Abstract
Objectives
To evaluate the concordance between Google Maps® application (GM®) and clinical practice measurements of ambulatory function (e.g., Ambulation Score (AS) and respective Expanded Disability Status Scale (EDSS)) in people with multiple sclerosis (pwMS).
Materials and methods
This is a cross-sectional multicenter study. AS and EDSS were calculated using GM® and routine clinical methods; the correspondence between the two methods was assessed. A multinomial logistic model is investigated which demographic (age, sex) and clinical features (e.g., disease subtype, fatigue, depression) might have influenced discrepancies between the two methods.
Results
Two hundred forty-three pwMS were included; discrepancies in AS and in EDDS assessments between GM® and routine clinical methods were found in 81/243 (33.3%) and 74/243 (30.4%) pwMS, respectively. Progressive phenotype (odds ratio [OR] = 2.8; 95% confidence interval [CI] 1.1–7.11, p = 0.03), worse fatigue (OR = 1.03; 95% CI 1.01–1.06, p = 0.01), and more severe depression (OR = 1.1; 95% CI 1.04–1.17, p = 0.002) were associated with discrepancies between GM® and routine clinical scoring.
Conclusion
GM® could easily be used in a real-life clinical setting to calculate the AS and the related EDSS scores. GM® should be considered for validation in further clinical studies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-021-05389-7.
Keywords: Digital health, e-Health, Google Maps, Ambulatory disorders
Introduction
In response to the coronavirus disease 2019 pandemic, many hospitals have implemented restriction policies to hospitals access [1]. These policies, as well as, social distancing, limitations for caregivers’ access to outpatient clinic, mobility restrictions measures, fear of contagion, and quarantining of health-care providers, have drastically reduced physician in-person visits during the pandemic [1]
This unprecedented situation has raised the need to establish an efficient service of telemedicine to replace face-to-face visits, monitor disease progression, and manage complications as soon as possible [2, 3]
Recently, the reliability of telemedicine tools as alternative to EDSS quantified through clinical in-person visits has been explored in several studies [2, 4, 5]. Bove and colleagues generated a telemedicine-based MS disability examination using a digital tool and a basic neuro kit and tested it against an EDSS assessment performed by a neurologist [4]. The authors showed that disability evaluation in MS is feasible using telemedicine without an aid at the patient’s location [4]. Further, a pilot study evaluated the reliability of a wearable biosensor to assess pwMS walking ability remotely, revealing that motor parameters derived from the accelerometer could be a reliable measure of motor disability in pwMS, suggesting it could be a useful tool to monitor MS patients’ endurance remotely [5].
Evaluation of patient’s walking ability is an important part of neurological examination [6], i.e. the maximum distance patients can walk without rest or the distance they can walk until symptoms occur (maximum walking distance, MWD) [7]. In clinical practice, due to time and space constraints, physicians frequently rely on patients’ responses on the distance covered. However, several studies have shown the inaccuracy of distance estimates and therefore the unreliability of disability assessment in clinical routine [8].
About 40–75% of people with MS (pwMS) have impairment of the walking ability [9]. Although all deficits contribute to overall disability, gait impairment weighs more than other functional systems (FS) in disability assessment [6] The Ambulation Score (AS) of the Expanded Disability Status Scale (EDSS) [6] is the most widely used method to assess gait impairment in pwMS; in clinical practice, the MWD to rate AS is assessed by asking pwMS how long they could walk without rest, and consequently, the AS and therefore the EDSS rating may be affected by subjective, hence possibly inaccurate, MWD estimate and reporting [10].
In the last 10 years, digital technology has facilitated exact distance measurement [11]. Google Maps® (GM®) has revolutionized distance calculation by simply using a digital device (e.g., a smartphone) and an internet connection. We used the GM® application to evaluate the MWD in pwMS and verified its correspondence with the estimates reported by these patients and the objective measures assessed by neurologists, in order to determine whether GM® provides an accurate and accessible method to evaluate walking distances in pwMS. The aim of the study was to investigate whether GM® might be used as an objective tool to evaluate the AS in pwMS and its potential implications on the accuracy of EDSS scoring.
Methods
Study population
This cross-sectional multicenter study was conducted in five MS clinical centers in Italy (two centers in Naples, one in Rome, Bari, and Palermo). PwMS were consecutively enrolled from February to May 2019. The inclusion criteria were diagnosis of clinically isolated syndrome or confirmed MS according to McDonald criteria 2010; EDSS score ≤ 6; mental functional system ≤ 1; disease duration (DD) ≥ 1 year; and agreement to display a route on GM®. Exclusion criteria were ongoing relapse or steroid treatment during the 6 months preceding enrollment; any other diseases or conditions which might influence ambulation (e.g., fractures, recent surgery, etc.); and patients who ask to display a route on GM® which was either uphill or downhill.
We did not include a control group as the MS-specific metrics do not apply to putative healthy controls. Indeed, while the MWD could be assessed in healthy subjects, we evaluated the correlation between the measures relying on AS and EDSS scores that do not apply to putative healthy controls.
Study variables
Demographic (age, sex, education) and clinical (DD, disease phenotype, ongoing disease-modifying therapies, DMTs) data were collected. Fatigue Severity Scale (FSS) [12] and Patient Health Questionnaire-9 (PHQ-9) [13] were used to evaluate fatigue and depression, respectively. For EDSS evaluation, pwMS were asked to estimate their MWD according to routine practice as reported above [1]. Patients’ perceived AS (pAS) and, consequently, the perceived EDSS score (pEDSS) were calculated based on the MWD reported by the patients. During the enrollment visit, a neurologist blinded to the previously collected data illustrated to the patients how to use GM® on a smartphone or a hospital computer. Using GM® satellite imagery (with the street maps and 360° panoramic Street View) and fixing their home address as starting point, all the recruited patients were asked to indicate a route usually followed from their home and the distance they could walk (adopting a moderate and regular walking pace) without stopping or needing any help. This was done to identify the arrival point on GM® and then measure the start-arrival distance. Based on the MWD evaluated by GM®, the GM®AS (gmAS) and the GM®EDSS (gmEDSS) were calculated.
In 75 pwMS, a blinded neurologist measured the MWD by walking the 20 m. The MWD objectively measured was used to calculate the objectiveAS (obAS) and the objective EDSS (obEDSS) to test the consistency of the pAS and with the pEDSS, respectively. Since EDSS is heavily influenced by walking ability particularly in the mid-range scores (4.0–6.0) [14–16], the overall study population was stratified into two subgroups according to mild (pEDSS ≤ 3.5) or moderate (4 ≤ pEDSS ≤ 6) disability.
Standard protocol approvals, registrations, and patient consent
According to the good clinical practice and the Declaration of Helsinki, all pwMS gave their written consent to participate in the study, which was approved by the Ethical Committee of each MS center involved.
Statistical analysis
Given the exploratory nature of this study, no sample size calculation was performed [17, 18]. Normal distribution of variables of interest was checked with graphical and statistical approaches. Continuous variables were described both as mean (M) and standard deviation (SD), median, and range interquartile (Q1–Q3); categorical variables were described as frequency and percentage. Descriptive statistics were performed where appropriate. Specifically, two-group comparisons were performed using the Student’s t test, the Mann–Whitney U test, and the Chi-square or Fisher’s exact test. Comparisons between multiple groups were performed employing the analysis of variance (ANOVA) or nonparametric Kruskal–Wallis test. Correlation analyses were performed using the Spearman correlation test [19]. To calculate agreement in measures estimated by the neurologist, pwMS, or GM® service, the intraclass correlation coefficient (ICC) [20] was employed using a two-way mixed effect model. ICC estimates and their 95% confidence intervals were calculated. The two-way mixed effect model was chosen as the neurologists who were blinded to the pAS and pEDSS scores identified to acquire the objective MWD were chosen before commencing the study [21].
The following two new subgroups were identified:
pwMS with pEDSS correspondent to gmEDSS
pwMS with pEDSS different (higher/lower) from gmEDSS
Univariate and multivariate logistic regression models were applied to evaluate whether age, level of education, DD, clinical phenotype, FSS score, and PHQ-9 score might have influenced the discrepancy between the pEDSS and the gmEDSS. Results were presented as weighted Kappa, odds ratio (OR), and corresponding 95 % confidence interval (CI), as appropriate. A p value of <0.05 was considered statistically significant. Model goodness of fit was assessed using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC).
Statistical analysis was performed by using SPSS software (SPSS, version 22.0; SPSS Inc., Chicago, IL). The only missing data were about the educational level for 13 patients. According to a previous study, outcomes with < 20% missing data were not excluded from the final analysis [22].
Results
Sample description
Two hundred forty-three pwMS were enrolled between February and May 2019. No patients were excluded due to uphill or downhill route reported on GM®.
Table 1 shows demographic and clinical data of the whole study sample. Education was available for 230/243 (94.6%) pwMS and the overall mean level of education was 12.4 ± 3.55 years. Most (42.4%) of the patients were treated either with interferon beta-1a (21.0%) or with dimethyl fumarate (21.4%). The clinical phenotype of the 243 pwMS involved in the study was the following: 1 clinically isolated syndrome (0.5%), 210 relapsing–remitting (RR, 86.5%), 23 secondary progressive (SP, 9.2%), 9 primary progressive (PP, 3.8%).
Table 1.
Whole population and subgroups stratified based on agreement between pEDSS and gmEDSS; demographic and clinical feature
| Total (n = 243) | pEDSS = gmEDSS (n = 169) | pEDSS < gmEDSS (n = 32) | pEDSS > gmEDSS (n = 42) | ||||
|---|---|---|---|---|---|---|---|
| Variable | P value | ||||||
| Sex | |||||||
| Female (n; %) | 173,00 (71,19) | 118 (69,82) | 24 (75%) | 31 (73.81) | 0.771 | ||
| Male (n; %) | 70,00 (28,81) | 51 (30,18) | 8 (25%) | 11 (26.19) | |||
| Age | |||||||
| Mean, sd | 39,77 (12,35) | 37,46 (12,14) | 45.03 (12.16) | 45.02 + 10.60 | < 0.001 | ||
| Median (Q1-Q3) | 39 (30–48) | 37 (27–45) | 45,5 (37,5–47,5) | 44 (39–51) | |||
| Disease type | |||||||
| CIS (n; %) | 1,00 (0,41) | 1 (0,59) | 0 (0) | 0 (0) | < 0.001 | ||
| RR (n; %) | 210,00 (86,78) | 156 (92,31) | 20 (62,50) | 34 (82,93) | |||
| SP (n; %) | 23,00 (9,09) | 10 (5,92) | 7 (21,88) | 5 (12,20) | |||
| PP (n; %) | 9,00 (3,72) | 2 (1,18) | 5 (15,62) | 2 (4,88) | |||
| Disease duration | |||||||
| mean, sd | 9,60 (7,83) | 8,68 (6,70) | 9.78 (10.28) | 13,16 (9,01) | 0.007 | ||
| median (Q1-Q3) | 7 (3–13) | 7 (3–12) | 5,5 (3–13) | 11 (6.8 -18.3) | |||
| Ongoing therapy | |||||||
| GA (n; %) | 19,00 (7,82) | 13 (7,69) | 3 (9,38) | 3 (7.14) | |||
| INF (n; %) | 51,00 (20,99) | 38 (22,49) | 7 (21,88) | 6 (14.29) | |||
| DMF (n; %) | 52,00 (21,40) | 39 (23,08) | 5 (15,62) | 8 (19.05) | |||
| TRF (n; %) | 16,00 (6,58) | 6 (3,55) | 6 (18,75) | 4 (9.52) | |||
| FTY (n; %) | 46,00 (18,93) | 37 (21,89) | 0 (0) | 9 (21.43) | |||
| NTZ (n; %) | 36,00 (14,81) | 25 (14,79) | 4 (12,50) | 7 (16.67) | |||
| ALZ (n; %) | 10,00 (4,12) | 5 (2,96) | 4 (12,50) | 1 (2.38) | |||
| None (n; %) | 13,00 (5,35) | 6 (3,55) | 3 (9,38) | 4 (9.52) | |||
| FSS | |||||||
| mean, sd | 30,63 (16,09) | 26.68 (14.27) | 39.65625 (17.80) | 39,61 (15,60) | < 0.001 | ||
| median (Q1-Q3) | 30 16–43 | 24 (14–37) | 44,5 (21–55,5) | 38 (30–53) | |||
| PHQ9 | |||||||
| mean, sd | 7,52 (6,07) | 5.86 (4.92) | 11.1875 (7.168198) | 11.35714 (6.49135) | < 0.001 | ||
| median (Q1-Q3) | 6 (3–11) | 5 (2–8) | 12 (5–15,5) | 11.5 (5–16) | |||
| pEDSS | |||||||
| < = 3,5 (n; %) | 142 (58.68) | 130 (76,92) | 11 (34,38) | 1 (2,44) | < 0.001 | ||
| > = 4 (n; %) | 101 (41.32) | 39 (23,08) | 21 (65.62) | 41 (97,56) | |||
GA glatiramer acetate, INF interferon, DMF dimethyl fumarate, TRF teriflunomide, FTY fingolimod, NTZ natalizumab, ALZ alemtuzumab, EDSS Expanded Disability Status Scale, pEDSS perceived EDSS, gmEDSS Google EDSS, FSS Fatigue Severity Scale, PHQ-9 Patient Health Questionnaire-9
Perceived EDSS (pEDSS) and GM® EDSS (gmEDSS)
Based on the data collected concerning pAS and gmAS, we calculated the pEDSS and the gmEDSS, respectively, as may be seen in Table 1.
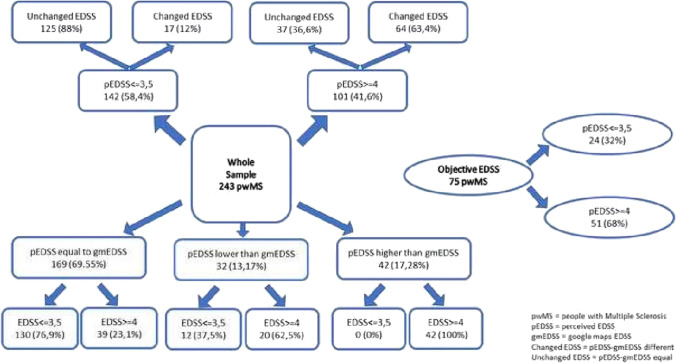
pEDSS and gmEDSS coincided in 169 pwMS and differed in 74/243 pwMS (30.45%) as shown in Fig. 1.
Fig. 1.
General flowchart on Expanded Disability Status Scale (EDSS) results
Due to the weight of other FS on EDSS calculation, only in 7 pwMS, the EDSS calculation was unaffected by the AS results. Comparison between pAS and gmAS was reported in supplementary material.
Based on the EDSS calculated with the pAS (pEDSS) and the EDSS calculated with the gmAS (gmEDSS) (weighted kappa: 0.82; almost perfect agreement), the results can be stratified into three subgroups: (1) pwMS with pEDSS coinciding with gmEDSS; (2) pwMS with pEDSS lower than gmEDSS; (3) pwMS with pEDSS higher than gmEDSS. In each of these subgroups, pwMS with mild and moderate perceived disability were included (Fig. 1).
PwMS with pEDSS that coincided with gmEDSS were significantly younger either than pwMS with pEDSS lower than gmEDSS (37.5 ± 12.14 vs 45.03 ± 12.16; p = 0.001) or than pwMS with pEDSS higher than gmEDSS (37.5 ± 12.14 vs 45.02 ± 10.60; p = 0.001). The three groups did not differ as regards education level (Kruskal–Wallis p = 0.607) but they were different for DD (Kruskal–Wallis p = 0.007). In particular, pwMS with pEDSS that coincided with gmEDSS had a significantly shorter DD (median: 7, Q1–Q3: 3–12) than pwMS with pEDSS higher than gmEDSS (median: 11, Q1–Q3: 6.8–18.3; Mann–Whitney Bonferroni adjusted p = 0.003).
Moreover, the three groups (pEDSS = gmEDSS, pEDSS < gmEDSS, and pEDSS > gmEDSS) differed greatly for FSS scores (Kruskal–Wallis p = 0.0001) and for PHQ-9 scores (Kruskal–Wallis p = 0.0001). Post-hoc comparisons (Table 1) showed that pwMS with pEDSS that was in line with gmEDSS had significantly lower FSS scores compared to pwMS with pEDSS higher than gmEDSS (Mann–Whitney Bonferroni adjusted p < 0.001) and to pwMS with pEDSS lower than gmEDSS (Mann–Whitney Bonferroni adjusted p < 0.001).
Similarly, pwMS with pEDSS that coincided with gmEDSS had significantly lower PHQ-9 scores than pwMS with pEDSS higher than gmEDSS (median: 11.5, Q1–Q3: 5–16; Mann–Whitney Bonferroni adjusted p < 0.001) and than pwMS with pEDSS lower than gmEDSS (Mann–Whitney Bonferroni adjusted p < 0.001) (Table 1).
Finally, pwMS with pEDSS that coincided with gmEDSS were more likely to have a pEDSS score < = 3.5 (130; 76.92%) compared to both pwMS with pEDSS lower than gmEDSS (11; 34.38%) and pwMS with pEDSS higher than gmEDSS (1; 2.44%) (Chi-square p < 0.001) (Table 1).
ObjectiveAS (obAS)
Demographic and clinical data from the subgroup of pwMS with obAS calculation are shown in Table 2.
Table 2.
obAS population; demographic and clinical features
| Variable | ||
|---|---|---|
| Age | ||
| Mean, sd | 41.3 (11.71) | |
| Median (Q1–Q3) | 41 (32–74) | |
| Sex | ||
| Female (n; %) | 58 (77.3) | |
| Male (n; %) | 17 (22.7) | |
| Level of education | ||
| Mean, sd | 11.1 (2.69) | |
| Median (Q1–Q3) | 13 (8–13) | |
| Disease duration | ||
| Mean, sd | 10.7 (8.4) | |
| Median (Q1–Q3) | 9 (4–14) | |
| FSS | ||
| Mean, sd | 36.4 (15.85) | |
| Median (Q1–Q3) | 37 (22–50) | |
| PHQ9 | ||
| Mean, sd | 8.64 (6.35) | |
| Median (Q1–Q3) | 7 (3–14) | |
| Concordance between ObAS, gmAS, and pAS | ||
| obAS = gmAS (n; %) | 45 (60) | |
| pAS = gmAS (n; %) | 39 (52) | |
| pAS = obAS (n; %) | 43 (45.2) | |
Abbreviations: pAS perceived Ambulation Score; pEDSS perceived Expanded Disability Status Scale; gmAS Google Maps Ambulation Score; gmEDSS Google Maps Expanded Disability Status Scale; obAS actual (objective) Ambulation Score; obEDSS actual (objective) Expanded Disability Status Scale; FSS Fatigue Severity Scale; PHQ-9 Patient Health Questionnaire-9
This group consisted of 75 pwMS (58 F [77.3%]; 17 M [22.7%]). The mean level of education was 11.1 ± 2.69 years (median: 13; Q1–Q3: 8–13 years). The mean age was 41.3 + 11.71 years (median: 41; Q1–Q3 = 32–47). The mean DD was 10.7 ± 8.40 years (median: 9; Q1–Q3: 4–14). In this subgroup, the obAS and gmAS are more frequently coincident (45 patients; 60%) either than obAS and pAS (34 patients; 45.3%) or than pAS and gmAS (39 patients; 52%).
Factors influencing EDSS variation (pEDSS different from gmEDSS)
Logistic regression (Table 3) models showed that the progressive phenotypes (p = 0.03) as well as higher FSS (p = 0.01) and PHQ-9 (p = 0.002) scores were associated with the discrepancy between pEDSS and gmEDSS.
Table 3.
Univariate and multivariate logistic models: factors influencing the discrepancy between pEDSS and gmEDSS
| Variables | Model I | Model II | Model III | Model IV | Model V | Model VI | Model VII | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | P value | OR | P value I | OR | P value | OR | P value | OR | P value | OR | P value | OR | P value | |
| Disease type | 3.34 | < 0.001 | 2.8 | 0.03 | ||||||||||
| Age, y | 1.05 | < 0.001 | 1.02 | 0.164 | ||||||||||
| Education, years | 0.9 | 0.381 | 0.95 | 0.321 | ||||||||||
| DD | 1.04 | 0.007 | 1.07 | 0.208 | ||||||||||
| FSS | 1.05 | < 0.001 | 1.06 | 0.01 | ||||||||||
| PHQ-9 | 1.16 | < 0.001 | 1.17 | 0.002 | ||||||||||
Abbreviations: PP primary progressive multiple sclerosis; SP secondary progressive multiple sclerosis; RR relapsing–remitting multiple sclerosis; FSS Fatigue Severity Scale; PHQ-9 Patient Health Questionnaire-9
Discussion
In neurology clinical practice, the evaluation of walking ability is frequently based on the question “how long can you walk without rest?” In the MS field, the EDSS requires patients to walk at least 500 m to obtain the AS. However, there are a number of limitations coming from both approaches. There are many variables that may influence the actual measurement of walking distance: (1) time and space constraints limiting an optimal examination; (2) daily variations in fatigue and overall functioning; (3) concomitant diseases and treatments. The same applies to self-reported measurements: (1) inaccuracy in providing an exact estimate of distances [23]; (2) depressed mood [24]; (3) being fatigued at question time [25]; (4) level of education [26]; and (5) impairment of mental functions [27].
We used the GM® application to evaluate the MWD in pwMS and therefore investigated whether GM® might be used as an objective tool to evaluate the AS in pwMS and the potential implications on EDSS scoring accuracy.
Our results showed that one-third of our patients indicated a pAS different from the gmAS. These data are consistent with a previous study showing that pwMS are inaccurate at estimating distances [9], suggesting that the estimates of walking distances covered are not reliable indicators for disability assessment. We found that the discrepancies between pAS and gmAS are more frequent in the subgroup of pwMS with moderate disability (63%), indicating a less reliable estimate of MWD in more disabled patients and consequently prompting clinicians to improve disability assessment to monitor disease course in clinical practice. In particular, once the EDSS milestone of 4.0 is reached, further progression in the EDSS score can be independent of decline or improvement in the other functional systems and determined solely by the MWD a MS patient can usually walk. Therefore, differences in MWD are very important in pwMS with moderate disability and should be monitored closely and carefully to improve EDSS assessment. Unfortunately, routine clinical examination for pwMS does not include an objective evaluation of the AS and the EDSS score is calculated based on what patients report to their neurologists.
To partially overcome the risk of inaccurate estimates of the MWD, we used GM® application to calculate the gmAS and the gmEDSS. Those pwMS with pEDSS corresponding to the gmEDSS were found to be younger and with a shorter disease duration (DD) but, above all, these patients had significantly lower FSS and PHQ-9 scores. A previous study reported that age does not influence the patient estimation of MWD [28]. Our data confirmed this result but showed a better correspondence between pEDSS and gmEDSS in younger pwMS with significantly lower DD, probably reflecting a milder disease [29]. In pwMS where pAS and gmAS were not concordant, we found that 42% had a pAS lower than the gmAS and 58% had a pAS higher than the gmAS. Distinguishing these subgroups of pwMS with mild and moderate disability, we found that pwMS with a moderate disability tend to overestimate MWD (with respect to GM® output). In these groups, pwMS with unchanged EDSS (pEDSS = gmEDSS) and those with discrepant EDSS (pEDSS < or > gmEDSS), we found that progressive phenotype and higher scores in FSS and PHQ-9 increased the probability of inaccurate estimates of MWD and therefore belonged to the discrepant group. These data are consistent with the findings of Skjearbak et al. [30], showing that the extent of misclassification of MWD increases in pwMS with fatigability and progressive phenotype and that depressive symptoms are related to self-perception of MWD; these findings emphasize the need to improve disability assessment especially in pwMS with moderate disability. Furthermore, a subgroup analysis conducted on the 75 patients in whom the obAS was calculated showed that GM® seems to effectively improve the accuracy of the MWD. According to our results, GM® technology may represent a feasible tool to measure MWD increasing the agreement with objective measures. Ideally, a large-scale clinical trial should be focused on pwMS who are at higher risk of making false estimates of MWD (i.e., fatigued and depressed) or in those pwMS with a moderate disability or transitional forms of the disease where the prompt close monitoring of disability progression is necessary for therapy adjustment and ultimately to improve pwMS management. The results of the subgroup obAS/obEDSS strengthen the hypothesis that GM® may represent an objective tool to evaluate the AS in pwMS, and could result in greater accuracy of EDSS scoring.
In conclusion, our results suggest that GM® application may be easily and accurately used in a real-life clinical setting to improve the evaluation of the AS and the related EDSS, thus making it also suitable to overcome the difficulties imposed by the ongoing health-care restrictions due to the pandemic.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 26 kb)
(DOCX 14 kb)
Declarations
Ethical approval
The Ethical Commitee of University of Campania Luigi Vanvitelli (Coordinator Center) approved the project and the protocol numner is 0014460/i.
Competing interest
The authors declare no competing interests.
Footnotes
The original online version of this article was revised: Originally, the article has been published online with a spelling error in author name. The correct author name is Paolo Ragonese and not Paolo Raganose.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luigi Lavorgna and Pietro Iaffaldano contributed equally to this work.
Change history
11/2/2021
A Correction to this paper has been published: 10.1007/s10072-021-05707-z
Contributor Information
Luigi Lavorgna, Email: luigi.lavorgna@policliniconapoli.it.
Pietro Iaffaldano, Email: pietro.iaffaldano@uniba.it.
Gianmarco Abbadessa, Email: gianmarcoabbadessa@live.com.
Roberta Lanzillo, Email: roberta.lanzillo@unina.it.
Sabrina Esposito, Email: sabrina.esposito1@unicampania.it.
Domenico Ippolito, Email: mimmoip88@gmail.com.
Maddalena Sparaco, Email: ms.86@hotmail.it.
Simone Cepparulo, Email: cepparulo.s@gmail.com.
Giacomo Lus, Email: giacomo.lus@unina2.it.
Rosa Viterbo, Email: rosa.viterbo@uniba.it.
Marinella Clerico, Email: marinella.clerico@unito.it.
Francesca Trojsi, Email: francesca.trojsi@unicampania.it.
Paolo Ragonese, Email: paolo.ragonese@unipa.it.
Giovanna Borriello, Email: giovanna.borriello@gmail.com.
Elisabetta Signoriello, Email: elisabetta.signoriello@gmail.com.
Raffaele Palladino, Email: raffaele.palladino@unina.it.
Marcello Moccia, Email: moccia.marcello@gmail.com.
Francesco Brigo, Email: dr.francescobrigo@gmail.com.
Maria Troiano, Email: maria.trojano@uniba.it.
Gioacchino Tedeschi, Email: gioacchino.tedeschi@unicampania.it.
Simona Bonavita, Email: simona.bonavita@unicampania.it.
References
- 1.Chatterji P, Li Y. Effects of the COVID-19 pandemic on outpatient providers in the United States. Med Care. 2021;59(1):58–61. doi: 10.1097/MLR.0000000000001448. [DOI] [PubMed] [Google Scholar]
- 2.Moccia M, Lanzillo R, Brescia Morra V, et al. Assessing disability and relapses in multiple sclerosis on tele-neurology. Neurol Sci. 2020;41:1369–1371. doi: 10.1007/s10072-020-04470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bombaci A, Abbadessa G, Trojsi F, et al. Telemedicine for management of patients with amyotrophic lateral sclerosis through COVID-19 tail. Neurol Sci. 2021;42(1):9–13. doi: 10.1007/s10072-020-04783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bove R, Bevan C, Crabtree E, Zhao C, Gomez R, Garcha P, Morrissey J, Dierkhising J, Green AJ, Hauser SL, Cree BA, Wallin MT, Gelfand JM. Toward a low-cost, in-home, telemedicine-enabled assessment of disability in multiple sclerosis. Mult Scler. 2019;25(11):1526–1534. doi: 10.1177/1352458518793527. [DOI] [PubMed] [Google Scholar]
- 5.Abbadessa G, Lavorgna L, Miele G, et al. Assessment of multiple sclerosis disability progression using a wearable biosensor: a pilot study. J Clin Med. 2021;10(6):1160. doi: 10.3390/jcm10061160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 7.Kruidenier LM, Nicolai SPA, Willidgendael EM, et al. Functional claudication distance: a reliable and valid measurement to assess functional limitation in patients intermittent claudication. BMC Cardiovasc Disord. 2009;2(9):9. doi: 10.1186/1471-2261-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharrack B, Hughes RCA. Reliability of distance estimation by doctors and patients: cross sectional study. BMJ. 1997;315:1652–1654. doi: 10.1136/bmj.315.7123.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohart J, Lamping D, Fitzpatrick R, et al. The multiple sclerosis impact scale: a new patients based outcome measures. Brain. 2001;124:962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 10.PiriÇinar B, GüvenYorgun K. What we learned from the history of multiple sclerosis measurement: expanded disability status scale. Arch Neuropsychiatry. 2018;55(Supplement 1):69–75. doi: 10.29399/npa.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparaco M, Lavorgna L, Conforti R, et al. The role of wearable devices in multiple sclerosis. Mult Scler Int. 2018;2018:7627643. doi: 10.1155/2018/7627643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 13.Hind D, Kaklamanou D, Beever D, et al. The assessment of depression in people with multiple sclerosis: a systematic review of psychometric validation studies. BMC Psychiatry. 2016;16:278. doi: 10.1186/s12888-016-0931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman MD, Motl RW, Rudick RA. Possible clinical outcome measures for clinical trials in patients with multiple sclerosis. Ther Adv Neurol Disord. 2010;3:229–239. doi: 10.1177/1756285610374117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Confavreux C, Vukusic S, Adeline P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: an amnesic process. Brain. 2003;126:770–782. doi: 10.1093/brain/awg081. [DOI] [PubMed] [Google Scholar]
- 16.Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and progression of disability in multiple sclerosis. N Eng J Med. 2000;343:1430–1438. doi: 10.1056/NEJM200011163432001. [DOI] [PubMed] [Google Scholar]
- 17.Kimmelman J, Mogil JS, Dirnagl U. Distinguishing between exploratory and confirmatory preclinical research will improve translation. Plos Biol. 2014;12(5):e1001863. doi: 10.1371/journal.pbio.1001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67. doi: 10.1186/1471-2288-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukaka MM. A guide to appropriate use of Correlation coefficient in medical research MM Mukaka. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 21.Mandrekar JN. Measures of interrater agreement. J Thorac Oncol. 2011;6(1):6–7. doi: 10.1097/JTO.0b013e318200f983. [DOI] [PubMed] [Google Scholar]
- 22.Dong Y, Peng CY (2013) Principled missing data methods for researchers. Springerplus 2(1):222. Published 2013 May 14. 10.1186/2193-1801-2-222. [DOI] [PMC free article] [PubMed]
- 23.Herbolsheimer F, Riepe M, Peter R. Cognitive function and the agreement between self-reported and accelerometer-accessed physical activity. BMC Geriatr. 2018;18:56. doi: 10.1186/s12877-018-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalron A, Aloni R. Contrasting relationship between depression, quantitative gait characteristics and self-report walking difficulties in people with multiple sclerosis. Mult Scler Relat Disord. 2018;19:1–5. doi: 10.1016/j.msard.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Dalgas U, Langeskov-Christensen M, Skjerbæk AG. Is the impact of fatigue related to walking capacity and perceived ability in persons with multiple sclerosis? A multicenter study. J Neurol Sci. 2018;387:179–186. doi: 10.1016/j.jns.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Berger W, Payne MWC, Morrow SA. Self reported maximum walking distance in persons with MS may affect the EDSS. Neurol Sci. 2017;379:77–80. doi: 10.1016/j.jns.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Neil BA, Kenneth EG, Darryl GT, et al. Self-Reported walking ability predicts functional mobility performance in frail older adults. JAGS. 2000;48:1408–1413. doi: 10.1111/j.1532-5415.2000.tb02630.x. [DOI] [PubMed] [Google Scholar]
- 28.Giantomaso T, Makowsky L, Ashworth N, et al. The validity of patient and physician estimate of walking distance. Clin Rehabil. 2003;17:394–401. doi: 10.1191/0269215503cr626oa. [DOI] [PubMed] [Google Scholar]
- 29.Minden SL, Frankel D, Hadden L, et al. Disability in elderly people with multiple sclerosis: an analysis of baseline data from the Sonya Slifka longitudinal multiple sclerosis study. Neurorehabilitation. 2004;19(1):55–67. doi: 10.3233/NRE-2004-19107. [DOI] [PubMed] [Google Scholar]
- 30.Skjerbæk AG, Boesen F, Petersen T, Rasmussen PV, Stenager E, Nørgaard M, Feys P, Kjeldgaard-Jørgensen ML, Hvid LG, Dalgas U. Can we trust self-reported walking distance when determining EDSS scores in patients with multiple sclerosis? The Danish MS hospitals rehabilitation study. Mult Scler. 2019;25(12):1653–1660. doi: 10.1177/1352458518795416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 26 kb)
(DOCX 14 kb)