseases have an animal origin, with more than 70% of emerging infectious diseases that affect humans initially crossing over from animals8. Generating wider knowledge of how pathogens behave in animals can give indications of how to develop control strategies for human diseases, and vice versa. ‘One Health vaccinology’, a concept in which synergies in human and veterinary immunology are identified and exploited for vaccine development, could transform our ability to control such emerging infectious diseases. Due to similarities in host–pathogen interactions, the natural animal hosts of a zoonotic infection may be the most appropriate model to study the disease and evaluate vaccine performance9. This could result in a scenario where a cross-species vaccine is feasible, such as Louis Pasteur’s live attenuated rabies vaccine that was protective in dogs and humans10 — although different products are now used for rabies vaccination in humans and dogs11 — or our own group’s Rift Valley fever vaccine, which is in co-development for use in humans and multiple livestock species12. Effective control of zoonotic diseases may require vaccination within reservoir animal hosts to break transmission to humans, and in humans to prevent disease13, making One Health vaccinology relevant for disease control policy. This strategy is already used for prevention and elimination of rabies, where mass dog vaccination remains the most cost-effective strategy for breaking disease transmission to humans14,15. However, due to the difficulty in predicting spillover events for new infections from animals to humans16, implementing a cross-species vaccination programme may only be feasible where the domestic animal reservoir of human infection is known (see Table 1 for some examples), but a cost–benefit analysis would be necessary to inform implementation.
Table 1.
Key diseases where cross-species vaccination programmes may be feasible
| Human disease | Key domestic animal hosts | Licensed human vaccines available? | Licensed veterinary vaccines available? |
|---|---|---|---|
| Rabies | Dogs | Yes | Yes |
| Rift Valley fever | Sheep, goats, cattle, camels | No | Yes |
| Brucellosis | Sheep, goats, cattle, camels | No | Yes |
| Crimean–Congo haemorrhagic fever | Sheep, goats, cattle, camels | No | No |
| Middle East respiratory syndrome | Camels | No | No |
| Tuberculosis | Cattle | Yes | No |
| Q fever | Sheep, goats, cattle, camels | Yes | No |
| Nipah virus infection | Pigs | No | No |
| Hendra virus infection | Horses | No | Yes |
For non-zoonotic illnesses, the natural course of infection and acquisition of immunity against closely related pathogens may be similar between animals and humans, allowing accelerated development of vaccines that target similar protective mechanisms. For example, bovine and human respiratory syncytial viruses, which cause pneumonia in young calves and children, respectively, are closely related genetically and are targeted by the same types of immune mechanisms, suggesting that vaccine strategies exploiting the same underlying mechanism of immunity may work for both species17,18. The most widely used human vaccine, bacille Calmette–Guérin (BCG) against tuberculosis, is essentially an attenuated strain of the cattle-infecting bacterium Mycobacterium bovis that is avirulent in a wide range of animal species19. BCG was developed through close collaboration between medical and veterinary practitioners more than 100 years ago19, and the extensive experience of its use in humans is now informing vaccination strategies to control tuberculosis in cattle20. A recent study found that BCG vaccination in humans also confers protection against other non-tuberculous infections in early childhood21; we are aware of no studies of non-specific effects of BCG vaccination in cattle, but this clearly warrants investigation. Edward Jenner’s observation that milkmaids exposed to the cowpox virus were protected against smallpox is perhaps the earliest example of exploiting pathogen relatedness for vaccine development and was the basis of the vaccine that was used for eradication of smallpox22.
Early manufacture of Jenner’s smallpox vaccine involved serial propagation of the cowpox virus in calves reared in ‘vaccine farms’23. Vaccine manufacture has advanced considerably but animal-sourced materials are still used for production of human vaccines; for example, embryonated chicken eggs are routinely used for the manufacture of influenza and yellow fever vaccines24,25. New highly scalable platform technologies and delivery systems are accelerating vaccine development such that it is now possible to go from the pathogen genetic sequence encoding an immunogen of choice to a vaccine candidate in a matter of weeks26. Bioinformatic analyses, X-ray crystallography and cryo-electron microscopy continue to be leveraged for the identification and optimization of protective antigens for human and veterinary vaccines27–29. These new technologies are being applied to emerging infectious diseases such as COVID-19 or stubborn persistent challenges, including malaria and brucellosis30–33.
In this Perspective, we highlight some key areas of synergy in human and veterinary vaccinology that could be exploited to accelerate the development and deployment of effective vaccines against zoonotic diseases. We focus on comparative immunology, applications of current vaccine technologies, and regulatory and operational considerations for vaccine deployment.
Immune systems of different species
The overall structure and composition of the innate and adaptive immune systems of humans and animal species are broadly similar, and comparing their responses to inoculation or infection with similar antigens or pathogens can inform vaccine development34. Allometric scaling is an important consideration, and the body size and physiology of livestock species are more similar to those of humans than to those of rodents. While rodents may be convenient for laboratory studies due to the ready availability of specific immunological reagents, lower purchase and maintenance costs and ease of handling, they may not reproduce the pathology and immunological attributes that would be observed in a natural animal host of infection9. The similarities between humans and livestock species may be most important when one is comparing the responses to aerosol delivery of antigens or pathogens35. Clearly, non-human primates are ideal species to predict responses in humans, but their availability is limited and certainly not possible for field studies. Nevertheless, the differences between the immune systems of humans and animals are important, and a cautious approach is justified when one is drawing detailed conclusions from animal studies.
Some of the most striking differences between the immune systems of humans and animals relate to their T cell populations and antibody structures (Fig. 2). Pigs are increasingly used to study vaccine candidates, in particular influenza vaccines36–38. However, there are key differences between pigs and humans that should be kept in mind. For example, three distinct subpopulations of CD8+ T cells have been identified in pigs by flow cytometry: a bright-staining population that expresses the CD8αβ heterodimer, a population that expresses the CD8αα homodimer and a CD8+ population that co-expresses CD4 (refs39–41). Ongoing studies indicate that most memory T cells in pigs are present in the double-positive population and that this population is the predominant source of interferon-γ (IFNγ) in recall responses to live viral vaccines39,42. Peripheral CD4+CD8+ T cells have been characterized in many different species but the proportion of these cells in the total T cell population differs greatly, from 1–2% in humans to 10–20% in pigs. In humans, the number and function of this subpopulation change in response to a range of infectious and neoplastic diseases43. Recently, these double-positive human T cells were shown to exhibit a memory phenotype44, similar to the double-positive T cells in pigs. The impact on responses to vaccination and infection due to the marked difference in the proportion of these double-positive cells in different species is yet to be resolved.
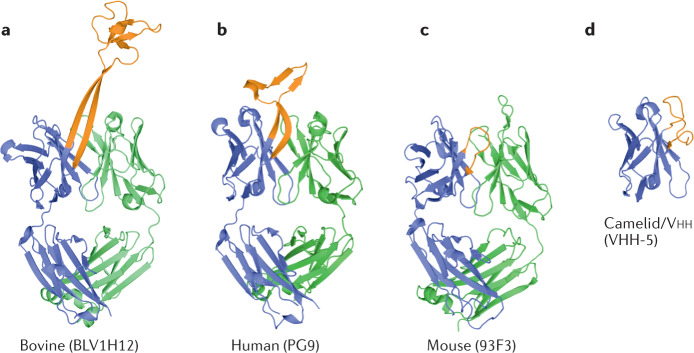
Fig. 2. The heavy chains of bovine antibodies can encode a very long CDR H3, which contrasts with the equivalent CDRs of human, mouse and heavy chain camelid antibodies.
Structures of antigen-binding fragment regions from bovine (BLV1H12, Protein Data Bank (PDB) ID 4K3D58; part a), human (PG9, PDB ID 3U2S86; part b), mouse (93F3, PDB ID 1T4K87; part c) and camelid (VHH-5, PDB ID 5U65 (ref.88); part d) antibodies (shown in cartoon representation). Heavy chains are coloured blue and light chains are coloured green. Heavy chain complementarity-determining region 3 (CDR H3; or CDR3 in the case of the camelid antibody) for each structure is coloured orange. PG9 contains a relatively long CDR H3 for human antibodies. Structures were rendered with PyMOL (version 1.8.6.0; Schrödinger LLC).
Another striking difference is the large percentage of circulating γδ T cells in young pigs and ruminants. γδ T cells constitute up to 60% of circulating lymphocytes in young cattle45 and pigs46. Even in adulthood, 30% of the peripheral blood mononuclear cells found in these species are γδ T cells47, whereas in humans only approximately 4% of peripheral blood mononuclear cells are γδ T cells48. Despite this difference between humans and ruminants, the results of protection studies in ruminants can provide valuable evidence to support the development of human vaccines; for example, the protection of calves from bovine respiratory syncytial virus by a stabilized prefusion F protein vaccine may guide the development of human vaccines against respiratory syncytial virus49. There is a case to be made that the results of vaccine efficacy and safety studies in large animals can provide important information to help shape vaccine development programmes in humans, but the precise immune mechanisms conferring that resistance may be different between species.
The similarities between human and bovine tuberculosis offer a potential opportunity for cross-species development of novel vaccines against the diseases. A BCG challenge model has been used to investigate the protective immune response in humans. Genes linked to protective responses included IFNG and IL17F, together with other genes associated with these cytokines that those two genes encode, such as NOD2, IL22, IL23A and FCGR1B50. A recent review highlights the potential role of IL-22 in the protective response to Mycobacterium tuberculosis infection in cattle and humans51. In cattle, IL-22 and IFNγ produced by purified protein derivative-stimulated peripheral blood mononuclear cells were identified as the primary predictors of vaccine-induced protection in an M. bovis challenge model52. Furthermore, BCG vaccination in children and young calves provides protection against tuberculosis, with activation of natural killer cells being a key immune mechanism in the induction of protective immunity in both species53,54. Therefore, there are elements of the protective immune response to tuberculosis that are consistent between cattle and humans, including humoral responses55. The similarities in different species of immune responses to closely related pathogens may help identify protective vaccine responses.
Another example where studying comparative immunology may improve our understanding of protective immune responses is in determining the role of antibodies with long heavy chain complementarity-determining region 3 (CDR H3) gene segments. Human antibody CDR H3 ranges between 8 and 16 amino acids in length, although antibodies with longer CDR H3 have been observed (for example, 18 amino acids56 and 28 amino acids57) and seem to play an important role in cross-protective immune responses to HIV-1 (refs56,57). However, such long CDR H3 antibodies are rare in humans, which makes some investigations more challenging58. By contrast, cattle have a larger population of antibodies (more than 10% of antibodies) with long and ultralong (more than 70 amino acids) CDR H3 gene segments59, and the study of these ultralong cattle antibodies may prove useful for the development of human interventions. For instance, immunizing cattle with HIV envelope glycoprotein results in rapid induction of broadly neutralizing ultralong CDR H3 antibodies, whereas it takes many years for such broadly neutralizing antibodies to develop in humans following HIV infection60. These bovine antibodies may be engineered for prophylactic or therapeutic use, and determining the immune mechanisms that underlie their induction could inform vaccine design.
Conversely, the antibody repertoire in dromedary camels is composed of both conventional IgG molecules and heavy chain-only IgG antibodies (HCAb) molecules, with the latter accounting for more than 70% of the repertoire61. These smaller HCAb molecules are better adapted for binding cryptic epitopes on pathogens that may be inaccessible to conventional IgG molecules62, allowing their use in diverse applications in diagnostics, therapy and research62. However, very little is known about the relative contribution of HCAbs to naturally acquired immunity to infections and whether vaccines could be tailored to elicit immune responses focused solely on either HCAbs or conventional IgG molecules. Dromedary camels are susceptible to infection with a wide range of pathogens that are also able to infect humans and domestic livestock such as cattle, sheep and goats; examples include Rift Valley fever virus, Brucella species (which cause brucellosis) and Crimean–Congo haemorrhagic fever (CCHF) virus63. They are also reservoirs of Middle East respiratory syndrome coronavirus, which emerged in 2012 and is associated with high case fatality in humans63. Understanding how different species are able to mount protective immunity against common pathogens, despite profound differences in IgG structures, could transform approaches to vaccine design and development. These differences in antibody structure can be exploited to identify different mechanisms of protection: for example, long CDR H3 antibodies penetrating viral glycan shields64. Importantly, a single vaccine platform can induce protection across multiple species12, including humans65, despite these differences in immune response. Veterinary vaccinology has made a substantial contribution to the broad knowledge base to develop vaccines and understand how they work, but there may be a difference in the response to similar vaccine platforms in different species. It may also be the case that some platforms may work well in humans and not some other species. However, within each species, individual heterogeneity in mounting immune responses does occur, and this might be due to factors such as chronic underlying illnesses, genetics and age66. Performing comparative studies to identify the common protective mechanisms across species, while accounting for individual within-species heterogeneity, will move the field beyond identifying correlates of protection to defining protective mechanisms.
Vaccine deployment in different species
The goals of vaccination programmes in humans and animals are similar, and include global disease eradication (permanent worldwide reduction of the incidence of a specific disease to zero), elimination of target diseases from a specified region with deliberate measures to prevent re-establishment, prevention of epidemic cycles and minimization of mortality and morbidity associated with infectious diseases. To date, vaccination has resulted in the eradication of smallpox in humans and rinderpest in cattle22,67. Although focused on two separate species, the two eradication programmes used similar vaccine deployment strategies combining mass vaccination campaigns to achieve herd immunity, intensive surveillance systems to identify and contain outbreaks promptly, and surveillance reporting and sharing of new knowledge that allowed eradication in stubborn pockets of each disease67,68. The endgame for these programmes required innovative strategies that included house-to-house case searches for smallpox and containment of outbreaks, and participatory community-based surveillance approaches to identify the hidden rinderpest disease pockets and deploy the vaccines. The key similarities in the deployment of vaccines and cross-learning from the medical and veterinary fields go beyond these disease eradication programmes to the control and elimination of current vaccine-preventable diseases.
Vaccination for the control of zoonoses has dual benefits for both human and animal health. A good example is the control of rabies, a viral zoonosis transmitted to humans through dog bites, which is responsible for about 60,000 human deaths annually15. A global elimination goal for human deaths from rabies has been set for 2030 (ref.14). The key strategies for achieving this goal are mass dog vaccinations to break the dog–dog and dog–human transmission cycles, prompt provision of postexposure prophylaxis to prevent clinical rabies among bite patients and enhanced surveillance systems to detect areas where the virus circulates and targeting the rabies interventions14. Whereas provision of rabies postexposure prophylaxis prevents clinical disease and death in people, elimination of human deaths from rabies is only cost-effective when combined with mass dog vaccinations69,70. Similarly, animal vaccination against brucellosis (a bacterial zoonosis transmitted to humans by livestock) reduces the incidence of human brucellosis, while improving milk production and other production indices among vaccinated livestock71.
Animal vaccination against epidemic zoonoses is a key strategy to limit human illness. The design and implementation of vaccine programmes for this purpose requires interaction between veterinary and public health personnel. Ideally, such collaboration needs to be in place before the occurrence of an epidemic, rather than being reactive72. In Kenya, the Zoonotic Disease Unit, a national entity co-led by medical and veterinary epidemiologists for the purpose of zoonotic disease surveillance, may provide an exemplar framework through which vaccine programmes to tackle endemic/epidemic zoonoses can be implemented73. For instance, through extant surveillance for key disease syndromes in livestock, the most recent Rift Valley fever outbreak in Kenya was detected in humans within a fortnight of confirmed livestock cases74. In such a scenario, Rift Valley fever vaccination could be implemented among susceptible animals (licensed vaccines are already available) and humans (when a vaccine is available) within a radius in proximity to the initial cases. Such a ‘ring vaccination’ approach has its roots in the control of disease outbreaks in livestock75 and has been used successfully to control Ebola virus disease epidemics76.
However, not all zoonoses of public health importance cause clinical disease in animals. For instance, domestic ruminants such as sheep and goats are key animal hosts of CCHF virus, which results in asymptomatic infection only in these species77. By contrast, CCHF is a highly fatal disease in humans and is among the diseases prioritized by the World Health Organization for urgent development of countermeasures72. Investigating the pathophysiology and immunology of CCHF virus in ruminant species may provide clues towards identifying therapeutic targets and aid the development of vaccines against human CCHF. Furthermore, assessment of vaccine efficacy against CCHF virus infection in livestock field trials could support development of human CCHF vaccines by providing a stringent test for ranking the performance of candidate vaccines outside a high-containment laboratory environment. Due to the lack of clinical disease in animals, estimation of vaccine efficacy following natural exposure could rely on serological detection of responses to virus components that are not part of a vaccine, thereby allowing distinction of infected animals from vaccinated animals, a concept well known in veterinary vaccinology. Following widespread COVID-19 vaccine use, a similar serological monitoring strategy could be useful for tracking population-level severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exposure, based on detection of antibodies against antigens absent from approved vaccines. There are several licensed veterinary vaccines against coronavirus infections in domestic animals (Box 1; Table 2); the experience with licensure and use of these products provides insights into the likely performance of vaccines against COVID-19 and other coronavirus infections in humans but has rarely been discussed in the medical debate on vaccine development.
Table 2.
Examples of licensed coronavirus vaccines for veterinary use
| Target species | CoV genus targeted by vaccine | CoV-induced disease | Licensed product | Technology | Formulation |
|---|---|---|---|---|---|
| Cattle | Betacoronavirus | Gastroenteritis, neonatal calf diarrhoea | Rotavec Corona | Inactivated plus adjuvant | Trivalent (CoV, rotavirus and Escherichia coli) |
| Bovigen Scour | Inactivated plus adjuvant | Trivalent (CoV, rotavirus and E. coli) | |||
| Calf-Guard | Live attenuated | Bivalent (CoV and rotavirus) | |||
| Poultry | Gammacoronavirus | Respiratory disease, reduced egg yields | Nobilis IB + ND + EDS | Live attenuated | Multivalent (infectious bronchitis virus, Newcastle disease virus and egg drop syndrome virus) |
| Pigs | Alphacoronavirus G | Gastroenteritis | ProSystem TGE/Rota | Live attenuated | Bivalent (TGE virus and rotavirus) |
| Dogs | Alphacoronavirus G | Gastroenteritis | Solo-Jec 6 | Live attenuated plus inactivated plus adjuvant | Multivalent (CoV, adenovirus, parainfluenza virus and parvovirus) |
| Nobivac Canine 1-Cv | Inactivated plus adjuvant | Monovalent (CoV) | |||
| Cats | Alphacoronavirus G | Peritonitis | Felocell FIP | Live attenuated | Monovalent (FIP virus) |
CoV, coronavirus; FIP, feline infectious peritonitis; TGE, transmissible gastroenteritis.
Box 1 Experience and lessons from the use of coronavirus vaccines in animals.
All major domestic animal species are susceptible to coronavirus (CoV) infection, typically resulting in clinical symptoms involving the respiratory system or the gastrointestinal system. Several licensed veterinary vaccines against CoV-associated disease are available (see Table 2 for examples), and these are predominantly composed of live attenuated CoV or whole inactivated virions that are administered in an adjuvant. However, subunit, viral-vectored and other types of recombinant vaccines are in development. Some examples of licensed animal CoV vaccines and some key immunological observations are summarized below, with further details available in recent reviews of animal CoV vaccines89–91.
Key observations from veterinary use of CoV vaccines89–91:
Virus-neutralizing antibodies directed to the surface spike (S) protein play a major role in protective immunity.
The duration of vaccine-induced immunity is variable but can last for at least 12 months, with annual boosters required to maintain protective levels of immunity.
Vaccines can be highly protective against severe illness resulting from CoV infection yet show limited protection against mild disease or infection.
Passive transfer of maternal antibodies from vaccinated dams can provide protective immunity against both enteric and respiratory CoV infections, as has been demonstrated in cattle.
T cell responses play an active role in the control of CoV infections. For instance, adoptive transfer of CD8+ T cells from immune chickens into unvaccinated chicks provides protection from acute infectious bronchitis, with epitopes mapped on the nucleocapsid and spike protein.
Different routes of administration can be used for CoV vaccination. Some veterinary vaccines have been deployed for use orally (for example, infectious bronchitis vaccines in poultry), intranasally (for example, bovine CoV vaccines in calves) or as an oral prime followed by an intramuscular boost (for example, transmissible gastroenteritis vaccines in pigs). Induction of mucosal immunity, mediated by IgA, is thought to increase the protective efficacy of vaccines.
The emergence of CoV spike protein variants may impact vaccine performance, resulting in insufficient protection and necessitating updates to vaccine immunogens. The strategies used to increase the breadth of the protective immune response against different CoV variants include (1) prime–boost regimens using vaccines incorporating different CoV variants (that is, vaccinating with one strain and boosting with another) and (2) inclusion of multiple CoV strains within a single vaccine.
Antibody-dependent enhancement of CoV infection following vaccination and virus exposure can be readily demonstrated in cats. This may provide a useful model to understand the antibody-dependent enhancement phenomenon.
Monitoring of CoV antibody seroprevalence in poultry has been used to inform decisions on whether to implement a vaccination programme on the basis of the levels of flock immunity. This is primarily aimed at achieving a cost-efficient disease control programme but could also be used in a scenario where vaccine supply is limited.
Operational considerations
The target product profile for any vaccine, whether human or veterinary, needs to incorporate an efficient manufacturing strategy, the design of optimal vaccination regimens and consideration of deployment requirements very early in the development pipeline. All these factors influence the final cost per vaccine dose and, after consideration of the potential benefit of using the product, inform go versus no-go decisions on vaccine implementation programmes and policy. The business case for the development of vaccines against many of the known zoonotic pathogens with epidemic potential is poor78. This is largely due to the sporadic nature of the epidemics they cause — for example, there are typically intervals of 5–15 years between epidemics of Rift Valley fever, and there are even longer intervals between epidemics of CCHF and other diseases — in addition to their restricted geography and poor data on their economic costs, which make the design of cost-effective vaccine implementation plans challenging. The costs associated with vaccine development and manufacture (Fig. 1) mean that returns on investments made by vaccine developers for such diseases are unlikely to be high.
Initiatives such as the Coalition for Epidemic Preparedness Innovations (CEPI) are de-risking the human vaccine development process through provision of funding to support early-stage to late-stage development of vaccines against epidemics, including clinical evaluation that is crucial for licensure79. Funding schemes to advance veterinary vaccine development are also available, although none exclusively targets epizootic diseases. The Global Alliance for Livestock Veterinary Medicines (GALVmed) was founded in 2004 with a primary focus on supporting the development and eventual registration of control interventions for a wide range of livestock diseases in low-income and middle-income countries (LMICs)80; the CEPI now plays a similar role for human vaccines.
For veterinary vaccines, the ideal cost per dose for ruminants should be less than US$0.5 to allow cost-effective use in LMICs81. The per dose cost of human vaccines tends to be much higher and highly variable from product to product82. However, although higher human vaccine costs can be tolerated, especially in high-income countries, vaccine cost remains a key factor that underlies the demand, affordability and implementation of immunization programmes in LMICs83. To further reduce deployment costs, most veterinary vaccines are multivalent, composed of different immunogens co-formulated to target two or more diseases with a single vaccination (see Table 2 for coronavirus vaccines as an example). Co-administration of different immunogens in a single product (for example, the childhood pentavalent vaccine that targets diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae type b) is commonplace for the Expanded Programme on Immunization in children, which accounts for the bulk of global vaccine use. Cross-learning between the veterinary and medical fields from their respective experiences relating to vaccine development and implementation of programmes based on multivalent or co-administered products could be of mutual benefit.
Where a single vaccine is developed for use in both animals and humans, the same vaccine master seed stock could be used to generate bulk material that is then processed in parallel for human and animal use in accordance with the respective manufacturing requirements to derive a livestock product and one for use in humans from the same manufacturing run. Bulk material could be stockpiled, with downstream processing initiated as soon as the need for vaccination arises. However, this strategy would be successful only if the bulk material was stable in the long term and a regulatory approval strategy incorporating veterinary and human considerations was in place. Harmonized regulatory processes allowing mutual recognition of vaccine registration procedures between countries are already in place, aiming to address operational bottlenecks that limit rapid access to licensed human and veterinary vaccines84,85.
Conclusions
Studying animal pathogens, diseases and protective immune responses has had a major impact on controlling human diseases over the past century. It is usually the case that new vaccine platforms are deployed more rapidly in veterinary species than in humans. Veterinary vaccines commonly undergo rigorous safety and efficacy testing in the target hosts using challenge models before registration and widespread use. Safety testing and pharmacovigilance are especially important in food-producing animals. Also, developing cost-effective manufacturing at scale is essential for animal vaccines. In the past few decades, the widespread use of safe and effective vaccines in livestock has given confidence to develop the same platforms or indeed the same active substances for use in human vaccines. However, there remain untapped opportunities to leverage advances in human and veterinary immunology for the development of vaccines, as well as operational experiences to inform vaccine deployment. Effective control of zoonotic infections, which account for the bulk of public health emergencies, require One Health approaches with complementary interventions in both animal hosts and humans. The success of the One Health approach in eliminating disease burden also requires attention to the challenges associated with eradication of zoonotic diseases in natural reservoirs. Indeed, the elimination of the risk associated with other natural hosts, such as bats, rodents and arthropods, is a long-standing challenge, which may be addressed only through improved understanding of reservoir species immunobiology and epidemiology. Research strategies and funding priorities need to be realigned to improve interactions between animal and human health communities.
Acknowledgements
The authors are grateful to P. Bejon and M. Hamaluba for helpful discussions and comments on the manuscript. G.M.W. is supported by a fellowship from the Oak Foundation and grants from the Wellcome Trust (220991/Z/20/Z and 203077/Z/16/Z). T.A.B. is supported by the UK Medical Research Council (MR/S007555/1) and by the Wellcome Trust through the Wellcome Centre for Human Genetics (203141/Z/16/Z). As the authors are supported by the Wellcome Trust, for the purpose of open access, the authors have applied a CC-BY public copyright licence to any author accepted manuscript version arising from this submission.
Author contributions
The authors contributed equally to all aspects of the article.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Immunology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130433. doi: 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh TL, Yoder J, Deboch T, McElwain TF, Palmer GH. Livestock vaccinations translate into increased human capital and school attendance by girls. Sci. Adv. 2016;2:e1601410. doi: 10.1126/sciadv.1601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth JA. Veterinary vaccines and their importance to animal health and public health. Procedia Vaccinol. 2011;5:127–136. doi: 10.1016/j.provac.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knueppel D, Cardona C, Msoffe P, Demment M, Kaiser L. Impact of vaccination against chicken Newcastle disease on food intake and food security in rural households in Tanzania. Food Nutr. Bull. 2010;31:436–445. doi: 10.1177/156482651003100306. [DOI] [PubMed] [Google Scholar]
- 5.McElwain TF, Thumbi SM. Animal pathogens and their impact on animal health, the economy, food security, food safety and public health. Rev. Sci. Tech. 2017;36:423–433. doi: 10.20506/rst.36.2.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UK Vaccine R&D Network. Vaccine development process map. Vaccine Developmenthttps://www.vaccinedevelopment.org.uk/ (2020). [DOI] [PMC free article] [PubMed]
- 7.Pollard AJ, et al. Human microbial challenge: the ultimate animal model. Lancet Infect. Dis. 2012;12:903–905. doi: 10.1016/S1473-3099(12)70292-X. [DOI] [PubMed] [Google Scholar]
- 8.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bean AG, et al. Studying immunity to zoonotic diseases in the natural host-keeping it real. Nat. Rev. Immunol. 2013;13:851–861. doi: 10.1038/nri3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berche P. Louis Pasteur, from crystals of life to vaccination. Clin. Microbiol. Infect. 2012;18:1–6. doi: 10.1111/j.1469-0691.2012.03945.x. [DOI] [PubMed] [Google Scholar]
- 11.Perez O, Paolazzi CC. Production methods for rabies vaccine. J. Ind. Microbiol. Biotechnol. 1997;18:340–347. doi: 10.1038/sj.jim.2900391. [DOI] [PubMed] [Google Scholar]
- 12.Warimwe GM, et al. Chimpanzee adenovirus vaccine provides multispecies protection against Rift Valley fever. Sci. Rep. 2016;6:20617. doi: 10.1038/srep20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abela-Ridder B, et al. 2016: the beginning of the end of rabies? Lancet Glob. Health. 2016;4:e780–e781. doi: 10.1016/S2214-109X(16)30245-5. [DOI] [PubMed] [Google Scholar]
- 15.Hampson K, et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morse SS, et al. Prediction and prevention of the next pandemic zoonosis. Lancet. 2012;380:1956–1965. doi: 10.1016/S0140-6736(12)61684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor G, et al. Efficacy of a virus-vectored vaccine against human and bovine respiratory syncytial virus infections. Sci. Transl. Med. 2015;7:300ra127. doi: 10.1126/scitranslmed.aac5757. [DOI] [PubMed] [Google Scholar]
- 18.Taylor G. Bovine model of respiratory syncytial virus infection. Curr. Top. Microbiol. Immunol. 2013;372:327–345. doi: 10.1007/978-3-642-38919-1_16. [DOI] [PubMed] [Google Scholar]
- 19.Luca S, Mihaescu T. History of BCG vaccine. Maedica. 2013;8:53–58. [PMC free article] [PubMed] [Google Scholar]
- 20.Waters WR, Palmer MV, Buddle BM, Vordermeier HM. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine. 2012;30:2611–2622. doi: 10.1016/j.vaccine.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Prentice S, et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(20)30653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenner F. Smallpox: emergence, global spread, and eradication. Hist. Philos. Life Sci. 1993;15:397–420. [PubMed] [Google Scholar]
- 23.Esparza J, Lederman S, Nitsche A, Damaso CR. Early smallpox vaccine manufacturing in the United States: introduction of the “animal vaccine” in 1870, establishment of “vaccine farms”, and the beginnings of the vaccine industry. Vaccine. 2020;38:4773–4779. doi: 10.1016/j.vaccine.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Settembre EC, Dormitzer PR, Rappuoli R. Bringing influenza vaccines into the 21st century. Hum. Vaccin. Immunother. 2014;10:600–604. doi: 10.4161/hv.27600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrett AD. Yellow fever in Angola and beyond—the problem of vaccine supply and demand. N. Engl. J. Med. 2016;375:301–303. doi: 10.1056/NEJMp1606997. [DOI] [PubMed] [Google Scholar]
- 26.Walsh EE, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porta C, et al. Rational engineering of recombinant picornavirus capsids to produce safe, protective vaccine antigen. PLoS Pathog. 2013;9:e1003255. doi: 10.1371/journal.ppat.1003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh CL, et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science. 2020;369:1501–1505. doi: 10.1126/science.abd0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crank MC, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019;365:505–509. doi: 10.1126/science.aav9033. [DOI] [PubMed] [Google Scholar]
- 30.Bundle DR, McGiven J. Brucellosis: improved diagnostics and vaccine insights from synthetic glycans. Acc. Chem. Res. 2017;50:2958–2967. doi: 10.1021/acs.accounts.7b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Groot AS, et al. Better epitope discovery, precision immune engineering, and accelerated vaccine design using immunoinformatics tools. Front. Immunol. 2020;11:442. doi: 10.3389/fimmu.2020.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 33.Duffy PE, Patrick Gorres J. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. 2020;5:48. doi: 10.1038/s41541-020-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mwangi W, Maccari G, Hope JC, Entrican G, Hammond JA. The UK Veterinary Immunological Toolbox website: promoting vaccine research by facilitating communication and removing reagent barriers. Immunology. 2020;161:25–27. doi: 10.1111/imm.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tungatt K, et al. Induction of influenza-specific local CD8 T-cells in the respiratory tract after aerosol delivery of vaccine antigen or virus in the Babraham inbred pig. PLoS Pathog. 2018;14:e1007017. doi: 10.1371/journal.ppat.1007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holzer B, et al. Immunogenicity and protective efficacy of seasonal human live attenuated cold-adapted influenza virus vaccine in pigs. Front. Immunol. 2019;10:2625. doi: 10.3389/fimmu.2019.02625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holzer B, et al. Comparison of heterosubtypic protection in ferrets and pigs induced by a single-cycle influenza vaccine. J. Immunol. 2018;200:4068–4077. doi: 10.4049/jimmunol.1800142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gauger PC, et al. Live attenuated influenza A virus vaccine protects against A(H1N1)pdm09 heterologous challenge without vaccine associated enhanced respiratory disease. Virology. 2014;471–473:93–104. doi: 10.1016/j.virol.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Pescovitz MD, Sakopoulos AG, Gaddy JA, Husmann RJ, Zuckermann FA. Porcine peripheral blood CD4+/CD8+ dual expressing T-cells. Vet. Immunol. Immunopathol. 1994;43:53–62. doi: 10.1016/0165-2427(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 40.Zuckermann FA, Husmann RJ. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology. 1996;87:500–512. [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Parkhouse RM. Differential expression of CD8 epitopes amongst porcine CD8-positive functional lymphocyte subsets. Immunology. 1997;92:45–52. doi: 10.1046/j.1365-2567.1997.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuckermann FA, Gaskins HR. Distribution of porcine CD4/CD8 double-positive T lymphocytes in mucosa-associated lymphoid tissues. Immunology. 1996;87:493–499. [PMC free article] [PubMed] [Google Scholar]
- 43.Overgaard NH, Jung JW, Steptoe RJ, Wells JW. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J. Leukoc. Biol. 2015;97:31–38. doi: 10.1189/jlb.1RU0814-382. [DOI] [PubMed] [Google Scholar]
- 44.Clenet ML, Gagnon F, Moratalla AC, Viel EC, Arbour N. Peripheral human CD4+CD8+ T lymphocytes exhibit a memory phenotype and enhanced responses to IL-2, IL-7 and IL-15. Sci. Rep. 2017;7:11612. doi: 10.1038/s41598-017-11926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackay CR, Hein WR. A large proportion of bovine T cells express the gamma delta T cell receptor and show a distinct tissue distribution and surface phenotype. Int. Immunol. 1989;1:540–545. doi: 10.1093/intimm/1.5.540. [DOI] [PubMed] [Google Scholar]
- 46.Takamatsu HH, et al. Porcine gammadelta T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet. Immunol. Immunopathol. 2006;112:49–61. doi: 10.1016/j.vetimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Rogers AN, et al. Gammadelta T cell function varies with the expressed WC1 coreceptor. J. Immunol. 2005;174:3386–3393. doi: 10.4049/jimmunol.174.6.3386. [DOI] [PubMed] [Google Scholar]
- 48.Groh V, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J. Exp. Med. 1989;169:1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang B, et al. Protection of calves by a prefusion-stabilized bovine RSV F vaccine. NPJ Vaccines. 2017;2:7. doi: 10.1038/s41541-017-0005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumiya M, et al. Gene expression and cytokine profile correlate with mycobacterial growth in a human BCG challenge model. J. Infect. Dis. 2015;211:1499–1509. doi: 10.1093/infdis/jiu615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ronacher K, Sinha R, Cestari M. IL-22: an underestimated player in natural resistance to tuberculosis? Front. Immunol. 2018;9:2209. doi: 10.3389/fimmu.2018.02209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhuju S, et al. Global gene transcriptome analysis in vaccinated cattle revealed a dominant role of IL-22 for protection against bovine tuberculosis. PLoS Pathog. 2012;8:e1003077. doi: 10.1371/journal.ppat.1003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton CA, Mahan S, Entrican G, Hope JC. Interactions between natural killer cells and dendritic cells favour T helper1-type responses to BCG in calves. Vet. Res. 2016;47:85. doi: 10.1186/s13567-016-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zufferey C, Germano S, Dutta B, Ritz N, Curtis N. The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNgamma response in bacille Calmette-Guerin (BCG)-immunized infants. PLoS ONE. 2013;8:e77334. doi: 10.1371/journal.pone.0077334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanner R, Villarreal-Ramos B, Vordermeier HM, McShane H. The humoral immune response to BCG vaccination. Front. Immunol. 2019;10:1317. doi: 10.3389/fimmu.2019.01317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saphire EO, et al. Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science. 2001;293:1155–1159. doi: 10.1126/science.1061692. [DOI] [PubMed] [Google Scholar]
- 57.Pejchal R, et al. Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1. Proc. Natl Acad. Sci. USA. 2010;107:11483–11488. doi: 10.1073/pnas.1004600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, et al. Reshaping antibody diversity. Cell. 2013;153:1379–1393. doi: 10.1016/j.cell.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanfield RL, et al. The unusual genetics and biochemistry of bovine immunoglobulins. Adv. Immunol. 2018;137:135–164. doi: 10.1016/bs.ai.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sok D, et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature. 2017;548:108–111. doi: 10.1038/nature23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamers-Casterman C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 62.Muyldermans S, Smider VV. Distinct antibody species: structural differences creating therapeutic opportunities. Curr. Opin. Immunol. 2016;40:7–13. doi: 10.1016/j.coi.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu S, Zimmerman D, Deem SL. A review of zoonotic pathogens of dromedary camels. Ecohealth. 2019;16:356–377. doi: 10.1007/s10393-019-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanfield RL, et al. Structural basis of broad HIV neutralization by a vaccine-induced cow antibody. Sci. Adv. 2020;6:eaba0468. doi: 10.1126/sciadv.aba0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brodin P, Davis MM. Human immune system variation. Nat. Rev. Immunol. 2017;17:21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mariner JC, et al. Rinderpest eradication: appropriate technology and social innovations. Science. 2012;337:1309–1312. doi: 10.1126/science.1223805. [DOI] [PubMed] [Google Scholar]
- 68.Henderson DA. The eradication of smallpox—an overview of the past, present, and future. Vaccine. 2011;29:D7–D9. doi: 10.1016/j.vaccine.2011.06.080. [DOI] [PubMed] [Google Scholar]
- 69.WHO Rabies Modelling Consortium The potential effect of improved provision of rabies post-exposure prophylaxis in Gavi-eligible countries: a modelling study. Lancet Infect. Dis. 2019;19:102–111. doi: 10.1016/S1473-3099(18)30512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zinsstag J, et al. Vaccination of dogs in an African city interrupts rabies transmission and reduces human exposure. Sci. Transl. Med. 2017;9:eaaf6984. doi: 10.1126/scitranslmed.aaf6984. [DOI] [PubMed] [Google Scholar]
- 71.Roth F, et al. Human health benefits from livestock vaccination for brucellosis: case study. Bull. World Health Organ. 2003;81:867–876. [PMC free article] [PubMed] [Google Scholar]
- 72.Mehand MS, Al-Shorbaji F, Millett P, Murgue B. The WHO R&D blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018;159:63–67. doi: 10.1016/j.antiviral.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mbabu M, et al. Establishing a One Health office in Kenya. Pan Afr. Med. J. 2014;19:106. doi: 10.11604/pamj.2014.19.106.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hassan A, et al. Epidemiological investigation of a rift valley fever outbreak in humans and livestock in Kenya, 2018. Am. J. Trop. Med. Hyg. 2020;103:1649–1655. doi: 10.4269/ajtmh.20-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdrakhmanov SK, et al. Spatiotemporal analysis of foot-and-mouth disease outbreaks in the Republic of Kazakhstan, 1955–2013. Transbound. Emerg. Dis. 2018;65:1235–1245. doi: 10.1111/tbed.12864. [DOI] [PubMed] [Google Scholar]
- 76.Gsell PS, et al. Ring vaccination with rVSV-ZEBOV under expanded access in response to an outbreak of Ebola virus disease in Guinea, 2016: an operational and vaccine safety report. Lancet Infect. Dis. 2017;17:1276–1284. doi: 10.1016/S1473-3099(17)30541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitehouse CA. Crimean–Congo hemorrhagic fever. Antivir. Res. 2004;64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 78.Plotkin SA, Mahmoud AA, Farrar J. Establishing a global vaccine-development fund. N. Engl. J. Med. 2015;373:297–300. doi: 10.1056/NEJMp1506820. [DOI] [PubMed] [Google Scholar]
- 79.Brende B, et al. CEPI—a new global R&D organisation for epidemic preparedness and response. Lancet. 2017;389:233–235. doi: 10.1016/S0140-6736(17)30131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bessell PR, et al. Assessing the impact of a novel strategy for delivering animal health interventions to smallholder farmers. Prev. Vet. Med. 2017;147:108–116. doi: 10.1016/j.prevetmed.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 81.Lyons NA, Jemberu WT, Chaka H, Salt JS, Rushton J. Field-derived estimates of costs for Peste des Petits Ruminants vaccination in Ethiopia. Prev. Vet. Med. 2019;163:37–43. doi: 10.1016/j.prevetmed.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.WHO European Region Member States. Review of vaccine price data (WHO, 2013).
- 83.Wouters OJ, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.GALVmed. A policy scoping study on harmonization of registration requirements for veterinary products for mutual recognition among East African community partner states (Global Alliance for Livestock Veterinary Medicines, 2016).
- 85.Ndomondo-Sigonda M, et al. Harmonization of medical products regulation: a key factor for improving regulatory capacity in the East African community. BMC Public Health. 2021;21:187. doi: 10.1186/s12889-021-10169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McLellan JS, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu X, et al. The origin of enantioselectivity in aldolase antibodies: crystal structure, site-directed mutagenesis, and computational analysis. J. Mol. Biol. 2004;343:1269–1280. doi: 10.1016/j.jmb.2004.08.102. [DOI] [PubMed] [Google Scholar]
- 88.Koch K, et al. Selection of nanobodies with broad neutralizing potential against primary HIV-1 strains using soluble subtype C gp140 envelope trimers. Sci. Rep. 2017;7:8390. doi: 10.1038/s41598-017-08273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alluwaimi AM, Alshubaith IH, Al-Ali AM, Abohelaika S. The coronaviruses of animals and birds: their zoonosis, vaccines, and models for SARS-CoV and SARS-CoV2. Front. Vet. Sci. 2020;7:582287. doi: 10.3389/fvets.2020.582287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tizard IR. Vaccination against coronaviruses in domestic animals. Vaccine. 2020;38:5123–5130. doi: 10.1016/j.vaccine.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Francis MJ. Lessons from animal coronaviruses. Biologist. 2020;67:18–21. [Google Scholar]