Abstract
Introduction
Proteus mirabilis is a biofilm-forming agent that quickly settles on the urinary catheters and causing catheter-associated urinary tract infections. Thus, the spread of multidrug-resistant P. mirabilis isolates, with the ability to form a biofilm that carries integron, extended-spectrum β-lactamases (ESBLs), and plasmid-mediated colistin resistance genes (mcr), represents a severe threat to managing nosocomial infectious diseases. This study is aimed at surveying the prevalence of ESBL, integrase, and mcr genes of P. mirabilis, isolated from the catheter, to assess the differences in their antimicrobial susceptibility and clonal dissemination.
Method
Microtiter plate assay was adopted to measure biofilm formation. The antimicrobial susceptibility was assessed by the disk diffusion method. Antimicrobial resistance genes (intI1, intI2, intI3, blaTEM, blaCTX-M, blaSHV, mcr1, and mcr2) were detected by PCR. All of the isolates were characterized by repetitive sequence-based PCR.
Result
From 385 collected catheters in patients admitted to the intensive care unit (ICU), 40 P. mirabilis were isolated. All of the isolates could form a biofilm. Proteus spp. had intrinsic resistance to tetracycline (95%) and nitrofurantoin (92.5%), which explains the high resistance prevalence. The most widely resistant antibiotic was trimethoprim-sulfamethoxazole (75%). Thirty-three (82.5%) isolates were classified as multidrug resistance (MDR). The prevalence of intI1 and intI2 genes was 60% and 25%, respectively. In 6 (15%) isolates, both genes were detected. The most frequent ESBL gene detected in all of the isolates was blaTEM. Also, no detection for mcr1 and mcr2 antibiotic resistance genes was reported. Rep-PCR identified 39(GTG)5 types (G1–G39) of 40 isolates that 38 isolates had unique patterns.
Conclusion
In this study, 82.5% of isolates were MDR with high antibiotic resistance to trimethoprim-sulfamethoxazole. The intI1 and blaTEM were the most prevalent genes in the integrase and ESBL gene family. High diversity was seen in the isolates with Rep-PCR. The increasing rate of MDR isolates with a high prevalence of resistance genes could be alarming and demonstrate the need for hygienic procedures to prevent the increased antibiotic resistance rate in the future.
1. Introduction
Proteus mirabilis is a charming polymorphic swarming persistent colonizer bacterium that strongly correlates with catheter blockage and urinary stone development. Complicated urinary tract infections (UTIs) at an alarming rate are expanding healthcare challenges, and P. mirabilis is the pathogen that must be noticed for causing those particular catheter-associated urinary tract infections (CAUTIs). P. mirabilis is a usual cause for complicated UTIs, and it becomes intricate in patients undergoing long-term indwelling urinary catheterization who may develop CAUTIs [1]. Such disorders cause difficulties by the inimitable ability of P. mirabilis to create crystalline biofilms, eventually leading to encrusted and blocked catheters [2]. Likewise, they could result in urine retention and reflux and, in severe conditions, septicemia and endotoxic shock in addition to trauma to the urethra and bladder mucosa due to removing the catheter [3–5]. CAUTIs present challenges to treatment strategies for different reasons, including the biofilm formation of P. mirabilis on catheters and urolith formation in the bladder and urinary tract, which could be created by multidrug-resistant isolates. Multidrug resistance (MDR) may be mediated by resistance agents located on chromosomes or mutations in a resident gene. However, it may also expand by attaining resistance genes through horizontal transfer [6].
These resistance genes, which are widely present on plasmids, transposons, and integrons, lead to the problem of rapid spread and treatment failure. In the past, most P. mirabilis isolates were susceptible to common antibiotic classes, but recent studies in different countries have indicated that antibiotic resistance among P. mirabilis isolates is increasing. The β-lactam resistance patterns of the P. mirabilis isolates have reported the production of various classes of extended-spectrum β-lactamases (ESBLs) [7].
The prevailing type of integrons discovered in clinical isolates is class 1 integron, which is highly associated with antibiotic resistance; therefore, they have been extensively studied [8]. Class 2 integrons stay in the second significant type of integrons obtained from clinical isolates. Usually, class 2 integrons are inserted in the nonreplicative transposon [9].
The extension of ESBL indicates a severe threat to managing nosocomial infectious diseases, causing problems for remedial choices of antimicrobial applications. The prevalence of integrons and characterized gene cassettes in Gram-negative bacteria integron-associated multidrug resistance has been investigated. However, it is seldom addressed in P. mirabilis [10]. Colistin is one of the last-resort drugs to treat infections caused by MDR Gram-negative bacteria. Although this bacterium is intrinsically colistin-resistant, it can carry plasmid-mediated colistin-resistant (mcr) genes and is often overlooked and not screened for mcr. However, this bacterium can serve as a reservoir to transmit these genes to colistin-susceptible bacteria [11].
Various molecular methods, such as repetitive extragenic palindromic PCR (Rep-PCR), ribotyping, and pulse-field gel electrophoresis (PFGE), have been used to assess the genotypic diversity within several bacterial species [12].
Rep-based fingerprinting has been proved to be a fast and reliable tool to differentiate between Enterobacteriaceae populations. Rep-fingerprinting uses variations in conserved intergenic palindromic DNA sequences for PCR amplification and an isolate's characterization. These DNA elements are stable, noncoding intergenic repetitive sequences scattered across the genome and act as amplification targets to produce various bands [13, 14].
In this study, the prevalence of integrase genes (intI1, intI2, and intI3) and three ESBL genes (blaTEM, blaCTX-M, and blaSHV), as well as mcr1 and mcr2, was investigated, and the comparison of integron-carrying and non-integron-carrying MDR P. mirabilis isolated from the catheter was performed to assess the differences in their antimicrobial susceptibility and clonal dissemination. The clonal relationship for finding the origin of infection of the isolates was also evaluated with Rep-PCR.
2. Material and Methods
2.1. Bacterial Isolation
In this cross-sectional study from June 2019 to July 2020, 385 nonduplicate catheters (5 days-15 days) from intensive care unit (ICU) patients were collected from various hospitals in Isfahan. The inclusion criteria for this study were that the collected catheters were from the patients without a primary urinary tract infection at admitting time; the minimum time of catheterization was five days.
P. mirabilis was isolated from the catheters. For this purpose, we followed the procedures of Mandakhalikar et al., with some modifications [15]. Briefly, catheters were cut into 1 cm segments and dipped in phosphate buffer saline (PBS) to discard loosely attached planktonic bacteria. The sample was transferred to 10 ml PBS and vortexed vigorously for 1 min, then probe-based sonication was directed at 10 W (RMS) for 60-90 seconds, and another round of vortex for 1 minute at the highest speed was repeated. The catheter solution was then cultured on blood agar, Eosin Methylene Blue (EMB), MacConkey agar, and catalase, oxidase, IMViC, and urease are a few examples of conventional biochemical tests that were performed, and P. mirabilis suspected colonies were recultured to achieve a pure and single colony, and the ureG gene PCR was performed for genetic confirmation.
2.2. Antibiotic Susceptibility Testing, ESBLs, and MDR Detection
Antimicrobial resistance of the isolates to ampicillin-sulbactam (10/10 μg), amoxicillin-clavulanic acid (20/10 μg), ampicillin (10 μg), nitrofurantoin (300 μg), meropenem (10 μg), cefotaxime (30 μg), ceftazidime (30 μg), ceftazidime-clavulanic acid (30/10 μg), cefixime (5 μg), aztreonam (30 μg), amikacin (30 μg), norfloxacin (10 μg), ofloxacin (5 μg), ciprofloxacin (5 μg), tetracycline (30 μg), and trimethoprim-sulfamethoxazole (1.25/23.75 μg) was determined by the disk diffusion method on Mueller-Hinton agar overnight at 37°C. The breakpoints for each antimicrobial agent were interpreted according to guidelines provided by the Clinical and Laboratory Standards Institute (CLSI) standards [16]. Furthermore, MDR isolates were defined, according to Magiorakos et al., when the isolates resist at least one agent in ≥3 antimicrobial classes categorized in MDR isolates, the antibiotic classes that were considered for the definition of the MDR were cephalosporins (cefotaxime, ceftazidime, and cefixime), monobactams (aztreonam), carbapenems (meropenem), aminoglycosides (amikacin), quinolones (norfloxacin, ofloxacin, and ciprofloxacin), folate pathway antagonists (trimethoprim-sulfamethoxazole), penicillins (ampicillin), and beta-lactamase inhibitors (ampicillin-sulbactam and amoxicillin-clavulanic acid) [17]. In addition, the ESBL production of the isolates was detected by the double-disc diffusion synergy test [18].
2.3. Biofilm Formation Assay
For this purpose, an overnight culture of P. mirabilis isolates was diluted (1 : 100) in TSB to reach the 0.5 McFarland concentration, and 5 μl of the diluted culture was inoculated in each well of polystyrene microtiter 96-well plates (Greiner, Germany). After 24 h incubation of the plates at 37°C, the medium was removed, and the wells were washed carefully with double distilled water (DDW) and fixed by adding 200 μl of ethanol (96%) for 15 minutes. Next, the medium was removed, and the plate was left to dry. The biofilms were stained with 0.1% (w/v) crystal violet for 20 minutes at 37°C. Next, the biofilm was washed three times with DDW. Then, 200 μl of ethanol–acetic acid (90 : 10) was added to each well, and optical density (OD) was measured at 590 nm with an enzyme-linked immunosorbent assay (ELISA) microtiter plate reader. Each assay was done in triplicate, and the mean absorbance ± standard deviation was calculated for all repetitions of the tests. Escherichia coli K12 and Pseudomonas aeruginosa ATCC 27853 were used as negative (weakly biofilm-forming) and positive (strong biofilm-forming) control strains, respectively [19].
2.4. PCR Amplification of bla, mcr1, and mcr2 Genes and Integrase Gene Detection
DNA was extracted by the phenol-chloroform method described by Sambrook and Russell [20]. The purity and quality of the extracted DNA were evaluated by the NanoDropTM spectrophotometer (OD260/OD280 nm ratio ≥ 1.8). PCR amplification was performed for bla genes, which coding for the blaTEM, blaSHV, blaCTX-M, and integron family (intI1, intI2, and intI3) using specific primers (Table 1). We used DNA of E. coli isolated and sequenced from Iranian Kidney Transplant Patients as control positive for integrase and ESBL genes, and DNA of mcr positive genes was taken from Pasteur Institute of Iran [21].
Table 1.
Sequences of primers used in this study.
| Gene | Primer sequence (5′-3′) | Product (bp) | Reference |
|---|---|---|---|
| intI1 | F: GGT CAA GGA TCT GGA TTT CG | 483 | [22] |
| R: ACA TGC GTG TAA ATC ATC GTC | |||
| intI2 | F: CAC GGA TAT GCG ACA AAA AGG T | 789 | [23] |
| R: GTA GCA AAC GAG TGA CGA AAT G | |||
| intI3 | F: AGT GGG TGG CGA ATG AG | 600 | [24] |
| R: TGT TCT TGT ATC GCC AGG TG | |||
| bla CTX-M | F: TTT GCG ATG TGC AGT ACC AGT AA | 544 | [25] |
| R: CGA TAT CGT TGG TGG TGC CAT A | |||
| bla TEM | F: AGT ATT CAA CAT TTC CGT GTC R: GCT TAA TCA GTG AGG CAC CTA TC |
850 | [26] |
| bla SHV | F: ATG CGT TAT ATT CGC CTG TG | 862 | [27] |
| R: GTT AGC GTT GCC AGT GCT CG | |||
| mcr1 | F: CGG TCA GTC CGT TTG TTC | 309 | [28] |
| R: CTT GGT CGG TCT GTA GGG | |||
| mcr2 | F: TGT TGC TTG TGC CGA TTG GA | 567 | [29] |
| R: AGA TGG TAT TGT TGG TTG CTG | |||
| ureG | F: AGA ATA TAA TCA ACC ACT GCG TA | 514 | [30] |
| R: CAT TTT GGC TGT ATC CGC TTC | |||
| Rep-typing | GTG GTG GTG GTG GTG | Variable | [31] |
PCR conditions for all these genes were 3 min at 94°C; 30 cycles of 30 seconds at 94°C, 30 seconds at 54°C, and 30 seconds at 72°C; and finally, 5 min at 72°C. The amplicons were revealed by electrophoresis on a 1% agarose gel with 0.5x TBE (Tris–borate–EDTA) running buffer and subsequent exposure to UV light in the presence of a safe stain.
2.5. (GTG)5-Rep-PCR Fingerprinting Technique
We followed the methods of (GTG)5-PCR fingerprinting, which was described by Gevers et al. [31]. Analysis of amplicon (GTG)5-PCR patterns and construction of phylogenetic tree were carried out using the curve-based algorithm (Pearson correlation) (Applied Maths, Sint-Martens-Latem, Belgium) to create a similarity scale and an unweighted pair group using arithmetic average algorithm (UPGMA) for cluster analysis, the cut-off value in this GTGS typing predestinated 100% [32]. The band sizes were compared by using a 50 bp ladder. The 15-mer primer (5′-GTGG TGGTGGTGGTG-3′) was used to amplify the repetitive sequences present in the chromosomal DNA of P. mirabilis. PCR condition was 3 min at 95°C; 30 cycles of 45 seconds at 94°C, 30 seconds at 50°C, and 45 seconds at 72°C; and finally, 5 min at 72°C. The PCR products were revealed by electrophoresis on a 1.5% agarose gel with 0.5x TBE at 2.5 hours with 65 voltage. This experiment was carried out three times. The graphical abstract of methods which performed in this study is shown in Figure 1.
Figure 1.
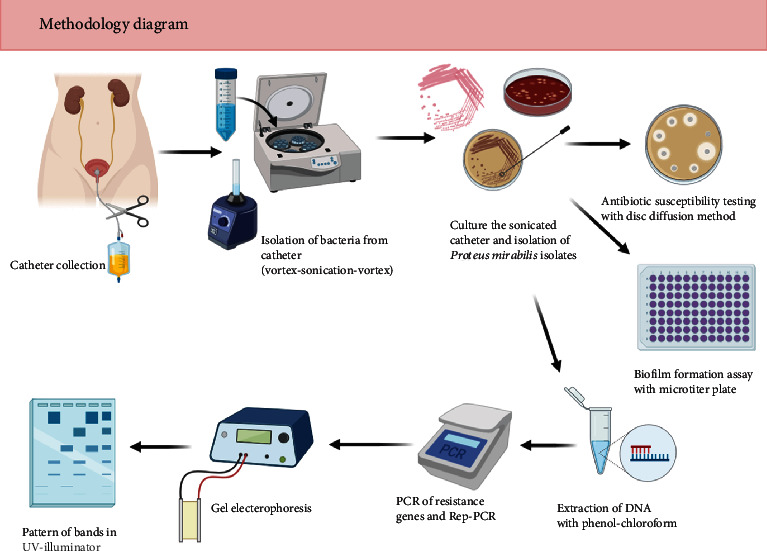
Graphical abstract of methodology.
2.6. Discriminatory Index Determination
A discriminatory index (D) was calculated to varying levels of similarity index according to the formula. In the formula, N represents the number of unrelated strains evaluated, S represents the number of distinct types, and xj represents the number of strains belonging to the jth category, assuming that strains are grouped into mutually exclusive groups.
| (1) |
2.7. Statistical Analysis
Genotypic diversity among the isolates was calculated using Nei's distances described earlier by Weir [33]. The Rep-fingerprinting methods' discriminatory ability was assessed by calculating Simpson's diversity index, as described previously [34]. Statistical analysis was performed by SPSS Statistics (Version 16). Chi-square or Fisher's exact test was used to determine any statistical association. Statistical significance was regarded as P values < 0.05.
3. Results and Discussion
From 385 collected catheters in patients admitted to ICU, 40 P. mirabilis were isolated. In this study, 72.5% of P. mirabilis were isolated from females, and 27.5% were from males. The median age was 42.5 years old (5-75 years). The duration of patients' catheterization, which bacterial isolation was performed, was 5-15 days. Furthermore, the most bacterial isolation was from the ten days of patient catheterization (50%). Patients who had a catheter for 10 to 15 days had moderate to strong biofilm formation; in addition, 50% of patients with short catheterization (7 days) had isolates with weak biofilm development (Table 2).
Table 2.
The relationship between the duration of catheterization and the strength of biofilm formation.
| Duration of catheterization | Number of isolation (%) | Strong biofilm (%) | Moderate biofilm (%) | Weak biofilm (%) |
|---|---|---|---|---|
| 15 days | 4 (10) | 3 (75) | 1 (25) | 0 (0) |
| 10 days | 20 (50) | 7 (35) | 13 (65) | 0 (0) |
| 7 days | 16 (40) | 2 (12.5) | 6 (37.5) | 8 (50) |
The antimicrobial-resistant patterns of isolates are shown in Figure 2. The resistant to tetracycline was 95% followed by nitrofurantoin (92.5%), trimethoprim/sulfamethoxazole (75%), ciprofloxacin (45%), cefotaxime (42.5%), ofloxacin (40%), ampicillin (35%), meropenem (30%), norfloxacin (25%), cefixime and amoxicillin-clavulanate (22.5%), aztreonam and amikacin (15%), ceftazidime (7.5%), and ampicillin-sulbactam (2.5%). P. mirabilis is intrinsically resistant to tetracycline and nitrofurantoin which explains its high tolerance to these drugs. Of the 40 P. mirabilis isolates, 82.5% were MDR. Furthermore, ESBL was found in 7 (17%) isolates.
Figure 2.
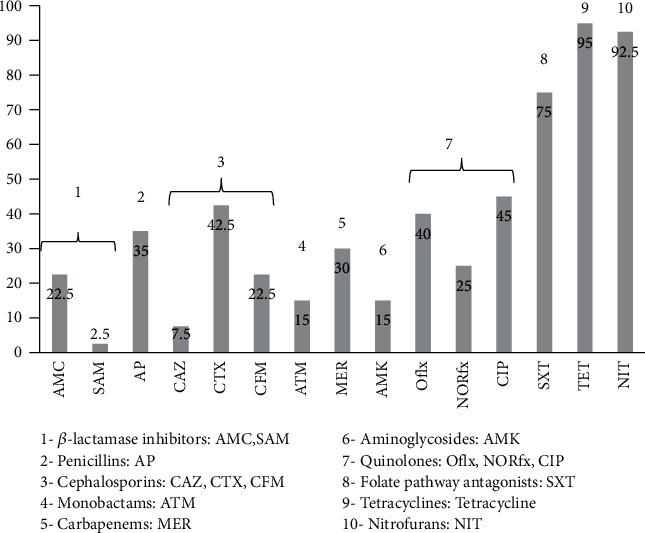
Frequency of antimicrobial resistance of Proteus mirabilis strains. Abbreviations: AMC: amoxicillin-clavulanate; SAM: ampicillin-sulbactam; AP: ampicillin; CAZ: ceftazidime; CTX: cefotaxime: CFM: cefixime; ATM: aztreonam; MER: meropenem; AMK: amikacin; Oflx: ofloxacin; NORfx: norfloxacin; CIP: ciprofloxacin; SXT: trimethoprim/sulfamethoxazole; TET: tetracycline; NIT: nitrofurantoin.
All P. mirabilis isolated from catheters were able to produce biofilm. Of 40 P. mirabilis, twelve isolates (30%) had strong biofilm ability (OD590 nm ≥ 2.5), twenty of them (50%) were moderate biofilm producer (1.5 ≤ OD590 nm < 2.5), and eight (20%) of them had weak biofilm (0.7 ≤ OD590 nm < 1.5) (Table 3).
Table 3.
The severity of biofilm and integrase prevalence.
| Biofilm | Integrase-1 positive No. (%) |
Integrase-2 positive No. (%) |
Total no No. (%) |
MDR No. (%) |
|---|---|---|---|---|
| Strong | 8 (66.6) | 3 (25) | 12 (30) | 11 (91.6) |
| Moderate | 13 (65) | 4 (20) | 20 (50) | 15 (75) |
| Weak | 4 (50) | 3 (37.5) | 8 (20) | 7 (87.5) |
| MDR | 22 (66.6) | 10 (30) | 33 (82.5) | — |
The frequency of three classes of the integron family is shown in Table 4. The most prevalent gene was intI1 with 60% (24) of isolates and 25% (10) of isolates had integrase 2 (intI2), and no detection for integrase 3; also, 15% (6) of isolates had both integrase 1 and integrase 2. The most prevalent gene in the bla ESBL family was observed in blaTEM genes with 100% (40) of isolates; 82.5% (33) of isolates had blaCTX-M genes and no detection of blaSHV. Also, PCRs targeting the mcr-1 and mcr-2 genes revealed no detection for these genes. Also, it demonstrated a significant relationship among resistance to trimethoprim/sulfamethoxazole, norfloxacin, and ofloxacin with a class 2 integron (P < 0.05).
Table 4.
Antibiotic resistance pattern of P. mirabilis isolates according to integrase 1, 2 positivity.
| Antibiotic | No. resistant integrase 1 positive No. (%) N = 24 |
No. resistant integrase 1 negative No. (%) N = 16 |
No. resistant integrase 2 positive No. (%) N = 10 |
No. resistant integrase 2 negative No. (%) N = 30 |
P value |
|---|---|---|---|---|---|
| AMC | 5 (20.8) | 4 (25) | 3 (30) | 7 (23.33) | NS |
| SAM | 0 (0) | 2 (12.5) | 1 (10) | 1 (3) | NS |
| ATM | 8 (25) | 3 (18.75) | 4 (40) | 7 (23.33) | NS |
| TET | 23 (95.8) | 15 (93.75) | 10 (100) | 28 (93.33) | NS |
| MEM | 8 (33.3) | 4 (25) | 4 (40) | 8 (26.66) | NS |
| NOR | 7 (29.1) | 5 (31.25) | 6 (60) | 6 (20) | <0.05 |
| CAZ | 9 (12.5) | 3 (18.75) | 2 (20) | 10 (33.33) | NS |
| Amp | 16 (62.5) | 6 (37.5) | 5 (50) | 10 (33.33) | NS |
| OFX | 10 (81) | 7 (43.75) | 8 (80) | 9 (30) | <0.05 |
| CFM | 7 (29.1) | 2 (12.5) | 2 (20) | 7 (23.33) | NS |
| SXT | 19 (79.1) | 11 (68.75) | 10 (100) | 20 (66.66) | <0.05 |
| CIP | 16 (50) | 9 (56.25) | 9 (90) | 16 (53.33) | NS |
| NIT | 24 (95.8) | 15 (93.75) | 10 (100) | 29 (96.66) | NS |
| AMK | 6 (83) | 2 (12.5) | 2 (20) | 6 (20) | NS |
| CTX | 14 (76) | 6 (37.5) | 4 (40) | 16 (53.33) | NS |
NS: not significant.
Rep-PCR or (GTG)5-PCR fingerprints of 40 isolates generated 7 to 13 bands with the molecular size ranging from 250 bp to more than 1 kb. (GTG)5-PCR amplification identified 39(GTG)5 types (G1–G39) of 40 isolates that 38 isolates had unique patterns (Figure 3). D was calculated from a constructed phylogenetic tree, and according to the formula described before, discriminatory power was calculated at 99.8%.
Figure 3.
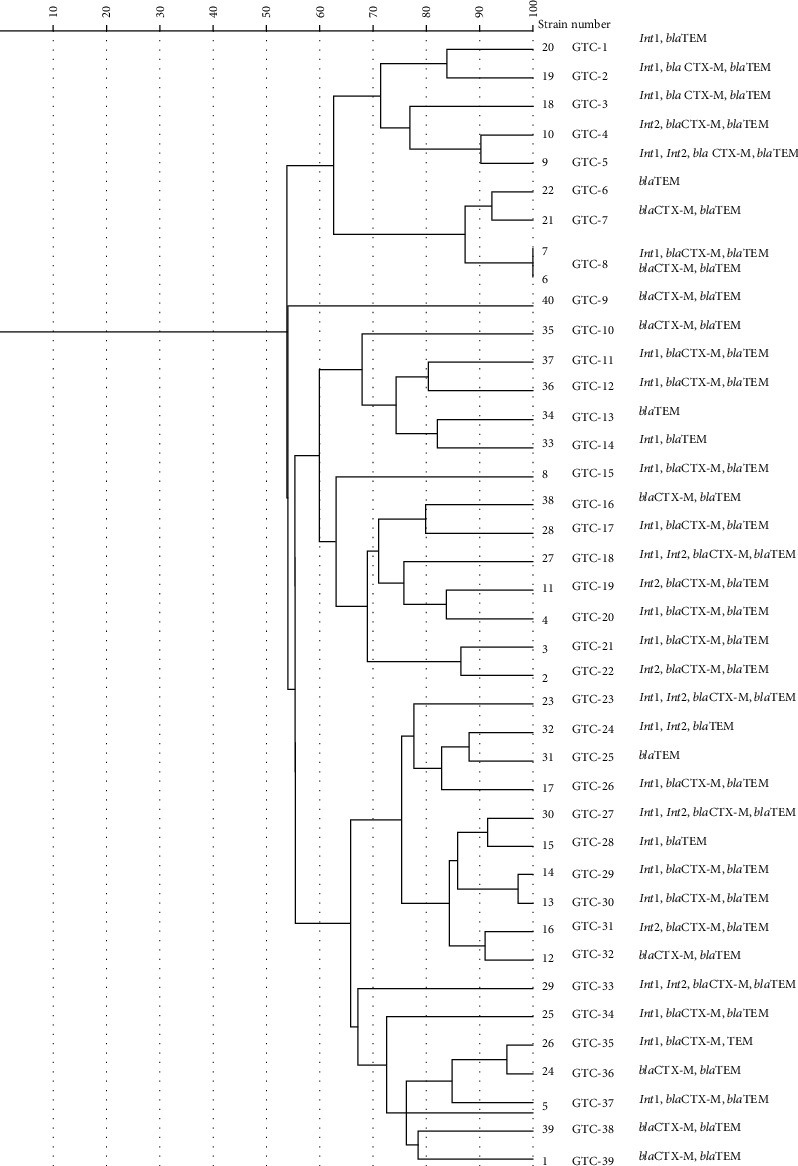
Dendrogram showing genetic relatedness of 40 strains of Proteus mirabilis determined by Rep-PCR analysis with Dice similarity coefficient and unweighted pair-group method with average linkages clustering method. Cut-off value: 100%.
P. mirabilis is a common cause of complicated UTI, particularly in patients with functional or anatomical urinary tract abnormalities. Swarming motility can promote the migration of P. mirabilis from the periurethral area along the catheter surface into the urinary bladder and initiate CAUTIs in patients undergoing long-term catheterization. There are limited data about the pathogenicity and antibiotic resistance of catheterized P. mirabilis in Iran. In this study, we evaluated the molecular characteristics of P. mirabilis isolated from CAUTI. The result of the current study showed that all of the isolates had the ability of biofilm formation, being in line with other studies [30, 35]. The severity of biofilm formation depends on the day of catheterization.
The survival and recovery strategy, engrossed by some microbial species in front of rough environmental situations, can form a biofilm, thereby causing antibiotic resistance boosting. The severity of the biofilm-associated antimicrobial resistance of microorganisms is higher than their planktonic form [36]. The present study revealed an increasing growth in antibiotic resistance prevalence in P. mirabilis over the past years [30, 37]. The ability of isolates to produce biofilm performs a matrix hindering the penetration of antimicrobial agents through biofilm layers. Moreover, the physiological attributes of microbial cells within biofilms, particularly persister cells, and acquiring of some resistance genes, including integrons family and ESBL genes, could explain biofilm resistance to antimicrobial agents [38]. Although Ojdana et al. [39] found the highest susceptibility to meropenem (100%) in P. mirabilis isolates, we found the highest susceptibility to ampicillin-sulbactam (95%); this may be due to differences in the types and amount of antibiotics used in different countries, geographical regions, years of study, and the fact that ampicillin-sulbactam was not used in the antibiotic test, which may explain the difference. The study also revealed a high prevalence of MDR isolates (82.5%), being consistent with the findings of one study by Alabi et al. [37]. In addition, we found a report that contradicts our findings [30] so that we could justify that these differences may be due to differences in the date and location of isolation, as well as differences in the source of isolation (catheter, urine, wound, and blood). In our study, the isolated species were from the biofilm of the catheter causing higher antibiotic resistance, leading to higher MDR percentage.
This study demonstrated a significant association between resistance to trimethoprim/sulfamethoxazole, norfloxacin, and ofloxacin and a class 2 integron (P < 0.05). In other words, the presence of the integrase 2 gene in species could lead to resistance to antibiotics, such as trimethoprim/sulfamethoxazole, norfloxacin, and ofloxacin.
Previous studies confirmed that the presence of these elements, mostly class 1 integron, could be considered the evidence for a multidrug-resistant phenotype and associated with the increased frequency of resistance to some antibiotics, such as ciprofloxacin, sulfamethoxazole, and cotrimoxazole [40, 41]. The results indicated a high prevalence of integron-positive isolates and a high antibiotic resistance level regarding the association between integron positive and antibiotic resistance.
In the light of the foregoing, the MDR phenomenon is often associated with the presence of integrons. The coexistence of the ESBL family and integrase genes increases the possibility of the emerging bacteria as a potential carrier of resistance determinants. Integrons can transfer, integrate, express, and distribute resistant agents, facilitating the MDR phenotype of the bacteria. Moreover, these elements entrap several resistance genes belonging to various classes. The prevalence of class 1, 2, and 3 integrons among isolates of P. mirabilis investigated in this work is consistent with that reported by others [42–44] and inconsistent with the study conducted by Fursova et al. [45]; this difference could be owing to the time and the source of isolation.
With bacteria's ability to produce ESBL enzymes, the phenomenon of broad-spectrum resistance to β-lactam antibiotics is observed and confirmed, but the presence of genes not necessarily leads to the phenotypical appearance of ESBLs as numerous reports have demonstrated [39, 46]. Our analysis revealed such a relationship; therefore, 17.5% of isolates had the phenotypical occurrence of ESBLs. However, the ESBL gene prevalence was higher, which might result in the lack of expression of these genes. According to CLSI reports [47], Proteus species bring only the TEM beta-lactamase gene for a long time. At present, the spread of CTX-M is replacing TEM and SHV genes. Hence, the prevalence of blaCTX-M is increasing, being in line with our study results [48] with this difference that blaTEM rampancy is one hundred percent; this could indicate the spread of ESBL genes among enteric bacteria and acquiring of those genes among the species as well as creation of new and more resistant species that is an alarming issue.
Surveying the epidemiological data over the past years could indicate a dramatic increase in antimicrobial resistance. Overall, the data present continually enhancing resistance to both β-lactams and other groups of antibiotics in the Enterobacteriaceae family's bacteria [26, 27]. Studies have demonstrated that the genes responsible for the production of CTX-M and TEM β-lactamases are more prevalent among tested strains than genes encoding SHV-type β-lactamases with no detection report. In this regard, the results of our study are in line with those of previous studies [37, 39, 45].
The steadfast intensify of concurrent resistance to different antibiotic classes significantly reduces the possibility of treatment of infections caused by ESBL producers and MDR isolates [49]. As a result, we aimed to analyze the level of resistance among ESBL-positive and MDR-tested strains. The different percent of resistance in other studies in different parts of the world is most likely due to variations in doctors' antibiotic prescribing prevalence and use in each country, which may be speculative. Other factors that may explain the disparities are the strains and sequence types that are the most prevalent ones in different regions of the world. Increased resistance to antibiotics has been observed in our study in proportion to other studies in Iran and other parts of the world. In this work, 63% of SXT-resistant isolates had the intI1 gene that was in contrast to further research reporting a 100% relationship between the SXT resistance and the presence of intI1 [50]. We can explain this issue or the existence of intI2 or other resistance genes. As indicated by the study conducted by Alabi et al. [37], 100% of MDR isolates had integrase genes, while this work revealed that 72.5% of MDR isolates had integrase genes. Until now, there is no reported existence of the intI3 gene in P. mirabilis isolates, being in line with other studies [37, 43]. As we know, P. mirabilis has the intrinsic chromosomal resistance to colistin, but emergence of plasmid-mediated genes for colistin resistance in this bacterium could be a disaster that previous studies reported it to be very low in mcr1 and mcr2 genes [11]. In this study, fortunately, there is not a report of those genes. To control the dissemination of these elements and resistance genes, molecular typing of isolates could be sufficient. Molecular typing of P. mirabilis is the typical assay conducted to inspect genetic connectedness, discriminate between isolates, and exhibit the source of infection and transmission route with adequate accuracy to recognize the origin of nosocomial outbreaks.
Two efficient methods for Proteus characterization at the species level and designation of single strains of P. mirabilis are ribotyping and PFGE [51–53]. However, these methods have limitations for use in ordinary clinical laboratories, including being laborious, expensive, and time-consuming. An alternative simple and cost-benefit molecular typing, PCR-based, has been successfully used to identify P. mirabilis isolates [54].
The constructed phylogeny tree from Rep-PCR typing effectively identified the genetic relatedness of P. mirabilis isolated from the catheter. Rep-PCR (GTG) typing of forty catheter isolates in this study showed 39 types, of which two isolates from one hospital were put in one GTG type (GTG8) with 100% identity, which could be due to the dissemination of the isolates from the hospital environment to patients by personnel or nurses or nosocomial infection, and other isolates were put in single GTG type, which demonstrated high diversity among isolates. Rep-typing results show high discriminatory power, being completely in agreement with the study conducted by Bedenic et al. [55]. The study typing did not confirm the previous research conducted by Michelim et al. [56]. This is most likely due to inconsistencies in the samples and DNA extraction preparation processes, and the most significant reason is the disparity in primers used in this analysis, which differs from our primers.
This GTG8 type, between two isolates, has a similar resistance gene, but the lack of the intI1 gene has been observed for one of them, which might because the gene release has not yet occurred. The high diversity of isolates in the phylogenic tree was seen in the previous study [30].
The limitation of this study was the lack of a modern technique, such as multilocus sequence typing (MLST) and PFGE, to determine the clonality of isolates due to limited financial sources.
4. Conclusions
In this study, 82.5% of isolates were MDR with high antibiotic resistance to trimethoprim-sulfamethoxazole. The intI1 and blaTEM were the most prevalent genes in the integron and ESBL gene family. High diversity was observed in isolates with Rep-PCR. The outcome of persistence MDR strains under antimicrobial selective pressure in the hospital environment will increase both the chance of an advantageous mutation and the opportunity to acquire additional resistance genes. Nevertheless, the existence of the same type of isolates could be an alarming issue for healthcare providers' hygiene conditions to eliminate these infections particularly spread of resistance genes and highlight the importance of sporadically epidemiological studies and methods with high discriminatory power.
Acknowledgments
This research benefited from a grant from Isfahan University of Medical Sciences. This work was supported by Isfahan University of Medical Sciences, Isfahan, I.R. Iran, through Grant No. 398353.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- 1.Jamil R. T., Foris L. A., Snowden J. Proteus mirabilis infections. StatPearls Publishing; 2017. [PubMed] [Google Scholar]
- 2.Jones S. M., Yerly J., Hu Y., Ceri H., Martinuzzi R. Structure of Proteus mirabilis biofilms grown in artificial urine and standard laboratory media. FEMS Microbiology Letters. 2007;268(1):16–21. doi: 10.1111/j.1574-6968.2006.00587.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen C.-Y., Chen Y.-H., Lu P.-L., Lin W.-R., Chen T.-C., Lin C.-Y. Proteus mirabilis urinary tract infection and bacteremia: risk factors, clinical presentation, and outcomes. Journal of Microbiology, Immunology, and Infection. 2012;45(3):228–236. doi: 10.1016/j.jmii.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Vaidyanathan S., Soni B. M., Hughes P. L., Singh G., Oo T. Severe Ventral Erosion of Penis Caused by Indwelling Urethral Catheter and Inflation of Foley Balloon in Urethra—Need to Create List of “Never Events in Spinal Cord Injury” in order to Prevent These Complications from Happening in Paraplegic and Tetraplegic Patients. Advances in urology. 2010;2010:5. doi: 10.1155/2010/461539.461539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasfi R., Hamed S. M., Abd Allah M. A. W., Ismail L. Proteus mirabilis biofilm: development and therapeutic strategies. Frontiers in Cellular and Infection Microbiology. 2020;10:p. 414. doi: 10.3389/fcimb.2020.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leverstein-van Hall M. A., Blok H. E. M., Donders A. R. T., Paauw A., Fluit A. C., Verhoef J. Multidrug resistance among Enterobacteriaceae is strongly associated with the presence of integrons and is independent of species or isolate origin. The Journal of Infectious Diseases. 2003;187(2):251–259. doi: 10.1086/345880. [DOI] [PubMed] [Google Scholar]
- 7.Chen C.-M., Lai C.-H., Wu H.-J., Wu L.-T. Genetic characteristic of class 1 integrons in proteus mirabilis isolates from urine samples. BioMedicine. 2017;7(2):p. 9. doi: 10.1051/bmdcn/2017070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluit A., Schmitz F. J. Class 1 integrons, gene cassettes, mobility, and epidemiology. European Journal of Clinical Microbiology & Infectious Diseases. 1999;18(11):761–770. doi: 10.1007/s100960050398. [DOI] [PubMed] [Google Scholar]
- 9.Wei Q., Hu Q., Li S., et al. A novel functional class 2 integron in clinical Proteus mirabilis isolates. The Journal of Antimicrobial Chemotherapy. 2014;69(4):973–976. doi: 10.1093/jac/dkt456. [DOI] [PubMed] [Google Scholar]
- 10.Bonomo R. A. Multiple antibiotic-resistant bacteria in long-term-care facilities: an emerging problem in the practice of infectious diseases. Clinical Infectious Diseases. 2000;31(6):1414–1422. doi: 10.1086/317489. [DOI] [PubMed] [Google Scholar]
- 11.Hmede Z., Kassem I. I. First report of the plasmid-borne colistin resistance gene (mcr - 1 ) in Proteus mirabilis isolated from a toddler in non-clinical settings. IDCases. 2019;18:p. e00651. doi: 10.1016/j.idcr.2019.e00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahm B.-K., Maldonado Y., Schreiber E., Bhunia A. K., Nakatsu C. H. Subtyping of foodborne and environmental isolates of Escherichia coli by multiplex-PCR, rep-PCR, PFGE, ribotyping and AFLP. Journal of Microbiological Methods. 2003;53(3):387–399. doi: 10.1016/S0167-7012(02)00259-2. [DOI] [PubMed] [Google Scholar]
- 13.Baldy-Chudzik K., Stosik M. Specific genomic fingerprints of Escherichia coli strains with repetitive sequences and PCR as an effective tool for monitoring freshwater environments. Polish Journal of Environmental Studies. 2005;14(5) [Google Scholar]
- 14.Mohapatra B. R., Mazumder A. Comparative efficacy of five different rep-PCR methods to discriminate Escherichia coli populations in aquatic environments. Water Science and Technology. 2008;58(3):537–547. doi: 10.2166/wst.2008.424. [DOI] [PubMed] [Google Scholar]
- 15.Mandakhalikar K. D., Rahmat J. N., Chiong E., Neoh K. G., Shen L., Tambyah P. A. Extraction and quantification of biofilm bacteria: method optimized for urinary catheters. Scientific reports. 2018;8(1):p. 8069. doi: 10.1038/s41598-018-26342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. CLSI supplement M100. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2018. [Google Scholar]
- 17.Magiorakos A.-P., Srinivasan A., Carey R. B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 18.Drieux L., Brossier F., Sougakoff W., Jarlier V. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clinical Microbiology and Infection. 2008;14:90–103. doi: 10.1111/j.1469-0691.2007.01846.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Toole G. A. Microtiter dish biofilm formation assay. Journal of visualized experiments: JoVE. 2011;47 doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J., Russell D. W. Purification of nucleic acids by extraction with phenol: chloroform. Cold Spring Harbor Protocols. 2006;2006(1) doi: 10.1101/pdb.prot4455. [DOI] [PubMed] [Google Scholar]
- 21.Halaji M., Shahidi S., Atapour A., Ataei B., Feizi A., Havaei S. A. Characterization of extended-spectrum β-lactamase-producing uropathogenic Escherichia coli among Iranian kidney transplant Patients. Infection and drug resistance. 2020;Volume 13:1429–1437. doi: 10.2147/IDR.S248572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazel D., Dychinco B., Webb V. A., Davies J. Antibiotic resistance in the ECOR collection: integrons and identification of a novel aad gene. Antimicrobial Agents and Chemotherapy. 2000;44(6):1568–1574. doi: 10.1128/AAC.44.6.1568-1574.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gündoğdu A., Long Y. B., Vollmerhausen T. L., Katouli M. Antimicrobial resistance and distribution of sul genes and integron-associated intI genes among uropathogenic Escherichia coli in Queensland, Australia. Journal of Medical Microbiology. 2011;60(11):1633–1642. doi: 10.1099/jmm.0.034140-0. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein C., Lee M. D., Sanchez S., et al. Incidence of class 1 and 2 integrases in clinical and commensal bacteria from livestock, companion animals, and exotics. Antimicrobial Agents and Chemotherapy. 2001;45(3):723–726. doi: 10.1128/AAC.45.3.723-726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelstein M., Pimkin M., Palagin I., Edelstein I., Stratchounski L. Prevalence and molecular epidemiology of CTX-M extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Russian hospitals. Antimicrobial Agents and Chemotherapy. 2003;47(12):3724–3732. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson J. R., Stell A. L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. The Journal of Infectious Diseases. 2000;181(1):261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 27.Wiegand I., Geiss H. K., Mack D., Stürenburg E., Seifert H. Detection of extended-spectrum beta-lactamases among Enterobacteriaceae by use of semiautomated microbiology systems and manual detection procedures. Journal of Clinical Microbiology. 2007;45(4):1167–1174. doi: 10.1128/JCM.01988-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y.-Y., Wang Y., Walsh T. R., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 29.Xavier B. B., Lammens C., Ruhal R., et al. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Eurosurveillance. 2016;21(27) doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 30.Mirzaei A., Habibi M., Bouzari S., Karam M. R. A. Characterization of antibiotic-susceptibility patterns, virulence factor profiles and clonal relatedness in Proteus mirabilis isolates from patients with urinary tract infection in Iran. Infection and drug resistance. 2019;Volume 12:3967–3979. doi: 10.2147/IDR.S230303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gevers D., Huys G., Swings J. Applicability of rep-PCR fingerprinting for identification of Lactobacillus species. FEMS Microbiology Letters. 2001;205(1):31–36. doi: 10.1111/j.1574-6968.2001.tb10921.x. [DOI] [PubMed] [Google Scholar]
- 32.Khare N., Kaushik M., Martin J. P., Mohanty A., Gulati P. Genotypic diversity in multi-drug-resistant E. coli isolated from animal feces and Yamuna River water, India, using rep-PCR fingerprinting. Environmental Monitoring and Assessment. 2020;192(11):1–13. doi: 10.1007/s10661-020-08635-1. [DOI] [PubMed] [Google Scholar]
- 33.Weir B. S. Genetic data analysis. Methods for discrete population genetic data. Sinauer Associates, Inc. Publishers; 1990. [Google Scholar]
- 34.Hunter P. R., Gaston M. A. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. Journal of Clinical Microbiology. 1988;26(11):2465–2466. doi: 10.1128/JCM.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Oliveira W. D., Barboza M. G. L., Faustino G., et al. Virulence, resistance and clonality of Proteus mirabilis isolated from patients with community-acquired urinary tract infection (CA-UTI) in Brazil. Microbial Pathogenesis. 2020;152 doi: 10.1016/j.micpath.2020.104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. International Journal of Antimicrobial Agents. 2010;35(4):322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Alabi O. S., Mendonça N., Adeleke O. E., da Silva G. J. Molecular screening of antibiotic-resistant determinants among multidrug-resistant clinical isolates of Proteus mirabilis from SouthWest Nigeria. African Health Sciences. 2017;17(2):356–365. doi: 10.4314/ahs.v17i2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng B. S., Zhang W., Harrison J. J., et al. The extracellular matrix protects Pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environmental Microbiology. 2013;15(10):2865–2878. doi: 10.1111/1462-2920.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojdana D., Sacha P., Wieczorek P., et al. The occurrence of blaCTX-M, blaSHV, and blaTEM genes in extended-spectrum β-lactamase-positive strains of Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in Poland. International Journal of Antibiotics. 2014;2014:7. doi: 10.1155/2014/935842.935842 [DOI] [Google Scholar]
- 40.Rezaee M. A., Sheikhalizadeh V., Hasani A. Detection of integrons among multi-drug resistant (MDR) Escherichia coli strains isolated from clinical specimens in northern west of Iran. Brazilian Journal of Microbiology. 2011;42(4):1308–1313. doi: 10.1590/S1517-83822011000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yekani M., Memar M. Y., Baghi H. B., Sefidan F. Y., Alizadeh N., Ghotaslou R. Association of integrons with multidrug-resistant isolates among phylogenic groups of uropathogenic Escherichia coli. Microbiology Research. 2018;9(1) doi: 10.4081/mr.2018.7484. [DOI] [Google Scholar]
- 42.White P. A., McIver C. J., Rawlinson W. D. Integrons and gene cassettes in theenterobacteriaceae. Antimicrobial Agents and Chemotherapy. 2001;45(9):2658–2661. doi: 10.1128/AAC.45.9.2658-2661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes A., Bello H., Domínguez M., Mella S., Zemelman R., González G. Prevalence and types of class 1 integrons in aminoglycoside-resistant Enterobacteriaceae from several Chilean hospitals. The Journal of Antimicrobial Chemotherapy. 2003;51(2):317–321. doi: 10.1093/jac/dkg083. [DOI] [PubMed] [Google Scholar]
- 44.Chang C.-Y., Chang L.-L., Chang Y.-H., LEE T.-M., CHANG S.-F. Characterisation of drug resistance gene cassettes associated with class 1 integrons in clinical isolates of Escherichia coli from Taiwan, ROC. Journal of Medical Microbiology. 2000;49(12):1097–1102. doi: 10.1099/0022-1317-49-12-1097. [DOI] [PubMed] [Google Scholar]
- 45.Fursova N. K., Astashkin E. I., Knyazeva A. I., et al. The spread of bla OXA-48 and bla OXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Annals of clinical Microbiology and Antimicrobials. 2015;14(1):1–9. doi: 10.1186/s12941-015-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nijssen S., Florijn A., Bonten M. J. M., Schmitz F. J., Verhoef J., Fluit A. C. Beta-lactam susceptibilities and prevalence of ESBL-producing isolates among more than 5000 European Enterobacteriaceae isolates. International Journal of Antimicrobial Agents. 2004;24(6):585–591. doi: 10.1016/j.ijantimicag.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Wayne P. A. Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20; 2010. [Google Scholar]
- 48.Musa H. A., Osman M. A. M., Abdelaziz Y. H., Mohamed S., Ibrahim-Saeed M. Distribution of extended-spectrum beta-lactamase TEM and CTX-M resistance genes among Proteus species isolated in Sudan. VacciMonitor. 2019;28(2):80–84. [Google Scholar]
- 49.Giamarellou H., Poulakou G. Multidrug-resistant gram-negative infections. Drugs. 2009;69(14):1879–1901. doi: 10.2165/11315690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 50.Leverstein-van Hall M. A., Paauw A., Box A. T. A., Blok H. E. M., Verhoef J., Fluit A. C. Presence of integron-associated resistance in the community is widespread and contributes to multidrug resistance in the hospital. Journal of Clinical Microbiology. 2002;40(8):3038–3040. doi: 10.1128/JCM.40.8.3038-3040.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pignato S., Giammanco G. M., Grimont F., Grimont P. A. D., Giammanco G. Molecular characterization of the genera Proteus, Morganella, and Providencia by ribotyping. Journal of Clinical Microbiology. 1999;37(9):2840–2847. doi: 10.1128/JCM.37.9.2840-2847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabbuba N. A., Mahenthiralingam E., Stickler D. J. Molecular epidemiology of Proteus mirabilis infections of the catheterized urinary tract. Journal of Clinical Microbiology. 2003;41(11):4961–4965. doi: 10.1128/JCM.41.11.4961-4965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfaller M. A., Mujeeb I., Hollis R. J., Jones R. N., Doern G. V. Evaluation of the discriminatory powers of the Dienes test and ribotyping as typing methods for Proteus mirabilis. Journal of clinical microbiology. 2000;38(3):1077–1080. doi: 10.1128/JCM.38.3.1077-1080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olive D. M., Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. Journal of Clinical Microbiology. 1999;37(6):1661–1669. doi: 10.1128/JCM.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedenić B., Firis N., Elveđi-Gašparović V., et al. Emergence of multidrug-resistant Proteus mirabilis in a long-term care facility in Croatia. Wiener Klinische Wochenschrift. 2016;128(11–12):404–413. doi: 10.1007/s00508-016-1005-x. [DOI] [PubMed] [Google Scholar]
- 56.Michelim L., Muller G., Zacaria J., Delamare A. P. L., da Costa S. O. P., Echeverrigaray S. Comparison of PCR-based molecular markers for the characterization of Proteus mirabilis clinical isolates. Brazilian journal of infectious diseases. 2008;12(5):423–429. doi: 10.1590/s1413-86702008000500014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


