Abstract
The monoamine oxidase A (MAOA) enzyme metabolizes monoamine neurotransmitters such as dopamine, serotonin and norepinephrine, and its genetic polymorphism (rs1137070) influences its activity level and is associated with smoking behaviors. However, the underlying neural mechanisms of the gene × environment interactions remain largely unknown. In this study, we aimed to explore the interactive effects of the rs1137070 and cigarette smoking on gray matter volume (GMV) and functional connectivity strength (FCS). A total of 81 smokers and 42 nonsmokers were enrolled in the present study. Voxel-based morphometry analysis showed a significant rs1137070 genotype × smoking effect on the GMV of the left orbitofrontal cortex (OFC), such that individuals with risk allele had greater GMV among nonsmokers but not smokers. Meanwhile, rs1137070 variant and nicotine dependence interactively altered the FCS of the right hippocampus, the left inferior parietal lobule (IPL), the left dorsolateral prefrontal cortex and bilateral OFC. In addition, the FCS in the left IPL was correlated with smoking initiation and smoking years in smokers with the risk allele. These findings suggest that MAOA rs1137070 contributes to the susceptibility to nicotine dependence through its influence on brain circuits involved in reward and attention, and interacts with smoking in the progression.
Keywords: Functional connectivity strength, Imaging genetics, Inferior parietal lobule, Nicotine dependence, Orbitofrontal cortex
Graphical Abstract
We investigated interactions between MAOA rs1137070 and smoking on the brain structure and function by using gray matter volume and functional connectivity strength measurements. Such interactions were observed on the OFC, hippocampus, DLPFC and IPL. Our findings suggest that MAOA rs1137070 contributes to the susceptibility to nicotine dependence through its influence on brain circuits involved in reward and attention, and interacts with smoking in the progression.
Introduction
Nicotine dependence (ND) is a complex disorder that develops due to multiple environmental and genetic risk factors. In this process, environmental factors contribute to the exposure and initial pattern of use, whereas genetic factors play a more salient role in the transition to problematic use (Ducci & Goldman, 2012). Twin studies suggested that the heritability estimate for smoking behaviors was about 50-70% (Goldman et al., 2005). Nicotine is the main addictive component in cigarettes that maintains smoking behaviors. According to the effects of nicotine and its metabolism in human body, a number of different genes have been reported to be related to ND, including CHRNA4, CHRNB2 in cholinergic system, DRD2 in dopaminergic system and CYP2A6 in metabolic pathway (Verde et al., 2011; Minica et al., 2017).
Besides these genes, the monoamine oxidase A (MAOA) gene is a promising candidate gene for the genetic susceptibility to ND. The MAOA gene is located on the X chromosome. It encodes MAOA, a critical enzyme involved in the degradation of biogenic amines, including neurotransmitters such as dopamine, norepinephrine and serotonin, via oxidative deamination. These neurotransmitters play an important role in arousal, emotions, mood and even affecting impulse control (Liu et al., 2016). Prior evidence suggests that MAOA gene is associated with addiction and a variety of other psychiatric disorders, including major depression disorder (Zhang et al., 2010; Liu et al., 2016) and attention deficit hyperactivity disorder (Liu et al., 2015; Ko et al., 2018), which have high rates of co-morbidity with addiction. The MAOA influences the neurobiology of anxiety, impulsivity and reward, which are involved in addiction to different agents and propensity to other psychiatric disorders (Ducci & Goldman, 2012). The activity levels of MAOA are highly variable among individuals. Lower MAOA activity leads to accumulation of these neurotransmitters in the synapses of the brain, causing persistent neuronal firing and reward effects. The long-term effects influence brain development and personality characteristics (i.e., anxiety and impulsivity) (Meyer-Lindenberg et al., 2006; Buckholtz et al., 2008), which may play an important role in the pathogenesis of addiction (Harro & Oreland, 2016). An animal study has shown that MAOA knockout mice exhibit impairments in nicotine preference but normal responses to novel stimuli (Agatsuma et al., 2006). Additionally, constituents of cigarette smoke other than nicotine inhibit MAOA activity, which contributes to the addictiveness of smoking by reducing the metabolism of dopamine (Fowler et al., 1996).
On the other hand, levels of MAOA enzyme activity are partly determined by polymorphisms of MAOA gene (Hotamisligil & Breakefield, 1991). Among these polymorphisms, rs1137070 is a C to T substitution at position 1460 of the complementary DNA sequence (c.1460C>T) in MAOA exon 14. It is a synonymous single nucleotide polymorphism (Asp470Asp); however, it does affect the restriction endonuclease site, thereby possibly regulating gene expression or protein translation (Hotamisligil & Breakefield, 1991). Prior work has shown that MAOA activity is relatively low in C allele carriers (Hotamisligil & Breakefield, 1991; Zhang et al., 2010). This genetic variation is associated with substance abuse, including alcohol, tobacco and heroin addiction (Hsu et al., 1996; Jin et al., 2006; Sun et al., 2017). Animal studies revealed that both acute and chronic MAOA inhibition resulted in decreased dopamine metabolism accompanied by an increase in dopamine levels (Brannan et al., 1995; Finberg, 2014). Thus, cigarette smoking and C allele together decrease MAOA activity and increase brain dopamine concentrations, which contribute to the addictiveness of smoking and enhance the likelihood of smoking persistence (Jin et al., 2006).
Taken together, both MAOA rs1137070 and smoking modulate the dopamine concentration in brain, which underlies the reinforcing properties of nicotine. Chronic nicotine exposure is associated with brain structural and functional alterations (Brody et al., 2004; Claus et al., 2013; Shen et al., 2016; Stoeckel et al., 2016; Sutherland et al., 2016). The susceptibility difference induced by genetic variation may also have its neural substrate, which presents before disease onset or interacts with long-term drug use. Understanding how the genetic variation influences brain in nicotine addicts may help to indicate the complex underlying neurobiology of the disorder. Neuroimaging studies have demonstrated MAOA gene × addiction interactions on brain structure and function in heroin and cocaine addicts (Alia-Klein et al., 2011; Verdejo-Garcia et al., 2013; Sun et al., 2017), consistently showing such effects on the orbital frontal cortex (OFC) rather than other areas. However, the impact of MAOA polymorphism in ND remains unexplored. Therefore, we combined gray matter volume (GMV) and functional connectivity strength (FCS) analysis to examine the interactions between the MAOA rs1137070 and cigarette smoking on brain structure and function. And we hypothesized that both GMV and FCS of the OFC are influenced by the interactions.
Materials and methods
Participants
A total of 124 healthy volunteers, including 82 smokers (aged 22-54 years) and 42 nonsmokers (aged 25-56 years) were recruited by posted flyers and online advertisements (Table 1). All participants were male, Han Chinese and right-handed. Smokers were defined as individuals who smoked more than 10 cigarettes per day in the last one year and met the DSM-IV criteria of ND as determined by structured clinical interview. While nonsmokers were defined as individuals who had smoked fewer than 20 cigarettes in their lifetime and none in the past ten years. Exclusion criteria for all participants were as follows: 1) a history of neurological or psychiatric diseases; 2) systemic diseases (i.e., diabetes, hypertension); 3) previous or current use of psychotropic medications or concurrent substance abuse, such as alcohol and heroin; 4) MRI contraindications like claustrophobia and metal implants. The screening was done through their medical records and clinical evaluations performed by an experienced psychiatrist. All of the participants were right-handed as measured by the Edinburgh Handedness Inventory. The study conformed with World Medical Association Declaration of Helsinki published on the website of the Journal of American Medical Association. All aspects of the research protocol were reviewed and approved by the Institutional Review Boards of the Second Affiliated Hospital of Zhejiang University School of Medicine. All subjects provided signed informed consents prior to study participation.
Table 1.
Characteristics of smokers and nonsmokers.
| Smoker |
Nonsmoker |
p | |||
|---|---|---|---|---|---|
| T | C | T | C | ||
| Number | 48 | 33 | 28 | 14 | 0.423 |
| Age | 37.98±7.50 | 38.21±7.07 | 38.04±8.07 | 37.57±8.75 | 0.995 |
| Education | 13.77±2.89 | 13.42±2.84 | 15.12±4.16 | 15.58±5.58 | 0.123 |
| Smoking initiation | 20.42±4.60 | 19.88±3.46 | -- | -- | 0.571 |
| Smoking years | 17.56±7.12 | 18.33±7.83 | -- | -- | 0.647 |
| Cigarettes/day | 23.46±10.47 | 23.58±11.18 | -- | -- | 0.962 |
| Pack years | 20.86±13.75 | 22.69±17.10 | -- | -- | 0.596 |
| FTND | 5.29±2.32 | 4.65±2.04 | -- | -- | 0.205 |
| FD | 0.24±0.12 | 0.22±0.11 | 0.21±0.07 | 0.19±0.09 | 0.323 |
Pack-years=cigarettes/day*smoking years/20; FD: framewise displacement (before scrubbing); FTND: Fagerström Test for Nicotine
Demographic and smoking data (i.e., smoking initiation, smoking years, cigarettes per day) were obtained from all participants by a questionnaire prior to scanning. Nicotine dependence severity was assessed using Fagerström Test for Nicotine Dependence (FTND).(Heatherton et al., 1991) Exhaled carbon monoxide was measured to confirm participants’ smoking status (smoker ≥ 10 ppm, nonsmoker ≤ 6 ppm). To reduce withdrawal effect, smokers smoked their last cigarette approximately ten minutes before scanning. A subgroup of these subjects’ imaging data has been published previously (Shen et al., 2016).
Genotyping
Genomic DNA was extracted from peripheral blood samples using QIAamp DNA Blood Mini Kit (Qiagen, Berlin, Germany). After reviewing the association between single-nucleotide polymorphism (SNP) and smoking, 48 SNPs of CHRNB2(n=1), SLC6A3(n=1), CHRNB3(n=2), BDNF(n=7), DRD2(n=3), CHRNA5(n=4), CHRNA3(n=4), CHRNB4(n=2), CYP2A6(n=6), CYP2B6(n=6), CHRNA4(n=7), COMT(n=3), MAOA(n=1) and MAOB(n=1) were selected. The SNPs were genotyped using a custom-by-design 48-Plex SNPscan™ Kit (Genesky Biotechnologies Inc., Shanghai, China) (Chen et al., 2012; Du et al., 2014). This kit was developed according to patented SNP genotyping technology which is based on double ligation and multiplex fluorescence PCR. In this study, we focus on the MAOA rs1137070.
Image acquisition
Imaging data were collected with a 3.0 T MRI scanner (GE Medical Systems, Signa EXCITE, Milwaukee, WI, United States) with a birdcage head coil. Conventional T1- and T2-weighted images were performed to rule out structural abnormalities. Resting-state functional scans consisted of 185 echo-planar imaging volumes with the following parameters: 30 slices (thickness/gap = 4/1 mm), repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, matrix = 64×64, field of view (FOV) = 240×240 mm2, flip angle = 80°. During fMRI scanning (370s), participants were instructed to lie still, keep eyes closed, and not to fall asleep. Ear plugs and foam padding were used to reduce scanner noise and head motion. After the scan, the subjects were asked whether or not they remained awake or experienced any withdrawal symptoms during the whole procedure. No one reported falling asleep or experiencing any withdrawal symptoms. Additionally, a set of high-resolution anatomical T1-wighted images were obtained using 3D fast spoiled gradient echo sequence with following parameters: 136 sagittal slices (thickness/gap = 1.2/0 mm), TR = 5.06 ms, TE = 1.12 ms, matrix = 256×256, FOV = 240×216 mm2, flip angle = 15°. All MRI data were visually inspected for image artifacts and anatomical abnormalities or pathologies by an experienced neuroradiologist.
Voxel-based morphometry (VBM) analysis
The T1-weighted images were preprocessed and analyzed with the Computational Anatomy Toolbox (CAT12, http://dbm.neuro.uni-jena.de/cat/) and Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm). The images were bias-corrected, tissue-classified (GM, white matter (WM) and cerebral spinal fluid (CSF)), and registered using linear (12 parameter affine) and non-linear transformations (warping) within the CAT12 default pre-processing pipeline. Registered images and preprocessing parameters exported were quantitatively assessed and data with weighted overall quality measure (IQR) lower than C+ were excluded from further analysis (one smoker was excluded). The remaining modulated normalized GM images were smoothed with an 8 mm full-width-at-half-maximum (FWHM) isotropic Gaussian kernel via a standard module of SPM12. The overall GM, WM, CSF volume, and total intracranial volume (TIV) were then obtained using the CAT12 estimating TIV function.
Resting-state fMRI image analysis
Resting BOLD data were preprocessed with Data Processing & Analysis for Brain Imaging (DPABI v3.0, http://rfmri.org/dpabi). The first ten volumes were discarded to reduce magnetization disequilibrium, followed by slice-timing correction and head motion correction. Exclusion criteria on head motion was exceeding more than 2 mm/degree (none was excluded). After segmentation of T1 images, resting images were co-registered to T1 images and then registered to the standard Montreal Neurological institute template and resampled into 3×3×3 mm3 cubic voxels. Finally, linear detrending and temporal band-pass filtering (0.01-0.08 Hz) were performed to remove low- and high-frequency noise. To remove any residual effects of motion and other non-neuronal factors, nuisance covariate regression was performed using the Friston-24 head motion parameters (six head motion parameters, six head motion parameters from the previous time point, and the 12 corresponding squared items), as well as signals of WM and CSF. We also calculated the mean framewise displacement (FD) (Power et al., 2012) of each participant. Given the potential for spurious signal changes from head micromovements, we removed frames with FD>0.5mm (‘scrubbing’) (Power et al., 2014).
FCS, a data-driven graph theoretical approach, is measured with degree centrality of the weighted functional network at the voxel level (Wang et al., 2015). In the present study, whole-brain functional connectivity analysis was performed as follows. First, Pearson’s correlations between the time series of all pairs of voxels were computed to construct a whole-brain connectivity matrix for each participant. This computation was constrained within a GM mask that was generated by setting a threshold of 0.2 on the mean map of all GM maps involving all subjects. To improve normality, individual correlation matrices were transformed to a z-score matrix using a Fisher r-to-z transformation. For a given voxel, FCS was computed as the sum of its connections (z-values) with all other voxels. This computation was conservatively restricted to positive correlations above a threshold of r = 0.25. Such a threshold was chosen to eliminate the weak correlations possibly arising from signal noise (Buckner et al., 2009). The FCS maps were further smoothed with a 6-mm FWHM Gaussian kernel.
Statistical analysis
Group differences in baseline demographic and smoking characteristics were analyzed using SPSS 19.0. Genotype frequency comparison between smokers and nonsmokers was evaluated by chi-square test. Age, years of education and FD were compared between smokers and nonsmokers using one-way analysis of variance. Smoking data were compared between smokers with different genotypes using independent-sample t tests. All tests were two-tailed and results were considered significant at p<0.05.
The interactions between rs1137070 and smoking on GMV were assessed with a 2×2 full factorial design, with group (smoker vs. nonsmoker) and genotype (C vs. T) as between-participant factors. Age, years of education and TIV were employed as covariates. The threshold was set at an uncorrected p<0.001 at a voxel level combined with a minimum cluster extent of 100 voxels. The mean GMV in regions showing significant genotype × smoking interactions was extracted, and its associations with smoking behavior data (i.e., smoking initiation, smoking years, cigarettes per day, pack-years and FTND scores) were tested using Pearson’s correlation analyses.
The interactions between rs1137070 and smoking on FCS were assessed with a 2×2 full factorial design, with group (smoker vs. nonsmoker) and genotype (C vs. T) as between-participant factors. Age, years of education and TIV were employed as covariates. A voxel-wise threshold of p<0.01 and a spatial extent of 45 contiguous voxels were determined based on Gaussian random field theory to provide a corrected threshold of p<0.05. The mean FCS in regions showing significant genotype × smoking interactions was extracted, and its associations with smoking behavior data (i.e., smoking initiation, smoking years, cigarettes per day, pack-years and FTND scores) were tested using Pearson’s correlation analyses.
Results
Participant characteristics
Finally, 81 smokers and 42 nonsmokers were enrolled in the present study. Based on the genotyping results, 47 participants were classified as having the C allele (33 smokers and 14 nonsmokers) and 76 as having the T allele (48 smokers and 28 nonsmokers). There was no significant difference in the frequency distribution of the rs1137070 genotype between smokers and nonsmokers (χ2=0.643, df=1, p=0.423), probably due to insufficient statistical power of the small sample. As shown in Table 1, there was no significant difference in age, education and FD between smokers and nonsmokers (p>0.05). The two smoker subgroups did not differ with regard to smoking initiation, smoking years, pack-years, cigarettes per day or FTND scores (p>0.05).
GMV results
The rs1137070 variant interacted with smoking status to affect GMV in the left medial OFC (264voxels; peak at X, Y, Z =−4.5, 66, −16.5; F=19.49; p<0.001). The C allele and T allele differentially affected the region in smokers and nonsmokers. Nonsmokers with the C allele had higher measures of GMV than those with the T allele, whereas smokers with the different genotypes displayed a similar GMV (Fig. 1). Correlation analysis showed that the GMV in the left medial OFC did not correlate with any measure of cigarette use.
Fig.1.
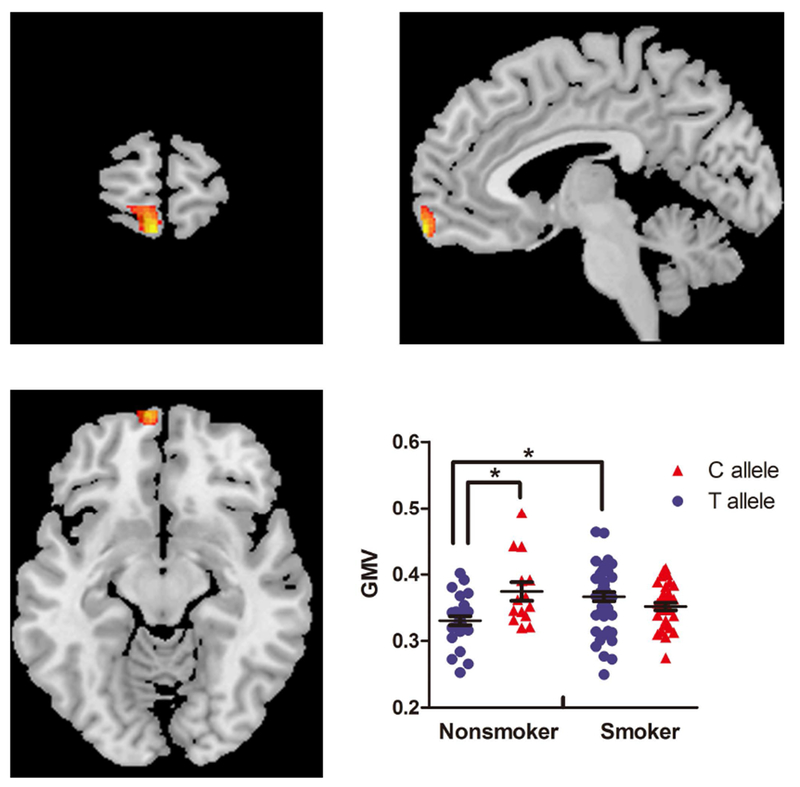
Interactions between MAOA rs1137070 and smoking on GMV of the left OFC (X=−4.5, Y=66, Z=−16.5; 264voxels). Scatter plot demonstrates the influence of MAOA rs1137070 genotype and smoking on OFC GMV. *p< 0.05; GMV, gray matter volume; OFC, orbitofrontal cortex.
FCS results
The full factorial models revealed significant group × gene interaction effects in the right hippocampus, left inferior parietal lobule (IPL), left dorsolateral prefrontal cortex (DLPFC) and bilateral OFC. The patterns of the effects varied from region to region. Compared with C allele carriers, T allele carriers presented a higher FCS in smokers, but a lower FCS in nonsmokers in the right hippocampus. An opposite pattern was presented in the left DLPFC. Nonsmokers with C allele showed a lower FCS than the other three groups in the left IPL, while an opposite pattern was presented in the bilateral OFC (Fig. 2; Table 2). Correlation analysis showed that FCS in the left IPL was positively correlated with smoking initiation (r=0.556, p=0.001) and negatively correlated with smoking years (r=−0.425, p=0.014) in C allele carriers (Fig. 3). To determine the brain circuits contributing to the above genotype × smoking interaction on FCS, we next used the identified OFC, DLPFC, hippocampus and IPL regions as seeds in a functional connectivity analysis (see Supplementary Materials).
Fig.2.
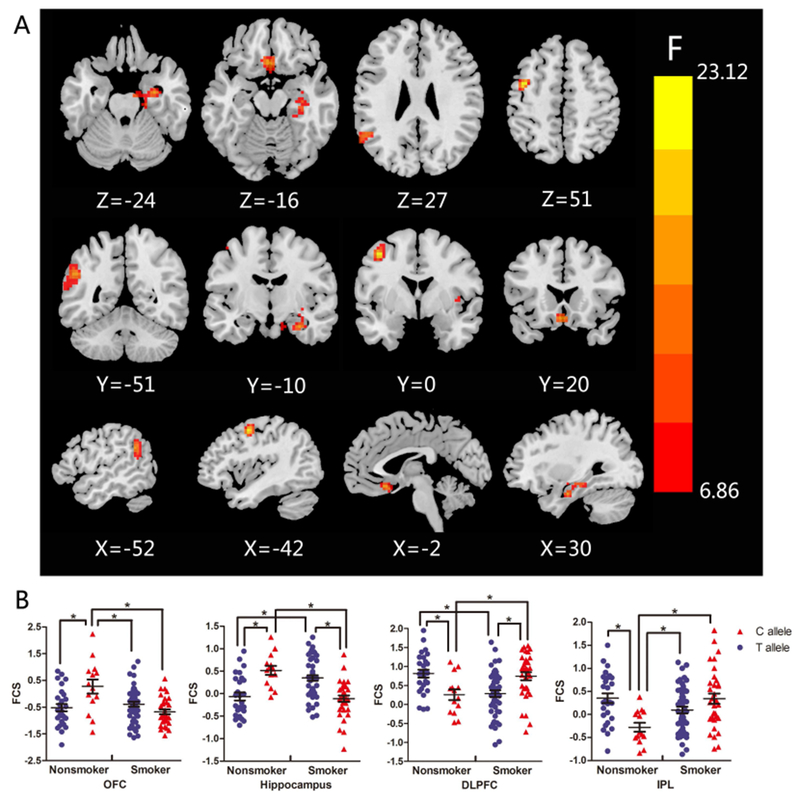
Interactions between MAOA rs1137070 and smoking on FCS. (A) Brain areas with statistical significance were the left DLPFC, left IPL, right hippocampus and bilateral OFC. (B) Scatter plots demonstrate the influence of MAOA rs1137070 genotype and smoking on the FCS of DLPFC, IPL, hippocampus and OFC. *p< 0.05; DLPFC, dorsolateral prefrontal cortex; FCS, functional connectivity strength; IPL, inferior parietal lobule; OFC, orbitofrontal cortex.
Table 2.
Clusters interactively modulated by MAOA rs1137070 and smoking.
| Brain Region | L/R | MNI coordinate |
Cluster Size | F | p | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Hippocampus | R | 30 | −9 | −24 | 112 | 15.79 | <0.001 |
| OFC | Bilateral | 0 | 21 | −15 | 48 | 16.72 | <0.001 |
| IPL | L | −51 | 51 | 30 | 98 | 15.79 | <0.001 |
| DLPFC | L | −42 | 0 | 51 | 58 | 23.12 | <0.001 |
DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule; OFC, orbitofrontal cortex
Fig.3.
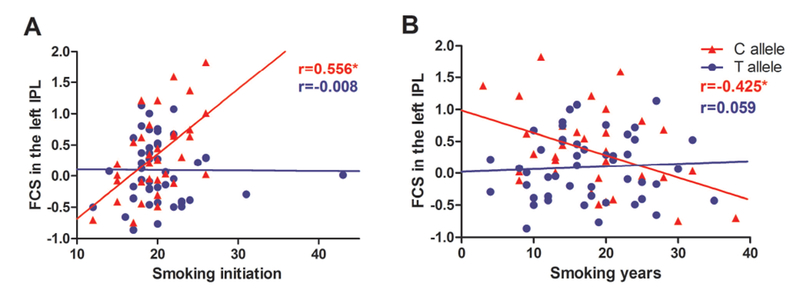
Scatter plots showing the correlation between the FCS of the left IPL and smoking data. (A) The FCS in the left IPL is positively correlated with smoking initiation in smokers with C allele (r=0.556, p=0.001). (B) The FCS in the left IPL is negatively correlated with smoking years in smokers with C allele (r=−0.425, p=0.014). FCS, functional connectivity strength; IPL, inferior parietal lobule.
Discussion
In the current study, we investigated the interactions between MAOA rs1137070 and smoking behaviors on brain structure and function. Consistent with our hypothesis, the results showed that a polymorphism (rs1137070) in MAOA, previously reported to be associated with ND, influenced both the GMV and FCS in the medial OFC, though there was no overlap of the two areas. Additionally, the interactions were observed on the FCS in the right hippocampus, left DLPFC and left IPL. Furthermore, the FCS in the left IPL was positively correlated with smoking initiation and negatively correlated with smoking years in smokers with C allele. To the best of our knowledge, this study provides the first evidence of MAOA rs1137070-by-smoking interactions and reveals the role of the candidate gene in the neurobiology of ND.
Gene × smoking interactions on the GMV and FCS of OFC
VBM has been widely used to detect the anatomical abnormalities in ND for two decades, while the results vary from study to study (Brody et al., 2004; Gallinat et al., 2006; Yu et al., 2011; Zhang et al., 2011; Liao et al., 2012; Franklin et al., 2014; Fritz et al., 2014; Wetherill et al., 2015; Hanlon et al., 2016; Stoeckel et al., 2016). Our finding, the MAOA rs1137070-smoking interplay in the GMV of the left OFC, suggested that the inconsistency might in part be attributable to genetic variations. Similar to our finding, prior studies also indicated increased OFC volume in subjects with low MAOA gene activity (Meyer-Lindenberg et al., 2006; Cerasa et al., 2008).
Besides the structural evidence, the present study also provided functional evidence of the rs1137070-by-smoking interactions on the OFC. As a hub of reward system, the OFC is involved in the whole cycle of addiction (preoccupation/anticipation, binge/intoxication, and withdrawal/negative affect), and abnormalities of OFC have been frequently implicated in ND (Goldstein & Volkow, 2002; Volkow & Baler, 2014; Herman & Roberto, 2015). Structural MRI studies commonly demonstrated GM reduction in the region in smokers (Gallinat et al., 2006; Kuhn et al., 2010; Morales et al., 2012; Franklin et al., 2014), which was correlated with lifetime nicotine exposure (Kuhn et al., 2010). Anatomically, the OFC is connected with other prefrontal areas and the mesolimbic dopamine system, and is critical for the rewarding effects of drugs (Koob & Bloom, 1988; Ongur & Price, 2000). Animal studies suggested that the OFC is involved in making stimulus-reward associations and the reinforcement of behavior (Walton et al., 2010). In human, functional MRI studies demonstrated activation of OFC together with other limbic areas when smokers were exposed to smoking-related cues (David et al., 2005; McClernon et al., 2008; Buhler et al., 2010; Kang et al., 2012), which was positively correlated with cue-induced craving (Kang et al., 2012). Meanwhile, OFC is involved in high-order cognitive functions, including inhibitory control, decision-making and working memory, which are affected by ND (Spinella, 2002). Our previous resting-state fMRI study also reported dysfunction in the OFC-amygdala pathway in smokers (Shen et al., 2017). As such, the OFC is the most susceptible to ND, due primarily to its role in nicotine reinforcement and motivation through the actions of dopamine induced by nicotine use. On the other hand, there is a high expression of MAOA protein in the OFC (Fowler et al., 1987), and MAOA activity levels were variable among humans in the frontal region (Balciuniene et al., 2002), partly due to DNA variants like rs1137070. Given the role of MAOA in degrading dopamine, its activity impacts the concentration of the neurotransmitter dopamine in the brain and hence the nicotine effects. Both structural and functional evidence have highlighted the influence of MAOA genotype on the OFC (Meyer-Lindenberg et al., 2006; Cerasa et al., 2008; Cerasa et al., 2010; Verdejo-Garcia et al., 2013). Taken together, both smoking and MAOA genotype affects the orbitofrontal structure and function. Thus, it is not surprising that we found the interactions between the two factors on the GMV and FCS in the OFC, which may be a reflection of the interactions on neurotransmitter dopamine.
MAOA × addiction interactions on the GMV of OFC were also observed in cocaine and heroin addicts with a similar pattern (Alia-Klein et al., 2011; Sun et al., 2017), in which decreased MAOA activity is associated with increased GMV in the OFC in healthy controls (Meyer-Lindenberg et al., 2006; Cerasa et al., 2008) but interacts with drug abuse to show an opposite trend. The phenomenon is specific to male, and the mechanism remains unknown. As a neurotransmitter, dopamine released in the brain plays an important role in brain development. MAOA modulates the levels of dopamine and hence leads to personality characteristics associated with drug abuse, which is reflected in the brain structure. Therefore, the modulating effect of the MAOA genotype on structural variability may start during early brain development, before addiction onset, and possibly turn to another direction by interplaying with drug abuse. Combining our findings with those of previous studies, we propose that the MAOA-by-environment interactions on the OFC indicate a shared mechanism for modulating drug reinforcement, which is responsible for the susceptibility to drug abuse. Notably, MAOA showed much more extensive effects on brain areas associated with cognitive control, such as ACC, insula, hippocampus, DLPFC and temporal cortex, in cocaine and heroin addicts (Alia-Klein et al., 2011; Sun et al., 2017). The discrepancy may result from the different addictive drugs. Recent studies indicated that some components of tobacco smoke (not nicotine) can inhibit MAOA and thus potentiate nicotine’s effects(Talhout et al., 2007; Leroy et al., 2009). Therefore, both genetic polymorphisms and some inhibitory compounds in tobacco reduce MAOA activity and increase dopamine level, resulting in enhanced addictive potential of tobacco.
Gene × smoking interactions on the FCS of DLPFC and hippocampus
The MAOA rs1137070-by-smoking interactions were also observed in the FCS of the left DLPFC and right hippocampus, regions also implicated in cocaine addicts with MAOA variants (Alia-Klein et al., 2011). Both animal and human studies have reported structural and functional connection between OFC and DLPFC (Carmichael & Price, 1995; Petrides & Pandya, 1999; Cavada et al., 2000). A prior study revealed DLPFC and OFC interactions during self-control of cigarette craving, which proposed a neuropsychological model that drug cue-induced craving in the OFC was modulated by context evaluation in the DLPFC (Hayashi et al., 2013). Through this process, the two regions may guide goal-directed behavior, cigarette smoking. The OFC also has direct reciprocal connections with hippocampus, which together may be the neural substrates underlying the learning and retrieval of drug-environment associations (Cavada et al., 2000; Kringelbach, 2005; Ross et al., 2011; McClernon et al., 2016). Thus, both neural pathways play a role in the transition from recreational use to a compulsive nicotine-use pattern. As MAOA rs1137070 interacted with smoking on the OFC, it may further alter the DLPFC and hippocampus regions through its connectivity.
On the other hand, our results revealed rs1137070 × smoking interactions on the FCS of four different brain regions, including the hippocampus, IPL, DLPFC and OFC. However, the patterns varied from region to region. This discrepancy may be due to the modulation by smokers’ status (satiated or abstinent). The MRI images in the present study were acquired in satiated state. As mentioned in our prior paper (Shen et al., 2016), acute nicotine effects on functional circuits may be a function of circuits, resulting in decreased functional connectivity, increased functional connectivity or no effect (Hong et al., 2009; Sutherland et al., 2013; Fedota et al., 2018). As such, it is possible that the four different functional circuits have their own FCS patterns. However, we just examined FCS in satiated state. Future studies examining FCS both in satiated and abstinent state are needed to validate our speculation.
Gene × smoking interactions on the FCS of IPL
In addition, the FCS of the left IPL was modulated by the MAOA rs1137070 × smoking interactions and correlated with smoking initiation and duration in smokers with risk allele. The IPL, as well as the hippocampus, is an important component of the default mode network implicated in working memory, attention bias and inhibition control, which has been reported to be associated with ND (Jacobsen et al., 2007; Luijten et al., 2011; de Ruiter et al., 2012). Meanwhile, the IPL, along with the DLPFC, forms a part of attention network, receives visual, auditory and somatosensory inputs from adjacent regions and integrates the modalities to reorient attention and detect target (Andersen, 2011; Igelstrom & Graziano, 2017). Its involvement in bottom-up sensory processing extracts information from environmental factors, which may interact with gene. Previous studies demonstrated smoking cue induced activation in the IPL (Engelmann et al., 2012; Yalachkov et al., 2013), which correlated with dependence severity (Yalachkov et al., 2013). A recent study indicated that reduced regional cerebral blood flow in the IPL predicted the onset of alcohol use and future dinking patterns (Ramage et al., 2015). Combining these findings, the IPL-related networks reflected the MAOA gene × environment interactions, suggesting that its integrity in the involvement of behavior traits was related to the predisposition for ND initiation, as well as its progression.
The longer smoking, the lower FCS in the left IPL. However, when combined with the relatively higher FCS in smokers than nonsmokers with C allele, the results seem a bit counter-intuitive. One possibility is that the sample size of nonsmokers with C allele is relatively small, which may not represent the FCS value distribution of this population. Another possibility is attributed to the cross-sectional design in this study. The value of FCS may change with different stages of ND and is affected by smokers’ smoking status. Although the sequential nature of the smoking years suggests a progressive FCS alterations in smokers, our one time point data may result in the correlation by accident. This simple mathematical correlation cannot reflect the complex process in ND. Therefore, future studies with large population and longitudinal design are needed to see if the results are reproducible.
Limitations
We have to admit that the liberal threshold is a weakness of our study. Once the paper (Eklund et al., 2016) published, the issue (the threshold of multiple comparison correction) has been widely discussed. The authors found that liberal threshold resulted in high false positive, so they recommended to use stringent correction to reduce false positive. However, this brings another problem, i.e., high false negative. How to balance the type I and type II error is a big issue in the field. Recently, a study (Jia et al., 2018) used the meta-analysis results as the robust results and found that the between-group design results of each original study showed high false negative rates (median 99%), high false discovery rates (median 86%) and low accuracy (median 1%), regardless of whether stringent or liberal multiple comparison correction was used. These observations suggest that multiple comparison correction does not control for false discoveries across multiple studies when the effect sizes are relatively small. In our study, although we used liberal threshold, the brain areas with significance, especially OFC, were also reported in drug addicts in prior studies, which might indicate a real effect.
Other limitations also should be noticed. First, because the vast majority of smokers in China are male (Chen et al., 2015) and there are interactions between MAOA and gender (Buckholtz et al., 2008; Harro & Oreland, 2016), only male participates were included in our study. Therefore, the findings may not be extended in female subjects. Second, because of the cross-sectional design, we were unable to determine the casual relationship between ND and brain abnormalities. Third, as a preliminary study with small sample size, we did not detect significant differences in the allele distribution of MAOA rs1137070 between smokers and nonsmokers. Further, ND is a complex syndrome, not attributable to a single genotype variant, and influenced by multiple genetic and environmental factors. Due to the small sample size, we did not take other factors into consideration. Future studies with large population should be conducted to validate the results.
Conclusion
In the present study, we found that the polymorphism of MAOA (rs1137070) played a moderating role in the effect of smoking on the OFC, DLPFC, IPL and hippocampus. In addition, the gene × environment interactions in the IPL was associated with smoking initiation and progression only among participants with C genotype of MAOA. This result sheds insight on the neural mechanism for the association between ND and the MAOA polymorphism.
Supplementary Material
Acknowledgements
This research was supported by grants from National Natural Science Foundation of China (81171310, 81701647), Zhejiang Provincial Natural Science Foundation (LQ18H180001), Medical and Health Scientific Research Fund Project of Zhejiang Province (2017KY080), Scientific Research Project of Zhejiang Province (2011C23094) and FCTC special project of the State Bureau (552016CQ0030). YY is supported by the Intramural Research Program of the National Institute on Drug Abuse, the National Institutes of Health.
Abbreviations
- CSF
cerebral spinal fluid
- DLPFC
dorsolateral prefrontal cortex
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders
- FCS
functional connectivity strength
- FD
framewise displacement
- fMRI
functional magnetic resonance imaging
- FTND
Fagerström Test for Nicotine Dependence
- FOV
field of region
- FWHM
full-width half-maximum
- GMV
gray matter volume
- IPL
inferior parietal lobule
- IQR
image and preprocessing quality
- MAOA
monoamine oxidase A
- MRI
magnetic resonance imaging
- ND
nicotine dependence
- OFC
orbitofrontal cortex
- TE
echo time
- TIV
total intracranial volume
- TR
repetition time
- WM
white matter
- VBM
voxel-based morphometry.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
Data Accessibility
This study is part work of ongoing research designed to investigate the influence of gene on brain structure and function in smokers. The supporting data will be available upon request by mailing to corresponding author (Minming Zhang).
References
- Agatsuma S, Lee M, Zhu H, Chen K, Shih JC, Seif I & Hiroi N (2006) Monoamine oxidase A knockout mice exhibit impaired nicotine preference but normal responses to novel stimuli. Hum Mol Genet, 15, 2721–2731. [DOI] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND & Goldstein RZ (2011) Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry, 68, 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RA (2011) Inferior Parietal Lobule Function in Spatial Perception and Visuomotor Integration. Comprehensive Physiology, 483–518. [Google Scholar]
- Balciuniene J, Emilsson L, Oreland L, Pettersson U & Jazin E (2002) Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet, 110, 1–7. [DOI] [PubMed] [Google Scholar]
- Brannan T, Prikhojan A, Martinez-Tica J & Yahr MD (1995) In vivo comparison of the effects of inhibition of MAO-A versus MAO-B on striatal L-DOPA and dopamine metabolism. J Neural Transm Park Dis Dement Sect, 10, 79–89. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G & London ED (2004) Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry, 55, 77–84. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, Egan MF, Mattay VS, Weinberger DR & Meyer-Lindenberg A (2008) Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol Psychiatry, 13, 313–324. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA & Johnson KA (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci, 29, 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Buchel C & Smolka MN (2010) Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry, 67, 745–752. [DOI] [PubMed] [Google Scholar]
- Carmichael ST & Price JL (1995) Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol, 363, 642–664. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ & Reinoso-Suarez F (2000) The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex, 10, 220–242. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Cherubini A, Quattrone A, Gioia MC, Magariello A, Muglia M, Manna I, Assogna F, Caltagirone C & Spalletta G (2010) Morphological correlates of MAO A VNTR polymorphism: new evidence from cortical thickness measurement. Behav Brain Res, 211, 118–124. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Labate A, Lanza P, Magariello A, Muglia M & Quattrone A (2008) MAO A VNTR polymorphism and variation in human morphology: a VBM study. Neuroreport, 19, 1107–1110. [DOI] [PubMed] [Google Scholar]
- Chen X, Li S, Yang Y, Yang X, Liu Y, Liu Y, Hu W, Jin L & Wang X (2012) Genome-wide association study validation identifies novel loci for atherosclerotic cardiovascular disease. J Thromb Haemost, 10, 1508–1514. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peto R, Zhou M, Iona A, Smith M, Yang L, Guo Y, Chen Y, Bian Z, Lancaster G, Sherliker P, Pang S, Wang H, Su H, Wu M, Wu X, Chen J, Collins R & Li L (2015) Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet, 386, 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, Mayer AR & Hutchison KE (2013) Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology, 38, 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM & Walton RT (2005) Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry, 58, 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Oosterlaan J, Veltman DJ, van den Brink W & Goudriaan AE (2012) Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depend, 121, 81–89. [DOI] [PubMed] [Google Scholar]
- Du W, Cheng J, Ding H, Jiang Z, Guo Y & Yuan H (2014) A rapid method for simultaneous multi-gene mutation screening in children with nonsyndromic hearing loss. Genomics, 104, 264–270. [DOI] [PubMed] [Google Scholar]
- Ducci F & Goldman D (2012) The genetic basis of addictive disorders. Psychiatr Clin North Am, 35, 495–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE & Knutsson H (2016) Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A, 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL & Cinciripini PM (2012) Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage, 60, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Ding X, Matous AL, Salmeron BJ, McKenna MR, Gu H, Ross TJ & Stein EA (2018) Nicotine Abstinence Influences the Calculation of Salience in Discrete Insular Circuits. Biol Psychiatry Cogn Neurosci Neuroimaging, 3, 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finberg JP (2014) Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: focus on modulation of CNS monoamine neurotransmitter release. Pharmacol Ther, 143, 133–152. [DOI] [PubMed] [Google Scholar]
- Fowler JS, MacGregor RR, Wolf AP, Arnett CD, Dewey SL, Schlyer D, Christman D, Logan J, Smith M, Sachs H & et al. (1987) Mapping human brain monoamine oxidase A and B with 11C-labeled suicide inactivators and PET. Science, 235, 481–485. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I & Wolf AP (1996) Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A, 93, 14065–14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H & Childress AR (2014) The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One, 9, e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, Hosten N & Lotze M (2014) Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology, 39, 2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F & Staedtgen M (2006) Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci, 24, 1744–1750. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G & Ducci F (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet, 6, 521–532. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ & Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry, 159, 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT & Hartwell KJ (2016) Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol, 21, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro J & Oreland L (2016) The role of MAO in personality and drug use. Prog Neuropsychopharmacol Biol Psychiatry, 69, 101–111. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ko JH, Strafella AP & Dagher A (2013) Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci U S A, 110, 4422–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC & Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Herman MA & Roberto M (2015) The addicted brain: understanding the neurophysiological mechanisms of addictive disorders. Front Integr Neurosci, 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK & Stein EA (2009) Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry, 66, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS & Breakefield XO (1991) Human monoamine oxidase A gene determines levels of enzyme activity. Am J Hum Genet, 49, 383–392. [PMC free article] [PubMed] [Google Scholar]
- Hsu YP, Loh EW, Chen WJ, Chen CC, Yu JM & Cheng AT (1996) Association of monoamine oxidase A alleles with alcoholism among male Chinese in Taiwan. Am J Psychiatry, 153, 1209–1211. [DOI] [PubMed] [Google Scholar]
- Igelstrom KM & Graziano MSA (2017) The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia, 105, 70–83. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M & Pugh KR (2007) Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl), 193, 557–566. [DOI] [PubMed] [Google Scholar]
- Jia X-Z, Zhao N, Barton B, Burciu R, Carriere N, Cerasa A, Chen B-Y, Chen J, Coombes S, Defebvre L, Delmaire C, Dujardin K, Esposito F, Fan G-G, Federica DN, Feng Y-X, Fling BW, Garg S, Gilat M, Gorges M, Ho S-L, Horak FB, Hu X, Hu X-F, Huang B, Huang P-Y, Jia Z-J, Jones C, Kassubek J, Krajcovicova L, Kurani A, Li J, Li Q, Liu A-P, Liu B, Liu H, Liu W-G, Lopes R, Lou Y-T, Luo W, Madhyastha T, Mao N-N, McAlonan G, McKeown MJ, Pang SY, Quattrone A, Rektorova I, Sarica A, Shang H-F, Shine J, Shukla P, Slavicek T, Song X-P, Tedeschi G, Tessitore A, Vaillancourt D, Wang J, Wang J, Wang ZJ, Wei L-Q, Wu X, Xu X-J, Yan L, Yang J, Yang W-Q, Yao N-L, Zhang D-L, Zhang J-Q, Zhang M-M, Zhang Y-L, Zhou C-H, Yan C-G, Zuo X-N, Hallett M, Wu T & Zang Y-F (2018) Small effect size leads to reproducibility failure in resting-state fMRI studies. bioRxiv. [Google Scholar]
- Jin Y, Chen D, Hu Y, Guo S, Sun H, Lu A, Zhang X & Li L (2006) Association between monoamine oxidase gene polymorphisms and smoking behaviour in Chinese males. Int J Neuropsychopharmacol, 9, 557–564. [DOI] [PubMed] [Google Scholar]
- Kang OS, Chang DS, Jahng GH, Kim SY, Kim H, Kim JW, Chung SY, Yang SI, Park HJ, Lee H & Chae Y (2012) Individual differences in smoking-related cue reactivity in smokers: an eye-tracking and fMRI study. Prog Neuropsychopharmacol Biol Psychiatry, 38, 285–293. [DOI] [PubMed] [Google Scholar]
- Ko CH, Hsieh TJ, Wang PW, Lin WC, Chen CS & Yen JY (2018) The Altered Brain Activation of Phonological Working Memory, Dual Tasking, and Distraction Among Participants With Adult ADHD and the Effect of the MAOA Polymorphism. J Atten Disord, 22, 240–249. [DOI] [PubMed] [Google Scholar]
- Koob GF & Bloom FE (1988) Cellular and molecular mechanisms of drug dependence. Science, 242, 715–723. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci, 6, 691–702. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Schubert F & Gallinat J (2010) Reduced thickness of medial orbitofrontal cortex in smokers. Biol Psychiatry, 68, 1061–1065. [DOI] [PubMed] [Google Scholar]
- Leroy C, Bragulat V, Berlin I, Gregoire MC, Bottlaender M, Roumenov D, Dolle F, Bourgeois S, Penttila J, Artiges E, Martinot JL & Trichard C (2009) Cerebral monoamine oxidase A inhibition in tobacco smokers confirmed with PET and [11C]befloxatone. J Clin Psychopharmacol, 29, 86–88. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, Chen X & Hao W (2012) Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol, 17, 977–980. [DOI] [PubMed] [Google Scholar]
- Liu L, Cheng J, Su Y, Ji N, Gao Q, Li H, Yang L, Sun L, Qian Q & Wang Y (2015) Deficiency of Sustained Attention in ADHD and Its Potential Genetic Contributor MAOA. J Atten Disord, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Liu Z, Huang L, Luo XJ, Wu L & Li M (2016) MAOA Variants and Genetic Susceptibility to Major Psychiatric Disorders. Mol Neurobiol, 53, 4319–4327. [DOI] [PubMed] [Google Scholar]
- Luijten M, Veltman DJ, van den Brink W, Hester R, Field M, Smits M & Franken IH (2011) Neurobiological substrate of smoking-related attentional bias. Neuroimage, 54, 2374–2381. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Conklin CA, Kozink RV, Adcock RA, Sweitzer MM, Addicott MA, Chou YH, Chen NK, Hallyburton MB & DeVito AM (2016) Hippocampal and Insular Response to Smoking-Related Environments: Neuroimaging Evidence for Drug-Context Effects in Nicotine Dependence. Neuropsychopharmacology, 41, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV & Rose JE (2008) Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology, 33, 2148–2157. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B,A,RH, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V & Weinberger DR (2006) Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A, 103, 6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minica CC, Mbarek H, Pool R, Dolan CV, Boomsma DI & Vink JM (2017) Pathways to smoking behaviours: biological insights from the Tobacco and Genetics Consortium meta-analysis. Mol Psychiatry, 22, 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O’Neill J & London ED (2012) Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend, 125, 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D & Price JL (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex, 10, 206–219. [DOI] [PubMed] [Google Scholar]
- Petrides M & Pandya DN (1999) Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci, 11, 1011–1036. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL & Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL & Petersen SE (2014) Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage, 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AE, Lin AL, Olvera RL, Fox PT & Williamson DE (2015) Resting-state regional cerebral blood flow during adolescence: associations with initiation of substance use and prediction of future use disorders. Drug Alcohol Depend, 149, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RS, Sherrill KR & Stern CE (2011) The hippocampus is functionally connected to the striatum and orbitofrontal cortex during context dependent decision making. Brain Res, 1423, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z, Huang P, Qian W, Wang C, Yu H, Yang Y & Zhang M (2016) Severity of dependence modulates smokers’ functional connectivity in the reward circuit: a preliminary study. Psychopharmacology (Berl), 233, 2129–2137. [DOI] [PubMed] [Google Scholar]
- Shen Z, Huang P, Wang C, Qian W, Luo X, Guan X, Qiu T, Yang Y & Zhang M (2017) Altered function but not structure of the amygdala in nicotine-dependent individuals. Neuropsychologia, 107, 102–107. [DOI] [PubMed] [Google Scholar]
- Spinella M (2002) Correlations between orbitofrontal dysfunction and tobacco smoking. Addict Biol, 7, 381–384. [DOI] [PubMed] [Google Scholar]
- Stoeckel LE, Chai XJ, Zhang J, Whitfield-Gabrieli S & Evins AE (2016) Lower gray matter density and functional connectivity in the anterior insula in smokers compared with never smokers. Addict Biol, 21, 972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu L, Feng J, Yue W, Lu L, Fan Y & Shi J (2017) MAOA rs1137070 and heroin addiction interactively alter gray matter volume of the salience network. Sci Rep, 7, 45321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE & Stein EA (2013) Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry, 74, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA & Laird AR (2016) Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct, 12, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhout R, Opperhuizen A & van Amsterdam JG (2007) Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacol, 17, 627–636. [DOI] [PubMed] [Google Scholar]
- Verde Z, Santiago C, Rodriguez Gonzalez-Moro JM, de Lucas Ramos P, Lopez Martin S, Bandres F, Lucia A & Gomez-Gallego F (2011) ‘Smoking genes’: a genetic association study. PLoS One, 6, e26668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Albein-Urios N, Molina E, Ching-Lopez A, Martinez-Gonzalez JM & Gutierrez B (2013) A MAOA gene*cocaine severity interaction on impulsivity and neuropsychological measures of orbitofrontal dysfunction: preliminary results. Drug Alcohol Depend, 133, 287–290. [DOI] [PubMed] [Google Scholar]
- Volkow ND & Baler RD (2014) Addiction science: Uncovering neurobiological complexity. Neuropharmacology, 76 Pt B, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Buckley MJ, Rudebeck PH & Rushworth MF (2010) Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning. Neuron, 65, 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xia M, Li K, Zeng Y, Su Y, Dai W, Zhang Q, Jin Z, Mitchell PB, Yu X, He Y & Si T (2015) The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum Brain Mapp, 36, 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H & Franklin TR (2015) Cannabis, Cigarettes, and Their Co-Occurring Use: Disentangling Differences in Gray Matter Volume. Int J Neuropsychopharmacol, 18, pyv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Gorres A, Seehaus A & Naumer MJ (2013) Sensory modality of smoking cues modulates neural cue reactivity. Psychopharmacology (Berl), 225, 461–471. [DOI] [PubMed] [Google Scholar]
- Yu R, Zhao L & Lu L (2011) Regional grey and white matter changes in heavy male smokers. PLoS One, 6, e27440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen Y, Zhang K, Yang H, Sun Y, Fang Y, Shen Y & Xu Q (2010) A cis-phase interaction study of genetic variants within the MAOA gene in major depressive disorder. Biol Psychiatry, 68, 795–800. [DOI] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y & Stein EA (2011) Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage, 54, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


