Abstract
The safety and efficacy of anticoagulation for venous thromboembolism (VTE) at times of severe thrombocytopenia is unclear. In this retrospective study, we evaluated patients with hematologic malignancy and either 1) acute or chronic VTE on anticoagulation before platelet count dropped below 50×109/L or 2) acute VTE occurring while platelets were <50×109/L. In 78 eligible patients, the primary outcomes of time to recurrent VTE or clinically significant bleeding within 100 days were compared by management strategy. Bleeding occurred in 27% of patients receiving anticoagulation versus 3% when anticoagulation was held (IRR 10.1, 95% CI 1.5–432.6). Recurrent VTE occurred in 2% of patients receiving anticoagulation versus 15% when anticoagulation was held (IRR 0.17, 95% CI 0.0–1.51). Most bleeding occurred before day 31(11/13), but recurrent VTE mostly occurred after day 40 (5/6). Our findings suggest that temporarily withholding anticoagulation for VTE during severe thrombocytopenia in patients with hematologic malignancies might reduce adverse outcomes.
Keywords: Anticoagulation, Hematologic Malignancies, Hemorrhage, Thrombocytopenia, Venous Thromboembolism
Introduction:
Cancer-associated thrombosis increases the risk of morbidity and mortality in hospitalized patients and is a leading cause of death in outpatients [1, 2]. Disease or chemotherapy-induced thrombocytopenia does not prevent the formation or progression of venous thromboembolism (VTE) [3, 4, 5] and when they occur together, data supporting optimal management strategies are limited [6, 7, 8]. In clinical practice, therapeutic doses of anticoagulation in the setting of VTE are typically thought to outweigh the risks of bleeding when platelet counts exceed 50 × 109/L [9, 10, 11, 12, 13, 14]. Under this platelet threshold, however, the dose, duration, and anticoagulant of choice have not been well studied and existing guidelines are conflicting.
The occurrence of VTE in patients with hematologic malignancies ranges from 2–12% [15, 16] and management can be challenging due to the severity and often extended duration of severe thrombocytopenia (<50 × 109/L). During the treatment of hematologic malignancies, clinically significant bleeding, independent of whether the patients are on anticoagulation or not, occurs in 19–50% of patients and 2–10% experience major bleeding [17, 18]. Findings from two large retrospective reviews of transplanted patients indicate that bleeding risk outweighs VTE risk at a ratio of approximately 3:1 [5, 19]. Furthermore, clinically significant bleeding, but not thrombotic events were associated with reduced survival.
The safety and efficacy of providing anticoagulation during severe thrombocytopenia have not been well established [20, 21]. The challenge of both recurrent VTE and bleeding in patients with hematologic malignancy and severe thrombocytopenia highlight the need to better define the course of VTE and evaluate management strategies. To determine the outcomes associated with various management strategies for VTE we examined recurrent VTE and clinically significant bleeding events from the onset of severe thrombocytopenia.
Methods:
We searched patient records at the University of North Carolina stored within a data repository containing clinical, research, and administrative data (Carolina Data Warehouse) from April 2004 – December 2015. We identified patients with ICD-9 codes for acute or chronic VTE (415.1, 416.2, 451.1, 451.2, 451.81, 451.83, 452, 453.xx), hematologic malignancies (200–208.99), and patients with platelets <50 × 109/L during a hospital admission. The accuracy of ICD-9 codes, initial VTE event date, and status of hematologic malignancy were confirmed by reviewing physician notes and/or imaging studies. VTE events were eligible for inclusion if acute VTE occurred during a period of severe thrombocytopenia or if a patient remained on anticoagulation for a prior VTE until the episode of severe thrombocytopenia. Eligible charts were reviewed and abstracted for primary and secondary outcomes and other variables. Patients were only included once when first eligible and could have more than one severe thrombocytopenic episode during the 100-day follow-up. Exclusion criteria were: age <18 years, hospice/comfort care treatments only, heparin-induced thrombocytopenia, and thrombotic thrombocytopenic purpura, or other contraindication to anticoagulation (except thrombocytopenia). Active hematologic malignancy was defined as initial diagnosis or immune/chemotherapy treatment for a blood cancer within the previous 12 months. At our institution in the absence of bleeding, the standard protocol for prophylactic platelet transfusion is to maintain platelet counts above 10 × 109/L, or greater than 20 × 109/L if febrile.
Primary outcomes were time to recurrent VTE or clinically significant bleeding within 100 days of follow-up from the first day of severe thrombocytopenia. Time to primary outcomes was determined by chart abstraction and corroborated with the appropriate imaging study. Recurrent VTE was defined as VTE occurring at a site other than the most recent VTE and included progressive DVT, defined as >5 cm increase in the length of previously evaluated DVT on Doppler ultrasound. Clinically significant bleeding events were defined as a Bleeding Severity Measurement Scale (BSMS) of 2 (2a – serious, 2b – major, 2c – fatal) [22]. Data for primary outcomes were considered complete at the time of last physician assessment. Anticoagulation management strategies were separated into groups based on initial hospital management during episodes of severe thrombocytopenia as follows: prophylactic, intermediate-dose, or therapeutic dose anticoagulation, or no anticoagulation. Duration of anticoagulation, transfusion practices, and duration and degree of thrombocytopenia were recorded. Secondary outcomes of the number of platelet and red blood cell transfusions were tabulated per patient during the 100 days of follow-up or until the clinically significant bleeding event or recurrent VTE occurred.
We examined the characteristics of the sample to assess the distribution of the variables and to assess any impact of missing data or extreme values. The means and standard deviation were computed for continuous variables and frequencies were tabulated for categorical variables. The chi-squared test was used to compare categorical variables. Where expected values were small, the p-values were based on simulation. The Wilcoxon rank sum test was used for uncensored, non-normal, continuous data. Kaplan-Meier curves were created for time to event data for recurrent VTE and clinically significant bleeding by anticoagulation strategy and were censored for death, lost to follow-up, and the opposite outcome. Incidence rates (IR) and incidence rate ratios (IRR) were calculated using total person time and person time (days) with platelets <50 × 109/L for recurrent VTE, clinically significant bleeding, and the combined incidence of bleeding or recurrent VTE (composite outcome). A quasi-Poisson generalized linear model was used to examine for differences in platelet transfusions among anticoagulation groups. The model was adjusted for the number of red blood cell transfusions, the total duration of severe thrombocytopenia, type of hematologic malignancy, and bone marrow transplant status. The study was approved by the University of North Carolina Institutional Review Board.
Results:
A search of the Carolina Data Warehouse identified 156 patients who met the initial search criteria. Chart review excluded 78 patients: 46 with VTE were not on anticoagulation prior to severe thrombocytopenia, 15 had an incorrect diagnosis code for VTE, 14 diagnosis codes for hematologic malignancy were either incorrect or disease history was remote, and 3 charts had insufficient documentation to determine inclusion/exclusion criteria or anticoagulation strategy (Figure 1). This left 78 patients, of whom 6 patients died and 3 were lost to follow-up before a thrombotic or clinically significant bleeding event occurred in the 100 days of follow-up.
Figure 1:
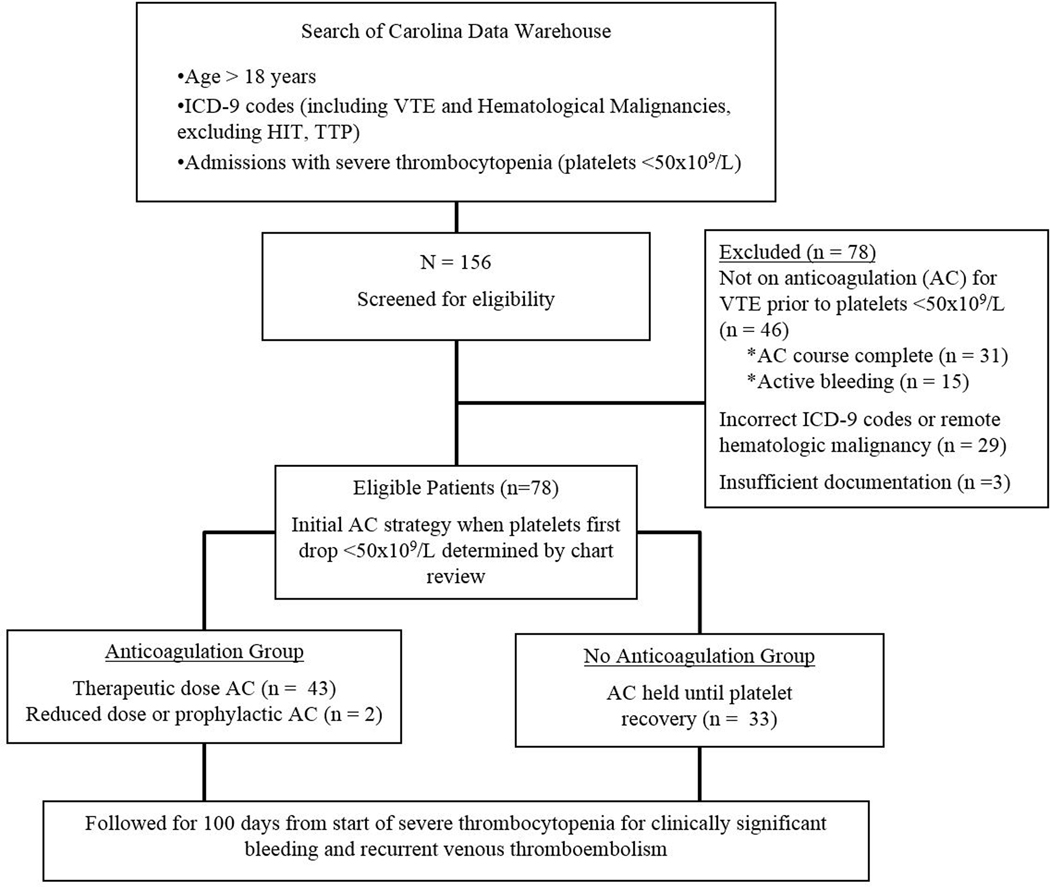
Flow diagram of selection criteria and follow-up
The mean age for the 78 patients included was 53 years (range 19–81) and mean BMI was 29.6 kg/m2 (range 19–48). Forty (51%) patients were being treated for acute leukemia, 22 (28%) for lymphoma, and 16 (21%) for a plasma cell dyscrasia. Over half of the patients (63%) underwent autologous (n=29) or allogeneic (n=20) transplant before or during follow-up. The median duration of severe thrombocytopenia was 14 days (IQR 6–35). Of the initial VTE, 12 (15%) events were PE, 7 (9%) were lower extremity DVT, and 58 (74%) were upper extremity DVT (52/58 were catheter-related). Therapeutic dose anticoagulation was the initial strategy when platelets dropped below 50 × 109/L in 43 (55%) patients and anticoagulation was held in 33 (42%). Two patients (3%) received lower doses of anticoagulation: 1 had prophylactic dosing and 1 had intermediate-dose enoxaparin. All patients treated with therapeutic anticoagulation had increased platelet transfusion thresholds of 40–50 × 109/L. Therapeutic anticoagulation during periods of severe thrombocytopenia was given either as intravenous heparin drip (70%) or as enoxaparin (30%).
Clinically significant bleeding occurred in 13 patients: 5 pre-transplant, 1 post-autologous, and 7 post-allogeneic transplant. Among these patients, the median number of days to clinically significant bleeding after the onset of severe thrombocytopenia was 8 (IQR 5–22) and the median platelet count that was most proximate to the bleeding event was 38 × 109/L (range 5–212 × 109/L). The sites of bleeding were: 8 mucocutaneous, 2 deep tissue, 2 pulmonary, 1 retroperitoneal. Overall 6 patients had recurrent VTE: 4 pre-transplant, 0 post-autologous, and 2 post-allogenic transplant. Among these patients, the median number of days to recurrence was 62.5 (IQR 40–91) and the median platelet count at the time of VTE recurrence was 225 × 109/L (range 21–407 × 109/L). Most clinically significant bleeding (n=11, 85%) occurred on or before day 30 while most of the recurrent VTE (n=5, 83%) occurred on or after day 40 (Figure 2). No recurrent VTE or bleeding resulted in death. Patients with acute leukemia/MDS accounted for 5/6 recurrent VTE and 10/13 bleeding events.
Figure 2:
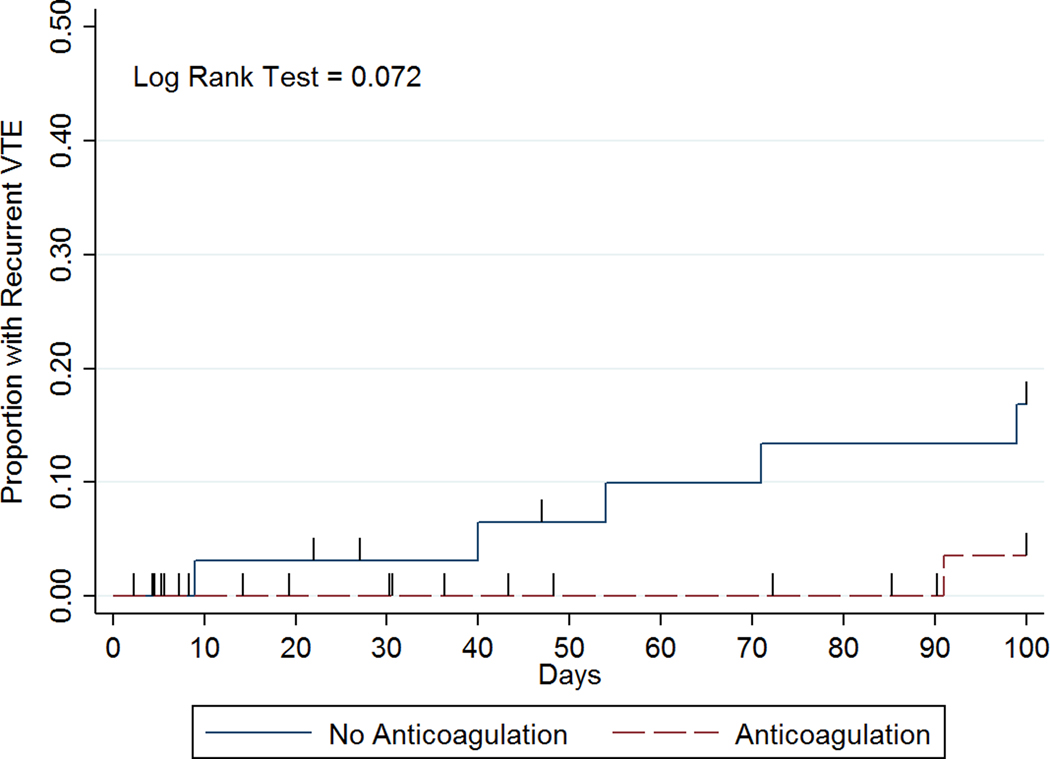
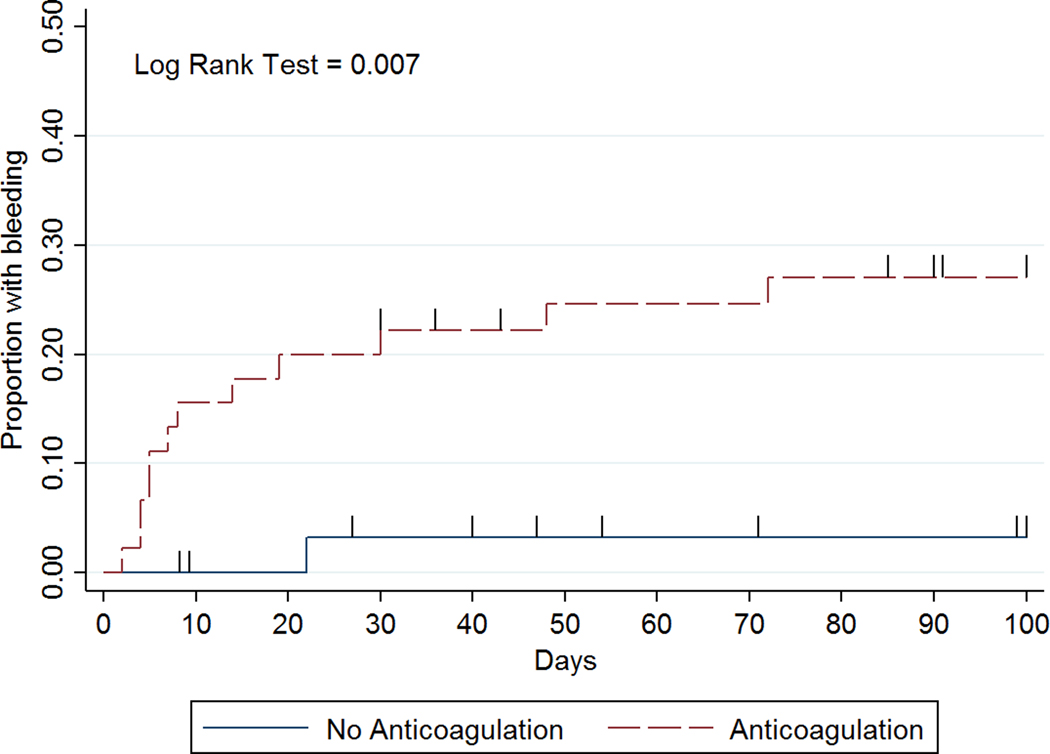
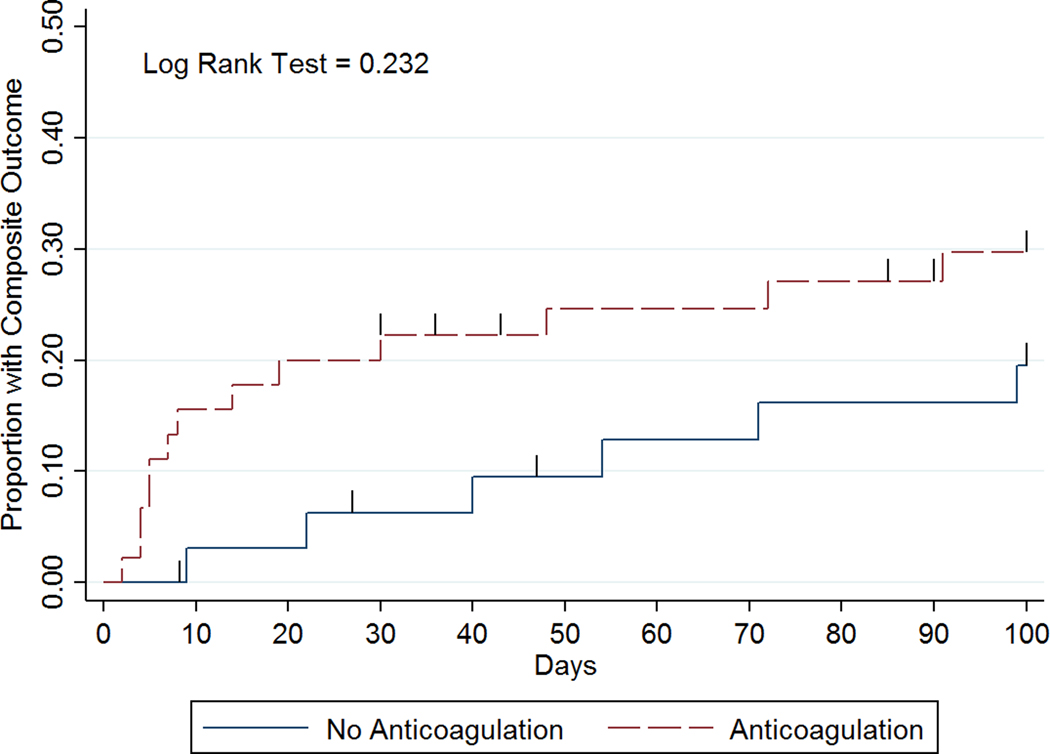
Time to event curves for (a) recurrent VTE, (b) clinically significant bleeding, and (c) composite outcome by anticoagulation management strategy.
For analysis purposes, the two patients treated with prophylactic or intermediate-dose anticoagulation were combined with patients receiving therapeutic anticoagulation for comparison to those with no anticoagulation during severe thrombocytopenic episodes. In total, 45 patients in the anticoagulation group had 3293 person-days of follow-up and 33 patients in the no anticoagulation group had 2777 person-days of follow-up. Person-time with severe thrombocytopenia was 843 days in the anticoagulation group versus 1244 days in the no anticoagulation group. Mean age and BMI were similar among patients who received any anticoagulation during severe thrombocytopenia compared to those with no anticoagulation (Table 1). The no anticoagulation group had a higher percentage of leukemia (67% vs. 40%) and pre-transplant patients (58% vs. 22%). More patients in the anticoagulation group had PE (20% vs. 9%) but fewer had LE DVT (2% vs. 18%). Liver and renal dysfunction were not significantly different between anticoagulation groups (data not shown). The percent of patients initially diagnosed with VTE during severe thrombocytopenia (27% vs. 31%, p=0.535: Table 1) and the median number of days from VTE diagnosis to severe thrombocytopenia (14 vs. 19 days, p=0.673: Table 2) were similar between each group.
Table 1:
Comparison of baseline patient characteristics by anticoagulation strategy during periods of severe thrombocytopenia. Abbreviations: IQR=interquartile range, BMI=body mass index (kg/m2), BMT=bone marrow transplant, PE=pulmonary embolism, VTE=venous thromboembolism, DVT=deep vein thrombosis, UE=upper extremity, LE=lower extremity, PCD=plasma cell dyscrasia, IVC=inferior vena cava. *Chi-squared test was used for all categorical variables and Wilcoxon Rank Sum test used to calculate p values for continuous variables.
| No Anticoagulation | Any Anticoagulation | P value* | |
|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | ||
| Total | 33 | 45 | |
| Age, years | 56 (46–66) | 54 (43–61) | 0.177 |
| BMI | 28 (24–29) | 30 (26–34) | 0.094 |
| Male | 18 (55) | 28 (62) | 0.642 |
| Malignancy | |||
| Lymphoma | 7 (21) | 15 (33) | 0.066 |
| Leukemia/MDS | 22 (67) | 18 (40) | |
| PCD | 4 (12) | 12 (27) | |
| BMT Status | |||
| None | 19 (58) | 10 (22) | 0.001 |
| Autograft | 5 (15) | 24 (53) | |
| Allograft | 9 (27) | 11 (24) | |
| Initial VTE | |||
| UE DVT | 23 (70) | 35 (78) | 0.021 |
| LE DVT | 6 (18) | 1 (2) | |
| PE | 3 (9) | 9 (20) | |
| Other | 1 (3) | 0 | |
| Time from VTE to PLT <50×109/L | |||
| 0 (concurrent) | 9 (27) | 14 (31) | 0.535 |
| 1–30 days | 10 (30) | 13 (29) | |
| 31–90 days | 5 (15) | 11 (24) | |
| >90 days | 9 (27) | 7 (16) | |
| Prior VTE | 1 (3) | 4 (9) | 0.389 |
| Recent Bleed (<1 month) | 2 (6) | 4 (9) | 0.691 |
| IVC filter | 3 (9) | 1 (2) | 0.305 |
Table 2:
Differences in treatments and laboratory assessments by anticoagulation strategy. Abbreviations: IQR=interquartile range, PRBC=packed red blood cells, PLT=platelet, Hgb=hemoglobin. *Chi-squared test was used for all categorical variables and Wilcoxon Rank Sum test used to calculate p values for continuous variables. ϕ For patients without a recurrent VTE or clinically significant bleeding event
| No Anticoagulation | Any Anticoagulation | P value* | |
|---|---|---|---|
| n (%) or mean | n (%) or mean | ||
| Catheter Removal | 19 (91) | 30 (88) | 1.0 |
| Mean PLT Count During Severe Thrombocytopenia (<50×109/L) | 32 | 48 | <0.001 |
| # days with PLTs <50×109/L | 37.7 | 18.7 | 0.004 |
| # days with PLTs <20×109/L | 8.9 | 1.5 | 0.001 |
| # days with PLTs <10×109/L | 2.9 | 0.4 | 0.01 |
| Median (IQR) | Median (IQR) | ||
| Minimum PLT Count (x109/L) | 6 (5–9) | 27 (14–32) | <0.001 |
| Minimum Hgb (g/dL) | 7.6 (7.0–8.2) | 8.0 (7.3–8.9) | 0.154 |
| PLT units per patient | 4 (2–8) | 4 (3–8) | 0.293 |
| PRBC units per patient | 5 (2–12) | 2 (0–4) | 0.001 |
| # days from VTE to PLT <50×109/L | 14 (0–83) | 19 (0–39) | 0.673 |
| Total Days of Anticoagulation from initial VTE ϕ | 40 (10–92) | 94 (71–112) | 0.022 |
Clinically significant bleeding within 100 days after the onset of severe thrombocytopenia occurred in 12/45 (27%) patients in anticoagulation group versus 1/33 (3%) patients in the no anticoagulation group (IRR 10.12, 95% CI 1.50–432.60; Table 3). Among those with clinically significant bleeding on anticoagulation at the time of their event (n=11), 9 had a platelet count <50 × 109/L (Figure 3). Among these 9 patients, 2 had major bleeds (BSMS 2b). In all cases of bleeding anticoagulation was held temporarily and in 7/11 anticoagulation was not re-started subsequently. In 2 patients, clinically significant bleeding was associated with a supratherapeutic level of anticoagulant. Recurrent VTE occurred in 1/45 (2%) patients in the anticoagulation group versus 5/33 (15%) patients in the no anticoagulation group (IRR 0.17, 95% CI 0.00–1.51; Table 3). Most recurrent VTE events were upper extremity DVTs (5/6) and 1 was a proximal lower extremity DVT. Among patients with recurrent VTE not on anticoagulation at the time of their event (n=5), only 2 occurred when platelets were below 50 × 109/L (Figure 3). The composite outcome occurred in 13/45 (29%) patients in the anticoagulation group versus 6/33 (18%) patients in the no anticoagulation group (IRR 1.83, 95% CI 0.65–5.86). In patients with acute VTE during severe thrombocytopenia, 1 had recurrent VTE at day 71. In those with VTE 1–30 days preceding severe thrombocytopenia, 3 had recurrent VTE (day 40, 91, 99). If VTE occurred 31–90 days beforehand, no subsequent VTE was identified, and for those with VTE occurring >90 days beforehand, 2 recurrent VTE were identified (day 9 & 54). The IRR for recurrent VTE for acute VTE (≤30 days) versus subacute/chronic VTE (>30 days) is 1.77 (95% CI 0.254–19.582). The IRR for clinically significant bleeding for acute VTE (≤30 days) versus subacute/chronic VTE (>30 days) is 10.63 (95% CI 1.573–454.329).
Table 3:
Incidence rates and incidence rate ratios for recurrent VTE, clinically significant bleeding, and composite outcome for patients in any anticoagulation vs. no anticoagulation group. Incidence rates (per 100 days) are scaled by total person time (the number of days from the initial VTE until an event) and by Plt <50 time (the number of days the patient had a platelet count <50 × 109/L). The number, incidence rates, and incidence rate ratio for Plt <50 time reflect only events that occurred when platelets were <50 × 109/L. The parenthetical values are exact 95% confidence intervals.
| Recurrent VTE | N | Person Time | Incidence by Person Time | N | Pit <50 | Incidence by Pit <50 Time |
|---|---|---|---|---|---|---|
| Any Anticoagulation | 1 | 3293 | 0.03 (0.00, 0.17) | 0 | 843 | 0.00 (0,00, 0.44) |
| No Anticoagulation | 5 | 2777 | 0.18 (0.06, 0.42) | 2 | 1244 | 0.16(0,02, 0.58) |
| Incidence Rate Ratio: | 0.17 (0.00, 1.51) | 0.00 (0,00, 7,86) | ||||
|
Clinically Significant Bleeding | ||||||
| Any Anticoagulation | 12 | 3293 | 0.36 (0.19, 0.64) | 10 | 843 | 1.19 (0.57, 2.18) |
| No Anticoagulation | 1 | 2777 | 0.04 (0,00, 0,20) | 1 | 1244 | 0.08 (0.00, 0.45) |
| Incidence Rate Ratio: | 10.12 (1.50, 432.60) | 14.76(2.10, 640.41) | ||||
|
Composite Outcome | ||||||
| Any Anticoagulation | 13 | 3293 | 0,39 (0.21,0.68) | 10 | 843 | 1.19(0.57, 2.18) |
| No Anticoagulation | 6 | 2777 | 0,22 (0.08, 0.47) | 3 | 1244 | 0.24(0.05, 0.70) |
| Incidence Rate Ratio: | 1.83 (0.65, 5.86) | 4.92 (1.27, 27,81) | ||||
Figure 3:
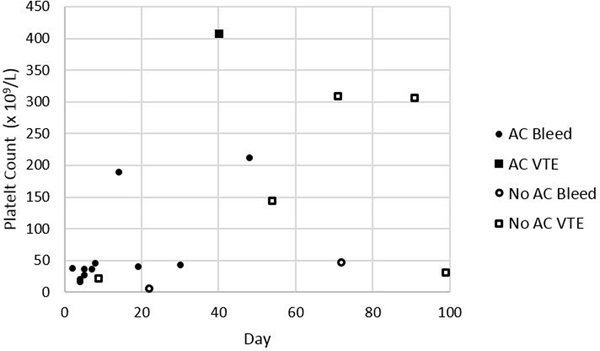
Platelet Count and Anticoagulation Status at Time of Recurrent VTE or Bleeding Event. Abbreviations: AC=anticoagulation
The strategy of transfusing platelets at higher thresholds in the anticoagulation group did result in a higher mean platelet count during episodes of severe thrombocytopenia (48 vs. 32 × 109/L, p<0.001: Table 2). The anticoagulation group also had fewer days with severe thrombocytopenia than the no anticoagulation group (18.7 vs. 37.7 days, p=0.002) and fewer days with platelet counts <20 × 109/L (1.5 vs. 8.9 days, p=0.001) and <10 × 109/L (0.4 vs. 2.9 days, p=0.01). The median number of platelet transfusions was similar (4 vs. 4, p=0.293) between the groups. However, when the number of platelet transfusions per group was adjusted for duration of severe thrombocytopenia, the number of red blood cell transfusions, the type of hematologic malignancy, and the bone marrow transplant status, patients treated with anticoagulation received 1.9 (95% CI 1.28–2.78) times the number of platelet transfusions than those who did not receive anticoagulation during severe thrombocytopenia.
Discussion:
In this observational study of hematologic malignancy patients with predominately catheter-related upper extremity (CRUE) DVT, we compared both bleeding and recurrent VTE outcomes associated with anticoagulation or no anticoagulation during periods of severe thrombocytopenia. We found that anticoagulation during severe thrombocytopenia resulted in a significantly higher rate of clinically significant bleeding compared to no anticoagulation. Furthermore, by evaluating time to bleeding and recurrent VTE we found that clinically significant bleeding predominately occurred in the first 30 days after the onset of severe thrombocytopenia whereas most recurrent VTE occurred after this period. Anticoagulation significantly contributed to this increased bleeding risk despite maintaining higher platelet counts with platelet transfusions.
Strategies for anticoagulation of thrombocytopenic patients with VTE vary significantly by clinician and institution [3, 8, 23, 24, 25, 26]. The 2013 International Society of Thrombosis and Haemostasis (ISTH) and the 2015 Canadian guidance statements both recommend therapeutic dose anticoagulation with platelet transfusions to maintain platelet counts >50 × 109/L for acute VTE (<30 days) but less aggressive management for more remote events [9, 14]. The initial anticoagulation strategies at our institution primarily varied between therapeutic dose anticoagulation with supportive platelet transfusions (55%) or no anticoagulation until the platelet count recovered (42%); in contrast to the guidelines, similar amounts of acute VTE were in each group. The type of most recent VTE and bone marrow transplant status differed between the groups indicating these factors may have influenced the anticoagulation decision. Most of the initial VTE in our sample was CRUE DVT but these were excluded from the recommendations made in the 2013 ISTH guidance statement [9]. CRUE DVT is not inconsequential, with concurrent PE occurring in 10–25% of patients [27, 28], however, the short-term recurrence/progression of VTE in the absence of anticoagulation and when modified by severe thrombocytopenia is unknown. In a separate ISTH clinical practice guideline [29] for patients with CRUE, they recommend anticoagulation for a minimum of 3 months (with or without catheter removal), but make no recommendations on anticoagulation during periods of thrombocytopenia. Our analysis cannot adequately determine if CRUE has a higher or lower risk of recurrent VTE, but does suggest that even for acute VTE, the risk of clinically significant bleeding exceeds the risk of recurrent VTE during severe thrombocytopenia.
The high rate of clinically significant bleeding in patients on anticoagulation with severe thrombocytopenia is similar to findings from a retrospective review of bone marrow transplant patients [19]. Within that cohort, 62 patients with VTE and severe thrombocytopenia (<50 × 109/L) were treated with anticoagulation and supportive platelet transfusions to maintain a platelet count above 50 × 109/L. That study reported a high rate of clinically significant bleeding (37%) and fatal bleeding (5%). In another study, 47 patients with hematologic malignancy and platelets <50 × 109/L were treated for VTE with prophylactic doses of LMWH (47%), therapeutic LMWH (30%), or observation (17%) with a reported incidence of clinically significant bleeding of 11% after a median follow-up of 24.6 months [24]. Other small case series also suggest that prophylactic or low dose anticoagulation can be safely used for the treatment of VTE during severe thrombocytopenia [6, 7, 30, 31].
Therapeutic dose anticoagulation with an elevated platelet transfusion threshold is a resource intensive strategy [32] and in our analysis lead to 1.9 times the number of platelet transfusions compared to the no anticoagulation group after adjustment. The strategy of transfusing platelets at higher thresholds for patients in the anticoagulation group was successful. Numerous platelet parameters were improved, including number of days with platelets <50, <20, <10×109/L. The type of hematologic disease and the chemotherapy regimen used can cause varying degrees of thrombocytopenia and would be expected to influence bleeding rates, however, platelet parameters have not been found to be associated with bleeding in this population after multivariable adjustment [3, 24]. In our study, multiple platelet parameters indicated a higher degree and longer duration of thrombocytopenia in the no anticoagulation group, yet only 1/13 bleeding events occurred in this group. Although not systematically recorded, difficulties in maintaining platelet counts above 50 × 109/L were noted in many patients in our cohort leading to increased platelet transfusions and on some occasions, discontinuation of this strategy. Elevated platelet count alone does not appear to be protective from bleeding, indicating either a higher platelet threshold is necessary or perhaps that transfused platelets are less functional. Alternatively, bleeding may have less to do with platelet counts and more to do with other factors such as endothelial integrity or hyperfibrinolysis [33, 34]. Interestingly, in patients with acute leukemia, VTE has been associated with disseminated intravascular coagulopathy (HR 11.08, 95% CI 3.23–38.06) and an elevated d-dimer (HR 12.3, 95% CI 3.39–42.64), but not major bleeding [35].
Although the strategy of no anticoagulation during severe thrombocytopenia in this study resulted in fewer overall adverse events, this approach should be cautioned in some patients with VTE based on our data. Since only three patients with PE were in the no anticoagulation group and none of the acute VTE that occurred during severe thrombocytopenia was PE or proximal lower extremity DVT, the optimal anticoagulation strategy cannot be determined. Therapeutic anticoagulation should still be considered in these situations. For patients with prolonged severe thrombocytopenia, transfusion of platelets to maintain counts >50 × 109/L is not feasible and indefinite delay of anticoagulation puts them at risk for recurrent VTE. One patient in our cohort that did not get anticoagulation suffered from recurrent VTE after platelets were <50 × 109/L for 99 days, highlighting the need for an alternative management strategy. We did not find evidence to suggest that the more aggressive management strategy of anticoagulation and supportive platelet transfusions to higher thresholds would be better for any subpopulation of hematologic malignancy or based on acuity of VTE, although meaningful comparison of subgroups in our population is limited due to sample size.
The increased (but not statistically significant) rate of recurrent VTE in patients who did not receive anticoagulation during severe thrombocytopenia may be explained by differences between the groups since we were not able to adjust for confounding. Patients with no anticoagulation during severe thrombocytopenia received more red blood cell transfusions (5 vs. 2 units) and had an overall increased number of cumulative days with severe thrombocytopenia (1244 vs. 843 days). This may indicate that the group with no anticoagulation during severe thrombocytopenia had more intense chemotherapy, greater severity of disease, or more comorbidities compared to those who received therapeutic anticoagulation and supportive platelet transfusions. For patients without a primary outcome, those who had no anticoagulation during severe thrombocytopenia also had significantly shorter courses of therapeutic anticoagulation compared to the anticoagulation group (median 40 vs. 94 days). This difference in overall duration of anticoagulation may also explain why VTE recurrences tended to occur later in this group. Re-initiation of anticoagulation when platelets recover to above 50 × 109/L and minimum 3-month anticoagulation (even if the central catheter was removed) may have prevented most (3/5) of the recurrent VTE in the no anticoagulation group.
Our inclusion/exclusion criteria may have actually selected for a cohort at lower risk for bleeding. Of the 46 patients excluded because they were not on anticoagulation, 15/46 either never started anticoagulation due to bleeding at the time of VTE diagnosis or discontinued anticoagulation due to bleeding before their platelets dropped below 50 × 109/L. This further underscores the bleeding risks in this population and argues that a platelet threshold of 50 × 109/L may not be that clinically meaningful in regards to the safety of anticoagulation. The differential impact of bleeding versus recurrent VTE is unclear from our analysis, as no deaths in our cohort were attributable to these events. We did not evaluate the duration of hospitalization or health care utilization which may be important considerations. Health-related quality of life and patient attitudes regarding bleeding and recurrent VTE should also be considered, but would need to be collected prospectively. Our results are limited by our sample size, and retrospective data collection may have missed events if not recorded in the chart or if events occurred at other hospitals and were not reported to the primary hematologist. The definition of outcomes, risk factors, and time to event data were strengthened by validation by physician chart review of imaging reports and clinical notes.
Findings from this study support a more conservative upfront approach in select patients, especially in those with upper extremity or CRUE DVT, which in the clinical setting could be monitored with repeat ultrasound and clinical evaluation to ensure no progression of known VTE. The dynamic risk of bleeding and recurrent VTE in patients with hematologic malignancies and severe thrombocytopenia illustrate that anticoagulation strategies should not remain fixed, but rather vary depending on where patients are in their treatment course. The significantly higher rate of clinically significant bleeding, higher platelet utilization, and overall increased adverse events in the anticoagulation group raises concerns over this treatment as the primary management strategy for this population and questions the appropriateness of recent guidelines on this topic. Safety and efficacy trials are needed to determine the best anticoagulation strategy for VTE in patients with hematologic malignancies and severe thrombocytopenia.
Acknowledgments:
Supported by NIH CTSA at the University of North Carolina (UL1TR001111) and UNC Hematology T32 (5T32HL007149-39).
Funding details: This work was supported by NIH CTSA at the University of North Carolina under grant [UL1TR001111] and the UNC Hematology T32 under grant [5T32HL007149-39].
Footnotes
Disclosure statement: The authors report no conflicts of interest.
Contributor Information
Damon E. Houghton, University of North Carolina, Division of Hematology/Oncology, 170 Manning Drive, 3rd Floor CB# 7305, Chapel Hill, NC 27599.
Nigel S. Key, University of North Carolina, Division of Hematology/Oncology, 120 Mason Farm Road, 1042 Genetic Medicine Building, CB#7035, Chapel Hill, NC 27599.
Neil A. Zakai, University of Vermont, Departments of Medicine and Pathology, 111 Colchester Avenue, Main Campus, Main Pavilion, Level 2, Burlington, VT 05401.
Jeffrey P. Laux, University of North Carolina, The North Carolina Translational and Clinical Sciences (NC TraCS) Institute, 160 N. Medical Drive, Brinkhous-Bullitt Building, 2nd Floor CB#7064, Chapel Hill, NC 27599
Thomas Shea, University of North Carolina, Division of Hematology/Oncology, 170 Manning Drive, 3rd Floor CB# 7305, Chapel Hill, NC 27599.
Stephan Moll, University of North Carolina, Division of Hematology/Oncology, 120 Mason Farm Road, 1042 Genetic Medicine Building, CB#7035, Chapel Hill, NC 27599.
References:
- 1.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–46. [DOI] [PubMed] [Google Scholar]
- 2.Khorana A, Francis C, Culakova E, Kuderer N, Lyman G. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Journal of Thrombosis and Haemostasis. 2007;5:632–4. [DOI] [PubMed] [Google Scholar]
- 3.Kopolovic I, Lee AY, Wu C. Management and outcomes of cancer-associated venous thromboembolism in patients with concomitant thrombocytopenia: a retrospective cohort study. Ann Hematol. 2015;94:329–36. [DOI] [PubMed] [Google Scholar]
- 4.Prandoni P, Lensing AWA, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–8. [DOI] [PubMed] [Google Scholar]
- 5.Labrador J, Lopez-Anglada L, Perez-Lopez E, Lozano FS, Lopez-Corral L, Sanchez-Guijo FM, Vazquez L, Rivera JAP, Martin-Herrero F, Sanchez-Barba M. Analysis of incidence, risk factors and clinical outcome of thromboembolic and bleeding events in 431 allogeneic hematopoietic stem cell transplantation recipients. Haematologica. 2013;98:437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drakos PE, Nagler A, Or R, Gillis S, Slavin S, Eldor A. Low molecular weight heparin for hickman catheter‐induced thrombosis in thrombocytopenic patients undergoing bone marrow transplantation. Cancer. 1992;70:1895–8. [DOI] [PubMed] [Google Scholar]
- 7.Herishanu Y, Misgav M, Kirgner I, Ben-Tal O, Eldor A, Naparstek E. Enoxaparin can be used safely in patients with severe thrombocytopenia due to intensive chemotherapy regimens. Leuk Lymphoma. 2004;45:1407–11. [DOI] [PubMed] [Google Scholar]
- 8.Campbell PM, Ippoliti C, Parmar S. Safety of anticoagulation in thrombocytopenic patients with hematologic malignancies: A case series. Journal of Oncology Pharmacy Practice. 2016;0:1–6. [DOI] [PubMed] [Google Scholar]
- 9.Carrier M, Khorana AA, Zwicker J, Noble S, Lee AY, Subcommittee on H, Malignancy for the SSCotI. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013;11:1760–5. [DOI] [PubMed] [Google Scholar]
- 10.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST Journal. 2012;141:419–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, Kakkar A, Kuderer NM, Levine MN, Liebman H, Mendelson D, Raskob G, Somerfield MR, Thodiyil P, Trent D, Francis CW, American Society of Clinical O. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–505. [DOI] [PubMed] [Google Scholar]
- 12.Mandala M, Falanga A, Roila F, Group EGW. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Annals of oncology. 2011;22:85–92. [DOI] [PubMed] [Google Scholar]
- 13.Farge D, Debourdeau P, Beckers M, Baglin C, Bauersachs RM, Brenner B, Brilhante D, Falanga A, Gerotzafias GT, Haim N, Kakkar AK, Khorana AA, Lecumberri R, Mandala M, Marty M, Monreal M, Mousa SA, Noble S, Pabinger I, Prandoni P, Prins MH, Qari MH, Streiff MB, Syrigos K, Bounameaux H, BÜLler HR. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Journal of Thrombosis and Haemostasis. 2013;11:56–70. [DOI] [PubMed] [Google Scholar]
- 14.Easaw JC, Shea-Budgell MA, Wu CMJ, Czaykowski PM, Kassis J, Kuehl B, Lim HJ, MacNeil M, Martinusen D, McFarlane PA, Meek E, Moodley O, Shivakumar S, Tagalakis V, Welch S, Kavan P. Canadian consensus recommendations on the management of venous thromboembolism in patients with cancer. Part 2: treatment. Current Oncology. 2015;22:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elice F, Rodeghiero F. Hematologic malignancies and thrombosis. Thromb Res. 2012;129:360–6. [DOI] [PubMed] [Google Scholar]
- 16.De Stefano V, Sora F, Rossi E, Chiusolo P, Laurenti L, Fianchi L, Zini G, Pagano L, Sica S, Leone G. The risk of thrombosis in patients with acute leukemia: occurrence of thrombosis at diagnosis and during treatment. Journal of Thrombosis and Haemostasis. 2005;3:1985–92. [DOI] [PubMed] [Google Scholar]
- 17.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, Cipolle MD, Cohn CS, Fung MK, Grossman BJ, Mintz PD, O’Malley BA, Sesok-Pizzini DA, Shander A, Stack GE, Webert KE, Weinstein R, Welch BG, Whitman GJ, Wong EC, Tobian AA, Aabb. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. [DOI] [PubMed] [Google Scholar]
- 18.Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, Bakrania L, Llewelyn C, Littlewood T, Soutar R, Norfolk D, Copplestone A, Smith N, Kerr P, Jones G, Raj K, Westerman DA, Szer J, Jackson N, Bardy PG, Plews D, Lyons S, Bielby L, Wood EM, Murphy MF, Investigators T. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368:1771–80. [DOI] [PubMed] [Google Scholar]
- 19.Gerber DE, Segal JB, Levy MY, Kane J, Jones RJ, Streiff MB. The incidence of and risk factors for venous thromboembolism (VTE) and bleeding among 1514 patients undergoing hematopoietic stem cell transplantation: implications for VTE prevention. Blood. 2008;112:504–10. [DOI] [PubMed] [Google Scholar]
- 20.Lim M, Enjeti AK. Safety of anticoagulation in the treatment of venous thromboembolism in patients with haematological malignancies and thrombocytopenia: Report of 5 cases and literature review. Critical Reviews in Oncology/Hematology. 2016;105:92–9. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim RB, Skewes MD, Kuriakose P. ‘Sailing in troubled waters’: a review of the use of anticoagulation in adult cancer patients with thrombocytopenia. Blood Coagulation & Fibrinolysis. 2016;27:615. [DOI] [PubMed] [Google Scholar]
- 22.Webert KE, Arnold DM, Lui Y, Carruthers J, Arnold E, Heddle NM. A new tool to assess bleeding severity in patients with chemotherapy-induced thrombocytopenia. Transfusion. 2012;52:2466–74. [DOI] [PubMed] [Google Scholar]
- 23.Samuelson BT, Gernsheimer T, Estey E, Garcia DA. Variability in management of hematologic malignancy patients with venous thromboembolism and chemotherapy-induced thrombocytopenia. Thrombosis Research. 2016;141:104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanal N, Bociek RG, Chen B, Vose JM, Armitage JO, Bierman PJ, Maness LJ, Lunning MA, Gundabolu K, Bhatt VR. Venous Thromboembolism in Patients with Hematologic Malignancy and Thrombocytopenia. American journal of hematology. 2016. Epub Aug 4, 2016. [DOI] [PubMed] [Google Scholar]
- 25.Oliver N, Short B, Thein M, Duong VH, Tidwell ML, Sausville EA, Baer MR, Kamangar F, Emadi A. Treatment of catheter-related deep vein thrombosis in patients with acute leukemia with anticoagulation. Leukemia & Lymphoma. 2015;56:2082–6. [DOI] [PubMed] [Google Scholar]
- 26.Napolitano M, Valore L, Malato A, Saccullo G, Vetro C, Mitra M, Fabbiano F, Mannina D, Casuccio A, Lucchesi A, Principe M, Candoni A, Raimondo F, Siragusa S. Management of venous thromboembolism in patients with acute leukemia at high bleeding risk: a multi-center study. Leukemia & Lymphoma. 2015;57:116–9. [DOI] [PubMed] [Google Scholar]
- 27.Verso M, Agnelli G. Venous Thromboembolism Associated With Long-Term Use of Central Venous Catheters in Cancer Patients. Journal of Clinical Oncology. 2003;21:3665–75. [DOI] [PubMed] [Google Scholar]
- 28.Winters JP, Callas PW, Cushman M, Repp AB, Zakai NA. Central venous catheters and upper extremity deep vein thrombosis in medical inpatients: the Medical Inpatients and Thrombosis (MITH) Study. Journal of Thrombosis and Haemostasis. 2015;13:2155–60. [DOI] [PubMed] [Google Scholar]
- 29.Debourdeau P, Farge D, Beckers M. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. Journal of Thrombosis and Haemostasis. 2013. [DOI] [PubMed] [Google Scholar]
- 30.Babilonia KM, Golightly LK, Gutman JA, Hassell KL, Kaiser JN, Kiser TH, Klem PM, Trujillo TC. Antithrombotic therapy in patients with thrombocytopenic cancer: outcomes associated with reduced-dose, low-molecular-weight heparin during hospitalization. Clin Appl Thromb Hemost. 2014;20:799–806. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim RB, Peres E, Dansey R, Abidi MH, Abella EM, Gumma MM, Milan N, Smith DW, Heilbrun LK, Klein J. Safety of low-dose low-molecular-weight-heparins in thrombocytopenic stem cell transplantation patients: a case series and review of the literature. Bone Marrow Transplantation. 2005;35:1071–7. [DOI] [PubMed] [Google Scholar]
- 32.Eikenboom JCJ, van Wordragen R, Brand A. Compliance with prophylactic platelet transfusion trigger in haematological patients. Transfusion medicine (Oxford, England). 2005;15:45–8. [DOI] [PubMed] [Google Scholar]
- 33.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. Journal of Thrombosis and Haemostasis. 2013;11:223–33. [DOI] [PubMed] [Google Scholar]
- 34.Franchini M, Frattini F, Crestani S, Bonfanti C. Bleeding complications in patients with hematologic malignancies. Seminars in thrombosis and hemostasis. 2012;39:94–100. [DOI] [PubMed] [Google Scholar]
- 35.Libourel EJ, Klerk C, Norden Yv, Maat M, Kruip MJ, Sonneveld P, Löwenberg B, Leebeek F. Disseminated intravascular coagulation at diagnosis is a strong predictor for both arterial and venous thrombosis in newly diagnosed acute myeloid leukemia. Blood. 2016;128:1854–61. [DOI] [PubMed] [Google Scholar]


