Abstract
Background and Purpose
Qualitative studies about the abnormalities appreciated on routine magnetic resonance imaging (MRI) sequences in prematurely born adults are lacking. This article aimed at filling this knowledge gap by (1) qualitatively describing routine imaging findings in prematurely born adults, (2) evaluating measures for routine image interpretation and (3) investigating the impact of perinatal variables related to premature birth.
Methods
In this study two board-certified radiologists assessed T1-weighted and FLAIR-weighted images of 100 prematurely born adults born very preterm (VP <32 weeks) and/or at very low birth weight (VLBW <1500 g) and 106 controls born at full term (FT) (mean age 26.8 ± 0.7 years). The number of white matter lesions (WML) was counted according to localization. Lateral ventricle volume (LVV) was evaluated subjectively and by measurements of Evans’ index (EI) and frontal-occipital-horn ratio (FOHR). Freesurfer-based volumetry served as reference standard. Miscellaneous incidental findings were noted as free text.
Results
The LVV was increased in 24.7% of VP/VLBW individuals and significantly larger than in FT controls. This was best identified by measurement of FOHR (AUC = 0.928). Ventricular enlargement was predicted by low gestational age (odds ratio: 0.71, 95% CI 0.51–0.98) and presence of neonatal intracranial hemorrhage (odds ratio: 0.26, 95% CI 0.07–0.92). The numbers of deep and periventricular WML were increased while subcortical WMLs were not.
Conclusion
Enlargement of the LVV and deep and periventricular WMLs are typical sequelae of premature birth that can be appreciated on routine brain MRI. To increase sensitivity of abnormal LVV detection, measurement of FOHR seems feasible in clinical practice.
Electronic supplementary material
The online version of this article (10.1007/s00062-020-00901-6) contains supplementary material, which is available to authorized users.
Keywords: Preterm birth, Diagnostic imaging, White matter diseases, Neuroradiology, Lateral ventricles
Introduction
Premature birth, defined as birth earlier than 37 gestational weeks, is estimated to have a prevalence of 11% worldwide and survival of preterm infants is increasing [1–3]. Preterm birth represents the major cause of infant mortality in high-income countries with increasing severity for lower gestational age [4]. Especially individuals born very prematurely, i.e. those born very preterm (VP <32 gestational weeks) or with very low birth weight (VLBW <1500 g) are at increased risk for long-term morbidity, for example with respect to neurocognitive impairment and psychiatric disorders [5–7]. Thanks to advances in neonatology, survival rates have increased in particular for very prematurely born children in the last decades [8].
Premature birth has been shown to be associated with an increased risk of white matter injury (WMI), either by relatively rare hemorrhagic or more commonly by nonhemorrhagic complications [2]. Perinatal WMI covers a spectrum ranging from its most severe form, cystic periventricular leukomalacia (PVL), to more diffuse forms caused by disturbed pre-oligodendrocyte maturation and axon myelination [9–11]. Punctate white matter lesions (PWML), ventriculomegaly due to white matter dysmaturation and diffuse excessive high signal intensity (DEHSI) on FLAIR-weighted images have been proposed as correlates of non-cystic WMI in prematurely born infants [10–12]; however, DEHSI has been shown to be a highly subjective imaging feature, dependent on the time of scanning and without reliable associations to outcomes of neurodevelopment [12, 13]. The pathophysiology underlying long-term structural brain alterations is under extensive investigation on a microstructural and macrostructural level using advanced neuroimaging methods [2, 14–17]. Due to the increased prevalence of survivors of premature birth, these individuals will become more likely to present to radiology departments and most likely undergo unrelated MRI examinations of the brain.
To date, there is a paucity of data regarding typical MRI findings in prematurely born adults that can be appreciated on routine sequences; however, this information will help the correct interpretation of presumably incidental findings. The purpose of this study was to qualitatively describe incidental findings with an emphasis on FLAIR-hyperintense PWML and ventricular enlargement in a large cohort of prematurely born adults and controls born at full term (FT). These findings were correlated with parameters of premature birth and it was established how to best measure ventricular size in order to identify ventriculomegaly in young adults in clinical practice. The impact of perinatal variables of premature birth on these findings was investigated and practical implications of the results in different diagnostic scenarios for the radiologist are discussed.
Material and Methods
Study Participants and Study Design
The participants examined in this study are part of the Bavarian Longitudinal Study, a geographically defined, whole population sample of neonatal at-risk children and healthy full-term controls who were followed from birth until adulthood [18, 19]. All participants underwent an MRI scan of the brain at approximately 26 years of age for research purposes only. For detailed information about recruitment of study participants, please see Fig. S1. The following conditions led to exclusion of participants before the MRI examination: claustrophobia, inability to lie still during the MRI, cerebral palsy, medical instability, epilepsy, tinnitus, previous central nervous system (CNS) trauma or disease, presence of MRI critical implants, and strong visual impairment. After exclusion of images with motion artifacts, FLAIR and T1 sequences were available for 100 VP/VLBW and 106 FT individuals. Papers about structural and functional imaging findings of subsamples of the present cohort of VP/VLBW were published before [16, 20–26]. The MRI examinations took place at two sites: The Department of Neuroradiology, Klinikum rechts der Isar, Technical University of Munich (n = 145) and the Department of Radiology, University Hospital Bonn (n = 67).
Birth-Related Variables
Gestational age (GA) was estimated from maternal reports on the first day of the last menstrual period and serial ultrasounds during pregnancy. In cases where the 2 measurements differed by more than 2 weeks, clinical assessment at birth with the Dubowitz method was applied [27]. Maternal age, birth weight (BW) and duration of hospitalization, and presence of intracranial hemorrhage, including the grade of periventricular hemorrhage (PVH) [28] were obtained from obstetric records.
MRI Data Acquisition
The MRI examinations were performed at both sites on either a Philips Achieva 3T or a Philips Ingenia 3T (Philips Medical Systems, Best, The Netherlands) system using 8‑channel SENSE head-coils. Subject distribution among scanners and sequence specifications are given in Table S1. Across all scanners, sequence parameters were kept identical for T1 and FLAIR sequences. Scanners were checked regularly to provide optimal scanning conditions. The MRI physicists at the University Hospital Bonn and Klinikum rechts der Isar regularly scanned imaging phantoms, to ensure within-scanner signal stability over time. Signal-to-noise ratio (SNR) was not significantly different between scanners (one-way ANOVA with factor “scanner-ID” [Bonn 1, Bonn 2, Munich 1, Munich 2]; F (3182) = 1.84, p = 0.11). For FLAIR sequences fast spin echo acquisition was used based on the variable refocusing 35° flip angle sweep technique [29].
MRI Data Analysis
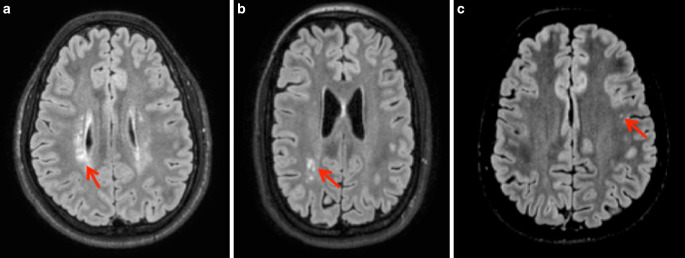
Two board-certified radiologists with 6 years experience evaluated FLAIR and T1-weighted images blinded to birth status. Supratentorial hyperintense lesions on FLAIR-weighted imaging were counted separately depending on their localization (periventricular, deep white matter, subcortical). Subcortical localization was defined as a distance <3 mm from the cerebral cortex, periventricular localization was defined by direct contact with the ventricular surface. Examples of periventricular, deep white matter and subcortical FLAIR hyperintense lesions are shown in Fig. 1. Lateral ventricle volume (LVV) was rated subjectively based on the radiologist’s impression in a yes or no fashion. Additionally, the Evans’ index (EI) and FOHR were measured as described previously [30]: the EI is defined as the ratio between the largest axial diameter of the frontal lateral ventricle horns and the biparietal diameter [31] and FOHR is defined as the sum of largest axial frontal horn and occipital horn diameter divided by double the biparietal diameter [30]. Due to the well-established excellent intrarater and interrater reliability [32], EI and FOHR were only measured by reader 1. Incidental findings were noted as free text.
Fig. 1.
Fluid-attenuated inversion recovery (FLAIR) lesion localization. Examples of FLAIR hyperintense white matter lesions in periventricular (a), deep white matter (b) or subcortical (c) localization. Periventricular FLAIR lesions abut the ventricular surface, subcortical FLAIR lesions lie within 3 mm of the cortical ribbon, without direct contact. Representative lesions are marked with a red arrow
In order to obtain fully automated and volumetric measures as a reference standard, T1-weighted scans of all participants were analyzed with the Freesurfer image analysis suite v6.0, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu) and volumes of subcortical structures were extracted [33]. The LVV was calculated as the sum of extracted left/right lateral ventricle and left/right inferior lateral ventricle volumes. The ratio between bilateral LVV and total intracranial volume (TIV) was calculated in order to correct for effects of different head sizes between individuals. Abnormal volume of the lateral ventricle was defined as two standard deviations above the bilateral LVV/TIV ratio in the control group. Youden’s index was calculated to determine the optimal cut-off for FOHR and EI.
Statistical Analysis
Statistical differences regarding the patient groups were tested by Student’s t‑test (age, maternal age, gestational age, birth weight) and χ2-test (sex). Logistic regression analysis was used to determine predictors of abnormal LVV, linear regression analysis was used to determine predictors of absolute LVV. Pearson correlation analysis was used to investigate the relationship between PVH grade and LVV. Interrater reliability for categorical variables was tested using Cohen’s kappa. Intraclass correlation coefficients were calculated to assess interrater reliability regarding flair lesion count for consistency from a 2-way mixed model. Statistical computations were performed with the SPSS software package (SPSS Statistics for Mac, version 25.0; IBM, Armonk, NY, USA). Statistical significance was set at p < 0.05.
Ethics Statement
The study was carried out in accordance with the Declaration of Helsinki and was approved by the local institutional review boards (#153/10). Written consent was obtained from all participants. All study participants received travel expenses and a small payment for participation.
Results
Study Cohort
Group demographic and clinical background variables are shown in Table 1. From 682 VP/VLBW neonates included in the initial study cohort, 260 participated in the psychological assessment at age 26 years and 101 underwent brain MRI (see flow diagram of study participants in Fig. S1). There were no significant differences between the VP/VLBW and FT group regarding age at scanning (p = 0.152), sex (p = 0.937), and maternal age (p = 0.868). By design, VP/VLBW subjects had significantly lower GA (p < 0.001) and lower BW (p < 0.001) and were hospitalized for a longer time after birth (p < 0.001).
Table 1.
Demographic data and perinatal variables
| VP/VLBW (n = 100) | FT (n = 106) | p value | |||||
|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | ||
| Sex (male/female) | 57/43 | – | – | 61/45 | – | – | 0.937 |
| Age (years) | 26.7 | ±0.6 | 25.7–28.3 | 26.8 | ±0.7 | 25.5–28.9 | 0.152 |
| GA (weeks) | 30.5 | ±2.1 | 25–36 | 39.7 | ±1.1 | 37–42 | <0.001 |
| BW (g) | 1328 | ±313 | 630–2070 | 3395 | ±448 | 2120–4670 | <0.001 |
| Hospitalization (days) | 72.3 | ±26.6 | 24–170 | 7.0 | ±3.0 | 2–26 | <0.001 |
| Maternal age (years) | 29.5 | ±4.7 | 16–41 | 29.4 | ±5.1 | 18–42 | 0.868 |
Statistical comparisons: sex, SES with χ2 statistics; age, GA, BW, Hospitalization, maternal age, IQ with two sample t‑tests
BW birth weight, FT full-term, GA gestational age, M Mean; maternal age, maternal age at birth, SD standard deviation, VP/VLBW very preterm and/or very low birthweight
Of 100 premature-born adults, 16 were diagnosed with intracranial hemorrhage (ICH) in the neonatal period (PVH grade 1: 5 (31.3%), grade 2: 7 (43.8%), grade 3: 3 (18.8%), grade 4: 1 (6.3%)).
Increased Rate of Ventricular Enlargement After Premature Birth
Automated lateral ventricle volumetry was successful in 186 of 206 cases and showed increased volumes for premature-born individuals (VLBW/VP: 21.5 ± 13.9 ml; FT: 14.1 ± 6.3 ml; p < 0.001). The LVV was also higher in premature-born adults if sides were considered separately (left LVV: VLBW/VP: 11.3 ± 8.4 ml; FT: 7.3 ± 3.4 ml; p < 0.001; right LVV: 10.1 ± 5.9 ml; FT: 6.8 ± 3.3 ml; p < 0.001) and if LVV was divided by total intracranial volume (TIV) (LVV/TIV ratio: VLBW/VP: 1.3 ± 0.8%; FT: 0.8 ± 0.4%; p < 0.001). In VLBW/VP individuals, LVV of 22 out of 87 (25.3%) were 2 standard deviations above the mean LVV in controls normalized to TIV and thus considered abnormal. The rate of abnormal LVV was 5.1% (5 out of 99) for FT individuals (p < 0.001). Premature birth was associated with an increased risk of adult ventricular enlargement (OR: 6.25, 95% CI: 2.25–17.35), while sex was not a significant predictor of ventricular enlargement (OR: 1.08, 95% CI: 0.46–2.56). Binary logistic regression analyses in prematurely born adults revealed that both GA at birth and presence of PVH at birth were significant predictors of abnormal adult LV enlargement (GA: OR: 0.71, 95% CI: 0.51–0.98; presence of ICH: OR: 0.26, 95% CI: 0.07–0.92), while BW and sex were not significant (BW: OR: 1.00, 95% CI: 0.999–1.002; sex: OR: 1.31, 95% CI: 0.43–4.00). In prematurely born individuals who were diagnosed with PVH during the neonatal period, grading of PVH neither predicted absolute adult LVV (OR: −0.32, p = 0.267) nor abnormal LVV (OR: 0.18, 95% CI = 0.2–1.46).
Subjective classification of LVV was abnormal in 25 out of 100 (25.0%) prematurely born individuals and in 2 out of 106 (1.9%) control individuals for reader 1 and in 16 out of 100 (16.0%) prematurely born individuals and in 2 out of 106 (1.9%) control individuals for reader 2. Cross-table analysis with the volumetric definition of abnormal LVV revealed a sensitivity and specificity of 55.6% and 93.8% and a positive and negative predictive value of 0.60 and 0.93, respectively for reader 1 and a sensitivity and specificity of 44.4% and 96.9% and a positive and negative predictive value of 0.71 and 0.91, respectively for reader 2. Interrater reliability was moderate (κ = 0.578; p < 0.001).
The ROC analysis revealed AUC of 0.928 (p < 0.001) and 0.880 (p < 0.001) for FOHR and EI, respectively. Youden’s J was calculated to identify the optimal cut-off for identifying abnormal LVV for both measures, leading to a value of 0.37 for FOHR and 0.27 for EI. These values were associated with sensitivities of 96.3% and 77.8% and specificities of 73.8% and 81.9% for FOHR and EI, respectively. The ROC curve is shown in Fig. 2.
Fig. 2.
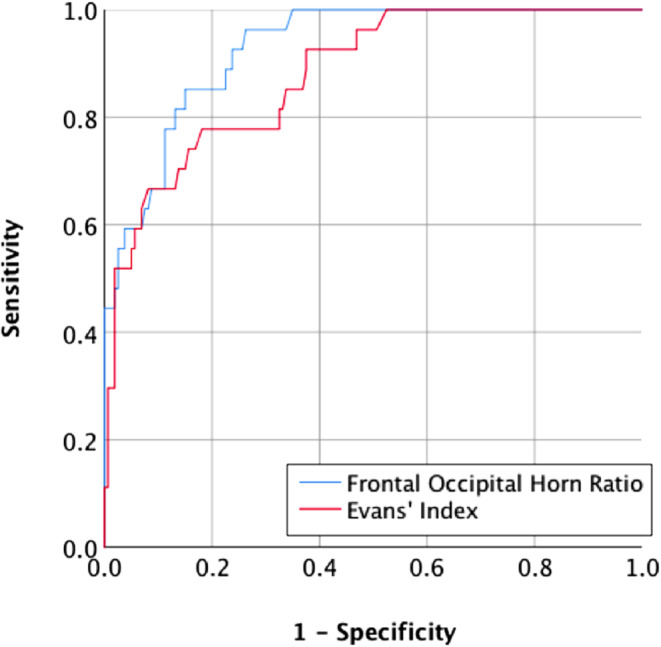
Accuracy of two-dimensional measurements of ventricular width. Receiver operating characteristics analysis showing the accuracy of Frontal Occipital Horn Ratio and Evans’ Index for classification of abnormal lateral ventricle volume
Prematurity is Associated with Deep White Matter Lesions on FLAIR Imaging in Adulthood
The FLAIR lesions were counted by two board-certified radiologists independently. Intraclass correlation coefficient (ICC) showed good to excellent agreement [34] (periventricular FLAIR lesions ICC = 0.945, 95% CI = 0.928–0.958; deep white matter FLAIR lesions ICC = 0.878, 95% CI =0.840–0.908; subcortical flair lesions ICC = 0.845, 95% CI = 0.796–0.882). All statistical analyses performed led to the same significant or insignificant results for reader 1 and 2. In the following, detailed numbers of the more experienced reader 1 are shown for reasons of simplicity and readability. A total of 115 individuals (55.8%) were positive for at least one FLAIR hyperintense WML in any location (VP/VLBW: 60/FT: 55; p = 0.259), 31 individuals (15.0%) were positive for periventricular FLAIR lesions (VP/VLBW: 24/FT: 7; p = 0.001), 49 individuals (23.8%) were positive for deep white matter FLAIR lesions (VP/VLBW: 33/FT: 16; p = 0.003) and 84 individuals (40.8%) were positive for subcortical FLAIR lesions (VP/VLBW: 35/FT: 49; p = 0.103). Detailed information on the distribution of periventricular, deep white matter, and subcortical FLAIR lesions can be found in Table 2, examples are shown in Fig. 1.
Table 2.
Localization patterns of hyperintense FLAIR lesions in prematurely born and term-born adults
| Localization | VP/VLBW | FT | p | ||||
|---|---|---|---|---|---|---|---|
| n | Median | IQR | n | Median | IQR | ||
| Periventricular | 24 | 2.0 | [1.0–4.0] | 7 | 1.0 | [1.0–2.0] | 0.053 |
| DWM | 33 | 4.0 | [2.0–6.5] | 16 | 1 | [1.0–2.0] | 0.004 |
| Subcortical | 35 | 3.0 | [1.0–9.0] | 49 | 2.0 | [1.0–5.0] | 0.447 |
Group differences of lesion numbers per localization were tested by Mann-Whitney U‑test. Cases without lesions in one of the three categories were not analyzed
DWM deep white matter, FT full-term, VP/VLBW very preterm and/or very low birthweight, IQR interquartile range
Miscellaneous Incidental Findings
Pineal cysts >10 mm, with or without solid components needing follow-up examinations were found in 10 individuals (4.9%; 4 VP/VLBW; 6 FT); Tornwaldt cysts were seen in 4 cases (1.9%; 1 VP/VLBW; 3 FT) and 2 individuals showed a pituitary intermediate lobe cyst (1.0%; 1 VP/VLBW; 1 FT). Arachnoidal cysts were seen in two FT subjects in retrocerebellar and anterior temporal pole localizations. Partially empty sella was diagnosed in three subjects (1.4%; 1 VP/VLBW, 2 FT). One FT subject showed a cerebellar developmental venous anomaly. One FT subject was diagnosed with megacisterna magna. Ecchordosis physaliphora was diagnosed in one VP/VLBW patient. One VP/VLBW individual showed widening of the cerebellar foliae consistent with cerebellar hypoplasia without involvement of the vermis. One VP/VLBW subject was diagnosed with a Chiari I malformation and associated long-segment syringomyelia of the cervical spine.
Suspicious FLAIR hyperintense mass lesions were found in three subjects (1.4%; 2 VP/VLBW, 1 FT) located in the right and left pulvinar, and the left parietal lobe. One FT patient showed a 9 mm meningioma adjacent to the left occipital pole. A 9 mm subependymal mass in the lateral ventricle found in one VP/VLBW subject, suggesting disturbed neuronal migration.
Discussion
This study qualitatively analyzed incidental findings in a large cohort of prematurely born adults and found increased rates of ventricular enlargement as well as an increased number of FLAIR hyperintense white matter lesions specifically located in the deep white matter. Significant predictors for abnormal lateral ventricle enlargement were low gestational age and history of periventricular hemorrhage in the neonatal period.
A previous study on a rather small subset of the present cohort investigated how well the neuroradiologist can distinguish between prematurely born adults and adults born at full-term [35]. They concluded that the discriminative power of visual inspection alone is suboptimal and can be increased by volumetric measurements. On the contrary, the present article aims at identifying characteristic incidental findings after premature birth in order to aid the radiologist at the correct classification of these imaging signs.
Increased Lateral Ventricle Volume After Premature Birth
Ventriculomegaly is a mostly idiopathic and unspecific neuroradiological imaging finding potentially indicating either hydrocephalus or parenchymal atrophy. These two etiologies can be reliably distinguished by midsagittal morphological changes such as stretching and displacement of the corpus callosum, widening of the third ventricular recessus, and decreased mamillopontine distance [36]. We confirmed the expected low rate of ventricular enlargement in control individuals born at full-term; however, one out of four prematurely born adults exhibited enlargement of the LV, making this a common incidental finding after prematurity. In line with previous studies from prematurely born infants and adolescents, we demonstrated higher mean LVV in prematurely born adults [37, 38]. Larger LVV was significantly predicted by lower GA and incidence of PVH in the neonatal period, suggesting a parenchymal white matter deficit caused by either white matter dysmaturation and/or direct damage by ICH. Comparing three approaches regarding the evaluation of LV size in young adults, we have identified the FOHR as the most accurate measure being feasible in clinical routine [30]. This superior accuracy of the FOHR is most likely caused by occipital horn dilatation in prematurely born adults which was described previously [39].
Increased FLAIR Hyperintense WMLs After Premature Birth in the Deep White Matter
Premature birth has been found to have an impact on the white matter either by causing direct damage and tissue necrosis or by inhibiting the development of pre-oligodendrocytes and subsequent altered axonal maturation [2, 10, 11]. This may result in visible or invisible alterations of the white matter on MRI ranging from cystic degeneration and parenchymal deficit to PWML and normal appearing white matter [10, 11]. The PWML are a common finding on brain MRI and are associated with increasing age and cardiovascular risk factors [40–42]. The presumed histopathological correlate of these PWML is astrogliosis following minor ischemia [43]. In the context of preterm birth, FLAIR hyperintense PWML reflect moderate WMI and have been described as a prognostic marker for adverse mental and psychomotor development in prematurely born infants at term-equivalent age [12]. One of the main findings of the study is that the total number of PWML is not increased in VP/VLBW adults; however, prematurely born adults were more likely to exhibit PWML in periventricular localization and in the deep white matter. This might indicate a pattern of prematurity affecting distinct white matter regions and reflect different underlying pathophysiological principles. Of course, besides particular vulnerability of the premature white matter towards hypoxic/ischemic events in the context of premature birth, other causes, such as infectious disease might lead to PWMLs visible on FLAIR imaging; however, the impact of severe CNS infections on the appearance of PWMLs can be considered small since a history of neurologic disease (including meningitis and encephalitis) served as exclusion criterion for MRI participation.
Miscellaneous Findings in the Context of Premature Birth.
Despite the rather sizable study cohort, its size is too small to deduct frequencies of rare incidental findings. One prematurely born individual exhibited a subependymal remnant of neuronal tissue. This is likely related to premature birth, since the neuronal migration period extends well into the third trimester and thus coincides with preterm birth [44]. We observed overt cerebellar hypoplasia in one preterm individual. The cerebellum is known to be vulnerable in preterm neonates and impaired growth after premature birth has been previously shown [9]. Other incidental findings in the VP/VLBW cohort such as ecchordosis physaliphora or Chiari I malformation are very unlikely to be related to premature birth [45].
Practical Considerations for the Radiologist
We have described increased rates of incidental findings on routine MRI after premature birth and think that this could have implications for radiological reporting in two distinct scenarios. Either the radiologist knows that a patient was born prematurely or it is unknown. If the radiologist knows, ventricular enlargement or increased periventricular or DWM punctate white matter lesion load can be interpreted as long-term sequelae of preterm birth and alternative explanations such as hypertension and/or chronic inflammatory CNS disease become less likely. If the radiologist is not aware of the patient’s birth history (which will be the case most of the times), findings such as ventricular enlargement or a certain distribution of PWMLs in periventricular or deep white matter locations should prompt thorough history taking before making a diagnosis.
Strengths and Limitations
Some points should be carefully considered when interpreting the results. The current sample is biased to VP/VLBW adults with less severe neonatal complications. Individuals with more severe birth complications and/or severe lasting impairments in the initial BLS sample were more likely to drop out of this study covering more than two and a half decades from the neonatal period until adulthood and to be excluded in initial screening for MRI due to exclusion criteria for MRI. This selection towards less serious cases should be kept in mind when interpreting the results.
Unfortunately, no structured physical examinations were performed at the 26-year follow-up visit, so that direct information about clinically evident findings is limited; however, due to the previously mentioned selection bias towards less severe cases and through the exclusion criteria for MRI participation as mentioned in the Material and Methods section (e.g. cerebral palsy, history of neurologic disease or CNS trauma), it seems justified that the analyzed imaging features in fact constitute incidental findings.
Unfortunately, no sequence to detect blood degradation products (T2* weighted or susceptibility weighted imaging) was included in the study protocol owing to the overall study design. We consider it a strength of our study that a relevant impact of patient age on MRI findings at the time of the scan is excluded because all participants had approximately the same age of 26 years.
Conclusion
Structural brain abnormalities visible on routine brain MRI sequences are commonly found in prematurely born adults. Prematurity at birth, in particular low GA and history of PVH is associated with ventricular enlargement, which could be best assessed by measuring the FOHR. The total number of PWML was not increased in prematurely born adults, however, the increased rate of deep white matter PWML suggested spatially distinct vulnerability of the white matter after premature birth.
Caption Electronic Supplementary Material
Flow chart diagram of study participants and MRI sequence parameter settings.
Abbreviations
- AUC
Area under the curve
- BW
Birth weight
- CI
Confidence interval
- EI
Evans’ index
- FLAIR
Fluid-attenuated inversion recovery
- FOHR
Frontal-occipital-horn ratio
- FT
Full term
- GA
Gestational age
- ICC
Intraclass correlation coefficient
- ICH
Intracranial hemorrhage
- LV
Lateral ventricle
- LVV
Lateral ventricle volume
- OR
Odds ratio
- PVH
Periventricular hemorrhage
- PVL
Periventricular leukomalacia
- PWML
Punctate white matter lesions
- SNR
Signal-to-noise ratio
- TIV
Total intracranial volume
- VLBW
Very low birth weight
- VP
Very preterm
- WMI
White matter injury
- WML
White matter lesion
Funding
Open Access funding provided by Projekt DEAL.
Conflict of interest
D.M. Hedderich, T. Boeckh-Behrens, J.G. Bäuml, A. Menegaux, M. Daamen, C. Zimmer, P. Bartmann, L. Scheef, H. Boecker, D. Wolke, C. Sorg and J.E. Spiro declare that they have no competing interests.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howson CP, Kinney MV, McDougall L, Lawn JE. Born too soon: preterm birth matters. Reprod Health. 2013;10(Suppl 1):S1. doi: 10.1186/1742-4755-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 5.Nosarti C, Reichenberg A, Murray RM, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69(6):1–8. doi: 10.1001/archgenpsychiatry.2011.1374. [DOI] [PubMed] [Google Scholar]
- 6.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry. 2013;70(11):1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 8.Patel RM, Rysavy MA, Bell EF, Tyson JE. Survival of infants born at periviable gestational ages. Clin Perinatol. 2017;44(2):287–303. doi: 10.1016/j.clp.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Back SA, Miller SP. Brain injury in premature neonates: A primary cerebral dysmaturation disorder? Ann Neurol. 2014;75(4):469–486. doi: 10.1002/ana.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volpe JJ. Confusions in nomenclature: “periventricular Leukomalacia” and “white matter injury”—identical, distinct, or overlapping? Pediatr Neurol. 2017;73:3–6. doi: 10.1016/j.pediatrneurol.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 11.van Tilborg E, Heijnen CJ, Benders MJ, et al. Impaired oligodendrocyte maturation in preterm infants: potential therapeutic targets. Prog Neurobiol. 2016;136:28–49. doi: 10.1016/j.pneurobio.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 12.de Bruïne FT, van den Berg-Huysmans AA, Leijser LM, et al. Clinical implications of MR imaging findings in the white matter in very Preterm infants: a 2-year follow-up study. Radiology. 2011;261(3):899–906. doi: 10.1148/radiol.11110797. [DOI] [PubMed] [Google Scholar]
- 13.Morel B, Antoni G, Teglas JP, Bloch I, Adamsbaum C. Neonatal brain MRI: how reliable is the radiologist’s eye? Neuroradiology. 2016;58(2):189–193. doi: 10.1007/s00234-015-1609-2. [DOI] [PubMed] [Google Scholar]
- 14.Dean JM, McClendon E, Hansen K, et al. Prenatal cerebral Ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5(168):1–22. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball G, Srinivasan L, Aljabar P, et al. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci. 2013;110(23):9541–9546. doi: 10.1073/pnas.1301652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng C, Bäuml JG, Daamen M, et al. Extensive and interrelated subcortical white and gray matter alterations in preterm-born adults. Brain Struct Funct. 2016;221(4):2109–2121. doi: 10.1007/s00429-015-1032-9. [DOI] [PubMed] [Google Scholar]
- 17.McClendon E, Shaver DC, Degener-O’Brien K, et al. Transient hypoxemia chronically disrupts maturation of preterm fetal ovine subplate neuron arborization and activity. J Neurosci. 2017;37(49):11912–11929. doi: 10.1523/JNEUROSCI.2396-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riegel K, Orth B, Wolke D, Österlund K. Die Entwicklung gefährdet geborener Kinder bis zum 5 Lebensjahr. Stuttgart: Thieme; 1995. [Google Scholar]
- 19.Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian Longitudinal Study. Dev Med Child Neurol. 1999;41(2):94–109. doi: 10.1017/S0012162299000201. [DOI] [PubMed] [Google Scholar]
- 20.Jurcoane A, Daamen M, Scheef L, et al. White matter alterations of the corticospinal tract in adults born very preterm and/or with very low birth weight. Hum Brain Mapp. 2016;37(1):289–299. doi: 10.1002/hbm.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daamen M, Bäuml JG, Scheef L, et al. Working memory in preterm-born adults : load-dependent compensatory activity of the posterior default mode network. Hum Brain Mapp. 2015;36:1121–1137. doi: 10.1002/hbm.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedderich DM, Bäuml JG, Berndt MT, et al. Aberrant gyrification contributes to the link between gestational age and adult IQ after premature birth. Brain. 2019 doi: 10.1093/brain/awz071.. [DOI] [PubMed] [Google Scholar]
- 23.Shang J, Bäuml JG, Koutsouleris N, et al. Decreased BOLD fluctuations in lateral temporal cortices of premature born adults. Hum Brain Mapp. 2018;39(12):4903–4912. doi: 10.1002/hbm.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grothe MJ, Scheef L, Bauml J, et al. Reduced cholinergic basal forebrain integrity links neonatal complications and adult cognitive deficits after premature birth. Biol Psychiatry. 2017;82(2):119–126. doi: 10.1016/j.biopsych.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Bauml JG, Daamen M, Meng C, et al. Correspondence between aberrant intrinsic network connectivity and gray-matter volume in the ventral brain of Preterm born adults. Cereb Cortex. 2015;25(11):4135–4145. doi: 10.1093/cercor/bhu133. [DOI] [PubMed] [Google Scholar]
- 26.Daamen M, Bäuml JG, Scheef L, et al. Neural correlates of executive attention in adults born very preterm. Neuroimage Clin. 2015;9:581–591h. doi: 10.1016/j.nicl.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77(1):1–10. doi: 10.1016/S0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- 28.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 29.Busse RF, Brau ACS, Vu A, et al. Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med. 2008;60(3):640–649. doi: 10.1002/mrm.21680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Hayon BB, Drake JM, Ossip MG, Tuli S, Clarke M. Frontal and occipital horn ratio : a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1999;8:245–249. doi: 10.1159/000028730. [DOI] [PubMed] [Google Scholar]
- 31.Evans WA., Jr. An encephalographic ratio for estimating the size of the cerebral ventricles: further experience with serial observations. Am J Dis Child. 1942;64(5):820–830. doi: 10.1001/archpedi.1942.02010110052006. [DOI] [Google Scholar]
- 32.Brix MK, Westman E, Simmons A, et al. The Evans’ Index revisited: New cut-off levels for use in radiological assessment of ventricular enlargement in the elderly. Eur J Radiol. 2017;95:28–32. doi: 10.1016/j.ejrad.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- 34.Koo TK, Li MY. A guideline of selecting and reporting Intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurcoane A, Daamen M, Keil VC, et al. Automated quantitative evaluation of brain MRI may be more accurate for discriminating preterm born adults. Eur Radiol. 2019 doi: 10.1007/s00330-019-06099-7. [DOI] [PubMed] [Google Scholar]
- 36.Segev Y, Metser U, Beni-Adani L, Elran C, Reider-Groswasser, Constantini S. Morphometric study of the midsagittal MR imaging plane in cases of hydrocephalus and atrophy and in normal brains. Am J Neuroradiol. 2001;22(9):1674–1679. [PMC free article] [PubMed] [Google Scholar]
- 37.Maunu J, Lehtonen L, Lapinleimu H, et al. Ventricular dilatation in relation to outcome at 2 years of age in very preterm infants: a prospective Finnish cohort study. Dev Med Child Neurol. 2011;53(1):48–54. doi: 10.1111/j.1469-8749.2010.03785.x. [DOI] [PubMed] [Google Scholar]
- 38.Nosarti C, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125(7):1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 39.Aukland SM, Elgen IB, Odberg MD, Chong WK, Eide GE, Rosendahl K. Ventricular dilatation in ex-prematures: Only confined to the occipital regionmri-based normative standards for 19-year-old ex-prematures without major handicaps. Acta Radiol. 2014;55(4):470–477. doi: 10.1177/0284185113497476. [DOI] [PubMed] [Google Scholar]
- 40.Habes M, Erus G, Toledo JB, et al. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain. 2016;139(Pt 4):1164–1179. doi: 10.1093/brain/aww008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Habes M, Sotiras A, Erus G, et al. White matter lesions: Spatial heterogeneity, links to risk factors, cognition, genetics, and atrophy. Neurology. 2018;91(10):e964–e975. doi: 10.1212/WNL.0000000000006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12(5):483–497. doi: 10.1016/S1474-4422(13)70060-7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medrano Martorell S, Cuadrado Blázquez M, García Figueredo D, González Ortiz S, Capellades Font J. Hyperintense punctiform images in the white matter: a diagnostic approach. Radiologia. 2012;54(4):321–335. doi: 10.1016/j.rx.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Kostovic I, Sedmak G, Judas M. Neural histology and neurogenesis of the human fetal and infant brain. Neuroimage. 2018;188:743–773. doi: 10.1016/j.neuroimage.2018.12.043. [DOI] [PubMed] [Google Scholar]
- 45.Shoja MM, Johal J, Oakes WJ, Tubbs RS. Embryology and pathophysiology of the Chiari I and II malformations: A comprehensive review. Clin Anat. 2018;31(2):202–215. doi: 10.1002/ca.22939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart diagram of study participants and MRI sequence parameter settings.