Abstract
The mechanisms that contribute to retinal tissue destruction during onset and progression of AIDS-related human cytomegalovirus (HCMV) retinitis remain unclear. Evidence for the stimulation of multiple cell death pathways including apoptosis, necroptosis, and pyroptosis during the pathogenesis of experimental murine cytomegalovirus (MCMV) retinitis in mice with retrovirus-induced immunosuppression (MAIDS) has been reported. Parthanatos is a caspase-independent cell death pathway mediated by rapid overactivation of poly (ADP-ribose) polymerase-1 (PARP-1) and distinct from other cell death pathways. Using the MAIDS model of MCMV retinitis, studies were performed to test the hypothesis that intraocular MCMV infection of mice with MAIDS stimulates parthanatos-associated mRNAs and proteins within the eye during the development of retinal necrosis that takes place by 10 days after MCMV infection. MCMV-infected eyes of MAIDS mice exhibited significant stimulation of PARP-1 mRNA and proteins at 3 days after infection, but declined thereafter at 6 and 10 days after infection. Additional studies showed the intraocular stimulation of mRNAs or proteins prior to MCMV retinitis development for two additional participants in parthanatos, polymer of ADP-ribose (PAR) and poly (ADP-ribose) glycohydrolase (PARG). These results provide new evidence for a role for parthanatos during MAIDS-related MCMV retinitis that may also extend to AIDS-related HCMV retinitis.
Keywords: Human cytomegalovirus, Murine cytomegalovirus, AIDS, MAIDS, retinitis, parthanatos
1. INTRODUCTION
Human cytomegalovirus (HCMV) is a β-herpesvirus that infects approximately 80% of the population worldwide [1]. Following primary infection that is typically asymptomatic in immunologically normal persons, the virus establishes a life-long latent infection in monocytes and bone marrow cells, but can reactivate to cause a wide spectrum of opportunistic diseases in immunocompromised patients including those with AIDS [2]. One such opportunistic infection in patients immunosuppressed by HIV is AIDS-related HCMV retinitis that results in vision loss or blindness in up to 30% of AIDS patients who do not have access to combination antiretroviral therapy (ART) or who do not respond to ART [3, 4]
Although the clinical and histopathologic features of AIDS-related HCMV retinitis are well characterized [2], the precise virologic and immunologic events that take place during onset and development of this sight-threatening disease during HIV-induced immunosuppression remain poorly understood. Toward this end, this laboratory has used for many years an animal model of experimental murine cytomegalovirus (MCMV) retinitis that develops in C57BL/6 mice with retrovirus-induced immunodeficiency syndrome (MAIDS) [5, 6]. The MAIDS model of MCMV retinitis has been used previously to provide evidence that multiple cell death pathways operate within the ocular compartment during development of retinal necrosis following intraocular infection with MCMV [5].
Parthanatos is a cell death pathway that is distinct from other known cell death pathways. This form of cell death is mediated by overactivation of the DNA nick-sensing enzyme poly (ADP-ribose) polymerase-1 (PARP-1) due to reactive oxygen species induced by oxidative stress. This rapid activation of PARP-1 stimulates a vast synthesis and toxic accumulation of polymer of ADP-ribose (PAR) causing its translocation from the nucleus to the cytosol that results in mitochondrial depolarization [7–9]. Importantly, PAR production during parthanatos is regulated by the glycosidic activity of poly (ADP-ribose) glycohydrolase (PARG) whose inhibitory function prevents the premature and toxic accumulation of PAR [10–12]. Migration of the PAR polymer leads to translocation of apoptosis-inducing factor (AIF) from the depolarized mitochondria to the nucleus where it interacts with cellular DNA, ultimately resulting in large-scale DNA fragmentation and eventual cell death. Death by parthanatos is unique from other forms of programmed cell death in that it is caspase-independent, it does not result in small-scale DNA fragmentation and the formation of apoptotic bodies as seen during apoptosis, nor does it cause cell swelling as seen during necroptosis and pyroptosis [9, 13].
Parthanatos has been reported recently to operate during the death of HCMV-infected immunocytes via HCMV-encoded proteins termed HCMV-associated insoluble substance (HCMVAIS) [14]. The HCMV-infected immunocytes displayed unique parthanatos-like features such as caspase-independent activation of PARP-1 and extensive DNA fragmentation. This finding, coupled with the finding that multiple cell death pathways are stimulated during the development of MAIDS-related MCMV retinitis [5], suggested that parthanatos may also be a contributing factor toward the retinal tissue damage that occurs following intraocular MCMV infection of mice with MAIDS. Studies were therefore performed to test the hypothesis that intraocular MCMV infection of mice with MAIDS stimulates parthanatos-associated mRNAs and proteins within the eye during the development of retinal necrosis.
2. MATERIALS AND METHODS
Adult female C57BL/6 mice with MAIDS of ten weeks’ duration and susceptible to MCMV retinitis [6, 13] were used during these studies. The left eyes of MAIDS mice were subretinally injected with 104 plaque-forming units (PFU) of MCMV [Smith] contained within 2 ul of Dulbecco’s modified Eagle’s medium (DMEM) as previously described [5]. The contralateral right eyes of each animal were injected with DMEM alone and served as controls. Both left and right eyes were collected from euthanized MAIDS mice at 3, 6, and 10 days after MCMV infection because previous studies have shown development of retinal necrosis by 10 days after MCMV infection [5]. All animal experiments were performed following the National Institutes of Health guide for the care and use of laboratory animals, and all the experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Georgia State University.
All MCMV-infected eyes and mock-infected eyes (controls) collected at 3, 6, and 10 days were analyzed and compared by quantitative RT-PCR (qRT-PCR) assay for detection and quantification of mRNAs for parthanatos-associated PARP-1 and PARG. An inability to identify appropriate primers for PAR mRNA prevented qRT-PCR assays for PAR mRNA in this investigation. All collected eyes were stored in RNAlater solution (Ambion, Austin, TX) at 4°C. At time of analysis, eyes were individually homogenized in 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA), total RNA was extracted from each homogenized eye using the PureLink total RNA purification system (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions, and the concentration of extracted total RNA for each eye was measured using Qubit 4 Fluorometer (Invitrogen, Carlsbad, CA). After normalization of total RNA for each sample, synthesis of cDNA was performed using the SuperScript III first-stand synthesis system (Invitrogen, Carlsbad, CA) per the manufacturer’s instructions. Detection and quantification was accomplished using the 7500 Fast Real-Time PCR System instrument (Applied Biosystems, Foster City, CA) with SYBR Green Master Mix. Primers used for detection and quantification of PARP-1, PARG, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNAs were obtained from Qiagen (Valencia, CA0. For statistical analysis of data, Student’s t test was used by comparing MCMV-infected eyes to their corresponding mock-infected eyes (controls). P-values of < 0.05 were considered statistically significant for the analysis.
PARP-1, PAR, and PARG protein production was determined by western blot analysis using MCMV-infected eyes and mock-infected control eyes collected at 3, 6, and 10 days. Whole eyes from each group were individually homogenized in phosphate-buffered saline (PBS) containing a protease inhibitor cocktail (Sigma, St. Louis, MO) and pooled. The concentration of extracted protein in each sample was determined using a Bradford protein assay (Bio-Rad, Hercules, CA). Following normalization of protein concentration of each sample, standard western blot analysis was performed to detect PARP-1, PAR, PARG and GAPDH proteins using rabbit anti-mouse PARP-1 antibody (1:1,000) (Abcam Biotechnology, Cambridge, United Kingdom), mouse anti-mouse PAR antibody (1:500) (Enzo Life Sciences, Farmingdale, NY), anti-mouse PARG antibody (1:500) (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-mouse GAPDH antibody (1:1,000) (Sigma, St. Louis, MO) as primary antibodies. After incubation with goat anti-rabbit polyclonal IgG antibody (heavy plus light chains [H + L]) (Abcam Biotechnology, Cambridge, United Kingdom) or goat anti-mouse antibody (heavy plus light chains [H + L]) (Invitrogen, Carlsbad, CA), transfer stacks nitrocellulose membrane for use with the Invitrogen power blotter was treated with enhanced chemiluminescence western blot detection reagents (Bio-Rad) and exposed to autoradiograph film (Denville Scientific, Holliston, MA).
3. RESULTS
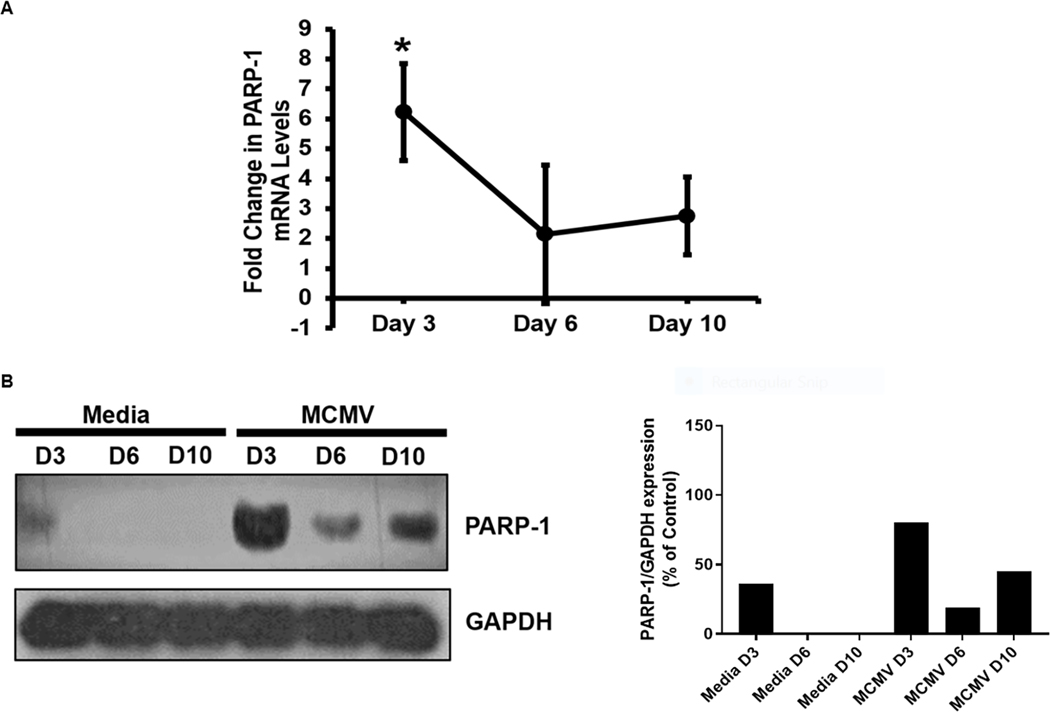
Initial experiments to determine if parthanatos operates during the onset and progression of MAIDS-related MCMV retinitis were directed at the intraocular detection and quantification of PARP-1 at different times during development of MCMV retinitis that is most severe at 10 days after intraocular MCMV infection [5]. MCMV-infected eyes from MAIDS mice were collected at 3, 6, and 10 days after MCMV infection and subjected to qRT-PCR assay and western blot analysis to determine the extent of PARP-1 mRNA and protein production, respectively, when compared with mock-infected eyes of MAIDS mice. Significant amounts of PARP-1 mRNA were observed within MCMV-infected eyes at 3 days after MCMV infection, but PARP-1 mRNA levels decreased at 6 and 10 days after MCMV infection albeit remaining at respectable levels (Fig 1 A). A similar pattern of PARP-1 protein production was observed within MCMV-infected eyes at days 3, 6, and 10 days after MCMV infection. While a modest amount of PARP-1 protein was detected in control eyes at 3 days following mock infection probably in response to needle-induced tissue injury, no PARP-1 protein could be detected within mock-infected eyes at 6 or 10 days when detectable and consistent amounts of this protein were detected within MCMV-infected eyes (Fig 1 B).
Figure 1.
Detection of PARP-1 mRNA (Fig 1A) and protein (Fig 1B) within MCMV-infected eyes of MAIDS mice at 3, 6, and 10 days after subretinal MCMV inoculation compared with mock-infected control eyes. PARP-1 mRNA expression values as determined by qRT-PCR assay represent means from n = 3 – 5 mice per group from two independent experiments, and error bars indicate standard errors of the mean (SEM). Statistical significance between groups per day (*, p < 0.05) was determined by Student’s t test. PARP-1 protein detection represents pooled whole eyes (n = 3 – 5 mice per group).
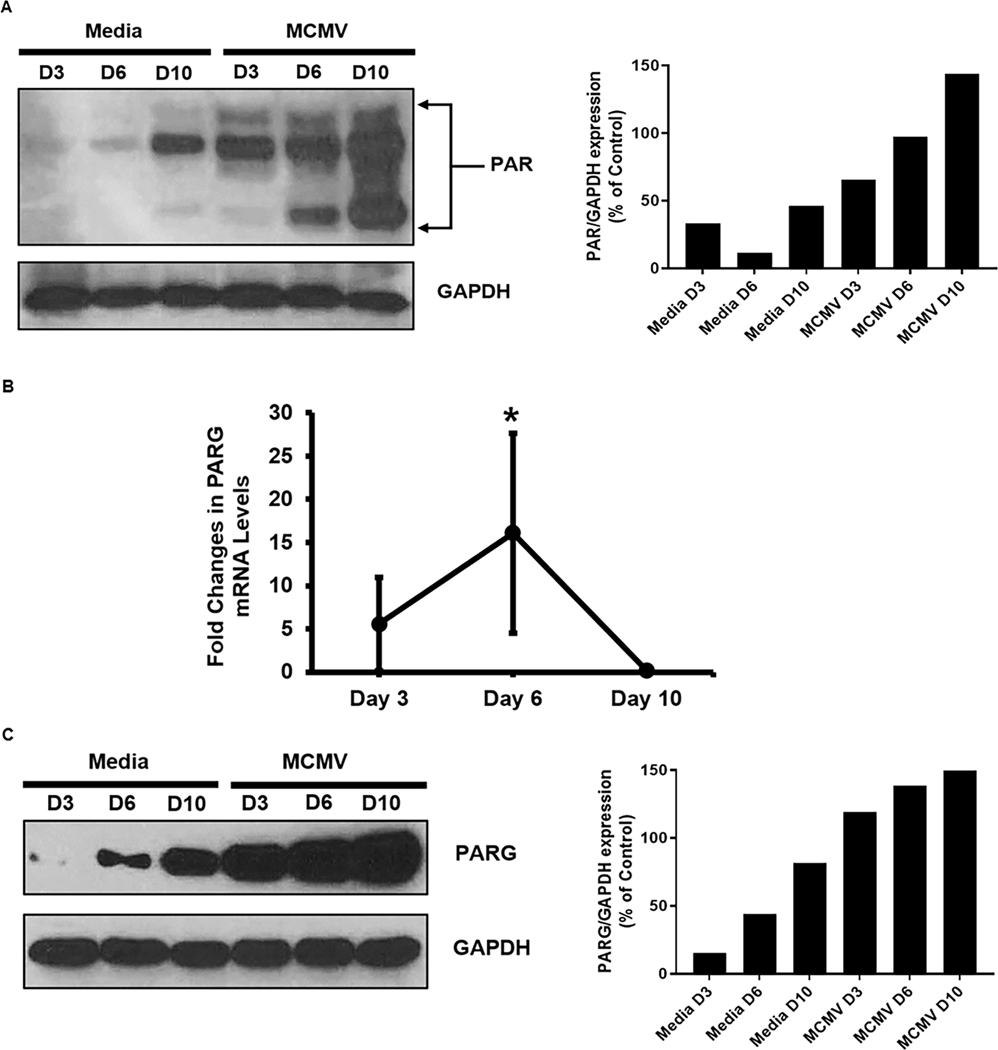
Because significant and consistent amounts of PARP-1 mRNA and protein were detected within MCMV-infected eyes when compared with mock-infected eyes of MAIDS mice, additional studies focused on PAR and PARG, two additional key parthanatos-associated proteins that participate in parthanatos cell death, but downstream of PARP-1 production [7, 8, 13]. Although qRT-PCR assay was not performed for detection and quantification of mRNA PAR due to an inability to identify appropriate primers for PAR mRNA, western blot analysis alone nonetheless demonstrated progressive increased synthesis of PAR proteins within MCMV-infected eyes of MAIDS mice from 3 days to 6 days and 10 days after MCMV infection at time of retinal necrosis development (Fig 2 A). Multiple PAR protein bands were observed due to the heterogeneity of the length of the ADP-ribose chain indicating PARylation had occurred [15, 16] consistent with previous studies [17, 18]. In comparison, mock-infected control eyes injected with maintenance medium only also showed increasing stimulation of PAR protein by 10 days, but not to the extent observed within MCMV-infected eyes (Fig 2 A). Similar to PARP-1 and PAR protein stimulation, western blot analysis demonstrated increasing stimulation of PARG protein within MCMV-infected eyes of MAIDS mice with peak amounts observed at 10 days after MCMV infection. (Fig 2 C) Unlike for PARP-1 mRNA synthesis, the pattern for PARG mRNA synthesis within MCMV-infected eyes of MAIDS mice when compared with mock-infected control eyes showed peak amounts at 6 days after MCMV infection (Fig 2 B). Peak mRNA synthesis prior to the development of severe retinal necrosis is not unique to PARP-1 mRNA, but has been reported repeatedly within MCMV-infected eyes for other key proteins involved in apoptosis, necroptosis, and pyroptosis [5].
Figure 2.
Detection of PAR protein (Fig 2A), PARG mRNA (Fig 2B), and PARG protein (Fig 2C) within MCMV-infected eyes of MAIDS mice at 3, 6, and 10 days after subretinal MCMV inoculation compared with mock-infected control eyes. PARG mRNA expression values as determined by qRT-PCR assay represent means from n = 3 – 5 mice per group from two independent experiments, and error bars indicate standard errors of the mean (SEM). Statistical significance between groups per day (*, p < 0.05) was determined by Student’s t test. PAR and PARG protein detection represents pooled whole eyes (n = 3 – 5 mice per group).
4. DISCUSSION
The mechanism by which full-thickness retinal necrosis develops during the onset and progression of AIDS-related HCMV retinitis involves far more than simply cell death due solely to virus replication within retinal cells and virus-induced cytopathology. Use of the experimental MAIDS-related MCMV retinitis model has identified many other players in this pathogenic process including the concerted efforts of the programmed cell death pathways apoptosis, necroptosis, and pyroptosis [5]. In the present communication, we extend the programmed cell death pathways involved in the pathogenesis of cytomegalovirus retinitis during retrovirus-induced immunosuppression to possibly include for the first time parthanatos. This conclusion is based on the findings that key mRNAs and/or proteins of the pathanatos pathway (PARP-1, PAR, and PARG) are stimulated within MCMV-infected eyes of MAIDS mice during onset and progression of MCMV retinitis. That parthanatos is stimulated during MCMV infection of the eye is in agreement with a recent publication showing that HCMV-infected immunocytes also appear to undergo parthanatos [14].
The term parthanatos was first used in a review in 2009 [13]. Since then, it has been associated with several diseases such as Parkinson’s disease, stroke, heart disease, and diabetes [9, 13] as well as an experimental mouse model of retinitis pigmentosis [19]. The present studies serve to possibly extend its role to another retinal disease, i.e., AIDS-related HCMV retinitis. While our findings are intriguing, they are preliminary. Firstly, it has not escaped our attention that parthanatos-associated proteins PARP-1, PAR, and PARG are also stimulated in mock-infected eyes of MAIDS mice, albeit to various amounts and to lesser degrees than found in MCMV-infected eyes of MAIDS mice during development of MCMV retinitis suggesting that parthantos may be a response to cellular injury associated with mild inflammation as occurs during injection of DMEM following corneal needlestick. Secondly, confirmation that parthanatos directly contributes to retinal tissue destruction during MAIDS-related MCMV retinitis must await use of appropriate knockout mice deficient in one or more key parthanatos pathway proteins. Finally, additional studies must be performed to explore the exciting possibility for cross-talk between parthanatos and other programmed cell death pathways during MAIDS-related MCMV retinitis. For example, stimulation of RIPK1 and RIPK3 activity during necroptosis has been shown to stimulate PARP-1 activity [20].
ACKNOWLEDGEMENTS
We thank the Biology Core Facilities, Department of Biology, Georgia State University, for technical assistance with real-time quantitative RT-PCR analysis. This work was supported by NIH/NEI grant EY024630, NIH/NEI core grant P30EY006360, and NIH/NEI Emory Eye Center Vision training grant T32-EY007092.
REFERENCES
- 1.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43(9):1143–51. Epub 2006/10/10. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 2.Heiden D, Ford N, Wilson D, Rodriguez WR, Margolis T, Janssens B, et al. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med. 2007;4(12):e334. Epub 2007/12/07. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledergerber B, Egger M, Erard V, Weber R, Hirschel B, Furrer H, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy: the Swiss HIV Cohort Study. JAMA. 1999;282(23):2220–6. Epub 1999/12/22. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson MA, Stanley H, Holtzer C, Margolis TP, Cunningham ET. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(1):231–3. Epub 2000/01/05. doi: 10.1086/313612. [DOI] [PubMed] [Google Scholar]
- 5.Chien H, Dix RD. Evidence for multiple cell death pathways during development of experimental cytomegalovirus retinitis in mice with retrovirus-induced immunosuppression: apoptosis, necroptosis, and pyroptosis. J Virol. 2012;86(20):10961–78. Epub 2012/07/28. doi: 10.1128/JVI.01275-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dix RD, Cray C, Cousins SW. Mice immunosuppressed by murine retrovirus infection (MAIDS) are susceptible to cytomegalovirus retinitis. Curr Eye Res. 1994;13(8):587–95. Epub 1994/08/01. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol. 2009;218(2):193–202. Epub 2009/04/01. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Kim NS, Haince JF, Kang HC, David KK, Andrabi SA, et al. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci Signal 2011;4(167):ra20. Epub 2011/04/07. doi: 10.1126/scisignal.2000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171(8):2000–16. Epub 2014/04/02. doi: 10.1111/bph.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Feng X, Koh DW. Activation of cell death mediated by apoptosis-inducing factor due to the absence of poly(ADP-ribose) glycohydrolase. Biochemistry. 2011;50(14):2850–9. Epub 2011/03/04. doi: 10.1021/bi101829r. [DOI] [PubMed] [Google Scholar]
- 11.Blenn C, Althaus FR, Malanga M. Poly(ADP-ribose) glycohydrolase silencing protects against H2O2-induced cell death. Biochem J. 2006;396(3):419–29. Epub 2006/03/11. doi: 10.1042/BJ20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidovic L, Vodenicharov M, Affar EB, Poirier GG. Importance of poly(ADP-ribose) glycohydrolase in the control of poly(ADP-ribose) metabolism. Exp Cell Res. 2001;268(1):7–13. Epub 2001/07/20. doi: 10.1006/excr.2001.5263. [DOI] [PubMed] [Google Scholar]
- 13.David KK, Andrabi SA, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Front Biosci (Landmark Ed). 2009;14:1116–28. Epub 2009/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, Kim J, Roh J, Park CS, Seoh JY, Hwang ES. Reactive oxygen species-induced parthanatos of immunocytes by human cytomegalovirus-associated substance. Microbiol Immunol. 2018;62(4):229–42. Epub 2018/01/20. doi: 10.1111/1348-0421.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Giammartino DC, Shi Y, Manley JL. PARP1 represses PAP and inhibits polyadenylation during heat shock. Mol Cell. 2013;49(1):7–17. Epub 2012/12/12. doi: 10.1016/j.molcel.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hossain MB, Ji P, Anish R, Jacobson RH, Takada S. Poly(ADP-ribose) Polymerase 1 Interacts with Nuclear Respiratory Factor 1 (NRF-1) and Plays a Role in NRF-1 Transcriptional Regulation. J Biol Chem. 2009;284(13):8621–32. Epub 2009/02/03. doi: 10.1074/jbc.M807198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, et al. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad Sci U S A. 2006;103(48):18308–13. Epub 2006/11/23. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucarini L, Durante M, Lanzi C, Pini A, Boccalini G, Calosi L, et al. HYDAMTIQ, a selective PARP-1 inhibitor, improves bleomycin-induced lung fibrosis by dampening the TGF-beta/SMAD signalling pathway. J Cell Mol Med. 2017;21(2):324–35. Epub 2016/10/06. doi: 10.1111/jcmm.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahaboglu A, Tanimoto N, Kaur J, Sancho-Pelluz J, Huber G, Fahl E, et al. PARP1 gene knock-out increases resistance to retinal degeneration without affecting retinal function. PLoS One. 2010;5(11):e15495. Epub 2010/12/03. doi: 10.1371/journal.pone.0015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19(12):2003–14. Epub 2012/07/21. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]