Abstract
Background—
Cardiovascular disease is often studied through patient self-report and administrative data. However, these 2 sources provide different information, and few studies have compared them.
Methods and Results—
We compared data from a longitudinal, nationally representative survey of older Americans with matched Medicare claims. Self-reported heart attack in the previous 2 years was compared with claims-identified acute myocardial infarction (AMI) and acute coronary syndrome. Among the 3.1% of respondents with self-reported heart attack, 32.8% had claims-identified AMI, 16.5% had non-AMI acute coronary syndrome, and 25.8% had other cardiac claims; 17.3% had no inpatient visits in the previous 2.5 years. Claims-identified AMIs were found in 1.4% of respondents; of these, 67.8% reported a heart attack. Self-reports were less likely among respondents >75 years of age (62.7% versus 74.6%; P=0.006), with less than high school education (61.6% versus 71.4%; P=0.015), with at least 1 limitation in activities of daily living (59.6% versus 74.7%; P=0.001), or below the 25th percentile of a word recall memory test (60.7% versus 71.3%; P=0.019). Both self-reported and claims-identified cardiac events were associated with increased mortality; the highest mortality was observed among those with claims-identified AMI who did not self-report (odds ratio, 2.8; 95% confidence interval, 1.5–5.1) and among those with self-reported heart attack and claims-identified AMI (odds ratio, 2.5; 95% confidence interval, 1.7–3.6) or non-AMI acute coronary syndrome (odds ratio, 2.7; 95% confidence interval, 1.8–4.1).
Conclusions—
There is considerable disagreement between self-reported and claims-identified events. Although self-reported heart attack may be inaccurate, it indicates increased risk of death, regardless of whether the self-report is confirmed by Medicare claims.
Keywords: acute coronary syndrome, Medicare, myocardial infarction, patient outcome assessment, survival analysis
Cardiovascular disease is the leading cause of death in the United States, with acute myocardial infarction (AMI) the focus of much research and numerous policy initiatives.1–4 Most of the information we have about the social and clinical epidemiology of the disease comes from self-report or administrative claims.5–10 However, there have been no nationally representative studies comparing the congruence between these 2 sources and assessing the potential implications of divergence, which may indicate poor understanding of medical history, for patient outcomes.
Inaccurate self-assessment of disease status may be particularly important among older patients, those with less education, those who have more severe disease, and patients with functional or cognitive impairments.11,12 Because physicians’ understanding of patient history often relies on self-report of previous conditions, such inaccuracies may have implications for treatment decisions. Furthermore, the extent and distribution of any incongruence between self-reported and claims-identified events may help inform the use of both data sources to study the clinical and social epidemiology of cardiovascular diseases.
In this study, we use data from the Health and Retirement Study (HRS), a nationally representative survey of older Americans, matched to Medicare claims to examine the congruence between self-reported heart attack and claims-identified AMI. We examine whether patient characteristics are associated with patients self-reporting a heart attack without a Medicare claim or not reporting a heart attack when claims indicate one. We also assess the extent to which other diagnoses, both cardiac and noncardiac, may account for self-reported heart attack. Finally, we assess the relationship between 1-year mortality and self-reported heart attack or claims-identified AMI.
Methods
Study Population
We analyzed data from the HRS, a longitudinal, nationally representative survey of older Americans.13 The majority of respondents have given permission to link their survey data to Medicare claims for research. The linkage between survey responses and Medicare claims was performed by HRS staff on the basis of the respondents’ Health Insurance Claim number. We used interview data from the years 1996 to 2008 because the questions on cardiac health history were nearly identical and there was sufficient follow-up to fully assess respondents’ survival. Because our analysis involved identifying hospitalizations up to 2.5 years preceding each interview, the study population was limited to respondents at least 67 years old who had >2.5 years of previous traditional (fee-for-service) Medicare coverage.
Study Variables
Demographic variables included age at the time of interview, sex, race, education, and wealth. We dichotomized education on the basis of whether respondents had completed high school (or GED [general education development test]). We used a measure of wealth that captured the sum of respondents’ total assets (including the value of one’s primary residence, investments, and retirement accounts, among others); this value was dichotomized based on the median. Wealth was chosen over income because our study population consisted of older adults who are more likely to be retired.
Health status variables included self-rated fair or poor health and limitation in any of 3 activities of daily living (ADLs): bathing, dressing, and eating. Mental status questions included self-rated fair or poor memory, a total word recall score (0–20), and a summary cognitive score (0–35).14 Total word recall was dichotomized on the basis of being below the 25th percentile among HRS respondents ≥67 years of age. Overall cognitive score was dichotomized on the basis of a cutoff suggestive of moderate to severe cognitive impairment (score, 0–7).15
Self-reported heart attack was defined as a positive answer to 1 of 2 questions: “Since [month and year of last interview], have you had a heart attack or myocardial infarction?” or “In the past 2 years, have you had a heart attack or myocardial infarction?” This question was asked only of respondents who had responded positively to the question, “Has a doctor ever told you that you had a heart attack, coronary heart disease, angina, congestive heart failure, or other heart problems?” Therefore, respondents who reported AMI comprise a subset of those who reported heart problems.
From Medicare claims, 2 types of cardiac hospitalizations were defined: acute coronary syndrome (ACS) and AMI. ACS is a wider diagnostic category, encompassing AMI and other emergent conditions with diminished cardiac blood supply.16 We included these related conditions because their clinical presentations may be indistinguishable from AMI; a patient may reasonably report having experienced a heart attack. We identified ACS visits by International Classification of Diseases, Ninth Revision (ICD-9) code 410.XX, 411.XX (other acute or subacute ischemic heart disease), or 413.XX (unstable angina) in any position17 on an inpatient hospital claim. Among these events, a narrower subcategory of AMI was identified by a primary or secondary ICD-9 diagnosis code of 410.X1 (X being any number 0–9), a definition validated with chart review.18 We purposely created a relatively broad definition for ACS and a relatively narrow one for AMI. For all claims-identified events, we included only hospitalizations with a length of stay >2 days, in accordance with validated definitions.18
Primary and secondary diagnosis codes for all hospitalizations were recorded among those with a positive self-report. Additionally, among those with claims-identified AMI, ICD-9 procedure codes for percutaneous stent placement (360X), coronary artery bypass graft (361X), and percutaneous imaging of coronary blood vessels (372X) were recorded, as were the overall and ICU lengths of stay. Medicare claims data are quite accurate in recording cardiovascular health events.18 There are strong incentives for hospitals to report events; they are paid only for what they report, and there are substantial penalties for fraudulent claims.19
Statistical Analyses
Study Population Characteristics
All statistical analyses were calculated using population weights and accounting for complex survey design. We assessed summary statistics of the study population: age, sex, race, education, wealth, health, mental status, and self-reported AMI and heart problems. We determined whether there were any significant differences in these characteristics across HRS respondents who were and were not included in the study cohort (those with <2.5 years of Medicare claims).
Self-Reported Heart Attack
There were 2 possible AMI definitions: self-reported and claims-identified. First, we determined the percent of each demographic subgroup who self-reported experiencing a heart attack in the previous 2 years. Among these individuals, we assessed the percent who had a claims-identified AMI or ACS in the 2.5 years before the interview (as defined above, claims-identified ACS is a broader category, including AMI). We used a longer time window to allow for respondents’ potential errors in remembering events slightly beyond the horizon about which they were asked. We used these data to calculate a kappa statistic to summarize the agreement between self-reported heart attack and claims-identified AMI.
Some respondents with self-reported heart attack may have actually experienced a different cardiac condition, not AMI or ACS. In an attempt to account for all self-reported events, we tabulated all cardiac and noncardiac diagnoses from inpatient claims from the previous 2.5 years for respondents with a positive self-report. We then assigned each respondent a single diagnosis that was based on relevance to AMI/ACS. Those with claims-identified AMI were labeled as such. Next, we identified those with non-AMI ACS. We then assigned respondents a single cardiac diagnosis, with those appearing most frequently assigned first. For those with only noncardiac diagnoses, we assigned either one of the most frequent noncardiac diagnoses or a designation of “other”.
Claims-Identified AMI
The second way we defined events was through Medicare claims. We determined the percent of each demographic subgroup with a claims-identified AMI. Here, we included only those with claims-identified AMI (not the broader ACS) occurring between 2 consecutive interviews or within 2 years. We then assessed the proportion of these respondents who self-reported a heart attack or reported having heart problems (respondents reporting heart problems make up a larger group, which includes all those who reported a heart attack). We compared how frequently respondents with a claims-identified AMI reported a heart attack across categories defined by self-rated health and memory, ADL difficulty, cognitive function indicators, and clinical treatment: overall length of stay, intensive care unit length of stay, and receipt of cardiac stent, coronary artery bypass graft, or percutaneous imaging.
Trends Over Time
Because of concerns about potential changes in AMI/ACS diagnosis rates, and patient education efforts, over the time period, we examined wave-specific rates of claims-identified AMI, ACS, self-reported heart attack, and wave-specific concordance.
Survival Analysis
We assessed differences in one-year mortality after interview across each category of self-reported or claims-identified events. One-year mortality was chosen because most deaths related to recent cardiac health history would likely occur sooner rather than later. Complete assessment of 1-year mortality was possible for virtually all respondents (99.9% of HRS respondents either had a valid date of death or were alive at the next wave), allowing the use of logistic regression. In these regressions, we first assessed the unadjusted odds of 1-year mortality associated with each of the following 5 categories: no claims-identified events but self-reported heart attack, claims-identified non-AMI ACS with or without self-report, and claims-identified AMI with or without self-report. The reference group was respondents with neither claims-identified ACS nor self-reported events. We then determined the same statistics controlling for age, sex, race, education, marital status, and household wealth.
Sensitivity Analyses
We performed several sensitivity analyses. First, the population weights, used in our main analyses, assign a value of zero to all institutionalized (eg, living in a nursing home) respondents, excluding a substantial minority of respondents with cardiac health history. We therefore reran all of our tabulations and regressions without weights. Additionally, adjusting regression analyses for the complex survey design precluded the simultaneous inclusion of longitudinal effects; each interview was treated as a separate observation. We addressed the potential for clustering of observations over time by running our regressions with robust standard errors that accounted for clustering within respondents.
In assessing 1-year mortality, we made assumptions regarding the 0.1% who neither had a reported date of death nor were ascertained to be alive at the next interview. We created alternative outcomes in which all of these respondents were assessed to have lived 1 year and in which all were assessed to have died. Additionally, although our main analysis used a dichotomized measure of wealth for consistency with the summary statistics we presented elsewhere, we tested whether controlling for alternative wealth measures, either quintiles or a continuous variable, affected our logistic regression findings. We also performed alternative survival analyses with a longer follow-up by assessing survival times up to 6 years for 2 cohorts of respondents (those who were respondents in 1998 or 2004) and then combining these respondents into a single analytic cohort. These data were then analyzed by use of Cox proportional hazards analysis.
Finally, we tested several alternative claims-based AMI definitions. The most stringent definition included only those inpatient visits with a diagnosis-related group code of 121, 122, or 123, whereas the broadest definition allowed an ICD-9 code of 410.XX (X being any digit 0–9) in any diagnosis field on the claim. Relatedly, in the main analyses of self-reported heart attack, all inpatient admissions from the 2.5 years before the interview date were analyzed (despite the interview question asking only about the previous 2 years). It is possible that respondents recalled an event correctly but had experienced it even earlier. We therefore reran this analysis, looking back either 3 or 3.5 years. Finally, although the validated definition we used had a minimum length of stay of 3 days, it is possible that patients with ACS, or even AMI, could be discharged sooner. Therefore, in this analysis, we included all inpatient claims for AMI and ACS events, with no minimum length of stay.
Data management of Medicare claims was performed with SAS version 9.3, and all analyses performed with Stata version 12.1. The study was approved by the local Institutional Review Board. All participants provided informed consent for the use of their survey responses and Medicare claims for research purposes.
Results
Respondent Characteristics
Table 1 presents demographic and health characteristics among HRS respondents ≥67 years of age included in our final study cohort. Among the 65 810 respondents ≥67 years of age, 45 335 (68.9%) were included in the study cohort. They gave permission for their Medicare claims to be used and had at least 2 years of continuous non-HMO (health maintenance organization) coverage before their interview date. Compared with those not in the cohort, there were no significant differences in the distribution of sex, educational achievement, wealth, self-rated health, or frequency of self-reported AMI (data not shown). Those in the study cohort were slightly older (76.7 versus 75.4 years; P<0.001), less likely to identify as black or “other” race (9.1% versus 12.2%; P=0.004), slightly more likely to report difficulty with at least 1 ADL (16.9% versus 15.7%; P=0.02), and more likely to report having heart problems (33.1% versus 28.9%; P<0.001).
Table 1.
Demographics and Health Characteristics Among HRS Respondents ≥67 Years of Age Included in the Study Cohort
| Total observations, n | 45 335 |
| Demographics | |
| Mean age, y | 76.7 |
| Male, % | 41.9 |
| Black/other race, % | 9.1 |
| Married, % | 53.6 |
| Less than high school education, % | 28.5 |
| Above median wealth, % | 54.4 |
| Health status, % | |
| Self-reported health fair/poor | 32.6 |
| 1 + ADL | 16.9 |
| Self-reported AMI | 3.1 |
| Self-reported heart problems | 33.1 |
| Mental status, % | |
| Self-rated memory fair/poor | 29.0 |
| Below 25th percentile word recall | 19.4 |
| Moderate/severe cognitive impairment | 1.5 |
The study population included only those HRS respondents who had agreed to the use of their Medicare claims for research purpose and had at least 2 years of continuous traditional (fee-for-service) Medicare claims before the interview date. Each interview contributed an observation to the analysis. 1+ADL indicates difficulty with at least 1 of the following activities: eating, dressing, or bathing. Quartile of word recall was determined among the overall (unweighted) population ≥67 years of age. Moderate to severe cognitive impairment was determined on the basis of a score of ≤7 on a 35-point overall cognitive score. Only those respondents who reported heart problems were asked about taking heart medications. All percentages were calculated with population weights. ADL indicates activities of daily living; AMI, acute myocardial infarction; and HRS, Health and Retirement Study.
Self-Reported Heart Attack
In Table 2, we present the proportion with self-reported heart attack across demographic subgroups and the proportion of these individuals who had a claims-identified AMI or ACS within the previous 2.5 years (AMI is a subset of ACS). Overall, 3.1% self-reported a heart attack; of these, 32.3% had a claims-identified AMI, and 48.7% had a claims-identified ACS. Were we to assume claims-identified events as a gold standard, this would indicate a positive predictive value of either 32.3% or 48.7% (depending on how generously one defines “heart attack” among the claims). The kappa statistic between self-reported heart attack and claims-identified AMI was 0.41, which is in the lower end of the range considered to indicate moderate agreement.20 Age of respondents was marginally associated with the likelihood of finding claims-identified AMI, but there were no other significant associations between demographics and the likelihood that a self-reported heart attack would be matched with a claims-identified event.
Table 2.
Comparison of Self-Reported and Claims-Identified AMI Among HRS Respondents
| Self-Reported AMI, % | Claims-Identified AMI, % | ||||||
|---|---|---|---|---|---|---|---|
| Of these, What Percent Had | Of these, What Percent Reported | ||||||
| Total, n | Percent of Respondents | Claims-Identified AMI | Claims-Identified ACS (Includes AMI) | Percent of Respondents | Heart Attack | Heart Problems | |
| Overall | 45 335 | 3.1 | 32.3 | 48.7 | 1.4 | 67.8 | 90.5 |
| ≤75 y old | 22 154 | 2.8 | 35.3 | 51.0 | 1.2 | 74.6 | 93.9 |
| >75 y old | 23 181 | 3.4 | 30.0 | 47.0 | 1.5 | 62.7 | 88.0 |
| P | 0.053 | 0.192 | 0.006* | 0.042* | |||
| Male | 18 943 | 3.9 | 34.4 | 50.2 | 1.8 | 68.5 | 92.2 |
| Female | 26 392 | 2.5 | 30.0 | 47.1 | 1.0 | 66.9 | 88.3 |
| P | 0.166 | 0.413 | 0.687 | 0.068 | |||
| White | 39 269 | 3.2 | 32.4 | 48.8 | 1.4 | 68.5 | 90.8 |
| Black/other | 6066 | 2.3 | 31.0 | 47.9 | 1.2 | 59.6 | 87.5 |
| P | 0.771 | 0.845 | 0.077 | 0.399 | |||
| Not married | 20 528 | 3.2 | 32.6 | 49.6 | 1.5 | 64.5 | 87.5 |
| Married | 24 780 | 3.0 | 32.1 | 47.8 | 1.3 | 71.0 | 93.5 |
| P | 0.874 | 0.626 | 0.108 | 0.058 | |||
| Less than high school | 14 080 | 4.1 | 29.0 | 47.0 | 1.8 | 61.6 | 89.2 |
| High school or greater | 31 253 | 2.7 | 34.3 | 49.8 | 1.2 | 71.4 | 91.3 |
| P | 0.142 | 0.341 | 0.015* | 0.415 | |||
| Below median wealth | 22 207 | 3.7 | 30.0 | 49.0 | 1.6 | 65.8 | 90.1 |
| Above median wealth | 23 128 | 2.6 | 35.0 | 48.5 | 1.2 | 69.9 | 90.9 |
| P | 0.093 | 0.857 | 0.282 | 0.772 | |||
All statistics are calculated accounting for population weights and complex survey design. Claims-identified ACS includes both AMI and non-AMI ACS. AMI is defined by MedPAR claims indicating a visit with a length of stay of at least 3 days, and International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code of 410.01, 410.11, 410.21, 410.31, 410.41, 410.51, 410.61, 410.71, 410.81, or 410.91 in the primary or secondary position. ACS is defined by MedPAR claims indicating a visit with a length of stay of at least 3 days, and ICD-9 diagnosis code of 410, 411, or 413 in any position. When respondents were selected on the basis of claims-identified AMI (the right side of the table), only events between interview waves (for those interviewed in 2 consecutive waves) or <730 days (2 years) before the interview date were counted. All respondents who reported heart problems were subsequently asked about heart attacks. In the validation of self-reported AMI (left half of the table), events within 910 days (2.5 years) were included. Each interview wave per respondent contributes 1 observation. ACS indicates acute coronary syndrome; AMI, acute myocardial infarction; and HRS, Health and Retirement Study.
Significant.
In Figure 1, we display an attempt to account for all self-reported heart attacks by tabulating all inpatient claims from the previous 2.5 years. Of respondents with self-reported heart attack, 32.3% had claims-identified AMI, and 16.5% had non-AMI ACS. An additional 25.8% of the respondents had alternative cardiac hospitalizations: 8.5% each heart failure and other ischemic heart diseases, 4.9% dysrhythmia, and 3.9% other cardiac diagnoses. Among the remaining respondents, 1.1% had an inpatient admission for urinary tract infection, 0.7% had an inpatient admission for pneumonia, and 6.3% had some other noncardiac admission. For 17.3% of those who self-reported a heart attack, we found no inpatient admissions of any type in the previous 2.5 years.
Figure 1.
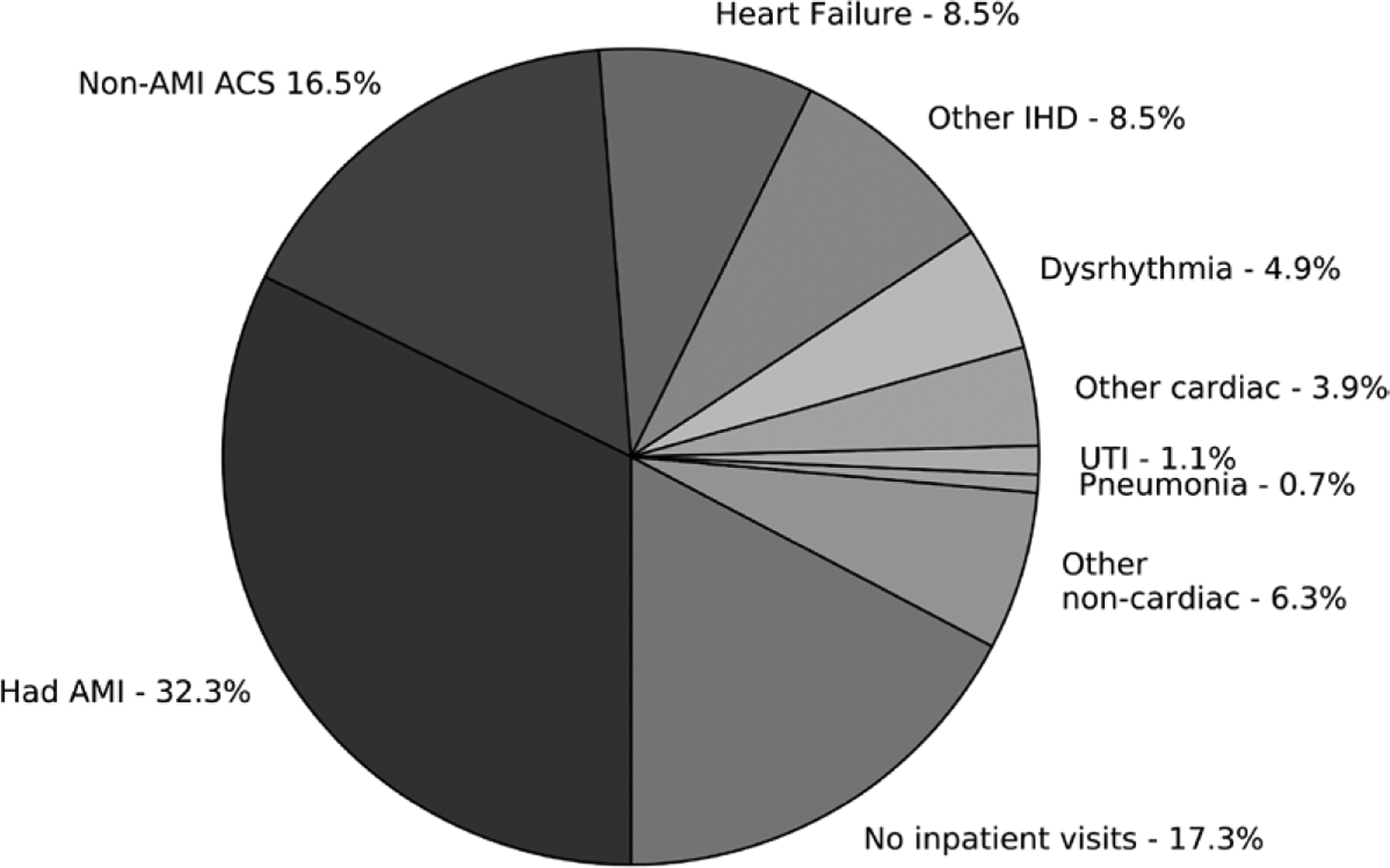
Claims-identified inpatient visit history among Health and Retirement Study (HRS) respondents with self-reported acute myocardial infarction (AMI). Data based on retrospective analysis of MedPAR claims from 1433 HRS respondents who reported experiencing a heart attack from 1996 to 2008. For each respondent, all claims from the previous 910 days (2.5 years) were assessed. AMI was defined by an International Classification of Diseases, Ninth Revision (ICD-9) code of 410.X1 in the first or second position on an inpatient claim for a visit with a length of stay (LOS) >3 days. Non-AMI acute coronary syndrome (ACS) was defined by ICD-9 code 411 or 413 in any position on an inpatient claim for a visit with an LOS >3 days. All other classifications were based on either the primary or secondary diagnosis, without any LOS restrictions. Respondents with multiple visits were assigned exclusively to one of the categories, with priority based first on clinical relevance (AMI before ACS, cardiac before noncardiac diagnoses) and then on the overall frequency with which the diagnoses appeared on all claims (eg, heart failure appeared more often than other ischemic heart disease [IHD]). UTI indicates urinary tract infection.
Claims-Identified AMI
We found claims-identified AMI during the previous 2 years in 1.4% of the population; of these, 67.8% self-reported a heart attack, and 90.5% reported heart problems in that time period. If claims were considered the gold standard in identifying heart attacks, this would suggest that self-report has a sensitivity of 67.8%. Respondents >75 years of age were less likely to report a heart attack (62.7% versus 74.6%; P=0.006) and heart problems (88.0% versus 93.9%; P=0.042), and those with less than a high school education were also less likely to self-report a heart attack (61.6% versus 71.4%; P=0.015).
In Figure 2, we demonstrate that respondents were equally likely to report an AMI if they had fair/poor self-rated health or memory (relative to not), but they were less likely to report one if they had at least 1 ADL limitation (59.6% versus 74.7%; P=0.001) or were below the 25th percentile of word recall (60.7% versus 71.3%; P=0.019). The only clinical treatments associated with the likelihood of self-report were the receipt of a cardiac stent (82.8% among those with stent versus 61.4%; P<0.001) and percutaneous imaging of cardiac vessels (74.1% versus 61.4%; P=0.018).
Figure 2.
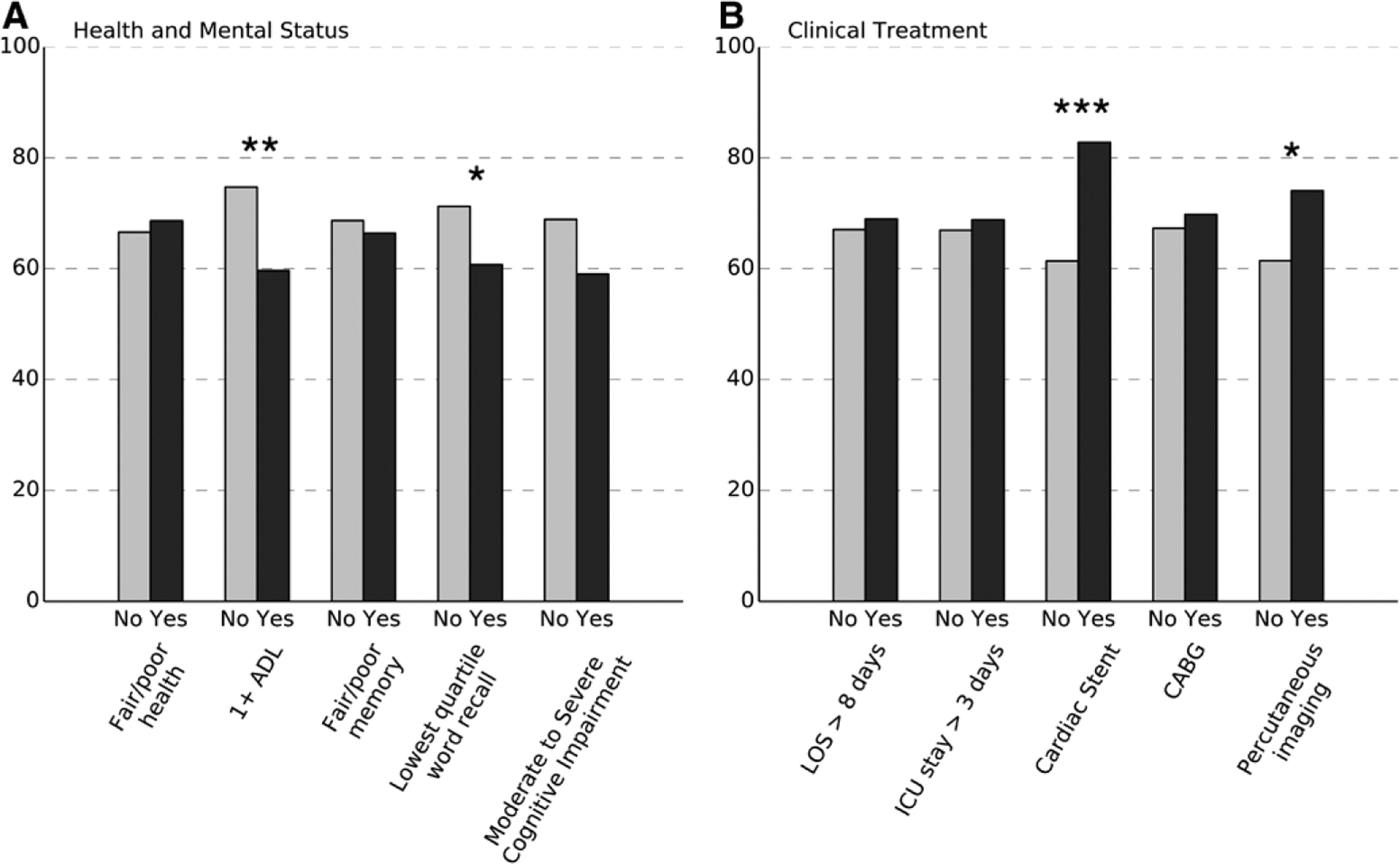
Percent of respondents with a claims-identified acute myocardial infarction (AMI) who also reported a heart attack by health, mental status, and documented clinical treatment. Fair/poor health and memory are self-rated by respondents. 1+ADL (activities of daily living) indicates that the respondent reported difficulty with at least 1 of the following tasks: bathing, eating, or dressing. Moderate to severe cognitive impairment indicates a score of 0 to 7 in a 35-point summary cognitive score. Clinical treatment assessed from all MedPAR claims dated since the previous interview (for those interviewed in 2 consecutive waves) or in the previous 730 days. *P<0.05; **P<0.01; ***P<0.001. CABG indicates coronary artery bypass graft; ICU, intensive care unit; and LOS, length of stay.
Trends Over Time
The frequency of claims-identified AMI and ACS fluctuated over time, and rates were somewhat lower in the final 3 waves; claims-identified AMI was between 1.1% and 1.2% in the waves corresponding to the years 2004, 2006, and 2008, down from rates between 1.3% and 1.7% in the waves from 1996 to 2002. Concordance between self-reported and claims-identified events, however, showed no clear trend over the time period; we therefore present the pooled estimates of concordance in Table 2.
Survival Analysis
In Figure 3, we present odds ratios of 1-year mortality associated with each of the 6 categories of self-reported versus claims-identified events (respondents with neither served as the reference). Unadjusted estimates are shown in Panel A, and in Panel B the same estimates adjusted for age, sex, race, marital status, education, and wealth.
Figure 3.
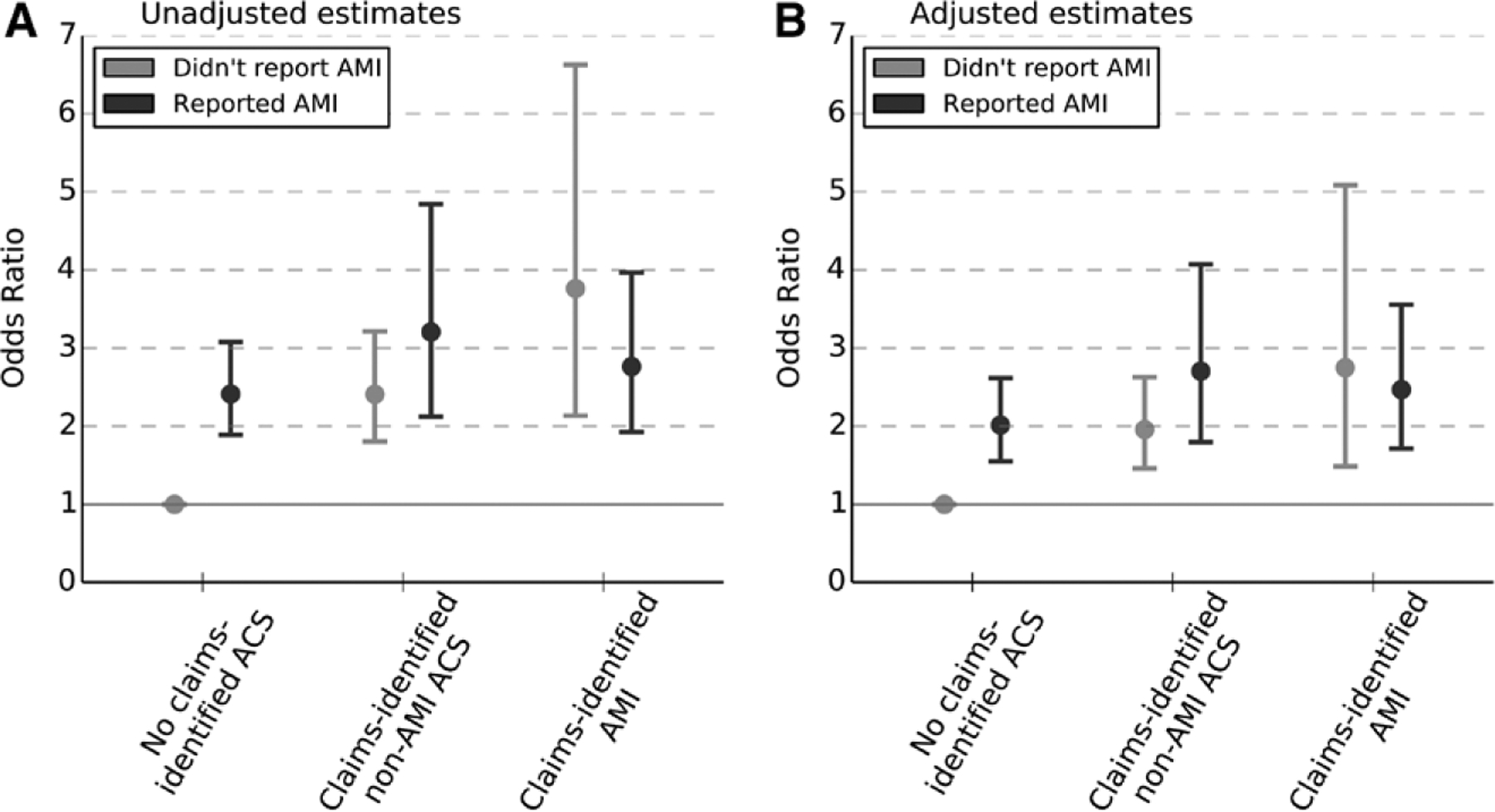
Unadjusted and adjusted odds of 1-year mortality associated with self-reported heart attack and claims-identified AMI or acute coronary syndrome (ACS) events. Estimated odds ratios from a logistic regression of 1-year mortality on category of self-reported or claims-identified cardiac events, with those who neither self-reported nor had a claims-identified event serving as the reference group. Adjusted estimates reflect controlling for age, sex, race, education, and wealth.
Even among those with no claims-identified ACS, self-reported heart attack was associated with increased 1-year mortality (unadjusted odds ratio [OR], 2.4; 95% confidence interval [CI], 1.9–3.1; adjusted OR, 2.0; 95% CI, 1.5 – 2.6). The highest odds of 1-year mortality were found among those who had a claims-identified AMI but did not report it (unadjusted OR, 3.8; 95% CI, 2.1–6.6; adjusted OR, 2.7; 95% CI, 1.5–5.1), as well as those with both self-reported heart attack and claims-identified non-AMI ACS (unadjusted OR, 3.2; 95% CI, 2.1–4.8; adjusted OR, 2.7; 95% CI, 1.8–4.1) or AMI (unadjusted OR, 2.8; 95% CI, 1.9–4.0; adjusted OR, 2.5; 95% CI, 1.7–3.6).
Sensitivity Analyses
Our results remained nearly identical when we ran our regressions under various specifications, including without weights, accounting for clustering of observations within individuals over time, and controlling for cognitive function. Specifically, our estimates of the odds of 1-year mortality associated with each self-reported/claims-identified event category changed little when we represented wealth as a continuous variable or as quintiles.
When different criteria were used to define AMI or ACS, a diagnosis-related group–based definition identified only about half as many admissions (338 compared with 712 admissions), whereas a broader definition identified ≈20% more claims (882 admissions). Our main conclusions remained unchanged with either definition. As the broader definition included claims that could possibly be used to indicate a read-mission, we believe the ICD-9 definition in our main analyses was the best available.
In looking back 3.5 years (relative to 2.5 years) and including visits with no minimum length of stay, we found that an additional 6.7% of the respondents experienced an AMI and 4.6% experienced a non-AMI ACS; overall, the total proportion of self-reports confirmed with either AMI or non-AMI ACS claims rose to 60.0% (<10 visits were excluded from our initial analyses on the basis of only length of stay). The proportion of respondents with self-reported AMI who had no inpatient claims in the previous 3.5 years was 12.3%.
Finally, in a Cox proportional hazards analysis with a 6-year follow-up, the hazard ratios associated with each of the self-reported or claims-identified cardiac events were somewhat smaller in magnitude than the odds ratios associated with 1-year mortality, likely reflecting higher mortality rates from causes unrelated to cardiac history as this elderly population aged. The largest hazard ratio was found among respondents with a claims-identified AMI but no self-reported heart attack (adjusted hazard ratio 1.86, 95% CI 1.42–2.45). The next highest hazard ratio was found among respondents with self-reported AMI but no claims-identified ACS (1.65, 95% CI 1.34–2.03); the other 3 exposure groups had smaller hazard ratios, all nearly identical (ranging from 1.46–1.49).
Discussion
In this analysis, we examined the congruence of self-reported heart attack, claims-identified AMI, and ACS. Among HRS respondents ≥67 years of age who reported a heart attack within the previous 2 years, only one-third had claims-identified AMI; an additional 16% had claims-identified non-AMI ACS (unstable angina or other acute ischemic heart disease). Overall, fewer than half of those who reported a heart attack had evidence of acute cardiovascular hospitalizations.
There were no associations between any identifiable demographic characteristics and the frequency with which self-reported heart attack was verified by Medicare claims. Of the half of respondents whose self-reports were not matched by a claims-identified ACS, another 25% had inpatient admissions for other cardiac diagnoses. However, 17% of those who reported experiencing a heart attack had no inpatient visits in the previous 2.5 years.
Among those with claims-identified AMI, only two thirds (68%) reported experiencing a heart attack (91% reported heart problems). Older respondents and those with less than a high school education were less likely to self-report. In addition, respondents with at least 1 ADL limitation, those with a worse memory, and those who had not received a cardiac stent or percutaneous coronary vessel imaging were also less likely to self-report.
The difference across whether respondents’ claims indicated percutaneous coronary vessel imaging suggests a few possible explanations for the discordance we document. First, some proportion of claims-identified AMI may result from borderline diagnoses; these claims may be less likely to be accompanied by percutaneous coronary vessel imaging. If respondents experienced such an event, they may be correct not to self-report. Alternatively, it is possible that receiving a stent makes the experience more salient. Interestingly, we found no difference in concordance among respondents who had received a more invasive coronary artery bypass graft. However, better candidates for coronary artery bypass graft often have more severe disease; these respondents may have been sicker or older (both associated with higher discordance).
In mortality analyses, self-reported AMI was associated with an increased risk of death regardless of claims-identified events. Even among those with no claims-identified ACS events, self-reported AMI was associated with doubled odds of 1-year mortality after controlling for age, sex, race, wealth, and education. The highest mortality was found among respondents who had a claims-identified AMI (regardless of self-report status) and those with self-reported heart attack that was concordant with a claims-identified ACS.
Although one might assume that the highest risk of death would be associated with congruent claims-identified AMI and self-reported heart attack, we found that discordant claims/self-reported status carried at least the same risk of death. We postulate that a potential increased risk could be due, at least in part, to a patient’s poor understanding of his or her medical conditions. It is also possible that other factors, perhaps lower socioeconomic status, could result in both poor understanding and worse health outcomes. Our regressions controlled for education and wealth (further including income did not change our findings), but other, more difficult to measure aspects of socioeconomic status may also contribute to this relationship.
In previous studies of the concordance between self-reported heart attack and medical records, self-reported events were verified at rates ranging from 40% to 75%.21–26 When evidence was available, investigators often found other cardiac diagnoses among the false-positives.22,23 The characteristics of the study population were likely associated with the accuracy of self-reports; the lowest concordance was found in a study of disabled elderly,24 and the highest concordance was documented among members of the general population recruited to participate in research about cardiac health.21
The fact that we found slightly lower congruence than previous studies may be attributable to a few factors. First, our study cohort is among the oldest of any of these studies; our results indicated that age was the only characteristic that was even marginally related to having a self-report confirmed by Medicare claims. Second, we used a relatively narrow definition of AMI; using the broader ACS definition to confirm self-reports would put our study within the range of previous ones. Third, although the HRS includes a wide range of questions, of which a small proportion relate to medical experiences, other studies were often focused exclusively on respondents’ previous illnesses, perhaps providing more appropriate cues to assist accurate recall of cardiac events.27
Our study is not without limitations. First, we did not have access to HRS respondents’ medical charts but rather relied on Medicare claims to identify clinical events. We recognize that neither self-report nor claims identification is a gold standard for documenting clinical events. To the extent that our use of Medicare claims resulted in misclassification of inpatient visits, this may have contributed to the lower congruence we found. However, Medicare claims allow the study of large populations and thus are often used to study AMI.6,8,9 Additionally, the criteria we used were recently validated.18 One way to further explore the discordance we document would be to compare both claims-identified and self-reported events with evidence from medical charts. Such an analysis would also allow further investigation of other factors related to concordance such as whether the patient received percutaneous coronary vessel imaging.
Second, our analyses are conditional on respondents having survived long enough after their AMI to be interviewed; we were unable to study those who died shortly after an AMI. However, this is representative of older, independently living patients who may present to a physician with a particular condition that needs to be understood in light of medical history.
Finally, the HRS is designed to assess overall health status and social and economic conditions. The study has only a few questions specific to cardiac health, and the number of respondents in some specific subgroups (eg, claims-identified AMI but no self report) was somewhat small, perhaps limiting the scope and power of some of our analyses. Nevertheless, the HRS is one of the few data sources that allow longitudinal follow-up along with a comprehensive study of both demographic and health status factors in conjunction with Medicare-linked claims data.
These results suggest that among older Americans, especially those who are older, sicker, or have worse memory, there may be considerable confusion about cardiac health history. At least a portion of patients are unable to correctly recall having experienced an AMI; these patients may be less likely to adhere to long-term medication regimens necessary for secondary prevention.
Our findings also have implications for research into cardiovascular disorders. Given that previous studies have shown that claims-identified events are more likely to be validated by medical record review than self-reported events,18,21–26 one could argue that claims-based definitions are superior and should be the first choice if medical records are unavailable. However, even self-reported heart attack without any concordant claims is associated with increased risk of death; therefore, self-reported events are still an important source of information about respondents’ health.
Our study raises some questions that could be fruitful avenues for further research. First, if at least some proportion of AMI patients are unable to correctly recall such a relatively remarkable event, then there may also be fairly widespread confusion about other cardiovascular diagnoses, with implications for patients’ ability to comply with the long-term medication regimens or lifestyle changes often recommended for secondary prevention. Second, it would be interesting to explore the extent to which patient confusion is associated with factors related to the provider versus the patient. We demonstrate that some patient factors are related to concordance, but systematic differences in concordance across providers could possibly be a measure of provider quality. Providers may vary in how well they educate their patients.
Conclusions
We found that older Americans, especially those with worse mental or health status, may have substantial difficulty in correctly identifying their own health history. However, both self-reported and claims-identified ACS events are important indicators of patient health.
CLINICAL PERSPECTIVE.
Much of what we know about the causes and long-term outcomes of cardiovascular disease comes from research using information that is either self-reported or identified through healthcare claims, yet little is known about the agreement between these sources of information. This study, the first nationally representative comparison of self-reported and claims-identified heart attack events, analyzes survey responses and matched Medicare claims from the Health and Retirement Study, a longitudinal survey of Americans >50 years of age. Among respondents who self-reported a heart attack, only about one-third (32.3%) had a claims-identified acute myocardial infarction, and an additional 16.5% had a non–acute myocardial infarction acute coronary syndrome hospitalization. Among those who had a claims-identified acute myocardial infarction, about two thirds (67.8%) reported experiencing a heart attack. Those who were older, were sicker, had less education, had worse performance on memory scores, or had not received percutaneous coronary imaging were less likely to self-report. Relative to those with no self-reported or claims-identified events, respondents with a self-reported heart attack but no claims-identified acute coronary syndrome events experienced twice the odds of 1-year mortality; the highest mortality was found among respondents who had a claims-identified acute myocardial infarction, regardless of whether it was self-reported. Further research comparing both self-reported and claims-identified events with medical charts could help clarify the relative accuracy of each source and associated clinical risk factors. These results suggest that there is considerable confusion among older Americans about their cardiac health history, with potential implications for adherence to the long-term medication regimens required for secondary prevention.
Acknowledgments
We thank William Ramsey for his assistance in acquiring the data and for administrative and technical support. We also thank Jonathan S. Skinner for his generous advice and assistance in interpreting the data. Dr Yasaitis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of all analyses.
Sources of Funding
Drs Yasaitis, Berkman, and Chandra acknowledge support from the National Institute on Aging (grants P01 AG19783-11, 1 R01 AG040248-01, and P01-AG005842-24 and P01-AG19783, respectively). The funding sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures
None.
References
- 1.Hoyert DL, Xu JQ. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–51. [PubMed] [Google Scholar]
- 2.Mehta RH, Montoye CK, Gallogly M, Baker P, Blount A, Faul J, Roychoudhury C, Borzak S, Fox S, Franklin M, Freundl M, Kline-Rogers E, LaLonde T, Orza M, Parrish R, Satwicz M, Smith MJ, Sobotka P, Winston S, Riba AA, Eagle KA; GAP Steering Committee of the American College of Cardiology. Improving quality of care for acute myocardial infarction: the Guidelines Applied in Practice (GAP) Initiative. JAMA. 2002;287:1269–1276. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis, Thrombosis, and Vascular Biology; Council on Cardiopulmonary, Critical Care, Perioperative, and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia; Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Frieden TR, Berwick DM. The “Million Hearts” initiative: preventing heart attacks and strokes. N Engl J Med. 2011;365:e27(1)–e27(4). [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 6.Greer SA, Nwaise IA, Casper ML. Atlas of Heart Disease Hospitalizations Among Medicare Beneficiaries. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Prevalence of coronary heart disease: United States, 2006–2010. Morb Mortal Wkly Rep. 2011;60:1377–1381. [PubMed] [Google Scholar]
- 8.Ketcham JD, Baker LC, MacIsaac D. Physician practice size and variations in treatments and outcomes: evidence from Medicare patients with AMI. Health Aff (Millwood). 2007;26:195–205. doi: 10.1377/hlthaff.26.1.195. [DOI] [PubMed] [Google Scholar]
- 9.Stukel TA, Lucas FL, Wennberg DE. Long-term outcomes of regional variations in intensity of invasive vs medical management of Medicare patients with acute myocardial infarction. JAMA. 2005;293:1329–1337. doi: 10.1001/jama.293.11.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J Gerontol B Psychol Sci Soc Sci. 2005;60:S93–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Servais MA. Overview of HRS Public Data Files for Cross-Sectional and Longitudinal Analysis. Ann Arbor, MI: Survey Research Center, Institute for Social Research; 2010. [Google Scholar]
- 14.Fisher GG, Hassan H, Rodgers WL, Weir DR. Health and Retirement Study: Imputation of Cognitive Functioning Measures: 1992–2010 Final Release Version. Ann Arbor, MI: Survey Research Center, Institute for Social Research; 2012. [Google Scholar]
- 15.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57: 830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 16.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA; GRACE Investigators. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291: 2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 17.Ko DT, Newman AM, Alter DA, Austin PC, Chiu M, Cox JL, Goodman SG, Tu JV; Canadian Cardiovascular Outcomes Research Team. Secular trends in acute coronary syndrome hospitalization from 1994 to 2005. Can J Cardiol. 2010;26:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services, Centers for Medicaid & Medicare Services. Medicare fraud & abuse: prevention, detection, and reporting. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Fraud_and_Abuse.pdf. Updated 2012. Accessed July 24, 2014.
- 20.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. Biometrics. 2010;26:129–134. [PubMed] [Google Scholar]
- 21.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Paganini-Hill A, Chao A. Accuracy of recall of hip fracture, heart attack, and cancer: a comparison of postal survey data and medical records. Am J Epidemiol. 1993;138:101–106. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell CJ, Glynn RJ, Field TS, Averback R, Satterfield S, Friesenger GC 2nd, Taylor JO, Hennekens CH. Misclassification and under-reporting of acute myocardial infarction by elderly persons: implications for community-based observational studies and clinical trials. J Clin Epidemiol. 1999;52:745–751. [DOI] [PubMed] [Google Scholar]
- 24.Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52:123–127. [DOI] [PubMed] [Google Scholar]
- 25.Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol. 1997;145:762–769. [DOI] [PubMed] [Google Scholar]
- 26.Walker MK, Whincup PH, Shaper AG, Lennon LT, Thomson AG. Validation of patient recall of doctor-diagnosed heart attack and stroke: a postal questionnaire and record review comparison. Am J Epidemiol. 1998;148:355–361. [DOI] [PubMed] [Google Scholar]
- 27.Bradburn NM, Rips LJ, Shevell SK. Answering autobiographical questions: the impact of memory and inference on surveys. Science. 1987;236:157–161. [DOI] [PubMed] [Google Scholar]


