Abstract
BACKGROUND.
Many patients with invasive urothelial cell cancer are poor candidates for cisplatin-based chemotherapy, and many are high risk for cystectomy. Southwest Oncology Group Trial 8733 was designed to address treatment for such patients.
METHODS.
Eligible patients had primary or recurrent muscle-invasive disease with transitional cell or squamous cell histology, a performance status from 0 to 2, no extrapelvic disease, a life expectancy >3 months, and adequate hematologic function. The treating clinician assigned patients to operable or inoperable groups. All patients received 2 cycles of 5-fluorouracil (5-FU) at a dose of 1000 mg/m2 per day × 4 starting concurrently with radiation at a dose of 200 centigrays per day × 10 each cycle. After 2 cycles, operable patients with positive biopsies underwent cystectomy, and patients with negative biopsies received a third cycle of chemoradiotherapy. Patients in the inoperable group received 3 cycles without interim biopsy.
RESULTS.
Eighteen of 24 eligible patients in the operable group were evaluable for response. Five patients had a complete response (CR), 9 patients had stable disease, 1 patient had progressive disease, and 3 patients were not assessable. The median progression-free survival was 10 months (95% confidence interval [95% CI], 4–14 months), and the median overall survival was 18 months (95% CI, 7–28 months). In the inoperable group, 35 of 37 eligible patients were evaluable for response with 17 CRs (49%; 95% CI, 31%–66%). The median progression-free survival was 13 months (95% CI, 10–17 months), and the median overall survival was 20 months (95% CI, 11–53 months). There were no episodes of grade 4 toxicity.
CONCLUSIONS.
In the current study, the combination of 5-FU and radiation was found to be tolerated well by patients with numerous comorbidities who could not tolerate cisplatin-based therapy or cystectomy.
Keywords: bladder cancer, urothelial cancer, bladder sparing, continuous infusion 5-fluorouracil, radiation
Standard treatment for patients with muscle-invasive transitional cell carcinoma of the bladder is cystectomy. The Southwest Oncology Group (SWOG) conducted a phase 3 trial of neoadjuvant chemotherapy followed by cystectomy versus cystectomy alone. The chemotherapy regimen consisted of methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (MVAC). This trial, S8710, demonstrated a survival benefit for patients who received chemotherapy in addition to cystectomy.1 During the accrual phase of the randomized trial, however, it was noted that many otherwise eligible patients were either poor surgical candidates or had impaired renal function and/or other comorbidities that made it difficult to treat with cisplatin or to administer prolonged general anesthesia for cystectomy. At that time, the majority of combined modality approaches for bladder cancer included cisplatin. 5-fluorouracil (5-FU), which generally is tolerated well when it is administered by 4-day continuous infusion, has no renal toxicity and is a radiosensitizer that has been used in combination with radiation in cancers of the head and neck, esophagus, stomach, pancreas, and anus. A phase I/II study2 of 34 patients with urothelial cancer of the bladder evaluated 5-FU and radiation in both operable patients and inoperable patients. This regimen had little toxicity and the complete response (CR) rate was 81% with a 5-year survival rate of 64%. Another small phase II trial of 20 patients also evaluated this combination.3 The CR rate in that trial was 74%, and the 5-year overall survival rate was 39%.
The SWOG sought to evaluate this approach further in patients who were poor surgical candidates or who otherwise were ineligible to receive cisplatin-based neoadjuvant therapy on S8710. In those patients who potentially could withstand surgery, cystectomy was performed only if there was persistent disease detected on biopsy after 2 cycles of chemotherapy and radiation. In all other patients, 3 cycles of chemotherapy and radiation were administered. The current report describes the tolerability of the regimen and the long-term outcome of these patients.
MATERIALS AND METHODS
Eligibility
All patients were recruited from participating SWOG institutions and had to have primary or recurrent muscle-invasive disease with transitional cell or squamous cell histology and could not be eligible for the ongoing SWOG trial S8710 of cystectomy versus MVAC followed by cystectomy. Pathologic review to confirm the diagnosis and presence of muscle invasion was performed by a single pathologist (W.A.S.). A SWOG performance status of 0 to 2 (Karnofsky score, 50–100), a life expectancy >3 months, and adequate hematologic function were necessary. A baseline computed tomography (CT) scan of the pelvis, a chest x-ray, and bone scans were required. Patients with disease outside the pelvis were not eligible. Patients who had received prior pelvic radiation and those with pre-existing gastrointestinal disorders, such as chronic diarrhea, malabsorption, extensive diverticular disease, or inflammatory bowel disease, also were ineligible. All patients signed informed consent approved by the local institutional review board.
At the time of initial registration, patients who were considered operable candidates with tumors classified as T2, T3, or T4 were assigned to the operable group. Patients with pelvic lymph node involvement, contraindications for cystectomy, or those who refused surgery were placed in the inoperable group. Descriptive information regarding transurethral bladder tumor resection (TURBT) status was collected.
The treatment schema is shown in Figure 1. After completing baseline staging studies and registration, patients were treated with 5-FU at a dose of 1000 mg/m2 per day (2000 mg maximum dose per day) by continuous intravenous infusion on Days 1 through 4 and started concurrent radiation at a dose of 200 centigrays (cGy) per day to the bladder and pelvic lymph nodes. Treatments were delivered on Days 1 through 5 and Days 8 through 12 through a 4-field arrangement involving parallel, opposed anteroposterior/posteroanterior and right/left lateral portals encompassing the bladder as well as the obturator, hypogastric, and external iliac lymph node basins. This was followed by another cycle of the same doses of chemotherapy and radiation beginning again on Day 22. Operable patients underwent a cystoscopy with at least 3 deep biopsies within 3 weeks after completion of 40 grays of radiation at approximately Week 8. After the biopsies, patients in the operable group underwent a second registration. The treatment course was based on pathologic findings. Patients who had persistent tumor were offered cystectomy. Patients who had no evidence of tumor received a third cycle of chemotherapy and radiation rather than cystectomy. During the third cycle, chemotherapy was administered as described above and 400 cGy were delivered to the pelvis with a boost of 1600 cGy delivered to the bladder with 1.5-cm margins. Patients in the inoperable group received 3 consecutive cycles of chemotherapy and radiation as described above for patients in the operable group, with the third cycle starting on Day 43 and finishing on Day 54. These patients underwent CT of the pelvis and cystoscopy 2 months after the completion of treatment (approximately Week 16) and were to undergo biopsies as described above for patients in the operable group. There was no second registration for the inoperable group. Thereafter, follow-up for both groups consisted of cystoscopy every 2 months for the first year, every 3 months for the second year, and every 6 months thereafter.
FIGURE 1.
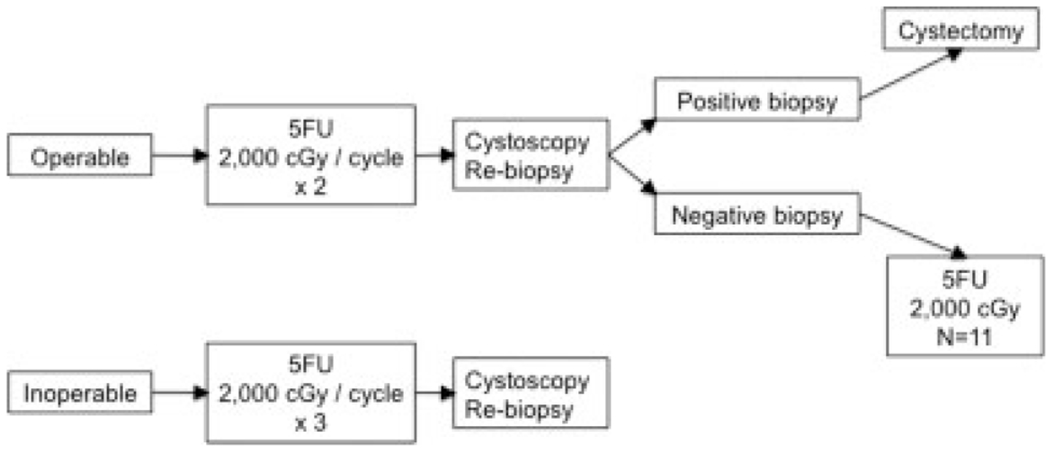
Treatment schema. 5FU indicates 5-fluorouracil; cGy, centigrays.
Dose modifications were based on laboratory values at the time of planned chemotherapy administration and on interim nonhematologic toxicities of the preceding cycle. 5-FU was administered at the full dose if the absolute neutrophil count was ≥1500 × 106/L and the platelet count was ≥100,000 × 109/L. If the absolute neutrophil count was <1500 × 106/L but ≥1000 × 106/L or if the platelet count was <100,000 × 109/L but ≥75,000 × 109/L, then the 5-FU dose was reduced to 750 mg/m2. If the absolute neutrophil count was <1000 × 106/L or the platelet count was <75,000 × 109/L, then the chemotherapy and radiation were held for 1 week, and the counts were repeated. If the counts remained low, then radiation was resumed without chemotherapy. All toxicities were graded according to the Cancer Clinical Trials Common Toxicity Criteria. For patients with grade 3 or 4 gastrointestinal toxicity, stomatitis, or skin toxicity, the chemotherapy was withheld in subsequent cycles.
Statistical Considerations
Descriptive factors that were collected at the time of registration were morphologic description (sessile vs papillary) and the extent of removal of lesion(s) (resected vs not resected) by TURBT. The primary endpoint was to estimate the probability of a CR, which was defined as a negative deep-muscle biopsy (cytology alone was not adequate); complete radiographic regression of initially positive regional lymph nodes, if applicable; and no new lesions. Secondary endpoints were progression-free survival and overall survival. If the tumor was resected completely by TURBT, then response criteria were not applicable and disease progression was defined as a positive biopsy of the bladder; a regional or distant disease site; or CT, bone scan, or plain film evidence of regional or distant disease recurrence. If the tumor was not resected completely by TURBT, then disease progression was defined as evidence of progression of the primary tumor by cystoscopy or radiographically or the development of new bladder lesions. Radiographic enlargement of prior adenopathy or the development of distant metastasis also constituted progression. Progression-free survival was defined as the time from registration until first evidence of disease progression or death from any cause. Overall survival was defined the time from registration to death from any cause. Patients were censored at their last known contact date.
Separate accrual goals were set for the operable and inoperable groups. For the operable group, a true probability of attaining a CR ≥30% was of interest, whereas further investigation would not be pursued if the response probability was ≤10%. A 2-stage design was used in which ≥2 responses observed in the first 20 patients would enable the accrual of 20 additional patients. Eight or more responses observed in 40 patients would provide strong enough evidence of treatment benefit to justify further study of the regimen. For the inoperable group, a true probability of attaining a CR ≥45% was of interest, whereas a response probability ≤20% was not. The inoperable group received an additional course of treatment, hence the requirement of a higher response rate. Twenty patients were to be accrued to the first stage and if 3 or more responses were observed, then an additional 15 patients were to be accrued. Twelve or more responses observed in 35 patients would warrant further study.
RESULTS
From 1988 to 1997, 81 patients were recruited from 23 SWOG institutions. There were 24 eligible patients and 9 ineligible patients in the operable group, and there were 37 eligible patients and 11 ineligible patients in the inoperable group. Patient characteristics and descriptive factors are listed in Table 1. Reasons for ineligibility in the operable group included no evidence of muscle invasion on pathology review (3 patients), prestudy biopsy performed >42 days before registration (2 patients), prior pelvic radiation (1 patient), missing initial prestudy forms (1 patient), missing evidence of prestudy bone scan or chest x-ray (1 patient), and insufficient materials for pathology review (1 patient). Reasons for ineligibility in the inoperable group were no evidence of muscle invasion on pathology review (8 patients), missing evidence of prestudy bone scan or chest x-ray (2 patients), and the presence of bone metastases at registration (1 patient).
TABLE 1.
Baseline Characteristics for All Eligible Patients
| No. of patients (%) |
||
|---|---|---|
| Characteristic | Operable group, n = 24 | Inoperable group, n = 37 |
| Median age [range], y | 71.5 [50.6–89.8] | 75.4 [46.4–93.3] |
| Sex | ||
| Men | 19 (79) | 21 (57) |
| Women | 5 (21) | 16 (43) |
| Race | ||
| Black | 3 (13) | |
| White | 21 (88) | 37 (100) |
| Hispanic | 0 (0) | 2 (5) |
| Performance status | ||
| 0 | 17 (71) | 13 (35) |
| 1 | 5 (21) | 18 (49) |
| 2 | 2 (8) | 6 (16) |
| Pretherapy TURBT | ||
| Yes | 17 (71) | 25 (68) |
| No | 7 (12) | 12 (32) |
| Morphology | ||
| Papillary | 9 (38) | 15 (41) |
| Sessile | 15 (62) | 22 (59) |
TURBT indicates transurethral bladder tumor resection.
Response Data
Figure 2 summarizes the results for patients in the operable group. Of 24 eligible patients in the operable group, 17 patients underwent pretreatment TURBT and 7 patients did not. Of the 17 patients who underwent TURBT, 6 patients achieved a complete resection and 11 patients had persistent disease. Response criteria could not be applied to the 6 patients who achieved complete resection with TURBT. Of the 18 patients who had evaluable disease, 5 patients attained a CR (28%; 95% confidence interval [95% CI], 10%–53%), 9 patients were stable, 1 patient developed disease progression, and 3 patients were not assessable because of early death (1 patient), off study before biopsy (1 patient), and insufficient data (1 patient), and we assumed that they were nonresponders. Of the 17 patients in the operable group who were eligible for postcystoscopy registration, 6 patients had a positive biopsy, and 11 patients had a negative biopsy after 2 cycles of therapy. Five of 6 patients who had positive biopsies underwent cystectomy. Liver metastases were discovered in the sixth patient, and cystectomy was cancelled for that patient. Kaplan-Meier plots of progression-free and overall survival are shown in Figure 3. The median progression-free survival for patients who had a negative biopsy was 28 months compared with 8 months for patients who had a positive biopsy (log-rank P = .004; curves not shown). For all patients in the operable group, the median progression-free survival was 10 months (95% CI, 5–14 months), and the median overall survival was 18 months (95% CI, 7–28 months).
FIGURE 2.
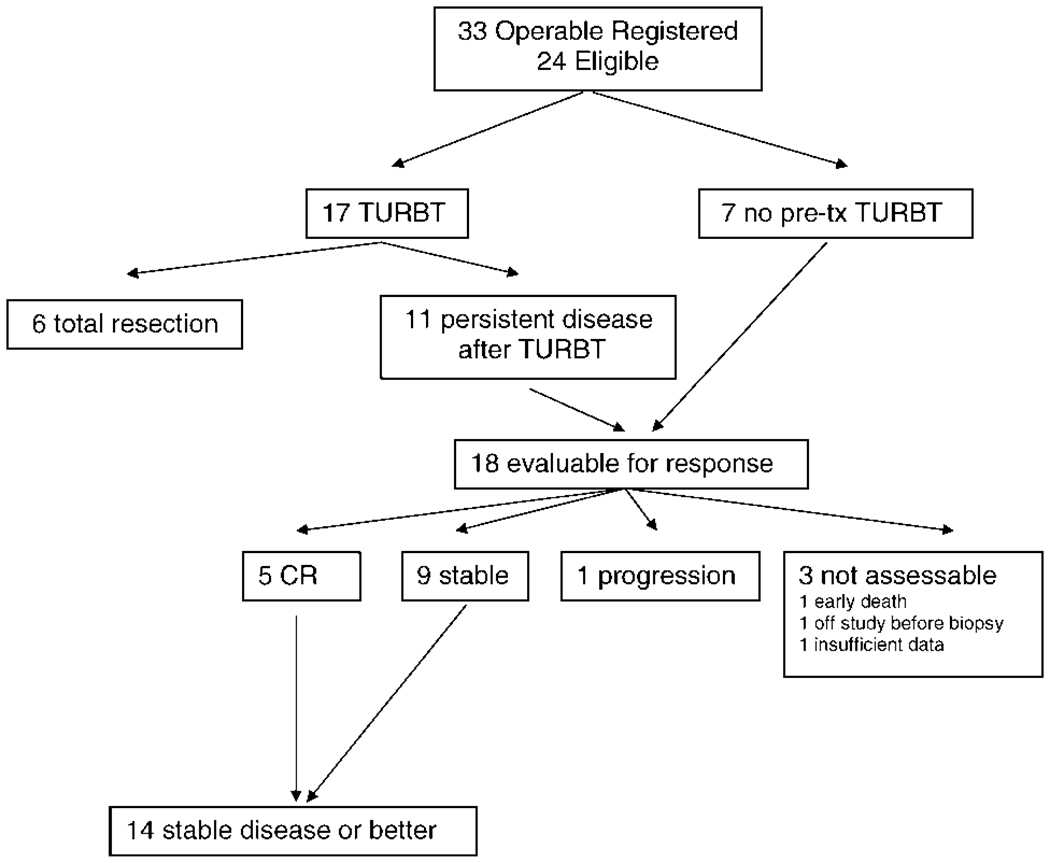
Operable group. TURBT indicates transurethral bladder tumor resection; pre-tx, pretreatment.
FIGURE 3.
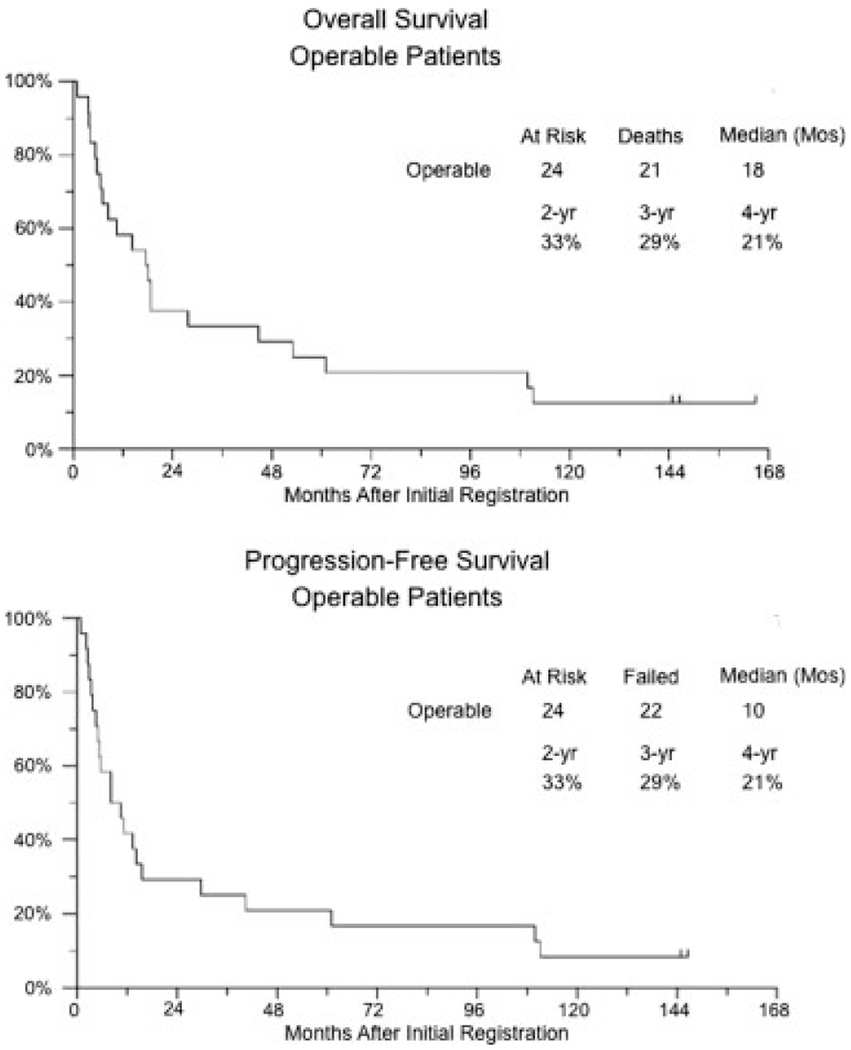
Kaplan-Meier plots of progression-free and overall survival for operable patients.
The response data for the inoperable group are summarized in Figure 4. Of 37 eligible patients, 25 patients underwent pretherapy TURBT, and 2 of those patients achieved complete resection of disease. Of 35 patients who were evaluable for response (23 patients with persistent disease after TURBT and 12 patients who did not undergo TURBT), 17 patients (49%) achieved a CR (95% CI, 31%–66%), 6 patients were stable, 2 patients developed disease progression, and 10 patients were not assessable for response (1 early death, 8 missing rebiopsies, and 1 with missing scans), and we assumed that they were nonresponders. Figure 5 shows the Kaplan-Meier progression-free and overall survival plots for this group. The median progression-free survival for all inoperable patients was 13 months (95% CI, 10–17 months), and the median overall survival was 20 months (95% CI, 11–53 months). Stratifying by CR status in the inoperable group, the median progression-free survival for non-CR responders was 9 months, and the median was 27 months for patients who achieved a CR (log-rank P = .0003).
FIGURE 4.
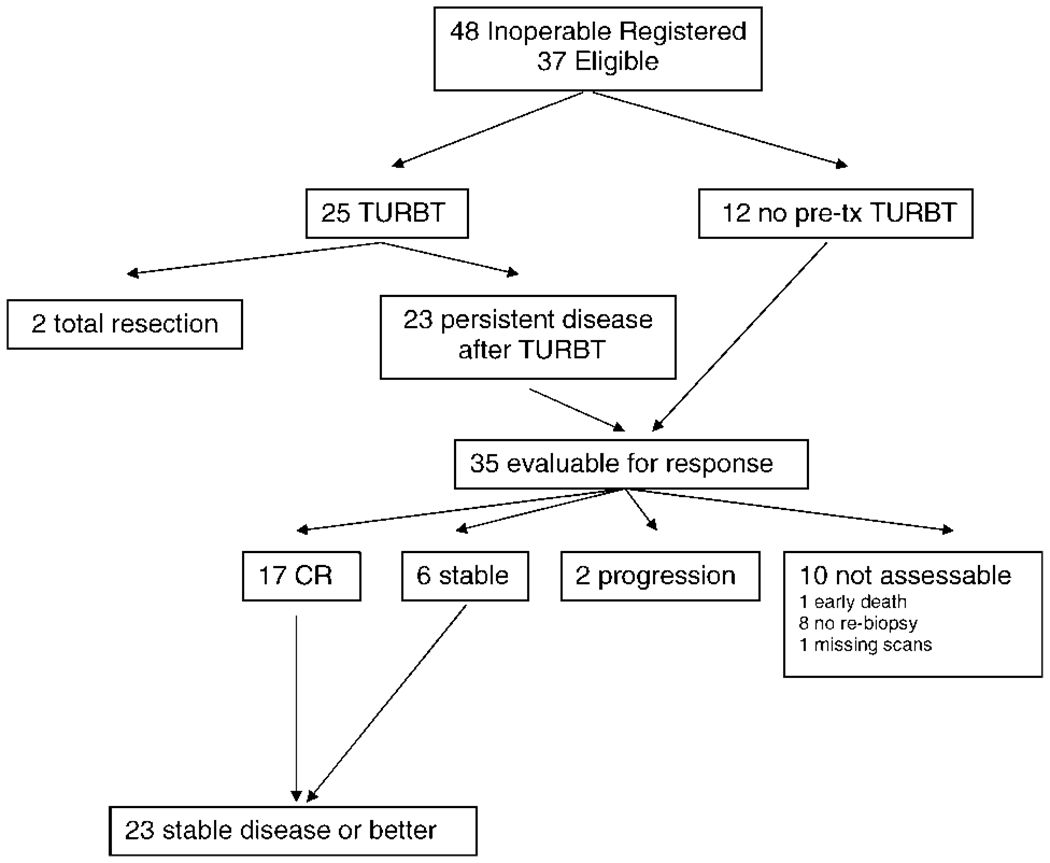
Inoperable group. TURBT indicates transurethral bladder tumor resection; pre-tx, pretreatment.
FIGURE 5.
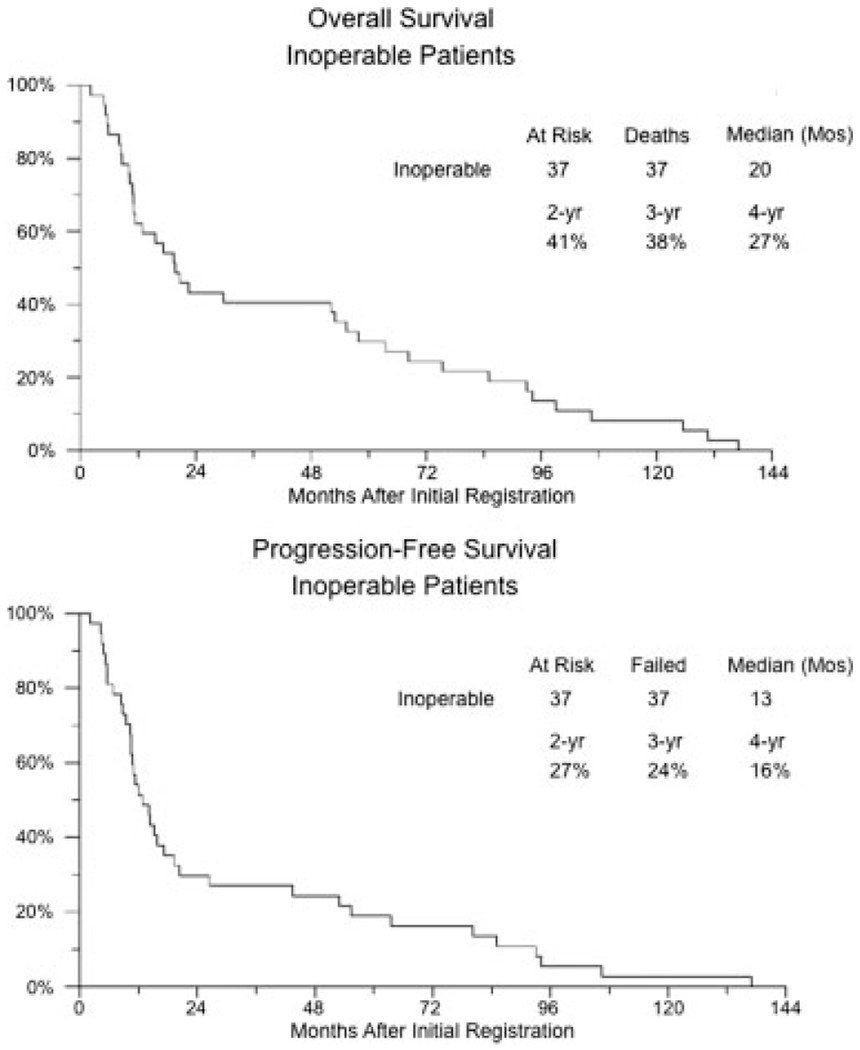
Kaplan-Meier plots of progression-free and overall survival for inoperable patients.
Entire Study Population
The median survival for all 61 eligible patients on the entire trial was 19 months, and the Kaplan-Meier estimated survival rate at 5 years was 28%. Of 58 deaths, the cause was coded in 34 patients. Death in 25 patients was because of cancer, including 22 patients with bladder cancer, 1 patient with nonsmall cell lung cancer, and 2 patients who had either bladder cancer and/or lung cancer. Two other patients were known to have metastatic disease but died of other causes (cardiac and lung). One patient died of an aneurysm with a CR in the bladder but a recurrence in the urethra. Three patients died of cardiac disease, and 3 patients died of other causes without evidence of recurrent bladder cancer. The cause of death could not be determined for an additional 24 patients.
Toxicities
There were no episodes of grade 4 or 5 toxicity in any patients on this trial. In the operable group, there was 1 episode of grade 3 granulocytopenia in 22 patients who received 2 cycles of chemotherapy and radiation before cystoscopy and second registration. The most common toxicities were grade 1 diarrhea (16 patients), urinary urgency and frequency (9 patients), stomatitis (7 patients), and fatigue (6 patients). Of the 5 patients who underwent cystectomy, grade 1 anemia was reported in 1 patient, and there were no other toxicities reported. For the 11 patients who received a third cycle of chemotherapy and radiation, 4 patients experienced grade 3 toxicity, including diarrhea, urinary urgency and frequency, dyspnea, and leukopenia.
In the inoperable group, 33 of 37 patients received all 3 cycles of chemotherapy and radiation. Fourteen patients experienced grade 3 toxicity as their worst grade, and the most common were dysuria (3 patients), diarrhea (2 patients), bladder other (2 patients), and urinary urgency and frequency (2 patients).
DISCUSSION
This pilot study of patients who were not good candidates for MVAC and/or surgery or who refused surgery was designed to evaluate the tolerability and response rate of 5-FU with radiation in this population of patients with multiple comorbidities. The study clearly demonstrates that this therapy is tolerated well. All but 3 patients were followed until death, and some patients lived for >10 years. The median survival was 18 months in the operable group and 20 months in the inoperable group with 5-year overall survival rates of 22% and 30%, respectively. The 5-year survival rates for more contemporary series of radiation therapy alone range from 20% to 40%.4–8
In another trial conducted by the SWOG, 56 patients who were not fit medically for surgery or who refused surgery and had T2, T3, or T4 tumors; NX, N0, N1, or N2 lymph node status; and metastasis-negative (M0) disease received cisplatin, 5-FU, and radiation.9 Treatment was completed as planned in 53% of those patients compared with 92% of patients in our operable group and 100% of patients in our inoperable group from the current study. There were no treatment-related deaths in either study; however, 45% of patients who were evaluable for toxicity in the other study experienced grade 3 or 4 events after treatment with cisplatin, 5-FU, and radiation compared with 28% in the current study. In the other study, the estimated median survival with treatment with cisplatin and 5-FU was 27 months, and the overall survival rate at 5 years was 32%, compared with a median survival of 19 months and an overall 5-year survival rate of 28% with 5-FU and radiation noted in the current trial.
We acknowledge that the current study had many limitations. First, it was designed as a pilot study to offer a bladder-sparing approach to patients who had multiple comorbidities and therefore were poor candidates for the available SWOG trial evaluating neoadjuvant chemotherapy with MVAC followed by cystectomy versus cystectomy alone. Because some patients in this category otherwise were operable, whereas others clearly were not, the study was complex and certainly would not be the design of choice by contemporary standards. The study was not designed to compare bladder sparing with cystectomy or to determine whether 5-FU with radiation was superior to radiation alone. Furthermore, it would have been ideal to compare biopsy status after 2 cycles of chemoradiotherapy in both the operable group and the inoperable group to establish the pathologic response; however, patients in the inoperable group generally were poor candidates for general anesthesia. Consequently, even the required restaging biopsy, which was to be performed after treatment was complete, was not performed in many patients. In this trial, there was no attempt to evaluate bladder function and patient bother, although the treatment appeared to be tolerated extremely well by these patients with several other comorbidities.
This trial was completed before conformal external-beam radiation or taxanes were available. Newer drugs and improved radiation techniques, coupled with a better selection of patients using prognostic markers, currently may provide this population of patients with reasonable, less invasive treatment options. A recently reported phase 1 trial performed in this same population of patients10 demonstrated that gemcitabine and concurrent radiation is tolerated well. Whether the addition of chemotherapy adds benefit to radiation alone should be studied further in this population of patients who are not good surgical candidates, and the importance of preventing local disease recurrence on quality of life should be addressed.
Acknowledgments
Supported in part by the following Public Health Service Cooperative Agreement grants awarded by the National Cancer Institute, Department of Health and Human Services: CA38926, CA32102, CA46441, CA20319, CA04919, CA16385, CA14028, CA27057, CA22433, CA35192, CA76447, CA12644, CA58686, CA58861, CA35176, and CA46282.
REFERENCES
- 1.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. [DOI] [PubMed] [Google Scholar]
- 2.Russell KJ, Boileau MA, Higano C, et al. Combined 5-fluorouracil and irradiation for transitional cell carcinoma of the urinary bladder. Int J Radiat Oncol Biol Phys. 1990;19:693–699. [DOI] [PubMed] [Google Scholar]
- 3.Rotman M, Aziz H, Porrazzo M, et al. Treatment of advanced transitional cell carcinoma of the bladder with irradiation and concomitant 5-fluorouracil infusion. Int J Radiat Oncol Biol Phys. 1990;18:1131–1137. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins BJ, Caulfield MJ, Fowler CG, et al. Reappraisal of the role of radical radiotherapy and salvage cystectomy in the treatment of invasive (T2/T3) bladder cancer. Br J Urol. 1988;62:343–346. [DOI] [PubMed] [Google Scholar]
- 5.Gospodarowicz MK, Hawkins NV, Rawlings GA, et al. Radical radiotherapy for muscle invasive transitional cell carcinoma of the bladder: failure analysis. J Urol. 1989;142:1448–1453; discussion 1453–1444. [DOI] [PubMed] [Google Scholar]
- 6.Mameghan H, Fisher R, Mameghan J, Brook S. Analysis of failure following definitive radiotherapy for invasive transitional cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys 1995;31:247–254. [DOI] [PubMed] [Google Scholar]
- 7.De Neve W, Lybeert ML, Goor C, Crommelin MA, Ribot JG. Radiotherapy for T2 and T3 carcinoma of the bladder: the influence of overall treatment time. Radiother Oncol. 1995;36:183–188. [DOI] [PubMed] [Google Scholar]
- 8.Pollack A, Zagars GZ. Radiotherapy for stage T3b transitional cell carcinoma of the bladder. Semin Urol Oncol. 1996;14:86–95. [PubMed] [Google Scholar]
- 9.Hussain MH, Glass TR, Forman J, et al. Combination cisplatin, 5-fluorouracil and radiation therapy for locally advanced unresectable or medically unfit bladder cancer cases: a Southwest Oncology Group study. J Urol. 2001; 165:56–60; discussion 60–51. [DOI] [PubMed] [Google Scholar]
- 10.Sangar VK, McBain CA, Lyons J, et al. Phase I study of conformal radiotherapy with concurrent gemcitabine in locally advanced bladder cancer. Int J Radiat Oncol Biol Phys. 2005;61:420–425. [DOI] [PubMed] [Google Scholar]


