Abstract
Background:
Examining handgrip strength (HGS) asymmetry could extend the utility of handgrip dynamometers for screening future falls.
Aims:
We sought to determine the associations of HGS asymmetry on future falls in older Americans.
Methods:
The analytic sample included 10,446 adults aged at least 65-years from the 2006–2016 waves of the Health and Retirement Study. Falls were self-reported. A handgrip dynamometer measured HGS. The highest HGS on each hand was used for determining HGS asymmetry ratio: (non-dominant HGS/dominant HGS). Those with HGS asymmetry ratio <1.0 had their ratio inverted to make all HGS asymmetry ratios ≥1.0. Participants were categorized into asymmetry groups based on their inverted HGS asymmetry ratio: 1) 0.0%−10.0%, 2) 10.1%−20.0%, 3) 20.1%−30.0%, and 4) >30.0%. Generalized estimating equations were used for the analyses.
Results:
Every 0.10 increase in HGS asymmetry ratio was associated with 1.26 (95% confidence interval (CI): 1.07–1.48) greater odds for future falls. Relative to those with HGS asymmetry 0.0%−10.0%, participants with HGS asymmetry >30.0% had 1.15 (CI: 1.01–1.33) greater odds for future falls; however, the associations were not significant for those with HGS asymmetry 10.1%−20.0% (odds ratio: 1.06; CI: 0.98–1.14) and 20.1%−30.0% (odds ratio: 1.10; CI: 0.99–1.22). Compared to those with HGS asymmetry 0.0%−10.0%, participants with HGS asymmetry >10.0% and >20.0% had 1.07 (CI: 1.01–1.16) and 1.12 (CI: 1.02–1.22) greater odds for future falls, respectively.
Discussion:
Asymmetric HGS, as a possible biomarker of impaired neuromuscular function, may help predict falls.
Conclusions:
We recommend that HGS asymmetry be considered in HGS protocols and fall risk assessments.
Keywords: Aging, Functional Laterality, Geriatrics, Geriatric Assessment, Muscle Strength Dynamometer
INTRODUCTION
Falls are a leading cause of injury and disability in older adults [1]. Poor muscle strength is a risk factor for several age-related adverse health outcomes including falls [2]. Handgrip strength (HGS) is a convenient and reliable assessment of muscle strength that is largely driven by neuromuscular function [3, 4]. Current HGS protocol guidelines focus exclusively on collecting maximum isometric grip force from a single hand across multiple trials, irrespective of hand dominance, for determining strength capacity [5]. Although low HGS is associated with increased fall risk during aging [6], valuable information regarding neuromuscular function could be lost by not examining hand dominance and the strength of both hands.
The motor skills and synchronous muscle contractions involved in HGS measurements are influenced by the neuromuscular system that mediates the control of coordinated movement [4]. Deteriorating neuromuscular system functioning could be reflected in the strength imbalances between limbs that increase the risk of falls [7]. For example, substantial leg extension power asymmetry between limbs encumbered walking and standing balance in older women [8]. Wide differences in strength between hands, as characterized by HGS asymmetry, has been shown to be associated with health outcomes linked to falls such as functional disability [9], low cognitive functioning [10], and early all-cause mortality [11]. Therefore, HGS asymmetry may represent the deficits in neuromuscular system functioning that leads to the strength imbalances that increase fall risk. Evaluating the associations between HGS asymmetry and future falls may improve the prognostic value of handgrip dynamometers, and improve our understanding of underlying pathways of age-related motor changes to improve screening for age-related disability.
While HGS asymmetry may help to predict future falls, the necessary degree of asymmetry for predicting falls remains opaque. A 10% difference in strength between the dominant and non-dominant hands has been postulated [12], but others have challenged this 10% difference in strength between hands [13], and detecting functional asymmetries between limbs may require a greater degree of asymmetry beyond 10% [14]. Providing clarity to the degree in which HGS asymmetry predicts outcomes such as falls will help to inform healthcare providers that have identified strength imbalances between limbs in their older adult patients. This may also help to establish HGS asymmetry thresholds for interventions aiming to preserve function during aging and screening for falls. Accordingly, we sought to determine the associations between HGS asymmetry and future falls in older Americans.
METHODS
Participants
A secondary analysis of publicly available data from 10,629 Americans aged ≥65-years who had at least one wave of HGS measured bilaterally with information about hand dominance (right, left), and one or more follow-up waves of falls assessed in the 2006–2016 waves of the Health and Retirement Study (HRS) were analyzed for this investigation. The HRS is a longitudinal-panel study that observes economic and health factors during aging. Core interviews in the HRS occur biennially and participants are followed until death. More details about the HRS are available elsewhere [15].
Starting in the 2006 wave, the HRS began conducting enhanced face-to-face interviews to include physical measures such as HGS. To minimize participant burden, the enhanced face-to-face interviews alternated completion at each wave, wherein a random half sample of participants were selected to complete the enhanced face-to-face and core interviews, while the other half sample only engaged in the core interview. Interview response rates for the HRS were consistently more than 80% at each wave [16]. Participants provided written informed consent before entering the HRS and the University’s Behavioral Sciences Committee Institutional Review Board approved study protocols.
Measures
Falls
At each wave, participants were asked if they had fallen down since their last interview (i.e., every 2-years). Those who reported that they experienced a fall were considered as having fell.
Handgrip Strength Asymmetry
A Smedley spring-type handgrip dynamometer (Scandidact; Odder, Denmark) measured HGS. Trained interviewers explained the HGS protocols before fitting the dynamometer to the hand size of the participants. Each person completed a practice trial with their arm at the side and elbow flexed at 90-degrees. Participants responded to the question “which is your dominant hand?” before testing, and beginning on the non-dominant hand, participants squeezed the dynamometer with maximal effort, twice on each hand, alternating between hands. Those unable to stand or properly position their arm while grasping the dynamometer could be seated and rest their upper arm on a supporting object during testing. Participants that had a surgical procedure in the last six-months, or swelling, inflammation, severe pain or an injury to both hands in the previous month before the enhanced face-to-face interview did not engage in HGS testing. More details about HGS testing in the HRS are available elsewhere [17].
The highest recorded HGS values from the non-dominant and dominant hand were used to calculate HGS asymmetry ratio: (non-dominant HGS (kilograms)/dominant HGS (kilograms)). Given that strength between hands may vary, previously published thresholds of approximately a 10% [12], 20% [10], and 30% [18] difference in strength between hands guided how we defined our HGS asymmetry groups. Those with HGS asymmetry ratio <1.0 had their ratio inversed to make all HGS asymmetry ratios ≥1.0 (inversed HGS asymmetry ratio; continuous variable). Participants were then categorized into HGS asymmetry groups based on their inverted HGS asymmetry ratio: 1) 0.0%−10.0%, 2) 10.1%−20.0%, 3) 20.1%−30.0%, and 4) >30.0%.
Covariates
Age, sex, race, height, and weight were self-reported. Body mass index was calculated as body weight in kilograms divided by height in meters-squared. Those with a body mass index ≥30 kilograms per meters-squared were considered obese. The single greatest HGS ascertained during HGS testing was included as maximal HGS. Respondents told interviewers if a healthcare provider had ever diagnosed them with hypertension, diabetes, cancer, lung disease, a heart condition, stroke, emotional or psychiatric problems, and arthritis. The number of affirmative morbid diagnoses were summed at each wave and those with multimorbidity had ≥2 health conditions. Participants that reported engaging in moderate-to-vigorous physical activity “once a week” or more were classified as participating in moderate-to-vigorous physical activity. Each participant also told interviewers if they had ever smoked more than 100 cigarettes in their lifetime (previous smoker) and if they were a current cigarette smoker. A single item measure of self-rated health determined if participants perceived their health as “excellent”, “very good”, “good”, “fair”, or “poor”.
An eight-item Center for the Epidemiologic Studies Depression scale assessed depressive symptoms [19]. Respondents revealed if they experienced any negative or positive emotions during the week prior to the interview. Scores ranged from 0–8 with higher scores indicating more depressive symptoms. Those with scores ≥3 were considered depressed. The 35-point adapted Telephone Interview of Cognitive Status, which is a well-validated screening tool designed from the Mini-Mental State Examination, evaluated cognitive function [20]. Assessments included immediate and delayed word recall from a list of 10 words, serial sevens subtraction test starting with the number 100, counting backward at maximal speed for 10 consecutive numbers beginning from the number 20, correctly identifying the current president and vice president of the United States, object naming, and date naming. Those with scores <11 were considered as having a cognitive impairment [21].
Statistical Analysis
All analyses were conducted with SAS 9.4 software (SAS Institute; Cary, NC). Current fall status and other covariates were assessed at each wave in which HGS was collected. The outcome was falls at the next available wave. Time to follow-up between waves was adjusted in which HGS was measured and the outcome was adjusted for in the analyses. A breakdown for when participants first entered our study and when falls were subsequently assessed are provided in Appendix 1. The descriptive characteristics of the participants were presented as mean±standard deviation for continuous variables or frequency and percentage for categorical variables.
Generalized estimating equations examined the associations between HGS asymmetry and future falls. First, a generalized estimating equation analyzed the association between inverted HGS asymmetry ratio and future falls. Thereafter, separate generalized estimating equations analyzed the associations of 1) HGS asymmetry 10.1%−20.0%, 2) HGS asymmetry 20.1%−30.0%, and 3) HGS asymmetry >30.0% for future falls. Individual generalized estimating equations similarly examined the associations of 1) HGS asymmetry >10.0%, and 2) HGS asymmetry >20.0% for future falls. The reference group was HGS asymmetry 0.0%−10.0% for each model with a HGS asymmetry category. The models were first run as crude, wherein falls at current wave and follow-up years were adjusted. Then, the models were fully-adjusted for maximal HGS, hand dominance, age, sex, race, multimorbidity, obesity, current smoking status, previous smoking status, self-rated health, depression, moderate-to-vigorous physical activity, cognitive impairment, falls at current wave, and follow-up years. Repeated measures were accounted for in the models and the outcome for the next waved participated was used. All covariates were pre-specified by the investigators and the results from the fully-adjusted models were included as our principal results.
Since HGS asymmetry and maximal HGS were both generated from HGS measurements, individual fully-adjusted generalized estimating equations determined if there was an interaction between maximal HGS and 1) inverted HGS asymmetry ratio, 2) HGS asymmetry 10.1%−20.0% (reference: HGS asymmetry 0.0%−10.0%), 3) HGS asymmetry 20.1%−30.0% (reference: HGS asymmetry 0.0%−10.0%), and 4) >30.0% (reference: HGS asymmetry 0.0%−10.0%) for future falls.
As a supplementary analysis, we examined HGS asymmetry dominance for the association between future falls, such that we did not invert any HGS asymmetry ratio. Specifically, participants were categorized into dominant HGS asymmetry groups based on their HGS asymmetry ratios: 1) 10.1%−20.0% (i.e., HGS asymmetry ratio 0.899–0.800), 2) 20.1%−30.0% (i.e., HGS asymmetry ratio 0.799–0.700), and 3) >30.0% (i.e., HGS asymmetry ratio <0.700). Participants were also categorized into non-dominant HGS asymmetry groups based on their HGS asymmetry ratios 1) 10.1%−20.0% (i.e., HGS asymmetry ratio 1.101–1.200), 2) 20.1%−30.0% (i.e., HGS asymmetry ratio 1.201–1.300), and 3) >30.0% (i.e., HGS asymmetry ratio >1.300). Separate crude and fully-adjusted generalized estimating equations analyzed the associations of 1) 10.1%−20.0% dominant and non-dominant HGS asymmetry (reference: HGS asymmetry 0.0%−10.0%), 2) 20.1%−30.0% dominant and non-dominant HGS asymmetry (reference: asymmetry 0.0%−10.0%), and 3) >30.0% dominant and non-dominant HGS asymmetry (reference: asymmetry 0.0%−10.0%) for future falls.
As another supplementary analysis, we conducted a receiver operating characteristic curve analysis with the inverted HGS asymmetry ratio values for future falls. Youden’s J, which is a function for optimizing sensitivity and specificity, was used to determine the inverted HGS asymmetry ratio cut-point [22, 23]. Youden’s J was defined as: J=maximum[sensitivity(c)+specificity(c)-1]. The results from all of the supplementary analyses were included as appendices. An alpha level of 0.05 was used for all analyses.
RESULTS
After exclusions (n=183) for missing or implausible covariates, there were 10,446 participants included and their baseline descriptive characteristics are presented in Table 1. Overall, participants were aged 72.8±6.7 years and spent 8.6±2.3 years in the study. Of these participants, 4,860 (46.5%) had HGS asymmetry 0.0%−10.0%, 3,459 (33.1%) had HGS asymmetry 10.1%−20.0%, 1,482 (14.2%) had HGS asymmetry 20.1%−30.0%, and 645 (6.2%) had HGS asymmetry >30.0%. To make comparisons between the HGS asymmetry groups, the means and 95% confidence intervals (CI) for the baseline descriptive characteristics of the participants are shown in Appendix 2. A histogram for inverted HGS asymmetry ratio is presented in Figure 1.
Table 1.
Baseline Descriptive Characteristics of the Participants.
| Overall (n=10,446) | HGS Asymmetry 0.0%–10.0% (n=4,860) | HGS Asymmetry 10.1%–20.0% (n=3,459) | HGS Asymmetry 20.1%–30.0% (n=1,482) | HGS Asymmetry >30.0% (n=645) | ||
|---|---|---|---|---|---|---|
| 30.0±10.4 | 30.4±10.5 | 30.4±10.2 | 29.0±9.2 | 26.8±10.3 | ||
| Age (years) | 72.8±6.7 | 72.5±6.5 | 72.8±6.7 | 73.0±6.6 | 74.9±7.4 | |
| Age Group (n (%)) | ||||||
| 65–74 years | 6,789 (65.0) | 3,241 (66.7) | 2,262 (65.4) | 936 (63.2) | 350 (54.3) | |
| 75–84 years | 2,895 (27.7) | 1,313 (27.0) | 942 (27.2) | 442 (29.8) | 198 (30.7) | |
| ≥85 years | 762 (7.3) | 306 (6.3) | 255 (7.4) | 104 (7.0) | 97 (15.0) | |
| Female (n (%)) | 6,015 (57.5) | 2,607 (53.6) | 2,028 (58.6) | 959 (64.7) | 421 (65.2) | |
| White Race (n (%)) | 8,004 (76.6) | 3,817 (78.5) | 2,614 (75.5) | 1,118 (75.4) | 455 (70.5) | |
| Right Hand Dominant | 9,635 (92.2) | 4,406 (90.6) | 3,237 (93.5) | 1,407 (94.9) | 585 (90.7) | |
| Health Conditions | 2.2±1.3 | 2.1±1.3 | 2.2±1.3 | 2.2±1.4 | 2.5±1.4 | |
| Multimorbidity (n (%)) | 7,142 (68.3%) | 3,237 (66.6%) | 2,405 (69.5%) | 1,011 (68.2%) | 489 (75.8%) | |
| Obese (n (%)) | 3,259 (31.2) | 1,528 (31.4) | 1,061 (30.6) | 479 (32.3) | 191 (29.6) | |
| Smoking Status (n (%)) | ||||||
| Never Smoked | 4,547 (43.5) | 2,073 (42.6) | 1,514 (43.8) | 672 (45.3) | 288 (44.7) | |
| Current Smoker | 1,023 (9.8) | 485 (10.0) | 353 (10.2) | 121 (8.2) | 64 (9.9) | |
| Previous Smoker | 4,876 (46.7) | 2,302 (47.4) | 1,592 (46.0) | 689 (46.5) | 293 (45.4) | |
| Depression (n (%)) | 1,880 (18.0) | 828 (17.0) | 607 (17.5) | 274 (18.4) | 171 (26.5) | |
| MVPA Participation (n (%)) | 6,031 (57.7) | 2,907 (59.8) | 1,969 (56.9) | 834 (56.2) | 321 (49.7) | |
| Cognitive Impairment (n (%)) | 242 (2.3) | 103 (2.1) | 74 (2.1) | 38 (2.5) | 27 (4.1) | |
| Self-Rated Heath (n (%)) | ||||||
| Excellent, Very Good | 4,242 (59.4) | 2,779 (57.2) | 2,052 (59.3) | 922 (62.2) | 451 (69.9) | |
| Good, Fair, Poor | 4,242 (40.6) | 2,081 (42.8) | 1,407 (40.7) | 560 (37.8) | 194 (30.1) | |
| Duration in Study (years) | 8.6±2.3 | 8.6±2.3 | 8.6±2.3 | 8.6±2.4 | 8.3±2.6 | |
| Fall at Current Wave (n (%)) | 3,513 (33.6) | 1,598 (32.8) | 1,129 (32.6) | 500 (33.7) | 286 (44.3) | |
| Fall at Next Wave (n (%)) | 3,623 (34.6) | 1,607 (33.0) | 1,195 (34.5) | 536 (36.1) | 285 (44.1) |
Note: HGS=handgrip strength; MVPA=moderate-to-vigorous physical activity.
Figure 1.
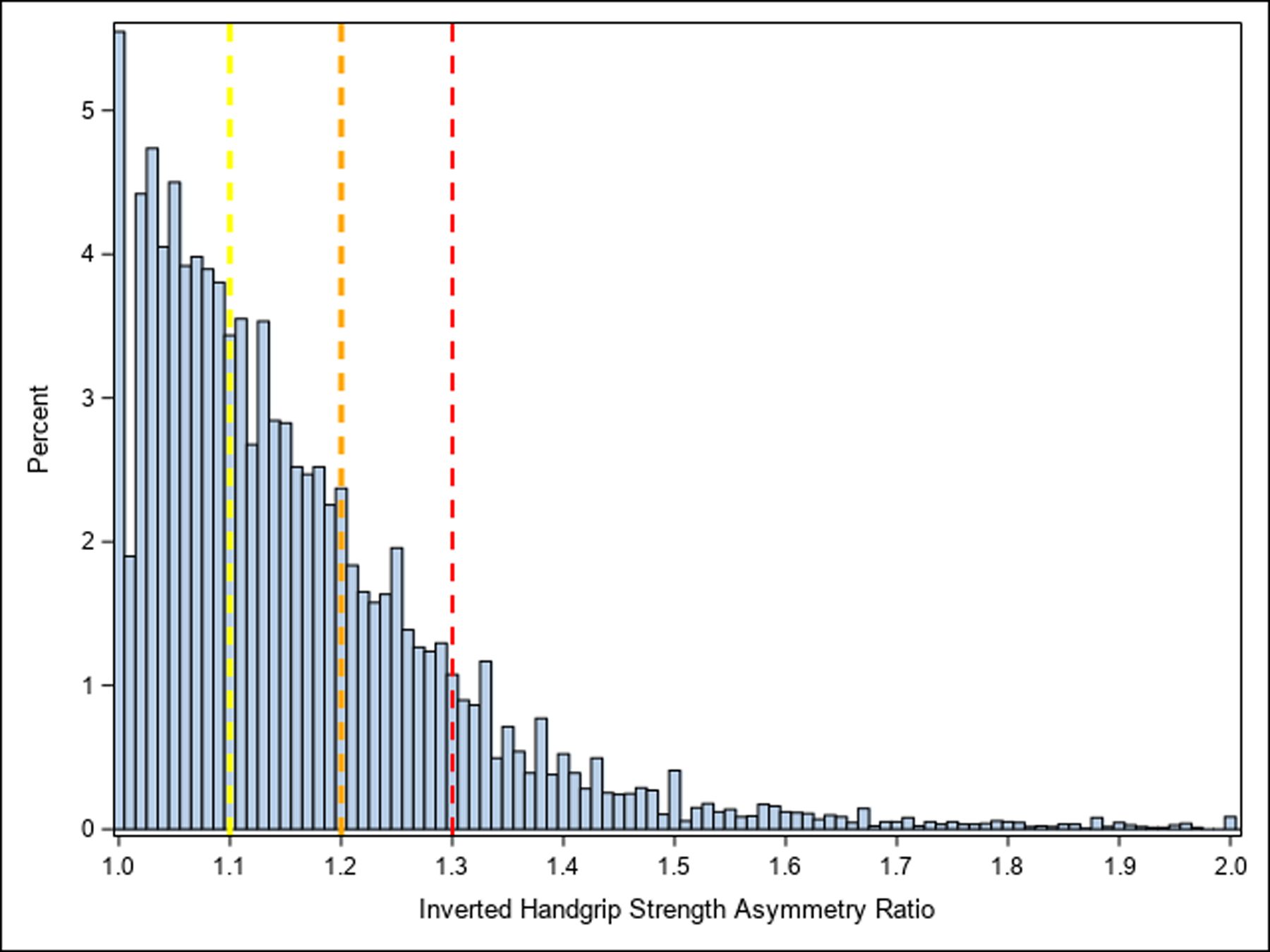
Histogram of Inverted Handgrip Strength Asymmetry Ratio.
Note: yellow reference line=handgrip strength asymmetry 10.1%−20.0%; orange reference line=handgrip strength asymmetry 20.1%−30.0%; red reference line=handgrip strength asymmetry >30.0%.
Inverted HGS asymmetry ratio did not interact with maximal HGS (p=0.53). Likewise, Maximal HGS did not interact with HGS asymmetry at 10.1%−20.0% (p=0.62), 20.1%−30.0% (p=0.35), and >30.0% (p=0.59). Table 2 provides the results for the associations between inverted HGS asymmetry ratio and future falls. Every 0.10 (i.e., 10%) increase in inverted HGS asymmetry ratio was associated with 1.26 (CI: 1.07, 1.48) greater odds for future falls. The results for the associations between the HGS asymmetry categories and future falls are presented in Table 3. The associations between those with HGS asymmetry 10.1%−20.0% (odds ratio: 1.06; CI: 0.98, 1.14) and 20.1%−30.0% (odds ratio: 1.10; CI: 0.99, 1.22) did not reach statistical significance. However, those with HGS asymmetry >30.0% had 1.15 (CI: 1.01, 1.33) greater odds for future falls. Table 4 shows the results for the associations between the overlapping HGS asymmetry categories and future falls. Participants with HGS asymmetry >10.0% and >20.0% had 1.07 (CI: 1.01, 1.16) and 1.12 (CI: 1.02, 1.22) greater odds for future falls, respectively.
Table 2.
Results for the Associations Between Inverted Handgrip Strength Asymmetry Ratio and Future Falls.
| Crude Model | Fully-Adjusted Model | ||||
|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | ||
| 1.40 | 1.14, 1.70 | 1.26 | 1.07, 1.48 | ||
Note: The crude model was adjusted for falls at current wave and follow-up years. The fully-adjusted model was adjusted for falls at current wave, maximal HGS, hand dominance, sex, age, race, multimorbidity, obesity, current smoking status, previous smoking status, self-rated health, depression, moderate-to-vigorous physical activity, cognitive impairment, and follow-up years. HGS=handgrip strength.
Table 3.
Results for the Associations Between the Handgrip Strength Asymmetry Categories and Future Falls.
| Crude Models | Fully-Adjusted Models | ||||
|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | ||
| 1.05 | 0.97, 1.13 | 1.06 | 0.98, 1.14 | ||
| HGS Asymmetry 20.1%–30.0%† (n=1,482) | 1.12 | 1.01, 1.23 | 1.10 | 0.99, 1.22 | |
| HGS Asymmetry >30.0%† (n=645) | 1.32 | 1.15, 1.51 | 1.15 | 1.01, 1.33 | |
Reference=HGS Asymmetry 0.0%−10.0% (n=4,860)
Note: Crude models were adjusted for falls at current wave and follow-up years. Fully-adjusted models were adjusted for falls at current wave, maximal HGS, hand dominance, sex, age, race, multimorbidity, obesity, current smoking status, previous smoking status, self-rated health, depression, moderate-to-vigorous physical activity, cognitive impairment, and follow-up years. HGS=handgrip strength.
Table 4.
Results for the Associations Between Overlapping Handgrip Strength Asymmetry Categories and Future Falls.
| Crude Models | Fully-Adjusted Models | ||||
|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | ||
| 1.10 | 1.02, 1.17 | 1.07 | 1.01, 1.16 | ||
| HGS Asymmetry >20.0%† (n=2,127) | 1.18 | 1.08, 1.28 | 1.12 | 1.02, 1.22 | |
Reference=HGS Asymmetry 0.0%−10.0% (n=4,860)
Note: Crude models were adjusted for falls at current wave and follow-up years. Fully-adjusted models were adjusted for falls at current wave, maximal HGS, hand dominance, sex, age, race, multimorbidity, obesity, current smoking status, previous smoking status, self-rated health, depression, moderate-to-vigorous physical activity, cognitive impairment, and follow-up years. HGS=handgrip strength.
The results for the associations between HGS asymmetry dominance and future falls are shown in Appendix 3, while Appendix 4 presents the results of the receiver operating characteristic curve analyses for the associations between inverted HGS asymmetry ratio and future falls. The inverted HGS asymmetry ratio cut-point was 1.2 (i.e., 20%).
DISCUSSION
The principal results of this investigation revealed that HGS asymmetry was associated with future falls in older Americans. Specifically, every 10% increase in inverted HGS ratio was associated with 26% greater odds for future falls. Those with HGS asymmetry >10%, >20%, and >30% had 7%, 12%, and 15% greater odds for future falls, respectively. These findings not only suggest that HGS asymmetry was associated with future falls, but the odds of falling generally increase as the degree of HGS asymmetry becomes severer. Healthcare providers should consider examining HGS asymmetry, as a possible biomarker of impaired neuromuscular function, alongside evaluations of maximal HGS in routine geriatric health and fall risk assessments.
Public health initiatives for preventing falls such as the Prevention of Falls Network Europe (ProFaNE) and Stopping Elderly Accidents, Deaths and Injuries (STEADI) evaluate a variety of factors that help to determine risk of falls [24, 25]. For example, STEADI fall risk criteria examine measures of physical function including the timed-up-and-go, 30-second chair stand, and 4-stage balance test [26]. Although these assessments of physical functioning are associated with an elevated risk of falls [27], these tests have a restricted capacity to evaluate within-individual variability in functional abilities and do not capture the multitude of physical attributes that contribute to fall risk [3, 28]. This may help to explain why examinations of physical performance, which are part of streamlined public health initiatives for preventing falls, are not frequently used by geriatricians during routine health assessments [29]. Given that our findings suggest HGS asymmetry was associated with future falls, incorporating measures of HGS asymmetry in geriatric health and fall risk assessments may help in overcoming the limitations of physical performance examinations.
Although evaluations of physical performance emphasize whole body functions related to mobility, deficiencies in neuromuscular function precede poor physical performance [3]. Thus, declining physical performance signifies being in a more advanced stage of the disabling process [3], which in turn, limits the efficacy potential for fall prevention interventions [30]. Low neuromuscular function, as measured by HGS and functional asymmetries, could represent the onset of the disabling process [31]. Screening for low neuromuscular function may help in timely identification of syndromes that elevate fall risk and the ability for fall prevention programs to intervene earlier.
We should also highlight the conceptual framework for why HGS asymmetries could be a discriminating factor for assessing fall risk (arguably more so than strength asymmetries in other muscle groups). As extensively discussed by Carson [4], individual variations in the ability to generate grip force are closely associated with a broad spectrum of markers that reflect neural system functioning. It has been shown that the changes in HGS across the lifespan are not solely attributable to declines in muscle mass [32]. Rather, grip force is a complex coordinated behavior that is mediated by integrated activity across distributed brain networks [4]. The capacity of the human hand for prehensile movement is a highly evolved function that involves 19 bones, 17 articulations, and 19 muscles within the hand, and another 20 tendons activated by forearm muscles [33]. Accordingly, gripping is complex task that requires coordinated engagement of a large number of motor units, and the quality of this coordination is a critical determinant of the level of grip force that can be achieved.
There is evidence that suggests the force generated during a maximum voluntary grip force task is around half of what would be expected if the skeletal musculature itself were fully activated by the nervous system [34, 35], due to reduced neural drive to the muscles [33]. Specifically, the maximum force that can be produced by each finger decreases in proportion to the number of other fingers that are engaged simultaneously, such that when four fingers contribute to the grip task, the maximum force that can be generated by each digit is typically less than half that produced when it is engaged in isolation (i.e., there is a force deficit) [35, 36]. Moreover, force deficits observed in HGS measurements are larger in older adults relative to young adults [35, 37]. Individual variations in the capacity to generate grip force are closely associated with a broad spectrum of markers that reflect neural function and brain health [4]. Thus, when it is considered that more than half of falls in older adults are linked to impaired motor function (e.g., incorrect weight shift, stumbles, trips) [38], we interpret our findings to suggest that HGS asymmetries are likely broadly reflective of motor functioning. Given the neuromuscular and motor factors that are ascertained in HGS assessments, functional asymmetries measured from HGS could provide insights for fall risk assessments that may not be observed when examining functional asymmetries in the lower extremities.
Lambach et al. [14] revealed that a large number of adults have >10% asymmetry in peak hip and knee flexion. The findings from this investigation suggest that detecting abnormal strength imbalances between limbs, including the upper extremities, may require a greater degree asymmetry. This notion corroborates with our results that suggest the odds of falling increase as the degree of asymmetry became severer. Improving our understanding of the motor change pathways that contribute to strength asymmetries between limbs could guide interventions aiming to preserve neuromuscular function during aging. The need to create robust HGS asymmetry cut-points could be warranted. Future research should continue evaluating HGS protocols in greater detail for uncovering aspects of neuromuscular function that are outside of maximal strength. Assessing muscle quality, localized or perhaps even non-localized, may provide additional insights into deteriorating health during aging [39], which may subsequently help to specify the neuromuscular system’s contribution to functional asymmetries and risk of falls. Examining how HGS asymmetry either overlaps or differs from maximal HGS, and assessing how HGS asymmetry factors into intrinsic capacity framework [40] may also inform fall risk assessments. Further, determining how functional asymmetries of the upper extremities could be linked to asymmetries in the lower extremities, and bimanual cognitive functioning tests such as the Trail Making Test-Part B, may further elucidate the role of HGS asymmetry on falls.
Some study limitations should be acknowledged. More precise operational definitions for falls such as those from the World Health Organization were not utilized by the HRS [41]. Including additional covariates that were not available in the HRS public dataset, but relevant for falls such as formal vision tests and identified environmental hazards may have influenced our findings. Low sampling limited our ability to determine how HGS asymmetry dominance was associated with future falls. Although self-report information from participants in the HRS is generally reliable and valid [42], self-report biases may have influenced our results. Specifically, handedness was self-reported and may not have captured lifespan changes in hand dominance. This is of particular importance because older populations may have experienced obligations to be right handed at younger ages, which may have compromised organic lateralization. Thus, footedness may factor into how functional asymmetries are examined [43]. The generalizability of our findings is limited to non-White races and left-handed persons because most of our sample identified as White race and right hand dominant. Those who were ambidextrous or only able to complete HGS testing on one hand were excluded.
Conclusions
This investigation revealed that HGS asymmetry was associated with future falls in older Americans. Severer asymmetries may exacerbate fall risk. Asymmetric HGS could be a biomarker that signifies impaired neuromuscular functioning. Thus, HGS asymmetry could have the potential to improve the predictive utility of handgrip dynamometers and operationalization of neuromuscular function. We suggest that HGS asymmetry be evaluated not only in HGS protocols, but also as part of fall risk assessments. Continued research into improving how we assess neuromuscular function with feasible protocols and modern technologies may not only improve their usage in clinical settings, but also strengthen neuromuscular-related screenings and fall risk assessments.
Supplementary Material
Funding
BCC’s effort on this manuscript was supported in part by a grant from the National Institutes of Health (R01AC044424 to BCC).
Conflicts of Interest:
In the past 5-years, BCC has received research funding from NMD Pharma, Regeneron Pharmaceuticals, Astellas Pharma Global Development Inc., and RTI Health Solutions for contracted studies that involved aging and muscle related research. In the past 5-years, BCC has received consulting fees from Regeneron Pharmaceuticals, Zev industries, and the Gerson Lehrman Group for consultation specific to age-related muscle weakness. BCC is a co-founder with equity of AEIOU Scientific.
REFERENCES
- 1.Jin J Prevention of Falls in Older Adults. JAMA. 2018;319(16):1734. doi: 10.1001/jama.2018.4396 [DOI] [PubMed] [Google Scholar]
- 2.Cawthon PM, Manini T, Patel SM, et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J Am Geriatr Soc. 2020;68(7):1429–1437. doi: 10.1111/jgs.16517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaudart C, Rolland Y, Cruz-Jentoft AJ, et al. Assessment of Muscle Function and Physical Performance in Daily Clinical Practice : A position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif Tissue Int. 2019;105(1):1–14. doi: 10.1007/s00223-019-00545-w [DOI] [PubMed] [Google Scholar]
- 4.Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 5.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 6.Bohannon RW. Grip Strength: An Indispensable Biomarker For Older Adults. Clin Interv Aging. 2019;14:1681–1691. Published2019 Oct 1. doi: 10.2147/CIA.S194543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portegijs E Asymmetrical lower-limb muscle strenght deficit in older people. https://hurusa.com/assets/uploads/casestudies/asymmetrical.pdf Accessed1 August 2020.
- 8.Portegijs E, Sipilä S, Alen M, et al. Leg extension power asymmetry and mobility limitation in healthy older women. Arch Phys Med Rehabil. 2005;86(9):1838–1842. doi: 10.1016/j.apmr.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 9.McGrath R, Vincent BM, Jurivich DA, et al. Handgrip Strength Asymmetry and Weakness Together are Associated with Functional Disability in Aging Americans [published online ahead of print, 2020 Apr 22]. J Gerontol A Biol Sci Med Sci. 2020;glaa100. doi: 10.1093/gerona/glaa100 [DOI] [PubMed] [Google Scholar]
- 10.McGrath R, Cawthon PM, Cesari M, Al Snih S, Clark BC. Handgrip Strength Asymmetry and Weakness Are Associated with Lower Cognitive Function: A Panel Study [published online ahead of print, 2020 May 30].J Am Geriatr Soc. 2020;10.1111/jgs.16556. doi: 10.1111/jgs.16556 [DOI] [PubMed] [Google Scholar]
- 11.McGrath R, Tomkinson GR, LaRoche DP, Vincent BM, Bond CW, Hackney KJ. Handgrip Strength Asymmetry and Weakness May Accelerate Time to Mortality in Aging Americans [published online ahead of print, 2020 Jun 28].J Am Med Dir Assoc. 2020;S1525–8610(20)30360–1. doi: 10.1016/j.jamda.2020.04.030 [DOI] [PubMed] [Google Scholar]
- 12.Armstrong CA, Oldham JA. A comparison of dominant and non-dominant hand strengths. J Hand Surg Br. 1999;24(4):421–425. doi: 10.1054/jhsb.1999.0236 [DOI] [PubMed] [Google Scholar]
- 13.Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444–447. doi: 10.5014/ajot.43.7.444 [DOI] [PubMed] [Google Scholar]
- 14.Lathrop-Lambach RL, Asay JL, Jamison ST, et al. Evidence for joint moment asymmetry in healthy populations during gait. Gait Posture. 2014;40(4):526–531. doi: 10.1016/j.gaitpost.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher GG, Ryan LH. Overview of the Health and Retirement Study and Introduction to the Special Issue. Work Aging Retire. 2018;4(1):1–9. doi: 10.1093/workar/wax032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crimmins E, Guyer H, Langa K, Ofstedal M, Wallace R, Weir D. Documentation of physical measures, anthropometrics and blood pressure in the Health and Retirement Study. https://hrs.isr.umich.edu/sites/default/files/biblio/dr-011.pdf.Accessed1 August 2020.
- 18.Incel NA, Ceceli E, Durukan PB, Erdem HR, Yorgancioglu ZR. Grip strength: effect of hand dominance. Singapore Med J. 2002;43(5):234–237. [PubMed] [Google Scholar]
- 19.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11(2):139–148. doi: 10.1017/s1041610299005694 [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Newman TT, Welsh KA, Helms M, Breitner JC. Properties of the telephone interview for cognitive status: application in epidemiological and longitudinal studies. Cog Behav Neurol. 1994;7:235–241. [Google Scholar]
- 21.Langa KM, Larson EB, Karlawish JH, et al. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity?. Alzheimers Dement. 2008;4(2):134–144. doi: 10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16(1):73–81. doi: 10.1097/01.ede.0000147512.81966.ba [DOI] [PubMed] [Google Scholar]
- 23.Dong R, Guo Q, Wang J. Optimal Cutoffs of Grip Strength for Definition as Weakness in the Elderly. J Biosci Med. 2014;2:14–18. [Google Scholar]
- 24.Centers for Disease Control and Prevention. STEADI-Older Adult Fall Prevention. https://www.cdc.gov/steadi/index.html.Accessed2 November 2020.
- 25.Prevention of Falls Network Europe. http://www.profane.eu.org/about.html.Accessed2 November 2020.
- 26.Centers for Disease Control and Prevention. STEADI-Older Adult Fall Prevention. Materials for Healthcare Providers. https://www.cdc.gov/steadi/materials.html.Accessed2 November 2020.
- 27.Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am. 2015;99(2):281–293. doi: 10.1016/j.mcna.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fasano A, Plotnik M, Bove F, Berardelli A. The neurobiology of falls. Neurol Sci. 33(6), 1215–1223. doi: 10.1007/s10072-012-1126-6 [DOI] [PubMed] [Google Scholar]
- 29.Bruyère O, Beaudart C, Reginster J-Y, et al. Assessment of muscle mass, muscle strength and physical performance in clinical practice: an international survey. Eur Geriatr Med. 7(3), 243–246. [Google Scholar]
- 30.Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med. 25, 172–183. doi: 10.1016/s0749-3797(03)00182-x [DOI] [PubMed] [Google Scholar]
- 31.Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011; 27(1), 1–15. doi: 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legrand D, Vaes B, Matheï C, Adriaensen W, Van Pottelbergh G, Degryse JM. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc. 2014;62(6):1030–1038. doi: 10.1111/jgs.12840 [DOI] [PubMed] [Google Scholar]
- 33.Ohtsuki T Inhibition of individual fingers during grip strength exertion. Ergonomics. 1981;24(1):21–36. doi: 10.1080/00140138108924827 [DOI] [PubMed] [Google Scholar]
- 34.Li ZM, Latash ML, Newell KM, Zatsiorsky VM. Motor redundancy during maximal voluntary contraction in four-finger tasks. Exp Brain Res. 1998;122(1):71–78. doi: 10.1007/s002210050492 [DOI] [PubMed] [Google Scholar]
- 35.Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. J Appl Physiol (1985). 2003;94(1):259–270. doi: 10.1152/japplphysiol.00643.2002 [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita H, Kawai S, Ikuta K. Contributions and co-ordination of individual fingers in multiple finger prehension. Ergonomics. 1995;38(6):1212–1230. doi: 10.1080/00140139508925183 [DOI] [PubMed] [Google Scholar]
- 37.Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. J Appl Physiol (1985). 2003;95(4):1361–1369. doi: 10.1152/japplphysiol.00070.2003 [DOI] [PubMed] [Google Scholar]
- 38.Robinovitch SN, Feldman F, Yang Y, et al. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study [published correction appears in Lancet. 2013 Jan 5;381(9860):28].Lancet. 2013;381(9860):47–54. doi: 10.1016/S0140-6736(12)61263-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsen B, Bellettiere J, Allison M, et al. Muscle area and density and risk of all-cause mortality: The Multi-Ethnic Study of Atherosclerosis [published online ahead of print, 2020 Jul 23].Metabolism. 2020;111:154321. doi: 10.1016/j.metabol.2020.154321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cesari M, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, et al. Evidence for the Domains Supporting the Construct of Intrinsic Capacity. J Gerontol A Biol Sci Med Sci. 2018;73(12):1653–1660. doi: 10.1093/gerona/gly011 [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Falls. https://www.who.int/news-room/fact-sheets/detail/falls#:~:text=A%20fall%20is%20defined%20as.Accessed2 November 2020.
- 42.Wallace RB, Herzog AR. Overview of the health measures in the Health and Retirement Study. J Hum Resour. 1995:S84–S107. [Google Scholar]
- 43.Elias LJ, Bryden MP, Bulman-Fleming MB. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia. 1998;36(1):37–43. doi: 10.1016/s0028-3932(97)00107-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


