Abstract
Background:
Early life stress (ELS) has been linked to health disparities across the human lifespan, particularly increased risk for depression and its recurrence. In this study we explore two plausible and competing pathways through which ELS may lead to depression via inflammation.
Methods:
Participants (ages 18–22; n = 41) completed the Early Trauma Inventory as a measure of ELS. Participants then completed consecutive daily diaries of mood and other sickness behavior for the 7 days prior to and 7 days after receiving the annual influenza vaccine. Circulating concentrations of plasma interleukin-6 (IL-6) were measured immediately before and 24 hr after vaccination.
Results:
ELS was not associated with the magnitude of change in IL-6 from pre- to post-vaccine, however, exposure to ELS moderated the association between change in IL-6 from pre- to post-vaccine and changes in both cognitive difficulty and depressed mood. Individuals exposed to greater ELS showed greater psychological sensitivity to increases in IL-6.
Conclusions:
Exposure to ELS may increase sensitivity to peripheral inflammation in the central nervous system. Future studies elaborating on the impact of ELS on the sensitivity of specific neural circuits and cells to inflammation are needed.
Keywords: depression, early life stress, IL-6, inflammation, influenza vaccine, interleukin-6, sickness behavior
1 |. INTRODUCTION
Cumulative exposure to early life stress (ELS), such as maltreatment, neglect, poverty, and family violence, is associated with elevated risk for psychiatric disorders (Kessler et al., 2010; McLaughlin et al., 2010), particularly depression (Chapman et al., 2004). Inflammation has been identified as a biological process through which depression and other internalizing psychiatric disorders develop (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008; Dooley et al., 2018; Miller & Raison, 2016). The purpose of this study was to explore two plausible, competing pathways through which ELS may lead to depressive symptoms via inflammation; whether exposure to ELS was associated with greater inflammatory responses to external stimulation or whether ELS was associated with greater sensitivity to the link between inflammation and mood. Several studies in preclinical animal models have provided promising experimental evidence to support the latter hypothesis, yet, no studies to our knowledge have interrogated this possibility in humans.
In response to injury and pathogen exposure, cells of the innate immune system produce soluble proteins called cytokines that cause inflammation (Janeway & Medzhitov, 2002). Increases in systemic inflammation lead to inflammation within the central nervous system (CNS) which lead to behavioral changes that reflect reorganization of priorities and energy expenditure to promote healing; this phenomenon is commonly referred to as sickness behavior (Dantzer & Kelley, 2007; Dantzer et al., 2008; Hart, 1988). Several manifestations of sickness behavior, including depressed mood, difficulty with attention and memory, and withdrawal from social activities overlap with depressive symptoms. Indeed, we recently demonstrated that mild increases in inflammation following receipt of the annual influenza vaccine were associated with corresponding increases in daily depressed mood, problems with memory and attention, and to a lesser extent social disconnection (Kuhlman et al., 2018). This is important because inflammation is considered to be one putative mechanism through which depression develops (Raison, Capuron, & Miller, 2006). For example, prospective longitudinal studies have shown that elevated systemic markers predict depression over time (Gimeno et al., 2009), acute activation of the inflammatory arm of the immune system can induce many of the behavioral features of depression (Dooley et al., 2018), and individuals with depression tend to have higher systemic inflammation than individuals without depression (Dowlati et al., 2010; Howren, Lamkin, & Suls, 2009).
There are several ways in which cumulative exposure to ELS could influence the link between inflammation and depression. First, more exposure to ELS could increase production of inflammatory cytokines following an immune stimulus, exogenous or endogenous. Evidence of this has been shown repeatedly (Carpenter et al., 2010; Danese et al., 2011, 2008; Danese & Lewis, 2017; Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Miller & Chen, 2010; Nusslock & Miller, 2016; Pace et al., 2006; Powell et al., 2013), specifically that individuals exposed to ELS have immune systems that are more prone to production of inflammatory cytokines. Thus, individuals exposed to greater ELS would produce more inflammation than non-exposed individuals and subsequently experience more depressive symptoms.
Another possibility is that ELS alters the sensitivity of the CNS to inflammatory signals in the body. Increased sensitivity of the CNS to peripheral inflammatory activity could occur through several mechanisms. These include increasing the permeability of the blood–brain barrier (BBB) (Kuvacheva et al., 2016; Menard et al., 2017) and increasing the size, density, and proinflammatory sensitivity of microglia, the resident inflammatory cells in the brain (Bilbo & Schwarz, 2012; Calcia et al., 2016; Delpech et al., 2016; Réus et al., 2017; Wang et al., 2017). Importantly, in both of these cases, individuals with a history of ELS would experience more neuroinflammation following the same peripheral stimulus, leading to more profound psychological and behavioral changes. Thus it is biologically plausible that increased risk for depression occurs in individuals exposed to ELS because those individuals are more sensitive to mild fluctuations in peripheral inflammation. Indeed, some studies have observed that the association between inflammation and depression is more tightly linked over time in individuals exposed to ELS (Danese et al., 2008; Miller & Cole, 2012). However, no study to our knowledge has translated these preclinical observations to a human sample using an exogenous immune stimulus.
In this study, we tested these competing hypotheses in a sample of healthy young adults. To do this, we examined three depression-related psychological domains associated with increases in peripheral inflammation following exposure to the annual flu vaccine: self-reported depressed mood, cognitive difficulty, and feelings of social disconnection (Kuhlman et al., 2018). Consistent with the literature demonstrating that ELS leads to exaggerated inflammatory responses, we hypothesized that individuals with greater self-reported exposure to ELS would demonstrate a larger increase in inflammation from pre- to post-vaccine. Consistent with the emerging preclinical animal literature on greater CNS sensitivity to peripheral inflammation in ELS-exposed individuals, we hypothesized that the association between changes in inflammation and changes in subjective features of depression would be moderated by ELS, such that participants with more reported exposure to ELS would be more sensitive to the psychological correlates of inflammation.
2 |. METHOD
2.1 |. Participants
Participants were recruited as part of a project on the feasibility of using the influenza vaccine as a model for interrogating the role of inflammation in depression (Kuhlman et al., 2018). Participants were recruited during the Fall of 2015 and 2016 using flyers on a university campus. All participants were screened over the phone to determine their eligibility for the study which included: being an undergraduate student (ages 18–22) who had not yet received the influenza vaccine that year, not being allergic to eggs, not currently using any medications (e.g., steroids, antidepressants) or substances (e.g., tobacco products) known to affect the immune system, and not currently experiencing influenza or upper respiratory symptoms, current depression, anxiety, any major medical condition (e.g., diabetes, asthma). A total of 46 individuals were enrolled in the study; 3 were removed due to illness after enrollment, and 2 were unable to provide one or both blood samples. The final analytic sample included 41 participants, Mage = 18.49 (SD = 0.75). These participants were predominantly female (73.2%) and Asian (58.5%).1
2.2 |. Procedures
All participants completed informed consent and self-report questionnaires, including our measure of ELS, and received training on how to complete daily diaries during the first visit to the laboratory. For the next 14 days, participants completed online daily diaries (3–5 min each) assessing mood and feelings of social disconnection each evening between 8:00 p.m. and bedtime. Compliance with daily diary completion was high, 97.0%.
Approximately 7 days after enrollment, participants returned to the laboratory for a blood draw, and then received their influenza vaccine. The participants returned to the laboratory the following day for their post-vaccine blood draw. This occurred 21–29 hr after the vaccination, MVaccine Delay = 24:35, SDVaccine Delay = 2:10. Participants continued to complete daily diaries for the remainder of the 14-days.
2.3 |. Measures
2.3.1 |. Early life stress
Exposure to ELS was measured via self-report using 37-items from the Early Trauma Inventory (ETI) (Bremner, Bolus, & Mayer, 2007; Bremner, Vermetten, & Mazure, 2000). These items reflected 19-items of general or nonintentional traumatic events (bereavement, natural disaster), 9-items reflecting physical abuse (hit with objects, frequently pushed or shoved), 8-items reflecting emotional abuse (often put down or ridiculed, often shouted at), and 1-item reflecting exposure to sexual abuse or assault. Participants indicated whether they had ever experienced a given item with either yes or no. The items marked yes were summed to create an index of cumulative ELS.
2.3.2 |. Depressed mood
Depressed mood was measured via daily diary using the 3-item Depressed mood subscale of the Profile of Mood States (POMS-15), which has been previously used in daily diary research (Cranford et al., 2006; Shrout, Herman, & Bolger, 2006). These items were sad, hopeless, and discouraged. Participants indicated the degree to which they experienced each mood state that day and responded to each item according to a 5-point Likert scale from 1 = not at all to 5 = extremely. For multi-item diary scales, reliability coefficients ranging from 0 to 1 for between-person (RKF) and day-to-day within-person change (RC) were computed using methods described for daily diary scales (Cranford et al., 2006). For the depressed mood subscale, RKF = 0.96 and RC = 0.75, which were comparable to prior work (Cranford et al., 2006).
2.3.3 |. Cognitive difficulty
Cognitive difficulty was measured via daily diary using the 3-item POMS-Confusion subscale (McNair, Lorr, & Droppleman, 1981). These items were unable to concentrate, forgetful, and confused. Participants indicated the degree to which they experienced each state that day and responded to each item according to a 5-point Likert scale from 1 = not at all to 5 = extremely. For the confusion subscale, RKF = 0.96 and RC = 0.47.
2.3.4 |. Social disconnection
Social disconnection was measured daily via a 12-item scale reflecting the degree to which participants felt disconnected from other people that day (Eisenberger, Inagaki, Mashal, & Irwin, 2010; Moieni et al., 2015). Participants responded according to a 5-point Likert scale from 1 = not at all, and 5 = very much so. For social disconnection, RKF = 0.97 and RC = 0.36.
2.3.5 |. Inflammation
Interleukin-6 (IL-6) was used to assess within-subject change in inflammation following vaccination because it is a widely used and reliable marker of systemic inflammation following immune challenges (e.g., Steptoe, Hamer, & Chida, 2007), and to optimize comparison of our results with existing literature on inflammation and ELS (Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, 2016; Kuhlman, Horn, Chiang, & Bower, 2019), depression (Dowlati et al., 2010), and inflammatory responses to the influenza vaccine (Carty et al., 2006; Christian, Iams, Porter, & Glaser, 2011; Edwards et al., 2006; Segerstrom, Hardy, Evans, & Greenberg, 2012; Tsai et al., 2005). Blood samples were collected in the morning, MBlood Draw 1 = 9:59 a.m. (SDBlood Draw 1 = 1:04 hr), by venipuncture into EDTA tubes, placed on ice, centrifuged for acquisition of plasma, and stored at −80°C for subsequent batch testing. Samples were assayed in duplicate using a high sensitivity ELISA (R&D Systems). The range of detection for this assay was 0.20–10.0 pg/ml, intra- and inter-assay CVs were <9%, and there were no undetectable values.
2.4 |. Data analysis
All statistics were run in SPSS version 24. For each participant, we computed mean depressed mood, cognitive difficulty, and social disconnection for the days before and after vaccination. We then subtracted each person’s mean score on days before the vaccination from their mean on days after the vaccination, such that higher values indicated increasing symptoms in that domain. We also computed a mean change in IL-6 (ΔIL-6) by subtracting each individual’s pre-vaccine IL-6 concentration from their post-vaccine IL-6 concentration. Again, higher values indicated an increase in IL-6 from pre- to post-vaccine.
All variables met assumptions of normality with the exception of ΔIL-6. The skew of the raw ΔIL-6 variable was 3.28. One participant demonstrated an extreme increase in IL-6 and was winsorized to three standard deviations from the mean ΔIL-6 to reduce their influence on our models. After winsorizing this value, the skew of ΔIL-6 was 2.17 and met the criteria for assumptions of normality.
Moderation analyses were conducted using PROCESS version 3.1 (Hayes, 2013). For our primary analyses, we ran three regression models predicting change in depressed mood, cognitive difficulty, and social disconnection as a function of ΔIL-6 from pre- to post-vaccine, ELS, and the interaction between ΔIL-6 and ELS. Each model covaried for pre-vaccine concentrations of IL-6, female sex, and BMI based on best practices in psychoneuroimmunology (O’Connor et al., 2009), as well as whether the participant was part of the first or second cohort (See Kuhlman et al., 2018 for details). It should be noted that the results of our models are similar and conclusions the same when post-vaccine IL-6 was our dependent variable while controlling for pre-vaccine IL-6 (See Wainer, 1991 for more on the theoretical and statistical importance of this issue).
To probe the regions of significance for our interactions, we used the Johnson–Neyman approach which reports a coefficient and 95% CI at each value of ELS (Preacher, Curran, & Bauer, 2006). For all analyses, p < .05 or 95% was considered statistically significant, although analyses where p < .10 are shown to facilitate comparisons with other studies.
3 |. RESULTS
Participants in this study reported exposure to between 0 and 16 early life stressors, METI = 4.85 (SDETI = 4.14). Only 4.9% of participants denied exposure to all of the events on the trauma exposure inventory, and 50% reported exposure to more than three events. These early life events were diverse, such that 80.5% of participants (n = 33) reported exposure to general or nonintentional traumatic events, 73.2% (n = 30) participants reported exposure to physical trauma, 61.0% (n = 25) participants reported exposure to emotional trauma, and 19.5% (n = 8) participants reported exposure to sexual trauma. The most common experiences reported in this sample were being spanked (n = 25, 61.0%), slapped in the face (n = 14, 34.1%), over-controlled (n = 14, 34.1%), or hit with objects (n = 14, 34.1%) by a caregiver.
Interleukin-6 increased from baseline to post-vaccine, t(40) = 2.77, p = .008. See Kuhlman et al., (2018) for more details. Table 1 provides the descriptive statistics for all study variables and the bivariate correlations between them. Notably, the bivariate associations between ELS and both circulating IL-6 prior to vaccination and ΔIL-6 from pre- to post-vaccine were nonsignificant, see Table 1.
TABLE 1.
Descriptive statistics for all key study variables and bivariate correlations between them
| Correlations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
| 1. Body Mass Index | 24.08 (3.84) | 1.00 | ||||||||
| 2. Early life stress | 4.85 (4.14) | 0.003 | 1.00 | |||||||
| 3. Pre-vaccine IL-6 (pg/ml) | 1.14 (0.95) | 0.45** | 0.04 | 1.00 | ||||||
| 4. ΔIL-6 (pg/ml) | 0.33 (0.75) | −0.03 | −0.19 | −0.01 | 1.00 | |||||
| 5. Pre-vaccine depressed mood | 1.45 (0.52) | −0.07 | 0.24 | 0.01 | −0.25 | 1.00 | ||||
| 6. Δ Depressed mood | −0.08 (0.48) | 0.04 | −0.06 | −0.04 | 0.10 | −0.70*** | 1.00 | |||
| 7. Pre-vaccine cognitive difficulty | 1.60 (0.52) | 0.02 | 0.21 | −0.01 | −0.33* | 0.70*** | −0.51** | 1.00 | ||
| 8. Δ Cognitive difficulty | −0.10 (0.39) | −0.09 | −0.29+ | −0.31 | 0.38** | −0.41** | 0.54** | −0.42** | 1.00 | |
| 9. Pre-vaccine social disconnection | 1.83 (0.45) | 0.02 | 0.35* | 0.18 | −0.14 | 0.44** | −0.30+ | 0.44** | −0.38* | 1.00 |
| 10. Δ Social Disconnection | 0.01 (0.28) | 0.07 | 0.05 | −0.01 | 0.28+ | −0.49** | 0.64*** | −0.42** | 0.54*** | −0.27+ |
p ≤ .10.
p ≤ .05.
p ≤ .01.
p ≤ .001.
We then conducted a multiple regression predicting changes in IL-6 from pre- to post-vaccine from ELS, while accounting for IL-6 at baseline, sex, and BMI, R2 = 0.11, p = .55. Exposure to more ELS was not associated with greater increases in IL-6 from pre- to post-vaccine, b = −0.03, SE = 0.03, t = −1.22, p = .23. Thus there was no evidence that individuals exposed to ELS exhibited a greater inflammatory response to the influenza vaccine.
In the model predicting changes in depressed mood from pre- to post-vaccine, exposure to ELS moderated the association between changes in IL-6 from pre- to post-vaccine and changes in depressed mood, b = 0.14, SE = 0.07, t = 2.21, p = .03, see Table 2. Specifically, the association between ΔIL-6 and change in depressed mood was nonsignificant for participants with low exposure to ELS (−1 SD), b = −0.22, SE = 0.24, t = −0.91, p = .37, whereas the association was positive and significant for participants with average, b = 0.37, SE = 0.14, t = 2.68, p = .01, or above average exposure to ELS, b = 0.97, SE = 0.35, t = 2.75, p = .01, see Figure 1a.
TABLE 2.
Coefficient estimates predicting changes in behavioral symptoms from pre- to post-vaccine from inflammation, early life stress, and their interactions
| Δ Depressed mood b (SE) | Δ Cognitive difficulty b (SE) | Δ Social disconnection b (SE) | |
|---|---|---|---|
| Intercept | 0.10 (0.23) | 0.21 (0.15) | 0.05 (0.14) |
| Baseline IL-6 | 0.07 (0.10) | −0.03 (0.07) | 0.00 (0.06) |
| Δ IL-6 | −0.33 (0.29) | −0.32 (0.19) | 0.08 (0.17) |
| Early life stress | −0.02 (0.02) | −0.05 (0.02)** | 0.01 (0.01) |
| Δ IL-6 × Early life stress | 0.14 (0.07)* | 0.14 (0.04)** | 0.02 (0.04) |
Note: Models covary for sex, cohort, and BMI.
Abbreviation: IL-6, interleukin-6.
p ≤ .05.
p ≤ .01.
FIGURE 1.
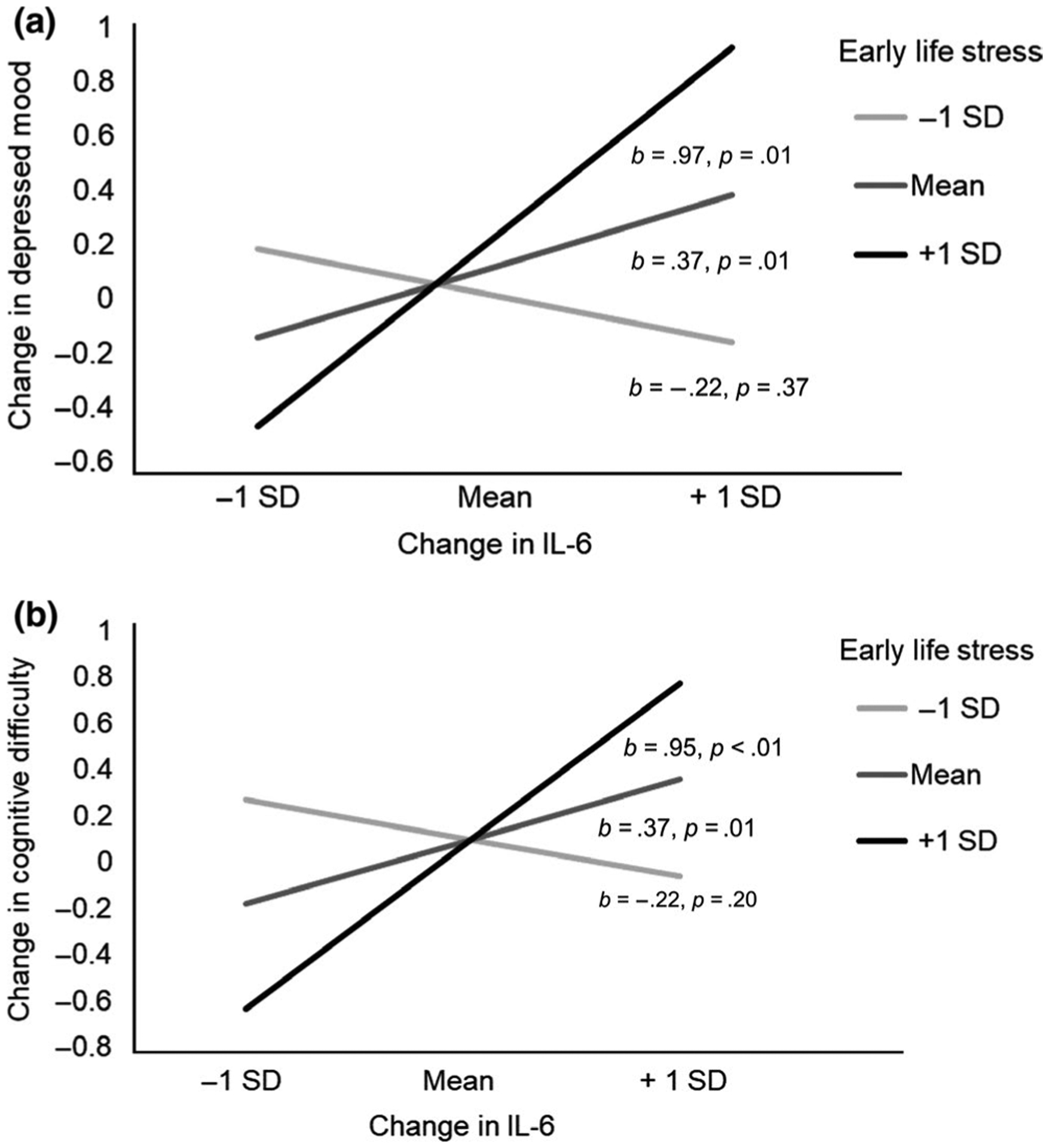
Change in IL-6 from pre- to post-vaccine as a predictor of (a) change in depressed mood and (b) change in cognitive difficulty by exposure to early life stress. Individuals exposed to average or above average (+1 SD) early life stress events exhibit a stronger association between change in inflammation and both increases in depressed mood and cognitive difficulty. IL-6, interleukin-6
We further determined the threshold of ELS after which the association between ΔIL-6 and change in depressed mood became significant. The association between ΔIL-6 and change in depressed mood for participants who reported four or fewer early trauma events was nonsignificant, all ps > .05, if ELS = 4.0 b = 0.25, SE = 0.13, 95% CI [−0.006, 0.51]. However, the association between ΔIL-6 and change in depressed mood was significant for individuals who reported exposure to 5 or more early trauma events, all ps < .018, if ELS = 4.8 b = 0.37, SE = 0.14, 95% CI [0.09, 0.65].
In the model predicting changes in cognitive difficulty from pre- to post-vaccine, exposure to ELS moderated the association between ΔIL-6 from pre- to post-vaccine and changes in cognitive difficulty, b = 0.14, SE = 0.04, t = 3.19, p < .01.2 Specifically, the association between ΔIL-6 and change in cognitive difficulty was nonsignificant for participants with low exposure to ELS (−1 SD), b = −0.22, SE = 0.17, t = −1.32, p = .20, whereas the association was positive and significant for participants with average, b = 0.37, SE = 0.09, t = 3.87, p < .01, or above average exposure to ELS, b = 0.95, SE = 0.24, t = 3.97, p < .01, see Figure 1b.
We further determined the threshold of ELS after which the association between ΔIL-6 and change in cognitive difficulty became significant. The association between ΔIL-6 and change in cognitive difficulty for participants who reported three or fewer early trauma events was nonsignificant, all ps > .10, if ELS = 3.20 b = 0.13, SE = 0.09, 95% CI [−0.05, 0.32]. However, the association between ΔIL-6 and change in cognitive difficulty was uniformly significant for individuals who reported exposure to four or more early trauma events, all ps < .007, if ELS = 4.0 b = 0.24, SE = 0.09, 95% CI [0.07, 0.42].
In the model predicting changes in social disconnection from pre- to post-vaccine, exposure to ELS did not moderate the association between ΔIL-6 from pre- to post-vaccine and changes in social disconnection, b = 0.02, SE = 0.04, t = 0.49, p = .63.
4 |. DISCUSSION
The purpose of this study was to examine the pathways through which inflammation may be linked to features of depression among individuals exposed to ELS. We tested two competing hypotheses: that ELS exaggerates peripheral inflammatory responses and that ELS sensitizes individuals to the psychological correlates of increases in inflammation. Exposure to more ELS was not associated with greater increases in inflammation from pre- to post-vaccine. Individuals exposed to more early trauma events, however, consistently demonstrated increases in depressed mood and cognitive difficulty that corresponded to the magnitude of their inflammatory response following the vaccine. These preliminary findings suggest that ELS may sensitize individuals to the psychological consequences of inflammation.
The main finding in this study was that individuals exposed to more ELS exhibited stronger associations between changes in inflammation and changes in depressed mood and cognitive difficulty following the influenza vaccine. Specifically, individuals exposed to five or more early trauma events (37.5% of our sample) consistently demonstrated increases in depressed mood that corresponded to the magnitude of their inflammatory response to the vaccine. Similarly, individuals exposed to four or more early trauma events consistently demonstrated increases in cognitive difficulty that corresponded to the magnitude of their inflammatory response to the vaccine. Of note, the larger epidemiological evidence linking adverse childhood events to greater health problems in adulthood also shows that health risks are greater for individuals exposed to four or more adversities (Chapman, Dube, & Anda, 2007; Chapman et al., 2004; Dube, Felitti, Dong, Giles, & Anda, 2003).
Our observation that individuals exposed to greater ELS were more sensitive to the psychological correlates of inflammation is consistent with the emerging literature suggesting that stress, in our case ELS, sensitizes the CNS to increases in peripheral inflammation. Our findings are more broadly consistent with prospective, longitudinal studies showing that the link between inflammation and depression is stronger among individuals exposed to ELS (Danese et al., 2008; Miller & Cole, 2012). There are several plausible explanations for this finding that are being explored using preclinical animal models. For example, peripheral immune activation may lead to symptoms of depressed mood and cognitive difficulty through activation of microglia. In preclinical animal models, exposure to ELS increases the density, size, and propensity to release inflammation within CNS by microglia (Bilbo & Schwarz, 2012; Calcia et al., 2016; Delpech et al., 2016; Réus et al., 2017; Wang et al., 2017), which has also been linked to behaviors associated with anxiety and depression in rodents (Johnson & Kaffman, 2018). Indeed, two of these experimental studies have explicitly linked ELS to behavioral phenotypes associated with depression in adulthood through this neuroinflammatory pathway (Réus et al., 2017; Wang et al., 2017) and one showed that the behavioral phenotype associated with depression could be resolved with pharmacological agents that blocked microglia activation (Wang et al., 2017). ELS may also lead to greater permeability of the BBB (Kuvacheva et al., 2016), which allows more peripheral inflammatory cytokines and cells to access the CNS. Indeed, experimental animal models have shown that vulnerability to the behavioral effects of stress are linked to greater permeability in the BBB (Menard et al., 2017). Through these and other mechanisms, neuroinflammation during critical phases of development may alter functional circuits in the brain (Ganguly & Brenhouse, 2015) and have profound implications for several neurotransmitters, including glutamate, serotonin, and dopamine (Dooley et al., 2018; Miller, Haroon, Raison, & Felger, 2013). Of course, these mechanisms are not mutually exclusive, and more translational research is needed to better characterize the development of functional circuits in the context of these developmental neuroimmune processes. This study constitutes compelling preliminary evidence that the psychological correlates of this neuroimmune phenomena can be observed at very low concentrations of inflammation and following immune stimulation that was relatively mild (See Dooley et al., 2018; Kuhlman et al., 2018 for more on the dose–response relationships between inflammation and behavior).
ELS was not associated with a larger increase in inflammation following our immune stimulus, despite previous observations to this effect in both nonhuman primates (Cole et al., 2012) and human adults (Carpenter et al., 2010; See Fagundes, Glaser, & Kiecolt-Glaser, 2013 for review; Miller et al., 2009; Pace et al., 2006), though never previously reported following influenza vaccination. While this finding contradicted our hypotheses, it may be theoretically consistent with studies that have found the association between ELS and inflammation to be either moderated or mediated by adulthood stress and other lifestyle factors (Raposa, Bower, Hammen, Najman, & Brennan, 2014; Slopen et al., 2010; Surtees et al., 2003; Taylor, Lehman, Kiefe, & Seeman, 2006). The absence of a main effect of ELS on change in inflammation in our data compared with previous studies may have also been due to heterogeneity in ELS or the form of immune stimulus used. Heterogeneity in ELS may play an important role in determining an individual’s proinflammatory phenotype (See Kuhlman, Chiang, Horn, & Bower, 2017 for review). For example, previous studies have operationalized ELS as low early life socio-economic status (Miller et al., 2009) and exposure to maltreatment during childhood (Carpenter et al., 2010). Our assessment of ELS was quite heterogeneous and may have obscured more nuanced associations. Alternatively, ELS may alter immune responses to stimuli that occur via sympathetic nervous system activation, such as stress. Indeed, social stressors specifically up-regulate inflammatory genes that are sensitive to ß-adrenergic signaling (Powell et al., 2013). The immune stimulus used in this paradigm, influenza vaccine, activates immune cells independent of sympathetic activation. This distinction will be important to further interrogate as the links between ELS and lifelong health disparities continue to be at the forefront of public health.
The results of this study should be considered in the context of some limitations. This was a relatively small, single-armed investigation aimed at better understanding within-person sensitivity to mild fluctuations in inflammation. For that reason, exposure to the influenza vaccine was not placebo-controlled and no causal inferences about links between inflammation and behavior can be drawn. Follow-up investigations of our observed phenomena could more strongly support our conclusions in a study that carefully over-sampled for ELS exposure and assessed this in more dimensions with interviews, self-report, and sociodeomographic factors. Additionally, our assessments of inflammation and psychological sensitivity were limited to IL-6 and daily reported mood. There are a number of indices of inflammatory signaling that are more sensitive than IL-6 alone, including multiplex assays that measure multiple circulating inflammatory proteins, expression of genes associated with the production and regulation of inflammation, and expression of RNA transcription factors that are linked to inflammation (Cole, 2009; Slavich & Cole, 2013). Similarly, psychological sensitivity to inflammatory signaling could be manifested at many levels of analysis, such as through structural and functional connectivity of neural circuits that govern mood, or through performance on behavioral and neuropsychological tasks (See Dooley et al., 2018 for review). Furthermore, our interpretations of psychological sensitivity to the influenza vaccine are limited by the imbalance in sampling frequency between our mood and inflammatory measures. Mood was measured daily for 14 days while inflammation was only measured immediately before and 1-day after influenza vaccine. It is possible that the greatest sensitivity was exhibited by individuals with larger inflammatory responses to the vaccine that were sustained over multiple days, but our study design precludes our interrogation of this possibility. Our sample also reported somewhat lower exposure to ELS compared with that of the original sample used to validate this measure (Bremner et al., 2007). Finally, our sample size precluded interrogation of other important factors in the link between inflammation and the behavioral features of depression, specifically sex (Lasselin, Lekander, Axelsson, & Karshikoff, 2018; Moieni et al., 2015) and ethnicity, which is an important future direction in this work. Indeed, our sample was predominantly female and Asian, the implications of which will need to be interrogated in a larger sample.
Ultimately, better understanding the biobehavioral pathways that link ELS to psychiatric symptoms and disorders will inform the development of effective treatments for this underserved population. To date, studies have focused on the hypothesis that ELS increases the peripheral inflammatory response to endogenous and exogenous stimulation (e.g., Nusslock & Miller, 2016). However, the present data are among the first to show that individuals reporting greater ELS were more psychologically sensitive to increases in inflammatory signaling from the periphery, independent of the main effect of ELS on their inflammatory response. These preliminary data underscore the potential for behavioral and pharmacological treatments that mitigate neural and neuroimmune pathways linking ELS and depression, such as neuroinflammation (Yirmiya, Rimmerman, & Reshef, 2015).
ACKNOWLEDGMENTS
This research would not be possible if not for the support of the George F. Solomon Endowed Term Chair in Psychobiology, Cousins Center for Psychoneuroimmunology, UCLA Semel Institute for Neuroscience and Human Behavior, as well as the participants who generously gave their time. The composition of this manuscript was made possible by the National Institute of Mental Health (K08MH112773) awarded to Dr. Kuhlman.
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Being female or having an Asian ethnic background was not associated with significant differences in change in IL-6 from pre- to post-vaccine, all ps > .53, change in depressed mood from pre- to post-vaccine, all ps > .45, or exposure to ELS, all ps > .77.
The interaction between ΔIL-6 and ELS when predicting change in cognitive difficulty remained significant when controlling for Δ depressed mood, b = 0.10 (SE = 0.04), while the interaction between ΔIL-6 and ELS when predicting Δ depressed mood did not remain significant when controlling for Δ cognitive difficulty, b = 0.04 (SE = 0.07). This may suggest that individuals exposed to greater ELS may be more sensitive to the cognitive effects of inflammation than the mood effects. Indeed, the main effect of change in IL-6 on confusion was larger with less variability, b = 0.20 (SE = 0.06), than on depressed mood, b = 0.17 (SE = 0.08) (Kuhlman et al., 2018). However, the relatively small sample size and high correlation between Δ depressed mood and Δ cognitive difficulty lead us to interpret the results of this sensitivity analysis with caution.
REFERENCES
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V (2016). Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry, 21(5), 642. 10.1038/mp.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, & Schwarz JM (2012). The immune system and developmental programming of brain and behavior. Frontiers in Neuroendocrinology, 33(3), 267–286. 10.1016/j.yfrne.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, & Mayer EA (2007). Psychometric properties of the Early Trauma Inventory-Self Report. The Journal of Nervous and Mental Disease, 195(3), 211–218. 10.1097/01.nmd.0000243824.84651.6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, & Mazure CM (2000). Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The Early Trauma Inventory. Depression and Anxiety, 12(1), 1–12. 10.1002/1520-6394(2000)12 [DOI] [PubMed] [Google Scholar]
- Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, & Howes OD (2016). Stress and neuroinflammation: A systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl), 233(9), 1637–1650. 10.1007/s00213-016-4218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, & Price LH (2010). Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology, 35(13), 2617–2623. 10.1038/npp.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty CL, Heagerty P, Nakayama K, McClung EC, Lewis J, Lum D, … Jarvik GP (2006). Inflammatory response after influenza vaccination in men with and without carotid artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(12), 2738–2744. 10.1161/01.ATV.0000248534.30057.b5 [DOI] [PubMed] [Google Scholar]
- Chapman DP, Dube SR, & Anda RF (2007). Adverse childhood events as risk factors for negative mental health outcomes. Psychiatric Annals, 37(5), 359–364. [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, & Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82(2), 217–225. 10.1016/j.jad.2003.12.013 [DOI] [PubMed] [Google Scholar]
- Christian LM, Iams JD, Porter K, & Glaser R (2011). Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine, 29(48), 8982–8987. 10.1016/j.vaccine.2011.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW (2009). Social regulation of human gene expression. Current Directions in Psychological Science, 18(3), 132–137. 10.1111/j.1467-8721.2009.01623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Conti G, Arevalo JMG, Ruggiero AM, Heckman JJ, & Suomi SJ (2012). Transcriptional modulation of the developing immune system by early life social adversity. Proceedings of the National Academy of Sciences, 109(50), 20578–20583. 10.1073/pnas.1218253109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, & Bolger N (2006). A procedure for evaluating sensitivity to within-person change: Can mood measures in diary studies detect change reliably? Personality and Social Psychology Bulletin, 32(7), 917–929. 10.1177/0146167206287721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, … Arseneault L (2011). Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry, 16(3), 244–246. 10.1038/mp.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & Lewis SJ (2017). Psychoneuroimmunology of early-life stress: The hidden wounds of childhood trauma? Neuropsychopharmacology, 42, 99–114. 10.1038/npp.2016.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, & Caspi A (2008). Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of General Psychiatry, 65(4), 409–415. 10.1001/archpsyc.65.4.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, & Poulton R (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, 104(4), 1319–1324. 10.1073/pnas.0610362104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, & Kelley KW (2007). Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity, 21(2), 153–160. 10.1016/j.bbi.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience, 9(1), 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech J-C, Wei L, Hao J, Yu X, Madore C, Butovsky O, & Kaffman A (2016). Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain, Behavior, and Immunity, 57, 79–93. 10.1016/j.bbi.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, & Bower JE (2018). The role of inflammation in core features of depression: Insights from paradigms using exogenously-induced inflammation. Neuroscience & Biobehavioral Reviews, 94, 219–237. 10.1016/j.neubiorev.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctôt KL (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67(5), 446–457. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, & Anda RF (2003). The impact of adverse childhood experiences on health problems: Evidence from four birth cohorts dating back to 1900. Preventive Medicine, 37(3), 268–277. 10.1016/S0091-7435(03)00123-3 [DOI] [PubMed] [Google Scholar]
- Edwards KM, Burns VE, Reynolds T, Carroll D, Drayson M, & Ring C (2006). Acute stress exposure prior to influenza vaccination enhances antibody response in women. Brain, Behavior, and Immunity, 20(2), 159–168. 10.1016/j.bbi.2005.07.001 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, & Irwin MR (2010). Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain, Behavior, and Immunity, 24(4), 558–563. 10.1016/j.bbi.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, & Kiecolt-Glaser JK (2013). Stressful early life experiences and immune dysregulation across the lifespan. Brain, Behavior, and Immunity, 27, 8–12. 10.1016/j.bbi.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, & Brenhouse HC (2015). Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Developmental Cognitive Neuroscience, 11, 18–30. 10.1016/j.dcn.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, … Ferrie JE (2009). Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine, 39(03), 413–423. 10.1017/S0033291708003723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL (1988). Biological basis of the behavior of sick animals. Neuroscience and Biobehavioral Reviews, 12(2), 123. 10.1016/S0149-7634(88)80004-6 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press. [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosomatic Medicine, 71(2), 171–186. 10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- Janeway CA, & Medzhitov R (2002). Innate immune recognition. Annual Review of Immunology, 20(1), 197–216. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- Johnson FK, & Kaffman A (2018). Early life stress perturbs the function of microglia in the developing rodent brain: New insights and future challenges. Brain, Behavior, and Immunity, 69, 18–27. 10.1016/j.bbi.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, … Williams DR (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197(5), 378–385. 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Chiang JJ, Horn S, & Bower JE (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neuroscience and Biobehavioral Reviews, 80, 166–184. 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Horn SR, Chiang JJ, & Bower JE (2019). Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain, Behavior, and Immunity, in press. 10.1016/j.bbi.2019.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Dooley LN, Boyle CC, Haydon MD, & Bower JE (2018). Within-subject associations between inflammation and features of depression: Using the flu vaccine as a mild inflammatory stimulus. Brain, Behavior, and Immunity, 69, 540–547. 10.1016/j.bbi.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuvacheva NV, Morgun AV, Malinovskaya NA, Gorina YV, Khilazheva ED, Pozhilenkova EA, … Salmina AB (2016). Tight junction proteins of cerebral endothelial cells in early postnatal development. Cell and Tissue Biology, 10(5), 372–377. 10.1134/S1990519X16050084 [DOI] [PubMed] [Google Scholar]
- Lasselin J, Lekander M, Axelsson J, & Karshikoff B (2018). Sex differences in how inflammation affects behavior: What we can learn from experimental inflammatory models in humans. Frontiers in Neuroendocrinology, 50, 91–106. 10.1016/j.yfrne.2018.06.005 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication II: Associations with persistence of DSM-IV disorders. Archives of General Psychiatry, 67(2), 124–132. 10.1001/archgenpsychiatry.2009.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF (1981). Profile of mood states. San Diego, CA: Educational and industrial testing service. [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, … Russo SJ (2017). Social stress induces neurovascular pathology promoting depression. Nature Neuroscience, 20(12), 1752. 10.1038/s41593-017-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, & Felger JC (2013). Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depression and Anxiety, 30(4), 297–306. 10.1002/da.22084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16(1), 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Chen E (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science, 21(6), 848–856. 10.1177/0956797610370161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, … Kobor MS (2009). Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences, 106(34), 14716–14721. 10.1073/pnas.0902971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72(1), 34–40. 10.1016/j.biopsych.2012.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, & Eisenberger NI (2015). Sex differences in depressive and socio-emotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacology, 40(7), 1709–1716. 10.1038/npp.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry, 80(1), 23–32. 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, … Irwin MR (2009). To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity, 23(7), 887–897. 10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, & Heim CM (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry, 163(9), 1630–1633. 10.1176/ajp.2006.163.9.1630 [DOI] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, … Cole SW (2013). Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences, 110(41), 16574–16579. 10.1073/pnas.1310655110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31(4), 437–448. 10.3102/10769986031004437 [DOI] [Google Scholar]
- Raison CL, Capuron L, & Miller AH (2006). Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology, 27(1), 24–31. 10.1016/j.it.2005.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposa EB, Bower JE, Hammen CL, Najman JM, & Brennan PA (2014). A developmental pathway from early life stress to inflammation: The role of negative health behaviors. Psychological Science, 25(6), 1268–1274. 10.1177/0956797614530570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réus GZ, Fernandes GC, de Moura AB, Silva RH, Darabas AC, de Souza TG, … Quevedo J (2017). Early life experience contributes to the developmental programming of depressive-like behaviour, neuroinflammation and oxidative stress. Journal of Psychiatric Research, 95, 196–207. 10.1016/j.jpsychires.2017.08.020 [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Hardy JK, Evans DR, & Greenberg RN (2012). Vulnerability, distress, and immune response to vaccination in older adults. Brain, Behavior, and Immunity, 26(5), 747–753. 10.1016/j.bbi.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Herman CM, & Bolger N (2006). The costs and benefits of practical and emotional support on adjustment: A daily diary study of couples experiencing acute stress. Personal Relationships, 13(1), 115–134. 10.1111/j.1475-6811.2006.00108.x [DOI] [Google Scholar]
- Slavich GM, & Cole SW (2013). The emerging field of human social genomics. Clinical Psychological Science, 1, 331–348. 10.1177/2167702613478594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, & Williams DR (2010). Early life adversity and inflammation in African Americans and Whites in the midlife in the United States survey. Psychosomatic Medicine, 72(7), 694–701. 10.1097/PSY.0b013e3181e9c16f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, & Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–912. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Surtees P, Wainwright N, Day N, Brayne C, Luben R, & Khaw K-T (2003). Adverse experience in childhood as a developmental risk factor for altered immune status in adulthood. International Journal of Behavioral Medicine, 10(3), 251–268. 10.1207/S15327558IJBM1003_05 [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, & Seeman TE (2006). Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry, 60(8), 819–824. 10.1016/j.biopsych.2006.03.016 [DOI] [PubMed] [Google Scholar]
- Tsai MY, Hanson NQ, Straka RJ, Hoke TR, Ordovas JM, Peacock JM, … Arnett DK (2005). Effect of influenza vaccine on markers of inflammation and lipid profile. Journal of Laboratory and Clinical Medicine, 145(6), 323–327. 10.1016/j.lab.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Wainer H (1991). Adjusting for differential base rates: Lord’s paradox again. Psychological Bulletin, 109(1), 147. 10.1037/0033-2909.109.1.147 [DOI] [PubMed] [Google Scholar]
- Wang H-T, Huang F-L, Hu Z-L, Zhang W-J, Qiao X-Q, Huang Y-Q, … Li C-Q (2017). Early-life social isolation-induced depressive-like behavior in rats results in microglial activation and neuronal histone methylation that are mitigated by minocycline. Neurotoxicity Research, 31(4), 505–520. 10.1007/s12640-016-9696-3 [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Rimmerman N, & Reshef R (2015). Depression as a microglial disease. Trends in Neurosciences, 38(10), 637–658. 10.1016/j.tins.2015.08.001 [DOI] [PubMed] [Google Scholar]


